Abstract
The short day lengths of late summer program the mosquito Culex pipiens to enter a reproductive diapause characterized by an arrest in ovarian development and the sequestration of huge fat reserves. We suggest that insulin signaling and FOXO (forkhead transcription factor), a downstream molecule in the insulin signaling pathway, mediate the diapause response. When we used RNAi to knock down expression of the insulin receptor in nondiapausing mosquitoes (those reared under long day lengths) the primary follicles were arrested in a stage comparable to diapause. The mosquitoes could be rescued from this developmental arrest with an application of juvenile hormone, an endocrine trigger known to terminate diapause in this species. When dsRNA directed against FOXO was injected into mosquitoes programmed for diapause (reared under short day lengths) fat storage was dramatically reduced and the mosquito's lifespan was shortened, results suggesting that a shutdown of insulin signaling prompts activation of the downstream gene FOXO, leading to the diapause phenotype. Thus, the results are consistent with a role for insulin signaling in the short-day response that ultimately leads to a cessation of juvenile hormone production. The similarity of this response to that observed in the diapause of Drosophila melanogaster and in dauer formation of Caenorhabditis elegans suggests a conserved mechanism regulating dormancy in insects and nematodes.
Keywords: forkhead transcription factor, insulin receptor, juvenile hormone
Culex pipiens, the mosquito that vectors West Nile virus in North America, overwinters in an adult diapause (dormancy) that is programmed by the short day length of autumn (1). In response to this environmental signal, females are not attracted to their avian hosts but instead seek sources of nectar used to generate the huge fat reserves that provide the energy source for winter survival (2, 3). Although females mate in the autumn before entering protected sites for overwintering, ovarian development is halted and does not resume until the females terminate diapause in the spring and seek a blood meal.
The endocrine basis for the diapause of C. pipiens, like that of other adult diapauses (4, 5), is a shutdown in the production of juvenile hormone (JH) by the corpora allata (6). This has been convincingly demonstrated by showing that an application of JH can terminate diapause in this species (6) and by the fact that removal of the corpora allata from a long-day mosquito (i.e., one not programmed for diapause) will halt reproduction and simulate a diapause-like state (7, 8). Yet we know little about the signaling pathway linking the clock mechanism that perceives day length to the ultimate endocrine signal regulating JH production.
This study tests the hypothesis that the insulin signaling pathway is a critical link in the regulation of mosquito diapause. Several previous studies suggest this possibility. Both the dauer state of nematodes, the dormancy equivalent of insect diapause, and the reproductive diapause of Drosophila melanogaster (9–11) appear to be mediated through the insulin pathway, and recent work with insulin signaling in mosquitoes (12–14) suggests that this pathway is critical for regulation of reproduction, a physiological feature that is key to a successful adult diapause. In this study, we evaluate the potential link between insulin signaling and the diapause of C. pipiens by focusing on genes encoding two components of this pathway: insulin receptor (InR), the receptor that mediates the insulin response, and FOXO (forkhead transcription factor), a factor that is normally suppressed in the presence of insulin (9, 15). When we use dsRNA to knock down expression of the gene encoding InR in adults reared under long day length (not programmed for diapause) we simulate the ovarian arrest of diapause, and we show that this arrest can be reversed by application of JH. Conversely, when we direct RNAi against FOXO in mosquitoes reared under short day length (programmed for diapause) the adults fail to stockpile the stores of fat normally associated with diapause, a result suggesting that expression of the gene encoding FOXO is essential for sequestering the lipids needed to fuel the overwintering period of dormancy. These lines of evidence thus point to a role for insulin signaling in the regulation of mosquito diapause and suggest that this pathway may be central to diverse forms of invertebrate dormancy.
Results
C. pipiens InR and FOXO.
The 324-bp cDNA fragment of the C. pipiens insulin receptor (cInR) shared highest identity (82%) with InR from a closely related mosquito, Aedes aegypti, and 76% and 71% identities to InR sequences from two other mosquitoes, Anopheles stephensi and Anopheles gambiae, respectively [supporting information (SI) Fig. S1]. The deduced amino acid InR sequence, based on a Pfam search, belongs to a family of tyrosine kinases (PF07714) with a predicted biological role in phosphorylation, a function essential for transducing the insulin signal to the insulin receptor substrate (16).
