Abstract
Focal adhesion kinase (FAK) is a nonreceptor tyrosine kinase that plays an important role in integrin-mediated signal transduction. To explore the role and mechanisms of FAK in cardiac development, we inactivated FAK in embryonic cardiomyocytes by crossing the floxed FAK mice with myosin light chain-2a (MLC2a) Cre mice, which expressed Cre as early as embryonic day 9.5 in the heart. The majority of conditional FAK knockout mice generated from MLC2a-Cre (CFKO-2a) died in the embryonic stage with thin ventricular wall and ventricular septal defects. A small fraction of CFKO-2a mice survived to adulthood with spontaneous eccentric right ventricle hypertrophy. Transmission electron microscopy analysis displayed swelling in the rough endoplasmic reticulum in CFKO-2a embryonic cardiomyocytes. We found that decreased cell proliferation, but not increased cell apoptosis or differentiation, is the reason for the thin ventricular wall in CFKO-2a mice. Microarray analysis suggests that myocyte enhancer factor 2a (MEF2a) can be regulated by FAK and that inactivation of FAK in the embryonic heart compromised MEF2a expression. Last, we found that Src, but not PI3K, is important in mediating signal transduction for the regulation of MEF2a by FAK. Together, these results identified the role and mechanisms of FAK in embryonic cardiac development.
Keywords: cardiac development, signal transduction
The heart is the first formed organ to begin functioning in a developing vertebrate embryo. Abnormalities of heart development induce congenital heart diseases, which are the most frequent form of birth defects in humans. Heart development and maturation depend on the interactions of integrins in cardiomyocyte with its surrounding extracellular matrix (ECM) (1). Integrins, which are cell surface receptors that mediate cellular adhesion to the ECM, are composed of noncovalently linked α- and β-subunits. β1 integrin knockout mice resulted in gastrulation defects and death by embryonic day 5.5 (E5.5) (2, 3). Chimeric mice constructed with a β1 integrin-null allele showed delayed development and differentiation of cardiac lineage, as well as abnormal sarcomergenesis of these cardiac-like cells (4). When β1 integrin was inactivated exclusively in ventricular cardiac myocytes, it resulted in myocardial fibrosis and cardiac failure (5). It is well established that focal adhesion kinase (FAK) is a key downstream kinase in the ECM–integrin signal transduction pathway (6–9). Therefore, these data suggest that FAK may play essential roles in heart development.
Previous studies have shown that FAK gene inactivation in mice resulted in an embryonic lethal phenotype with major defects in the axial mesodermal tissues and cardiovascular system. Neither a normal heart nor fully developed blood vessels were present in the FAK-null embryos (10, 11). Despite the abundant knowledge of FAK interaction with other proteins and its roles in cell signaling in vitro, still relatively little is known about the in vivo functions of FAK in embryonic development or in the adult organisms. In particular, the embryonic lethal phenotype of the FAK-null mice has limited its use for studies on the interesting questions of the roles and mechanisms of FAK in embryonic development and its functions in the adult. To overcome this problem, we and others have created floxed FAK (FAKflox/flox) mice with the FAK gene flanked by two loxP sites (12–14). In our previous study, we generated cardiomyocyte-specific FAK knockout mice and showed the important role of FAK in eccentric cardiac hypertrophy (15). However, because of the low FAK deletion efficiency in embryonic heart of these mice, the role of FAK in cardiac development remains unknown.
To decipher the role of FAK signaling in heart development, we used a mouse line expressing Cre under the control of the myosin light chain 2a promoter (MLC2a-Cre). Our results showed that inactivation of FAK in embryonic heart resulted in an embryonic lethal phenotype with thin ventricular walls and ventricular septal defects (VSD). Surviving knockout mice displayed spontaneous right ventricular hypertrophy, and this phenotype is related to the down-regulation of myocyte enhancer factor 2a (MEF2a)-mediated signal transduction.
Results
Cardiac-Restricted Deletion of FAK in Embryonic Development.
To determine the role of FAK in cardiac development, we first analyzed the temporal and spatial pattern of Cre activity in MLC2a-Cre (16) and MLC2vKICre (15) mice by crossing them with R26RstoplacZ mice (17). Analysis of the embryos at various gestations by X-gal staining showed a higher recombination efficiency of MLC2a-Cre than that of the MLC2vKICre in embryonic heart [supporting information (SI) Fig. S1 A–F]. We next crossed the MLC2a-Cre transgenic mice with the floxed FAK mice to obtain four types of mice: FAKflox/+:Cre−, FAKflox/+:Cre+ (designated as Cre), FAKflox/flox:Cre− (designated as control), and FAKflox/flox:Cre+ (designated as CFKO-2a for conditional FAK knockout mice from MLC2a-Cre). Screening of 351 pups showed that CFKO-2a mice were born but were underrepresented at weaning (6% of littermates rather than 25% as expected), suggesting that the majority of CFKO-2a mice died in the embryonic stage (Table S1). Analyses by immunofluorescence staining, Western blotting, and Southern blotting showed efficient and specific deletion of the FAK gene in the hearts of both CFKO-2a embryos and surviving adults compared with controls (Fig. S1 G–M).
