Abstract
The impact of anthropogenic climate change on terrestrial organisms is often predicted to increase with latitude, in parallel with the rate of warming. Yet the biological impact of rising temperatures also depends on the physiological sensitivity of organisms to temperature change. We integrate empirical fitness curves describing the thermal tolerance of terrestrial insects from around the world with the projected geographic distribution of climate change for the next century to estimate the direct impact of warming on insect fitness across latitude. The results show that warming in the tropics, although relatively small in magnitude, is likely to have the most deleterious consequences because tropical insects are relatively sensitive to temperature change and are currently living very close to their optimal temperature. In contrast, species at higher latitudes have broader thermal tolerance and are living in climates that are currently cooler than their physiological optima, so that warming may even enhance their fitness. Available thermal tolerance data for several vertebrate taxa exhibit similar patterns, suggesting that these results are general for terrestrial ectotherms. Our analyses imply that, in the absence of ameliorating factors such as migration and adaptation, the greatest extinction risks from global warming may be in the tropics, where biological diversity is also greatest.
Keywords: biodiversity, fitness, global warming, physiology, tropical
Global warming in this century may be the largest anthropogenic disturbance ever placed on natural systems (1, 2). Its impact on species is likely to vary geographically (2–4), but a mechanistic framework to predict its magnitude and global distribution has not yet been developed (5). One important determinant of biological responses to climate change will be the degree of warming itself, which will continue to be greater at high latitudes (6). Also relevant, however, is the physiological sensitivity of organisms to changes in the temperature of their environment (7, 8). The thermal tolerance of many organisms has been shown to be proportional to the magnitude of temperature variation they experience (9–11), a characteristic of climate that also increases with latitude. Evaluating the impacts of rapidly changing climates on population fitness and survival thus requires linking geographic patterns of the magnitude of temperature change with the physiological sensitivity of organisms to that change (12).
Ectotherms constitute the vast majority of terrestrial biodiversity (13) and are especially likely to be vulnerable to climate warming because their basic physiological functions such as locomotion, growth, and reproduction are strongly influenced by environmental temperature. The ability of ectotherms to perform such functions at different temperatures is described by a thermal performance curve (14), which rises gradually with temperature from a minimum critical temperature, CTmin, to an optimum temperature, Topt, and then drops rapidly to a critical thermal maximum, CTmax (Fig. 1). Critical temperatures CTmin and CTmax, operationally defined by the limits of organism performance, have been measured for diverse ectotherms (15–18) and usually covary with latitude, reflecting at least partial adaptation of ectotherms to their climate (9–11). Thermal performance curves index the direct effect of temperature on organism fitness (14–15), and thus provide a physiological framework for elucidating a fundamental component of the impact of global climate change in a spatially explicit and empirically constrained way.
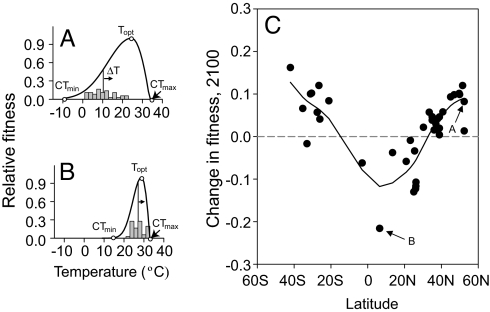
Fig. 1.
Fitness curves for representative insect taxa from temperate (A) and tropical (B) locations, and (C) the change in fitness because of climate warming for all insect species studied, as a function of latitude. (A and B) Fitness curves are derived from measured intrinsic population growth rates versus temperature for 38 species, including Acyrthosiphon pisum (Hemiptera), from 52°N (England) (A), and the same for Clavigralla shadabi (Hemiptera) from 6°N (Benin) (B). CTmin, Thab, Topt, and CTmax are indicated on each curve. Climatological mean annual temperature from 1950–1990 (Thab, drop lines from each curve), its seasonal and diurnal variation (gray histogram), and its projected increase because of warming in the next century (ΔT, arrows) are shown for the collection location of each species. For each of 38 species, fitness is integrated over both seasonal and diurnal temperature cycles for both the observed climate of the late 20th century (1950–1990) and for a model-simulated climate of the late 21st century (2070–2100) (23). (C) Predicted change in fitness of insects versus latitude is a measure of the impact of 21st century climate warming on population growth rates. Negative values indicate decreased rates of population growth in 2100 AD and are found mainly in the tropics. Positive values are found in mid- and high-latitudes. Line is a spline-fit with a span of 0.9.