The 432-bp cDNA fragment of the C. pipiens forkhead transcription factor (cFOXO) shared 85% identity with FOXO from the mosquito Ae. aegypti and 87% and 73% identities to FOXO sequences from the honey bee Apis mellifera and the mosquito An. gambiae, respectively (Fig. S2). The deduced amino acid sequence is a member of a protein family of forkhead transcriptional factors (PD485564), with predicted biological roles as transcription factors and regulators of the insulin signaling pathway.
dsInR Halts Ovarian Development in Nondiapausing (ND) Females.
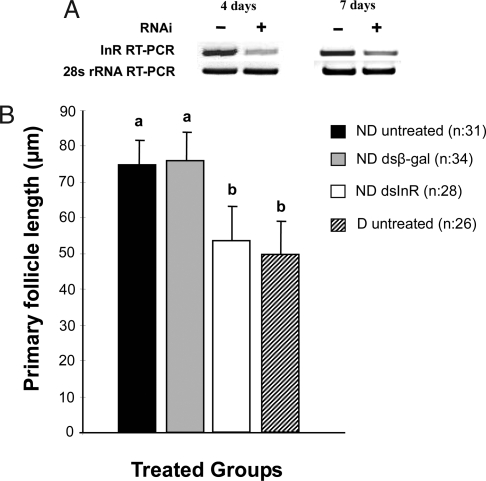
dsRNAi efficiency was first assessed by RT-PCR. In contrast to the relatively high induction of cInR in dsβ-gal-injected mosquitoes, only traces of cInR mRNA were detected in dsInR-injected ND mosquitoes, using RT-PCR and primers corresponding to the cInR gene (Fig. 1A), thus indicating that injection of dsInR successfully inhibited induction of the cInR gene. 28S ribosomal RNA (28S rRNA) was used as an internal control. RT-PCR analysis of 28S rRNA transcript levels at 4 and 8 days after injection detected no significant differences among the different treatments (Fig. 1A), thus indicating that the low expression levels observed for the cInR gene were related to the knockdown effect by dsRNAi rather than variation in sample loading.
Fig. 1.
RNA interference efficiency and the effect of dsInR on ovarian follicle length in C. pipiens. (A) cInR transcript levels in females injected with dsInR were reduced compared with the dsβ-gal controls. Expression levels were measured by RT-PCR 4 and 7 days after dsInR (RNAi) injection, using dsβ-gal as a control and expression of 28S rRNA (30 cycles) as a loading control. (B) Effect of dsInR knockdown on ovarian development of females programmed for nondiapause. Injections of dsInR (≈0.7 μg per female) were made into the thorax of cold-anesthetized mosquitoes within 1 day after eclosion (controls, dsβ-gal), and females were dissected 10 days later. ND, programmed by long day length for nondiapause; D, programmed by short day length for diapause. Both groups were maintained at 18°C. Bars (mean ± SD) with the same letters are not significantly different at P = 0.05, ANOVA.
Injection of dsInR prevented ovarian maturation in ND females and mimicked the diapause response (Fig. 1B). Ten days after adult eclosion, primary follicles in ND untreated females and dsβ-gal-injected controls were nearly 50% larger than follicles in diapausing (D) females reared under short day lengths. By contrast, when dsInR was injected into ND females, follicle length was greatly reduced and was not significantly different from that observed in D females.
When ovarian status was monitored by using the standard classification for diapause (17), the proportion of ND females that fell into the diapause category 10 days after injection was low for untreated mosquitoes and those injected with dsβ-gal, but a diapause-like status was high in the ND mosquitoes injected with dsInR and reached a level similar to that typically observed in D mosquitoes (Table 1).
Table 1.
Proportion of C. pipiens with diapause-type ovarian follicles after injection of respective dsRNAs and application of the JH analog methoprene
| dsRNA injection | n | Females with diapause-type ovarioles, % |
|---|---|---|
| Programmed for nondiapause | ||
| Untreated control | 30 | 13.3 |
| dsβ-gal | 31 | 9.7 |
| dsInR | 28 | 82.1* |
| Programmed for diapause | ||
| Untreated control | 26 | 92.3 |
| dsInR plus acetone | 24 | 79.2 |
| dsInR plus 5 ng of methoprene | 24 | 70.8* |
| dsInR plus 50 ng of methoprene | 28 | 7.1* |
| dsInR plus 500 ng of methoprene | 32 | 0* |
*Significant difference from untreated controls (χ2 goodness of fit test at P < 0.01 and df = 1).