Analysis of Cardiac Defects in CFKO-2a Embryo.
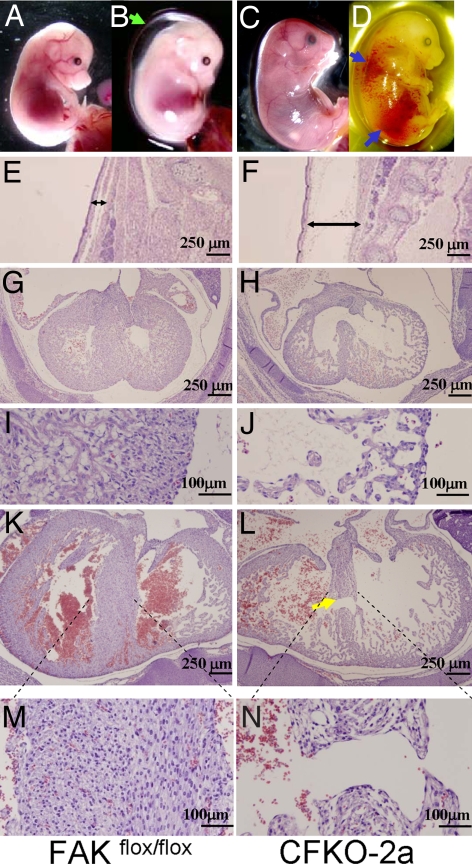
Because the majority of CFKO-2a mice died in the uterus, we harvested embryos at various ages between E11.5 and E18.5 to determine at which stage the CFKO-2a embryos died. Whole-mount analysis showed that the majority (90%) of CFKO-2a embryos appeared normal in early development stages, with no detectable external abnormality up to E13.5 (Table S1). CFKO-2a embryos were still viable at E14.5, as evidenced by cardiac pulsations and spontaneous movement. Approximately 60% of the CFKO-2a embryos at E14.5 or later exhibited progressive total body edema and nonspecific focal hemorrhages, which were associated with functional cardiac failure (Fig. 1 B and D). No visible defects were observed in E14.5 and E16.5 control embryos (Fig. 1 A and C). Histological analysis was performed on CFKO-2a and its littermate control embryonic hearts at E13.5, E14.5, and E16.5. Embryos were cut in the transverse plane and examined in serial sections from the cephalic to the caudal aspects of the specimens. At E13.5, the formation of the cardiac chambers was correct, and the compact as well as the trabecular zone of the heart appeared normal in the CFKO-2a embryo (data not shown). The histological sections of mutant embryos after E14.5 revealed marked edema in whole embryo, especially on the back (Fig. 1F), whereas no obvious edema was revealed in the control embryos (Fig. 1E). The ventricular myocardium, septum, and trabeculae of the control embryo consisted of tightly packed cells (Fig. 1 G and I). On the contrary, the CFKO-2a ventricular wall was significantly thinner and contained only a small number of loosely packed cells in the compact and trabecular zone (Fig. 1 H and J). At E16.5, a small fraction of CFKO-2a embryos still appeared normal in whole-mount analysis. However, histological serial sections stained with H&E showed that three of four of these embryos displayed muscular VSD (Fig. 1 L and N). In these embryos, the CFKO-2a cardiac ventricular walls were slightly thinner than those of the control embryos (Fig. 1 K and M), and the abnormalities are not as severe as that of the CFKO-2a embryos, which showed external edema on back (data not shown). Together, these results suggested that the thin ventricular walls were responsible for the embryonic lethality in the majority of the CFKO-2a embryos.
Fig. 1.
Thin ventricular wall and VSD in CFKO-2a embryos. (A–D) Gross examination of whole embryos at E14.5 (A and B) and E16.5 (C and D) of control (A and C) and CFKO-2a (B and D) mice. The green and blue arrows mark the edema on the dorsum and hemorrhages, respectively, of the mutant embryos. (E and F) Histopathological sections of skin from the dorsum of E14.5 embryos. Note the distinctive expansion of the s.c. tissue in CFKO-2a embryo due to edema. (G–N) Histological transverse sections through the hearts from E14.5 (G–J) and E16.5 (K–N) embryos. Note the significantly reduced thickness of the compact zone and VSD (yellow arrow) in the mutant embryos.
Ultrastructural Defects in CFKO-2a Myocardium.