Results
Estimating Impact on Insects.
Insects are the largest group of terrestrial organisms. The impact of temperature on intrinsic rates of population growth (r), a direct measure of Darwinian fitness, has been quantified experimentally for numerous insect species from around the globe (15). We use these data to construct fitness curves for each species to calculate the fractional change in population growth rate from the observed climate of the late 20th century (1950–1990) (19) to a model-simulated climate (20) of the late 21st century (2070–2100), where climate data were taken at the source site of each species (see supporting information (SI) Methods and Figs. S1 and S2). In both time periods, insect body temperature is assumed to track ambient air temperature, and fitness is averaged through diurnal and monthly temperature variations, implicitly accounting for potential shifts in organisms' preferred activity times (see SI Methods). The difference between population growth rates under current versus projected climates quantifies the direct thermal impact (positive or negative) of future warming on the fitness of organisms.
After a century of warming, population growth rates of insects change dramatically and exhibit a conspicuous latitudinal trend (Fig. 1). At mid- to high-latitudes, population growth rates are predicted to increase, indicating enhanced population fitness because of warming. In the tropics, however, intrinsic rates of population growth are expected to decrease by up to 20%, implying that warming will substantially reduce fitness. This latitudinal trend is robust: it is insensitive to how fitness is averaged through time, or to potential differences between environmental and body temperatures (see SI Methods and Figs. S3 and S4). Instead, the pattern of global warming's impact on insect fitness derives from fundamental geographic relationships between climate and physiological performance that can be distilled into two simple heuristic indicators, and that are observed among several other taxa.
Warming Tolerance and Thermal Safety Margin.
The impact of warming on insect fitness at each location depends on several factors, including the breadth of each performance curve, its position relative to the mean climate, and the spectrum of local temperature variability. However, the basic latitudinal trend in impact can be qualitatively elucidated by using two simple metrics that characterize the geographic covariations of fitness curves and climate. In addition to providing a heuristic explanation for the impact of warming across latitude, these two metrics allow impacts diagnosed for insects at point locations to be extrapolated globally and generalized to other ectotherm taxa.
In the context of long-term climate warming, a key characteristic of an ectotherm's performance curve is the difference between its critical thermal maximum and the current climatological temperature of the organism's habitat, Thab, here taken to be mean annual surface air temperature. This quantity approximates the average amount of environmental warming an ectotherm can tolerate before performance drops to fatal levels, and we refer to this difference as an organism's “warming tolerance,” (WT = CTmax − Thab). Although annual mean temperature provides a single robust climate statistic for referencing the position of performance curves, its use does not imply that mean temperature determines an organism's fitness, which will vary throughout the year.
For insect species, CTmax decreases slightly with latitude (11), but not as rapidly as does surface air temperature, either on an annual basis or during the hottest months (Fig. S1). The warming tolerance of tropical insects is, on average, only one-fifth that of mid-latitude insects (Fig. 2A). Tropical insects will thus approach near-lethal temperatures much faster than will insects from temperate climates, even though the rate of warming in the tropics is predicted to be half that of high latitudes (6). The latitudinal trend in warming tolerance alone will tend to increase the impact of tropical warming relative to that at higher latitudes.
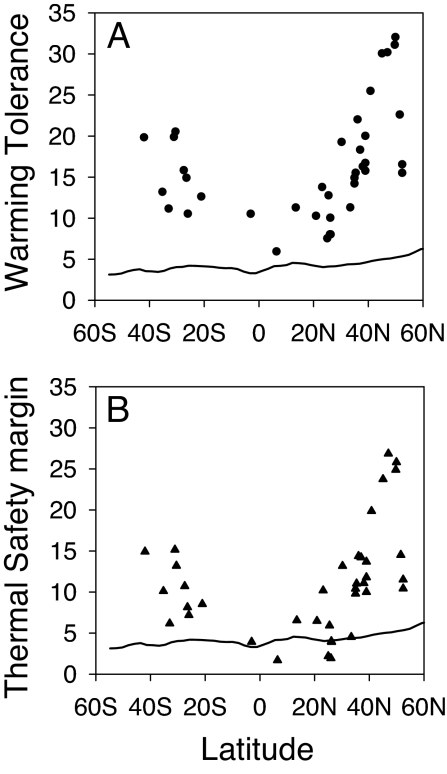
Fig. 2.