JH Rescues the Halt in Ovarian Development Caused by dsInR.
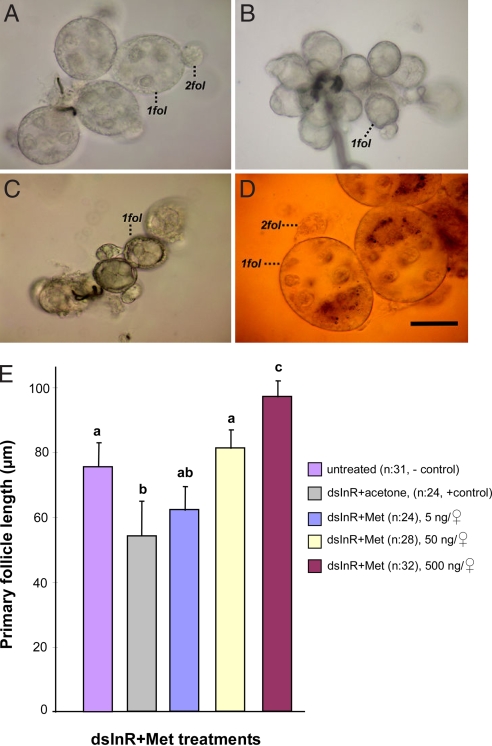
As demonstrated above, ovaries of dsInR-injected ND females halted development in a state simulating diapause. This is evident not only by differences in follicle length but also by distinctions in oocyte morphology. By day 10, the primary follicles in untreated ND females were robust and secondary follicles had already formed (Fig. 2A), whereas ovarian development was halted in untreated D females (Fig. 2B). Primary follicles in dsInR-injected ND females were arrested in a state similar to diapause (Fig. 2C). This arrest, however, could be rescued in dsInR-injected mosquitoes with a topical application of the JH analog methoprene (Fig. 2D). Primary follicle length in dsInR-injected mosquitoes increased in direct proportion to the concentration of methoprene (Fig. 2E). In addition, methoprene dramatically decreased the incidence of diapause in the dsInR-injected mosquitoes in a dose-dependent manner (Table 1).
Fig. 2.
Juvenile hormone rescue of ovarian development in InR knocked-down mosquitoes that were programmed by long day length for nondiapause. (A) Primary (1fol) and secondary (2fol) follicles from wild-type ND females, prepared 10 days after eclosion. (B) Primary follicles from wild-type D females, prepared 10 days after eclosion, showing the cessation of ovarian development. (C) Primary follicles from dsInR + acetone ND treated females dissected 10 days after eclosion. These follicles were arrested in Christopher's stage I, similar to the ovarian arrest observed in diapause. (D) Primary follicles from dsInR-treated ND females that received a 500 ng topical application of the JH analog methoprene in 0.5 μl of acetone, showing nondiapause characteristics, including secondary follicles. (Scale bar, 50 μm.) (E) Mean ± SD length of ovarian follicles in dsInR-injected ND females that subsequently received graded doses of the JH analog methoprene. Each n = 24–32 females. Bars with the same letters are not significantly different at P = 0.05, ANOVA.
dsFOXO Reduces Lipid Content in Diapausing Females.