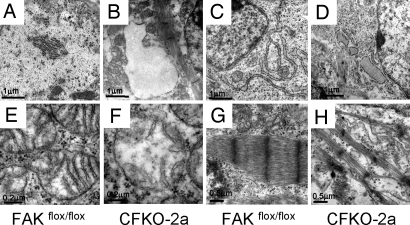
To further characterize the changes in CFKO-2a heart, electron microscopy analysis was carried out. The ventricular and atrial myocardium harvested from E14.5 CFKO-2a and control heart was examined. In the control atrial (Fig. 2A) and ventricular myocardium (Fig. 2C), rough endoplasmic reticulum (RER) showed orderly arrays of cisternae with small dense ribosomes attached to the membranes of the cisternae. However, in CFKO-2a atrial (Fig. 2B) and ventricular myocardium (Fig. 2D), the RER was greatly dilated, displacing the adjacent organelles with its empty lumen. The RER dilation was more dramatic in atrium than that of ventricle. In control ventricular myocardium, the mitochondrial cristae were regularly spaced and horizontally oriented (Fig. 2E). CFKO-2a ventricular mitochondria were affected and showed irregular spread and disrupted cristae with partially lucent matrix (Fig. 2F). The ventricular myofibrils of control heart exhibited an organized striation with clearly distinguishable Z lines (Fig. 2G). In contrast, the myofibrils were thinner and disorganized in CFKO-2a ventricular myocardium (Fig. 2H).
Fig. 2.
Electron microscopy analysis of the CFKO-2a heart. Myocardium was harvested from E14.5 control (A, C, E, and G) and CFKO-2a (B, D, F, and H) heart. Note the well preserved RER with dense ribosomes attached to the membranes of the cisternae in control atrial (A) and ventricular myocardium (C). The RER in CFKO-2a atrial (B) and ventricular myocardium (D) was dilated. Mitochondria have tightly packed, intact, horizontally oriented cristae in control ventricular myocardium (E), whereas in CFKO-2a ventricular myocardium the mitochondria showed irregular spread and disrupted cristae with lucent matrix (F). Myofibrils exhibited organized striation in control ventricular myocardium (G), whereas in CFKO-2a ventricular myocardium the myofibrils were thinner and disorganized (H).
Regulation of Cardiomyocyte Proliferation by FAK.
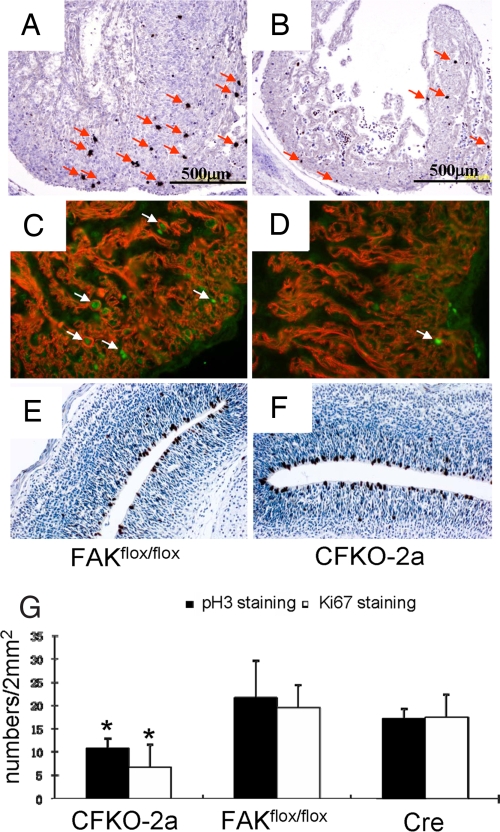
To investigate the causes of abnormality of heart development in CFKO-2a embryos, we tested whether reduced cell proliferation and/or enhanced apoptosis or differentiation contributed to the heart developmental defects. E14.5 embryo sections from both CFKO-2a and control mice were harvested and stained for the phosphorylated histone H3 (p-H3) to identify the mitotic cells. The number of p-H3-positive cardiac cells was reduced significantly in CFKO-2a embryos (Fig. 3B) compared with the controls (Fig. 3A). We also performed Ki67 and cardiac troponin T double immunofluorescence staining on E14.5 embryonic heart. The number of Ki67 and troponin T double-positive cells in CFKO-2a (Fig. 3D) was significantly decreased compared with the control (Fig. 3C), further confirming a role for FAK in embryonic cardiomyocyte proliferation. Quantitative analysis of the p-H3 and Ki67 labeling demonstrates an ≈50% decrease in the number of mitotic cells per unit area (2 mm2) in CFKO-2a embryos (Fig. 3G). To exclude the possibility that the decreased cardiomyocyte proliferation was simply a consequence of dying embryos due to cardiac failure, we performed p-H3 staining on cerebral cortex. Our results showed that the p-H3-positive cells in CFKO-2a cerebral cortex (Fig. 3F) were comparable to the control (Fig. 3E), suggesting that the decreased cardiomyocyte proliferation rate in CFKO-2a was due to the inactivation of FAK in cardiomyocyte. We also performed TUNEL and cleaved caspased-3 staining to determine whether an increased apoptosis also contributes to the thin ventricular wall. We found apoptotic cells in the valve formation zone and rarely in the compact zone. However, the relative apoptotic cell numbers are comparable between CFKO-2a and the control heart. Together, these results suggest that the reduced cardiomyocyte proliferation, but not differences in apoptosis, is the major reason for the thin ventricular wall in the CFKO-2a embryos.
Fig. 3.