Latitudinal trends in warming tolerance (CTmax − Thab; A) and thermal safety margin (Topt − Thab; B), the primary heuristic indicators of the impact of warming on the thermal performance of insects. The projected magnitude of warming by 2100 (ΔT; black line) erodes ≈50% of the warming tolerance of tropical insects and raises the temperature of their habitats above their thermal optima for much of the year, resulting in decreased thermal performance, whereas temperatures will remain below the thermal optimum for many high latitude species. Although these heuristic indicators are defined by using annual mean temperature (Thab), the calculated impact of warming on performance takes into account both seasonal and diurnal temperature variations.
A second important characteristic of performance curves is the difference between an organism's thermal optimum (Topt) and its current climate temperature, Thab. We call this difference the “thermal safety margin” (TSM = Topt − Thab). Species living in environments that are already close to their physiological optimum have small thermal safety margins, and thus even small amounts of warming will likely decrease performance. In contrast, species with large thermal safety margins are living in environments that are on average cooler than optimal, and should experience initial increases in population growth rates and performance as temperatures rise.
For insect species, thermal safety margins increase sharply with latitude (Fig. 2B). Tropical insects are already living at environmental temperatures very close to their physiological optimum (TSM ≈ 0; see Acyrthosiphon pisum in Fig. 2), whereas higher-latitude insects are experiencing environmental temperatures significantly below Topt, even during summer (TSM > 0, see Clavigralla shadabi in Fig. 2). The latitudinal trend in the impact of climate change inferred from warming tolerance alone is thus accentuated by the poleward increase in thermal safety margins, because it allows insect performance to increase in colder climates, at least initially. Taken together, warming tolerance and thermal safety margin thus provide simple but useful indicators for understanding the latitudinal trend in the impact of warming calculated from detailed climate data and individual insect performance curves (Fig. 1).
Global and Taxonomic Extrapolation.
We now combine these metrics into a simple conceptual model that we use first to extrapolate results for insects to the global scale, and next to estimate impacts of warming on other taxonomic groups for which only limited performance data are available. In this model, warming tolerance and thermal safety margins are assumed to capture the most salient geographic variations in the performance curves of ectotherms. Patterns of warming tolerance and thermal safety margins are estimated from their empirical relationships to the magnitude of the seasonal temperature cycle. Climate variability has long been considered a mechanistic driver of differences in thermal performance across latitude (10); high latitudes have greater climatic variability, which should favor organisms with a broad thermal tolerance. Indeed, seasonality is a strong predictor of both warming tolerance and thermal safety margin of insects (Fig. S5 and Table S1 ). We use these relationships to interpolate insect fitness curves from specific locations to the global scale. We then compute the impact of warming on fitness worldwide, accounting for both seasonal and diurnal temperature cycles (see SI Methods).
The global impact of 21st century warming on insects in this simplified model reproduces the qualitative pattern diagnosed above using actual fitness curves of individual insect species (Fig. 3A). This concordance confirms that the most important geographic variations in fitness curves are indeed captured by the heuristic indicators, warming tolerance, and thermal safety margin. The most deleterious impacts of warming on population growth rates are again predicted to occur in the tropics (Fig. 3B). This result depends only on the latitudinal increase in warming tolerance and is independent of the latitudinal increase in thermal safety margins (see SI Methods and Fig. S6). In contrast, the predicted increase in population growth rates at higher latitudes is due entirely to the relatively large thermal safety margins observed in cold climates and thus depends strongly on the poleward trend in thermal safety margins (see SI Methods and Fig. S6).
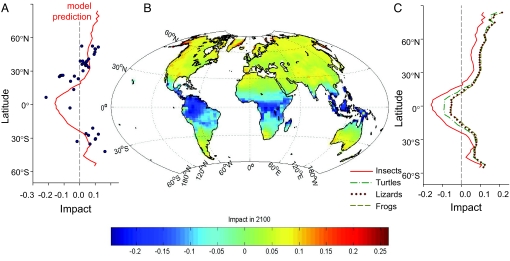
Fig. 3.