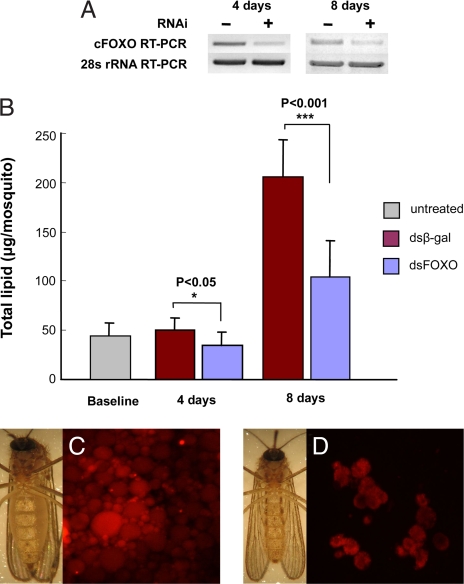
Diapausing females injected with ≈1 μg of cFOXO dsRNAs expressed only traces of cFOXO mRNA when examined 4 or 8 days later, whereas cFOXO was highly expressed in the dsβ-gal controls (Fig. 3A). Before injection, females contained a mean ± SD of 44.1 ± 9.4 μg of lipid per female, but 8 days later the lipid level increased >4-fold in the a dsβ-gal controls (Fig. 3B). In response to an injection of dsFOXO, diapausing females accumulated less lipid, a distinction that was already evident 4 days after injection. Although some lipid continued to accumulate in the dsFOXO-injected females, the level attained was approximately half that observed in the control females by day 8 (Fig. 3B). In addition, lipid content, as monitored by Nile Red staining, revealed a dramatic reduction of fat storage and number of fat body cells when RNAi was directed against cFOXO, and the untreated, diapausing females were conspicuously fatter (Fig. 3C) than their dsFOXO counterparts (Fig. 3D).
Fig. 3.
Knockdown of FOXO with RNAi reduced lipid stores in diapausing adults of C. pipiens. (A) RNAi efficacy in knocking down FOXO expression. Injections of dsFOXO (≈1.0 μg per female) were made into the thorax of cold-anesthetized mosquitoes within a day after eclosion (control, dsβ-gal), and expression was measured 4 and 8 days later (10 mosquitoes per group). (B) Lipid levels (mean ± SD) in females 4 and 8 days after injection with dsβ-gal (control) or dsFOXO. Baseline represents the lipid level 1 day after eclosion. Unpaired t test. (C) Fat females and Nile Red staining of fat body cells in diapausing adult females injected with dsβ-gal. (D) Slim females and Nile Red staining of fat body cells in diapausing adult females injected with dsFOXO.
Reduced Survival of Diapausing C. pipiens in Response to dsFOXO and Rescue with Mn(III)TBAP.
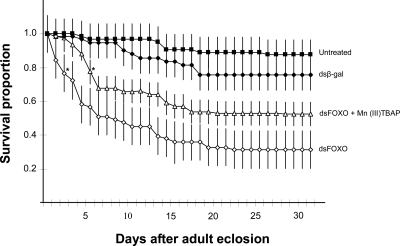
Whereas 80–90% of the wild-type and dsβ-gal-injected D mosquitoes survived 3 weeks, only 30% of the dsFOXO-injected females survived that long (Fig. 4). This suggests that reduced expression levels of the cFOXO gene significantly shortened the lifespan of diapausing C. pipiens, possibly a consequence of their reduced lipid stores. Loss of FOXO can also lead to a buildup of oxidative stress that may lead to early mortality (18, 19). The accumulation of oxidative stress can sometimes be effectively countered by administration of an exogenous substitute for oxidoreductase, such as Mn(III)TBAP (20). We tested this possibility in C. pipiens by injecting Mn(III)TBAP along with dsFOXO into D females, and our results demonstrated that Mn(III)TBAP rescued, at least partially, the phenotype suppressed by dsFOXO (Fig. 4). Survival of the coinjected females was intermediate between the controls and those injected with dsFOXO alone.
Fig. 4.
Survival (mean ± SD) of wild-type diapausing females of C. pipiens and mosquitoes injected with dsRNA targeting β-gal (control), cFOXO, and mosquitoes coinjected with dsFOXO and the oxidoreductase Mn(III)TBAP. The drop in survival caused by dsFOXO could be partially ameliorated by addition of the oxidoreductase. *, the first day when treated groups differ from dsβ-gal controls, (t test, P <0.05). Each n = 4 groups of 15 females.
Discussion
Insulin signaling is essential for normal growth in insects, and arguably it is the most important regulator of insect growth and size (21, 22). This pathway has been implicated in diverse roles including the immune response, apoptosis, longevity, and energy metabolism (22, 23). In addition, suppression of insulin signaling has been implicated in the induction of adult diapause in D. melanogaster (11, 24) and in dauer formation of the nematode Caenorhabditis elegans (25, 26). The results we report suggest that insulin signaling is integral to diapause in the mosquito C. pipiens as well. This common theme across taxa thus suggests a conserved role for the insulin signaling pathway for developmental and reproductive arrests among insects and other invertebrates.