Proliferation defects in CFKO-2a heart. (A and B) Comparable transverse heart sections from E14.5 control (A) and CFKO-2a (B) embryos were stained by p-H3 to mark the proliferating cells. (C and D) Immunofluorescence double staining of cardiac troponin T and Ki67 on E14.5 control (C) and CFKO-2a (D) hearts. (E and F) Immunohistological analysis of p-H3 on E14.5 control (E) and CFKO-2a (F) cerebral cortexes. (G) Quantitative analysis shows that the p-H3- and Ki67-positive cells per unit area were significantly decreased in CFKO-2a embryonic hearts compared with their flox FAK and Cre controls. *, P < 0.05.
In addition to functioning as an important regulator for cell proliferation and apoptosis, a recent report showed that inhibition of FAK functions in embryonic stem cells by overexpression of FAK-related non-kinase (FRNK) increased cardiomyocyte differentiation, suggesting a potential role of FAK in the regulation of cardiomyocyte differentiation (18). To determine whether FAK is essential for cardiomyocyte differentiation in vivo, we performed semiquantitative RT-PCR to examine the changes of cardiac differentiation markers. We found that cardiac transcription factors, including serum-response factor, Nkx2.5, GATA4, GATA5, GATA6, Hand 1, and Hand 2 were expressed at similar levels in the E14.5 embryonic hearts from CFKO-2a as those from control mice. Furthermore, the expression levels of contractile/cytoskeletal proteins such as α-myosin heavy chain, β-myosin heavy chain, myosin light chain-2a, and myosin light chain-2v were also comparable between CFKO-2a and the control (data not shown). These results suggested that inactivation of FAK in embryonic cardiomyocytes had no effect on cardiomyocyte differentiation in vivo.
Spontaneous Eccentric Right Ventricular Hypertrophy in Surviving CFKO-2a Mice.
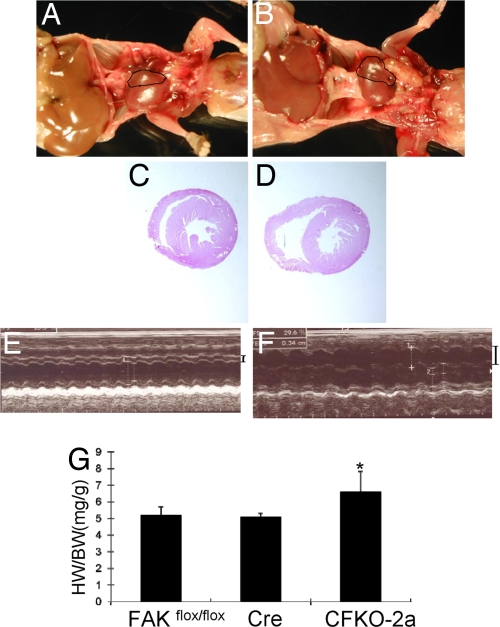
The small fraction of CFKO-2a mice that survived to adult stage allowed us to examine the influence of inactivation of FAK in embryonic heart on the cardiac growth and functions in adult mice. The live-born CFKO-2a mice are fertile and have a normal lifespan. However, autopsy revealed gross morphological differences in hearts between CFKO-2a mice and their control littermates (Fig. 4 A and B). CFKO-2a hearts were characterized by dramatically enlarged right ventricles, which were reproducibly observed in mice aged from 2 months to 10 months. H&E staining of cardiac transverse sections showed severe dilation of the right ventricle chamber in CFKO-2a mice compared with the control littermates (Fig. 4 C and D). H&E and trichrome-stained histological sections from 2- and 10-month-old mice did not reveal any other features typically associated with cardiomyopathies, such as myofiber hypertrophy, myocyte disarray, or interstitial fibrosis (data not shown). To further evaluate the morphology of mutant heart in vivo, echocardiography was performed on the CFKO-2a and control mice. Dilated right ventricles were observed in CFKO-2a mice (Fig. 4F and Table S2) compared with the normal control mice (Fig. 4E). Consistent with the histology and echocardiography results, the heart weight/body weight ratios were increased significantly in CFKO-2a mice (Fig. 4G). Taken together, these results suggested that inactivation of FAK in embryonic heart led to spontaneous eccentric right ventricular hypertrophy in the small fraction of surviving CFKO-2a mice.
Fig. 4.
Analysis of cardiac defects in the surviving CFKO-2a mice. (A and B) Gross examination of the whole heart in adult control (A) and CFKO-2a (B) mice. The right ventricle in CFKO-2a mice was significantly dilated. (C and D) Histological sections show obvious dilation in CFKO-2a (D) but not the control (C) mice. (E and F) Representative M-mode echocardiogram images of hearts of 12-week-old control (E) and CFKO-2a (F) mice. The right ventricle internal diameters are marked on the right. (G) Heart weight/body weight (HW/BW) ratios (mg/g) of 12-week-old CFKO-2a mice was increased compared with the floxed FAK control and Cre control, as indicated. *, P < 0.05.
Compromised MEF2a Expression in CFKO-2a Embryonic Heart.
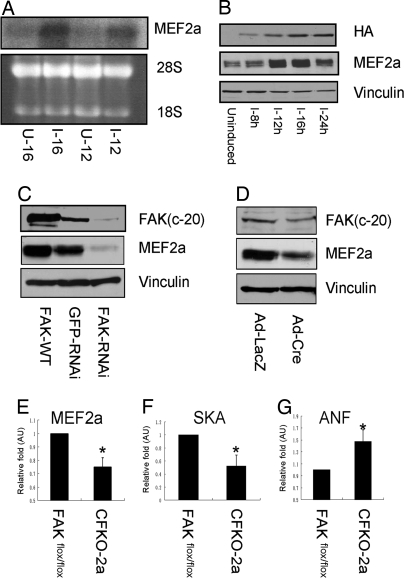
To identify potential gene targets regulated by FAK, we compared gene expression profiles by DNA microarray upon activation or disruption of the FAK signaling pathways. RNAs isolated from tet-off NIH 3T3 cells expressing the vectors alone (Mock cells) or wild-type FAK (FAK cells) were used for hybridization as described previously (19). Among the transcription factors that may play a role in heart development, MEF2a was found to be up-regulated in FAK cells compared with the control Mock cells. To verify the microarray results and provide independent evidence for MEF2a as a FAK target gene, we performed Northern blotting analysis to examine the effect of the overexpression of FAK on MEF2a. Total RNA was isolated from cultured FAK cells with or without tetracycline (removal of tetracycline and cultured for 12 or 16 h). In concordance with the microarray data, the MEF2a mRNA level was significantly increased after 12 h of FAK induction and further increased after 16 h of induction (Fig. 5A). Furthermore, up-regulation of MEF2a protein upon induction of FAK expression was confirmed by Western blotting analysis of lysates from the FAK cells (Fig. 5B). Together, these results identified MEF2a as a downstream target whose expression is up-regulated by FAK in fibroblasts.
Fig. 5.
Regulation of MEF2a by FAK. (A and B) FAK cells were incubated in media with (uninduced, U) or without (induced, I) 0.4 μg/ml tetracycline for various times as indicated. Total RNA or protein lysates were prepared and analyzed by Northern (A) or Western (B) blottings, respectively. Antibody against hemagglutin (HA) of influenza virus was used to measure the expression of exogenous HA-tagged FAK. (C) Lysates prepared from embryonic cardiomyocytes were infected with recombinant adenoviruses encoding FAK, GFP-RNAi, or FAK-RNAi as indicated. They were subjected to Western blotting with α-FAK, α-MEF2a, or α-vinculin antibodies. (D) Lysates prepared from FAKflox/flox embryonic cardiomyocytes were infected with recombinant adenoviruses encoding Cre and then were analyzed by Western blotting with various antibodies as indicated. (E–G) Total RNAs were extracted from E14.5 embryo control or CFKO-2a hearts. MEF2a (D), SKA (E), and atrial natriuretic factor (F) expression levels were examined by real-time RT-PCR and quantitated by comparative Ct method. The mean ± SD values of relative fold (normalized to the control heart) are shown in arbitrary units (AU). *, P < 0.05.
MEF2a has been shown to play an important role in myogenesis and cardiac development (20). Therefore, defective MEF2a expression may account for the cardiac defects observed in CFKO-2a embryos. To examine this possibility, we first examined whether MEF2a is regulated by FAK in cardiomyocytes. Embryonic cardiomyocytes were isolated from E14.5 embryos and then infected with recombinant adenoviruses encoding FAK (Ad-FAK), FAK siRNA (Ad-FAK-RNAi), or GFP siRNA (Ad-GFP-RNAi) as a control. Fig. 5C shows that overexpression of wild-type FAK increased the MEF2a expression whereas knockdown of FAK by FAK-RNAi decreased the MEF2a expression in cardiomyocytes. Next, we isolated FAKflox/flox embryonic cardiomyocytes and infected them with adenovirus-Cre to delete FAK. Consistent with results using siRNA and microarray, inactivation of FAK decreased MEF2a expression, providing further support that MEF2a is one downstream target of FAK (Fig. 5D). We next analyzed the expression of MEF2a as well as a number of target genes (21) regulated by MEF2a in CFKO-2a embryonic heart. Total RNAs were isolated from E14.5 embryonic hearts and subjected to real-time RT-PCR to quantify expression levels of MEF2a, α-skeletal actin (SKA), and atrial natriuretic factor (ANF). As shown in Fig. 5E, expression of MEF2a was significantly decreased in CFKO-2a hearts compared with the control hearts. Consistent with down-regulation of MEF2a, the expression level of SKA and ANF was down- and up-regulated, respectively, in CFKO-2a hearts (Fig. 5 F and G). Furthermore, we examined the influence of inactivation of FAK on other MEF2 family members on E14.5 CFKO-2a heart. Real-time PCR results showed that the deletion of FAK had no effect on MEF2b, MEF2c, and MEF2d transcription levels (data not shown). These results suggested that the compromised MEF2a signaling pathways may contribute to the defective cardiac development in CFKO-2a mice.
Regulation of MEF2a by FAK Depends on Src-Mediated Signal Transduction.