The fact that methoprene, a JH analog, can counter the ovarian arrest caused by the down-regulation of Culex InR indicates that insulin signaling has a significant role mediating JH synthesis in C. pipiens. Several lines of evidence indicate that JH synthesis is shut down during diapause in C. pipiens (6, 8), and our experiments rescuing the dsInR shutdown of development with the JH analog methoprene support a causative link between insulin signaling and JH production. The responsiveness of InR mutants in D. melanogaster to JH also supports such a connection (10, 24). In ND mosquitoes, the corpora allata synthesize JH immediately after adult eclosion, and JH titers reach peak activity during that first week (8). Knocking down the InR has likely blocked JH production in these long-day females, thus generating the diapause phenotype.
In C. elegans and D. melanogaster, insulin signals through a conserved PI3-kinase/Akt pathway to ultimately phosphorylate the FOXO protein and block the translocation of this protein into the nucleus (9, 27). Thus, suppression of the insulin signal likely causes the FOXO protein to be translocated into the nucleus to initiate transcription of its downstream genes, some of which are known to be involved in key diapause characters such as the metabolic switch toward lipid storage and protection from reactive oxygen species (15, 28, 29). Our results suggest that these functional roles for FOXO are evident in diapausing C. pipiens as well. Suppression of FOXO by RNAi in diapausing mosquitoes resulted in loss of two key characters essential for successful overwintering: fat hypertrophy and extended lifespan. An antioxidant role is also suggested by the results elicited by a coinjection of dsFOXO and Mn(III)TBAP, an exogenous substitute for oxidoreductase (20): coinjection increased the lifespan and countered the mortality observed by an injection of dsFOXO alone. This result suggests that adding the oxidoreductase function enables the mosquito to cope with the stressful conditions of food shortage and environmental stress evoked by suppression of FOXO. Down-regulating the FOXO gene possibly impairs expression of oxidoreductases or small heat-shock proteins that enhance survival during diapause (30). The introduction of exogenous Mn(III)TBAP may, at least partially, compensate for the function of stress-responsive proteins that may be missing in FOXO RNAi mosquitoes.
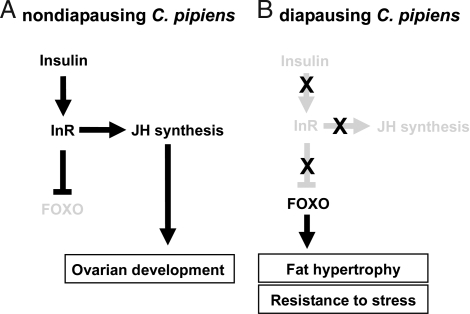
In summary, our data from C. pipiens support the hypothesis that the insulin signaling pathway and forkhead transcription factor control key characters of diapause, including the metabolic switch to lipid storage, the halt in ovarian development, and enhanced overwintering survival. We propose that, under long day lengths, insulin signaling leads to the production of JH needed to prompt ovarian development, and, concurrently, FOXO is suppressed, thus preventing accumulation of fat stores (Fig. 5A). By contrast, in response to short day lengths, the insulin signaling pathway is shut down, which in turn halts synthesis of the JH needed for ovarian development and releases the suppression of FOXO, leading to accumulation of lipid and the stress tolerance characteristic of diapause (Fig. 5B). The concurrence of these observations with the proposed involvement of the insulin signaling pathway in other forms of dormancy suggests a mechanism common to diverse forms of developmental arrest.
Fig. 5.
Model for diapause regulation in the mosquito C. pipiens. Insulin signaling pathway during the nondiapausing stage, resulting in ovarian development (A), and during diapause, resulting in arrested ovarian development, fat hypertrophy, and resistance to overwintering stress (B) is shown. Arrows indicate stimulation and T-bars indicate suppression. Black and gray indicate the ON and OFF activity states of the genes, respectively.
Materials and Methods
Insect Rearing.
The stock colony of C. pipiens (Buckeye strain) was reared at 25°C and 75% relative humidity under a 15-h light:9-h dark (L:D) photoperiod, as previously described (31). When larvae reached the second instar, rearing containers were placed under one of two environmental conditions: ND adults were generated by rearing at 18°C, 75% relative humidity, and 15:9 L:D. To induce diapause (D), mosquitoes were reared at 18°C, 75% relative humidity, and 9:15 L:D. To confirm diapause status, primary follicle and germarium lengths were measured, and the stage of ovarian development was determined according to the methods described by Christophers (32).
Identification and Bioinformatic Analysis of Culex InR and FOXO Sequences.
To retrieve sequences of Culex insulin receptor (cInR) and forkhead transcription factor (cFOXO), sequences of Drosophila InR and FOXO genes were used in discontinuous MEGA-BLAST searches on trace archives of genome data from the National Center for Biotechnology Information database (www.ncbi.nlm.nih.gov/blast/tracemb.shtml), and identity of the retrieved cInR and cFOXO sequences was confirmed by performing BLASTN searches against the nr (nonredundant) database (www.ncbi.nlm.nih.gov/BLAST/). Protein domains were identified by searching the Pfam database (http://pfam.sanger.ac.uk/). Multiple sequence alignments were performed by using ClustalW v1.81 (33).
dsRNA Preparation and Injection into Adult Female Mosquitoes.
dsRNA for the C. pipiens InR and FOXO genes was prepared by using the MEGAscript T7 transcription kit (Ambion) as previously described (34). Each PCR-derived fragment was sequenced and megablasted against the trace archive of C. pipiens quinquefasciatus genome sequences (www.ncbi.nlm.nih.gov/blast/tracemb.shtml) to validate the redundancy of the sequence and to confirm unique sequences. In knockdown experiment with ND mosquitoes, ≈0.5 μl of dsRNA of the cInR gene (1.5 μg/μl) or ≈0.5 μl of dsRNA of β-galactosidase (dsβ-gal, 1.5 μg/μl) was injected into the thorax of cold-anesthetized females by using a microinjector (Tritech Research). In knockdown experiments with D mosquitoes, we used ≈0.7 μl of dsRNA of the FOXO gene (1.5 μg/μl) or ≈0.7 μl of dsRNA of β-gal (1.5 μg/μl). Thus, ND females were injected with ≈0.7 μg of dsInR, and D females received ≈1 μg of dsFOXO. These selected concentrations of dsRNA were based on optimization experiments that evaluated a range of dsRNA concentrations.
RNAi Efficiency Evaluation Using RT-PCR.
RT-PCR of the dsRNA-injected mosquitoes was carried out as previously described (35). Briefly, total RNA samples were extracted with TRIzol (Invitrogen) from three batches of 15 adult female mosquitoes on various days after dsRNA injection. To remove genomic DNA contamination, RNA samples were treated with 1.0 μl of DNase I following the manufacturer's instructions (50–375 units/μl; Invitrogen). For reverse transcription, 5 μg of total RNA was reverse-transcribed with SuperScript III RNase H-reverse transcriptase (Invitrogen). From each cDNA, 2 μl of sample was amplified by PCR using recombinant TaqDNA polymerase (Invitrogen). To evaluate RNAi efficiency, primers were used to amplify endogenous cFOXO and cInR genes; 28S ribosomal RNA from C. pipiens, amplified for 30 cycles, was used as an internal control.
Follicle Assay After dsInR.
ND female mosquitoes within a day after eclosion were injected in the thorax with dsInR or dsβ-gal (control). Each treated cohort was kept in 8-cm-diameter × 12-cm cages. Cotton soaked in a 10% sucrose solution was provided 1 h after the dsRNA injection. Cages were placed at 18°C, 75% relative humidity, 15: 9 h L:D, and ovaries were assessed 10 days after injection. Ovaries were dissected in a drop of saline solution, disrupted with a needle, and examined at ×200 and ×400 magnifications. Mean follicle length for each female was calculated from measurements of 10 follicles, and data were colleted from ≈30 individuals. An unpaired t test was used to distinguish differences in follicle sizes among dsRNA and control groups. In addition, ovarian developmental stages were defined according to methods described by Spielman and Wong (17) with a slight adjustment: a mosquito was considered to be in diapause if follicle length did not exceed two times that of the germarium and if the primary follicles were <60 μm in length; the mosquito was classified as nondiapause if the length of the follicle was at least three times greater than that of the germarium.