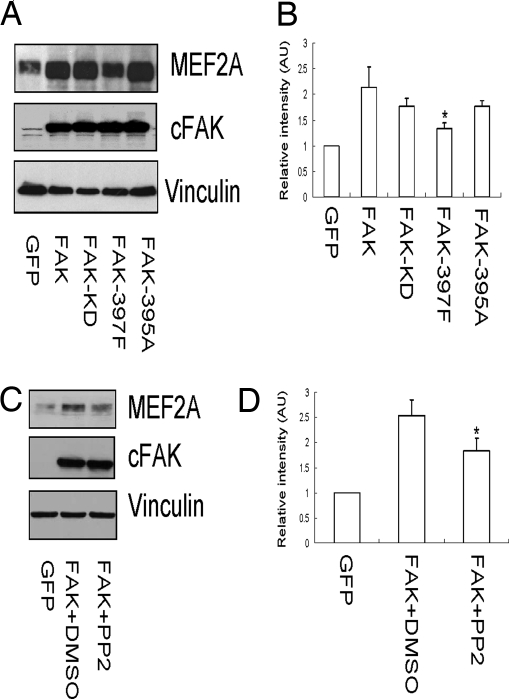
To explore how FAK regulates MEF2a expression, we generated recombinant adenoviruses encoding the kinase-defective FAK (Ad-KD), FAK Y397F (Ad-Y397F), and D395A (Ad-D395A) mutants and examined their effects on MEF2a in cardiomyocytes. Using an antibody that recognizes specifically the ectopically expressed chicken FAK, but not endogenous mouse FAK (22), we showed efficient and comparable levels of expression of the mutants upon adenovirus-mediated gene transfer into the cardiomyocyte (Fig. 6A Middle). As expected, wild-type FAK increased MEF2a expression in the cells (Fig. 6A Top). The Y397F mutant exhibited reduced ability to induce MEF2a expression, whereas the D395A and Ad-KD mutants showed activity similar to that of the wild-type FAK. Quantification and statistical analysis of MEF2a expression levels are shown in Fig. 6B. Previous studies have shown that Y397F mutant abolished FAK binding to both Src family kinases (23–26) and PI3K (27), whereas the D395A mutant disrupted only FAK binding to PI3K but retained its association with Src (28). Therefore, these results suggested that FAK association with Src, but not PI3K, was important in the regulation of MEF2a expression by FAK. Consistent with this, we found that treatment of cells with PP2 (an inhibitor for Src family kinases) reduced significantly the induction of MEF2a expression by FAK (Fig. 6C). Quantification data of MEF2a expression are shown in Fig. 6D. Taken together, these results suggested that FAK/Src complex formation and downstream signaling pathways play a critical role in the up-regulation of MEF2a by FAK.
Fig. 6.
Role of FAK/Src complex in regulation of MEF2a in cardiomyocytes. (A–D) Embryonic cardiomyocytes were infected with recombinant adenoviruses encoding GFP, FAK, or its mutants, as indicated (A). In C, the cells were treated with DMSO or PP2 as indicated. Lysates were prepared and analyzed by Western blotting using α-MEF2a, α-cFAK, or α-vinculin. The intensity of the bands was quantified from three independent experiments by densitometry. The mean± SD values of relative intensity (normalized to the cardiomyocyte infected with adenovirus encoding GFP) are shown in arbitrary units (AU) in B and D. *, P < 0.05 compared with FAK and FAK plus DMSO, respectively.
Discussion
There is increasing evidence of the importance of the integrin–FAK signaling pathway in cardiac development. Total deletion of FAK in the mouse embryo induces early embryonic lethality with mesoderm and cardiovascular development defects (10, 11). We have previously reported that postnatal deletion of FAK (using MLC2vKICre) did not affect cardiac morphology and functions under normal conditions in young mice (15). However, under challenged conditions, such as angiotensin II stimulation, transverse aortic constriction (TAC), and aging, inactivation of FAK promotes left ventricle eccentric hypertrophy. Because of the limitation of MLC2vKICre mice (in which FAK was not deleted in embryonic heart), these previous studies could not address the role and mechanisms of FAK in embryonic heart development. In the current study we took advantage of the MLC2a-Cre mice, which can delete FAK in embryonic heart, to show that inactivation of FAK in embryonic heart induced embryonic heart developmental defects and embryo lethality. A small proportion of CFKO-2a mice can survive to adulthood and show right ventricle eccentric hypertrophy at the age of 2 months (when CFKO-2v mice do not show any phenotype abnormality under the same conditions). Microarray and cell culture studies suggest that the deletion of FAK may compromise the MEF2a-mediated signal transduction and influence the right ventricle hypertrophy. Together, these results demonstrate that FAK plays a critical role in both embryonic development of the heart and cardiac hypertrophy in adult animals under stress conditions (Fig. S2).
The major reason for the thin ventricle wall and embryonic lethality in CFKO-2a mice, but not CFKO-2v mice, is likely due to the earlier and more efficient deletion of FAK by the MLC2a-Cre. A significant decrease in FAK expression level was found in E13.5 embryonic heart from CFKO-2a, but not MLC2vKICre. In addition, MLC2a-Cre expressed in both ventricles and atria, but MLC2vKICre expressed exclusively in ventricles. EM examination revealed that the deletion of FAK in embryonic heart resulted in defective atrial and ventricular myocardium of CFKO-2a mice. Therefore, disruption of atrial function in CFKO-2a may also contribute to the thin ventricle wall and embryonic lethality.