Methoprene Treatment.
The JH analog methoprene (Sandoz Pharmaceutical) was used to evaluate the mosquito's response to JH. ND females, within 1 day after adult eclosion, were injected with dsInR and then topically treated the same day with serial dilutions of methoprene (5, 50, and 500 ng per female) diluted in 0.5 μl of acetone. Ovaries were dissected and measured as described above. An ANOVA was used to distinguish differences in follicle sizes.
Lipid Assay After dsFOXO.
D females were injected in the thorax with dsFOXO or dsβ-gal (control) within 1 day after eclosion, and lipid levels were measured 4 and 8 days later using a slight modification of an assay previously described (36). Briefly, each mosquito was placed in a 2.0-ml tube, homogenized in 500 μl of chloroform-methanol (1:1), and centrifuged. The supernatant was transferred and placed in a 90°C incubator to evaporate the solvent. After 1 h, 0.2 ml of sulfuric acid was added and the sample was again heated for 10 min. After cooling, 5 ml of vanillin reagent (600 mg of vanillin, 100 ml of hot water, and 400 ml of 85% phosphoric acid) was added and mixed for 5 min. Samples were read directly in a spectrophotometer at 490 nm.
Fat Body Staining with Nile Red.
Nile Red powder (N-1142; Molecular Probes) was dissolved in acetone (500 μg/ml) and diluted in 1× PBS to a final concentration of 0.05 μg/ml, and fat bodies from dsInR-treated and dsβ-gal-treated mosquitoes, disrupted with a needle, were added. Subsequently, fat content of each mosquito was assessed by fluorescence microscopy (Zeiss Axioskop, ×400 with rhodamine filter).
Survival Assay After Injection of dsFOXO and Mn(III)TBAP.
To evaluate the knockdown effect of cFOXO on the survival rate of D mosquitoes, 15 females per cohort were intrathoracically injected with ≈0.7 μl of dsFOXO (1.5 μg/μl) or ≈0.7 μl of dsβ-gal (1.5 μg/μl) or remained untreated. For experiments involving Mn(III) tetrakis (4-benzoic acid) porphyrin (Mn(III)TBAP; Cayman Chemical), ≈0.8 μl of a 9:1 mixture of dsFOXO (1.5 μg/μl) and Mn(III)TBAP (1 mM) after dilution in PBS (1×) was coinjected into the thorax of D females by using a microinjector (Tritech Research). Thus, each mosquito was coinjected with ≈1.0 μg of dsFOXO and ≈0.8 μl of Mn(III)TBAP (0.1 mM). Mosquitoes were held at 18°C, 75% relative humidity, and a 9:15 L:D cycle, with access to sugar, and survival was assessed daily. Experiments were replicated four times.
Supplementary Material
Acknowledgments.
We appreciate helpful reviews of the manuscript provided by Drs. H. F. Nijhout (Duke University, Durham, NC) and M. Riehle (University of Arizona, Tucson). This work was supported in part by National Institutes of Health–National Institute of Allergy and Infectious Diseases Grant R01 AI058279.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. EU442282 (C. pipiens insulin receptor) and EU442283 (C. pipiens forkhead transcription factor)].
This article contains supporting information online at www.pnas.org/cgi/content/full/0802067105/DCSupplemental.