MEF2 transcriptional factors bind to A/T-rich DNA sequence, associate with most cardiac muscle structure genes, and have been shown to play an important role in the regulation of myogenesis and cardiac development (20). MEF2a is the predominant MEF2 gene product expressed in postnatal myocardium. Inactivation of MEF2a caused sudden death in most of the mice within the first week of life and displayed striking right ventricle dilation (21). In cultured neonatal rat ventricular myocytes, FAK plays an important role in stretch-induced MEF2 activation (29). Overexpression of MEF2a in cardiomyocytes induced dilated cardiomyopathy, suggesting that the amount of MEF2a in heart is critical for maintaining normal cardiac structure and function (30). In this report, we found that the MEF2a expression level can be regulated by FAK and was decreased in CFKO-2a embryonic heart. Interestingly, similar to MEF2a knockout mice, a small fraction of the CFKO-2a mice survived to adulthood, which also exhibited pronounced right ventricle dilation. Thus, it is possible that inactivation of FAK interfered with the MEF2a functions and subsequently caused right ventricle dilation. Taken together, our results suggested that MEF2a may be one of the important downstream targets of FAK signaling pathways in the regulation of embryonic heart development.
Hakim et al. (31) published an article very recently on the inactivation of FAK by NKX2.5-Cre in embryonic heart. Both the current study and the article by Hakim et al. showed that FAK is important for heart development and that inactivation of FAK resulted in VSD, leading to death of mice in the embryo or neonatal stage. However, there are some differences in structure and function between these two studies, such as time of death, severity of VSD, cardiomyocyte proliferation rate, and defects in right ventricle outflow. The most likely cause for these discrepancies is the different Cre mouse line (which has different Cre expression pattern and efficiency) used in these two studies. In the heart, it is possible that the MLC2a-Cre used in our study is stronger than NKX2.5-Cre, which would account for the observations that mice in our study died in the embryo stage whereas those in the Hakim et al. article (31) died in neonatal stage. In addition, it is known that NKX2.5-Cre not only expressed in heart but also expressed in neural crest cells, which are important for the development of right ventricle outflow and big vessel development (32, 33). In contrast, the MLC2a-Cre specifically expressed in cardiomyocytes (with only some leakage in embryonic tail part). Therefore, it is likely that the defective phenotype in the right ventricle outflow found by Hakim et al. (31) is induced by the inactivation of FAK not only in the cardiomyocytes, but also in neural crest cells. In addition, we noted that the mouse genetic backgrounds are different between those in our studies (a mixture of 129 and B6) and that of Hakim et al. (pure C57BL6). The mixed genetic background is likely the reason for the small amount of CFKO-2a mice to survive to adulthood and might also contribute to the differences in the phenotypes of the mice in these two studies.
Based on the current study and previous results (15), we suggest that FAK is a protecting factor for heart hypertrophy. In embryonic heart, the right ventricle is the major pump for circulation. However, after mice are born, the left ventricle becomes the dominant pump for the circulation (34). Because of the important function of right ventricle in embryonic development, it is not surprising that the inactivation of FAK in embryo heart induced spontaneous right ventricle hypertrophy as observed in the current study. However, the left ventricle is the dominant pump for circulation in adult mice, and stress conditions such as TAC are major challenges for the left ventricle in the adult mice. Therefore, it is possible that the differential roles of the right and left ventricles in the embryonic and adult hearts, respectively, could explain why knockout FAK in embryonic heart induces right ventricle hypertrophy, whereas deletion of FAK in adult heart causes left ventricle hypertrophy under the challenged conditions.
Materials and Methods
Generation and Identification of CFKO-2a Mice.
CFKO-2a mice were generated by crossing the floxed FAK mice with MLC2a-Cre mice (12, 16), and the genetic backgrounds of these mice are a mixture of 129 and B6 (see SI Materials and Methods for more details).
Southern, Northern, and Western Blots.
Southern, Northern and Western blots were performed essentially as described previously (15) (see SI Materials and Methods for more details).
RT-PCR and Real-Time PCR.
Total RNA was extracted from E14.5 embryonic hearts with TRIzol (Invitrogen) and purified with an RNeasy (Qiagen) kit as described previously (15). The RNAs were then reverse-transcribed to cDNA, amplified by PCR, and resolved on an agarose gel. For real-time PCR analysis, an equal amount of cDNA was mixed with Power SYBR Green PCR Master Mix according to the manufacturer's instructions, and real-time PCR was performed by using a 7500 Fast Real-Time PCR system (Applied Biosystems).
Morphological and Histological Analysis.
Embryos were harvested from time-crossed pregnant female mice, examined, and then photographed on a dissecting microscope using a camera (model DP70; Olympus). After taking the pictures, embryos were fixed in 4% paraformaldehyde overnight at 4°C and then submitted for paraffin wax embedding and sectioning following standard laboratory procedures. Tissue sections (4 μm thick) were stained with H&E, fibronectin (1:100), or cardiac troponin T (1:100) or stained by p-H3 (1:100).
Transthoracic Echocardiography.
Echocardiography was performed on control and CFKO-2a mice as described previously (15) (see SI Materials and Methods for more details).
Transmission Electronic Microscopy.
Transmission electronic microscopy analysis was performed as described previously (15).
Cardiomyocyte Isolation.
Time-crossed pregnant female mice were killed, and E14.5 or later embryonic hearts were harvested. Cardiomyocytes were then isolated from ventricles by trypsin digestion (see SI Materials and Methods for more details).
Generation and Infection of Recombinant Adenovirus.
Adenovirus encoding different FAK mutant has been described previously (12) (see SI Materials and Methods for more details).
Microarray.
Microarray analysis was performed by using standard protocols (see SI Materials and Methods for more details).
Statistical Analyses.
Data are presented as mean ± SD. Means were compared by Student's t test. P ≤ 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We are grateful to Laura Goodman of Cornell University for assistance with real-time PCR experiments. This research was supported by National Institutes of Health Grants HL73394 (to J.-L.G.) and HL064382 (to S.C.) and American Heart Association Scientist Development Grant 0735543T (to X.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802319105/DCSupplemental.
References
- 1.Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 2.Fassler R, et al. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephens LE, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 4.Fassler R, et al. Differentiation and integrity of cardiac muscle cells are impaired in the absence of beta 1 integrin. J Cell Sci. 1996;109:2989–2999. doi: 10.1242/jcs.109.13.2989. [DOI] [PubMed] [Google Scholar]
- 5.Shai SY, et al. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 6.Parsons JT. Focal adhesion kinase: The first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 7.Abbi S, Guan JL. Focal adhesion kinase: Protein interactions and cellular functions. Histol Histopathol. 2002;17:1163–1171. doi: 10.14670/HH-17.1163. [DOI] [PubMed] [Google Scholar]
- 8.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: In command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 9.Ilic D, Damsky CH, Yamamoto T. Focal adhesion kinase: At the crossroads of signal transduction. J Cell Sci. 1997;110:401–407. doi: 10.1242/jcs.110.4.401. [DOI] [PubMed] [Google Scholar]
- 10.Furuta Y, et al. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- 11.Ilic D, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 12.Shen TL, et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169:941–952. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beggs HE, et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLean GW, et al. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 2004;18:2998–3003. doi: 10.1101/gad.316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng X, et al. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wettschureck N, et al. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Galphaq/Galpha11 in cardiomyocytes. Nat Med. 2001;7:1236–1240. doi: 10.1038/nm1101-1236. [DOI] [PubMed] [Google Scholar]
- 17.Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakuno D, Takahashi T, Lammerding J, Lee RT. Focal adhesion kinase signaling regulates cardiogenesis of embryonic stem cells. J Biol Chem. 2005;280:39534–39544. doi: 10.1074/jbc.M505575200. [DOI] [PubMed] [Google Scholar]
- 19.Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 21.Naya FJ, et al. Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat Med. 2002;8:1303–1309. doi: 10.1038/nm789. [DOI] [PubMed] [Google Scholar]
- 22.Peng X, et al. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc Res. 2004;64:421–430. doi: 10.1016/j.cardiores.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Chan PY, Kanner SB, Whitney G, Aruffo A. A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125FAK. J Biol Chem. 1994;269:20567–20574. [PubMed] [Google Scholar]
- 24.Cobb BS, Schaller MD, Leu TH, Parsons JT. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol. 1994;14:147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaller MD, et al. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing Z, et al. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell. 1994;5:413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen HC, Guan JL. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiske HR, et al. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J Biol Chem. 1999;274:12361–12366. doi: 10.1074/jbc.274.18.12361. [DOI] [PubMed] [Google Scholar]
- 29.Nadruz W, Jr, et al. Focal adhesion kinase mediates MEF2 and c-Jun activation by stretch: Role in the activation of the cardiac hypertrophic genetic program. Cardiovasc Res. 2005;68:87–97. doi: 10.1016/j.cardiores.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, et al. Myocyte enhancer factors 2A and 2C induce dilated cardiomyopathy in transgenic mice. J Biol Chem. 2006;281:9152–9162. doi: 10.1074/jbc.M510217200. [DOI] [PubMed] [Google Scholar]
- 31.Hakim ZS, et al. Conditional deletion of focal adhesion kinase leads to defects in ventricular septation and outflow tract alignment. Mol Cell Biol. 2007;27:5352–5364. doi: 10.1128/MCB.00068-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilagan R, et al. Fgf8 is required for anterior heart field development. Development. 2006;133:2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- 33.Yelbuz TM, et al. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation. 2002;106:504–510. doi: 10.1161/01.cir.0000023044.44974.8a. [DOI] [PubMed] [Google Scholar]
- 34.Artman M, Mahoney L, Teitel DF. Neonatal Cardiology. 1st Ed. New York: McGraw-Hill; 2002. p. 45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.