References
- 1.Sanburg LL, Larsen JR. Effect of photoperiod and temperature on ovarian development in Culex pipiens pipiens. J Insect Physiol. 1973;19:1173–1190. doi: 10.1016/0022-1910(73)90202-3. [DOI] [PubMed] [Google Scholar]
- 2.Bowen MF. Patterns of sugar feeding in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae) females. J Med Entomol. 1992;29:843–849. doi: 10.1093/jmedent/29.5.843. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell CJ, Briegel H. Inability of diapausing Culex pipiens (Diptera: Culicidae) to use blood for producing lipid reserves for overwinter survival. J Med Entomol. 1989;26:318–326. doi: 10.1093/jmedent/26.4.318. [DOI] [PubMed] [Google Scholar]
- 4.Denlinger DL. Regulation of diapause. Annu Rev Entomol. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- 5.Denlinger DL, Yocum GD, Rinehart JP. In: Comprehensive Molecular Insect Science. Gilbert LI, Iatrou K, Gill SS, editors. Vol 3. Amsterdam: Elsevier; 2004. pp. 615–650. [Google Scholar]
- 6.Spielman A. Effect of synthetic juvenile hormone on ovarian diapause of Culex pipiens mosquitoes. J Med Entomol. 1974;11:223–225. doi: 10.1093/jmedent/11.2.223. [DOI] [PubMed] [Google Scholar]
- 7.Meola RW, Petralia RS. Juvenile hormone induction of biting behavior in Culex mosquitoes. Science. 1980;209:1548–1550. doi: 10.1126/science.209.4464.1548. [DOI] [PubMed] [Google Scholar]
- 8.Readio J, Chen MH, Meola R. Juvenile hormone biosynthesis in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae) J Med Entomol. 1999;36:355–360. doi: 10.1093/jmedent/36.3.355. [DOI] [PubMed] [Google Scholar]
- 9.Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- 10.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 11.Williams KD, et al. Natural variation in Drosophila melanogaster diapause due to the insulin-regulated PI3-kinase. Proc Natl Acad Sci USA. 2006;103:15911–15915. doi: 10.1073/pnas.0604592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riehle MA, Brown MR. Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti. Insect Biochem Mol Biol. 1999;29:855–860. doi: 10.1016/s0965-1748(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 13.Riehle MA, Brown MR. Insulin receptor expression during development and a reproductive cycle in the ovary of the mosquito Aedes aegypti. Cell Tissue Res. 2002;308:409–420. doi: 10.1007/s00441-002-0561-8. [DOI] [PubMed] [Google Scholar]
- 14.Brown MR, et al. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0800478105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Claeys I, et al. Insulin-related peptides and their conserved signal transduction pathway. Peptides. 2002;23:807–816. doi: 10.1016/s0196-9781(01)00666-0. [DOI] [PubMed] [Google Scholar]
- 17.Spielman A, Wong J. Environmental control of ovarian diapause in Culex pipiens. Ann Entomol Soc Am. 1973;66:905–907. [Google Scholar]
- 18.Essers MA, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy CT, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 20.Faulkner KM, Liochev SI, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- 21.Nijhout HF. The control of growth. Development. 2003;130:5863–5867. doi: 10.1242/dev.00902. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- 23.Nijhout HF. The control of body size in insects. Dev Biol. 2003;261:1–9. doi: 10.1016/s0012-1606(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 24.Tu MP, Yin CM, Tatar M. Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen Comp Endocrinol. 2005;142:347–356. doi: 10.1016/j.ygcen.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Beckstead RB, Thummel CS. Indicted: Worms caught using steroids. Cell. 2006;124:1137–1140. doi: 10.1016/j.cell.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell. 2001;1:841–851. doi: 10.1016/s1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- 27.Junger MA, et al. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arking R. Multiple longevity phenotypes and the transition from health to senescence. Ann NY Acad Sci. 2005;1057:16–27. doi: 10.1196/annals.1356.001. [DOI] [PubMed] [Google Scholar]
- 29.Oh SW, et al. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat Genet. 2006;38:251–257. doi: 10.1038/ng1723. [DOI] [PubMed] [Google Scholar]
- 30.Rinehart JP, et al. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc Natl Acad Sci USA. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robich RM, Denlinger DL. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proc Natl Acad Sci USA. 2005;102:15912–15917. doi: 10.1073/pnas.0507958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christophers SR. The development of the egg follicle in Anophelines. Paludism. 1911;1:73–88. [Google Scholar]
- 33.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim C, et al. Anopheles gambiae heat shock protein cognate 70B impedes o'nyong-nyong virus replication. BMC Genomics. 2007;8:231. doi: 10.1186/1471-2164-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sim C, et al. Modulation of Anopheles gambiae gene expression in response to o'nyong-nyong virus infection. Insect Mol Biol. 2005;14:475–481. doi: 10.1111/j.1365-2583.2005.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Handel E. Rapid determination of total lipids in mosquitoes. J Am Mosq Control Assoc. 1985;1:302–304. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.