Predicted impact of warming on the thermal performance of ectotherms in 2100. (A) Impact versus latitude for insects using thermal performance curves fit to intrinsic population growth rates measured for each species (black circles, from Fig. 1) and for a global model (red line) in which performance curves at each location are interpolated from empirical linear relationships between seasonality and both warming tolerance and thermal safety margin. (B and C) Results from the simplified conceptual model are shown globally for insects (B) for which performance data are most complete, and versus latitude for three additional taxa of terrestrial ectotherms: frogs and toads, lizards, and turtles (C), for which only warming tolerance was available. On the basis of patterns in warming tolerance, climate change is predicted to be most deleterious for tropical representatives of all four taxonomic groups. Performance is predicted to increase in mid- and high-latitudes because of the thermal safety margins observed there for insects, and provisionally attributed to other taxa.
To examine the generality of these results beyond insects, we investigated three groups of terrestrial vertebrates (frogs, lizards, and turtles), using published studies in which critical thermal limits were experimentally determined for at least 12 populations of closely related taxa across large climate gradients [(16–18), Table S1]. In each taxonomic group, warming tolerance of an organism increases strongly with the seasonal temperature variability of its habitat (Fig. S5). This consistent pattern indicates that warming will cause tropical vertebrate ectotherms to approach their critical maximum temperatures proportionately faster than similar high-latitude species, despite lower absolute rates of tropical warming. Consequently, tropical representatives of all four taxonomic groups will likely experience the most deleterious changes in thermal performance during warming (Fig. 3C).
In contrast, many mid- to high-latitude vertebrates should experience enhanced thermal performance because of warming, because they tend to inhabit climates that are currently cooler than optimal. Some high-latitude ectotherms may have narrower thermal safety margins than do insects, and such species would show more modest increases in performance than we have predicted (Fig. 3C). In addition, if global temperatures continue to rise beyond the 21st century, as projected under most climate scenarios (6), even high-latitude species will begin to experience decreased performance, as temperatures exceed their optimum temperatures. Expanded performance datasets are needed both to constrain the future fitness trajectories of high-latitude ectotherms and to further test the generality of the predicted impact patterns across taxa. Critical thermal limits of marine species may be especially illuminating (21–23), because the latitudinal profile of seasonal temperature variability in surface ocean waters exhibits minima in polar as well as tropical latitudes and may lead to strong thermal sensitivity and thus reduced future fitness in both climate extremes (21).
Discussion
The impact of increasing atmospheric temperature on the thermal performance of organisms is an important and direct biological consequence of climate change and can be evaluated on a global scale (Fig. 3). Individual organisms will react to changes in their performance, whether positive or negative, in a variety of ways including acclimation (10, 16, 24), adaptation (25–27), and dispersal and behavioral modification (3, 4). These responses and the resulting changes in ecosystem community structure will modulate the global patterns of impact predicted here. Evaluating these effects will require more fine-grained, species-specific models (28, 29), as well as expanded datasets. Even so, these processes seem unlikely to reverse our qualitative conclusion that tropical ectotherms are most at risk in a greenhouse future (11, 27, 30, 31).
Acclimation, adaptation, dispersal, and behavioral plasticity will all help mitigate the adverse impacts of climate change but are unlikely to completely offset the decreased fitness predicted here for tropical organisms. The capacity of ectotherms to acclimate their physiological performance curves is generally low in tropical species (10, 11, 16, 24). Adaptive evolutionary responses should also ameliorate the impacts of warming, but we currently lack a strong empirical or theoretical foundation upon which to assess the potential for evolutionary responses among tropical ectotherms to the rapid rates of projected warming (32, 33). Changes in behavior, including shifts in the timing of activity, and changes in range boundaries have been documented particularly at high latitudes (3, 4, 34, 35), but such phenological shifts may be caused by either enhanced or reduced fitness. The benefits of all these responses will be constrained by both physiological and ecological tradeoffs (5) and, in the case of migration, by increasing habitat fragmentation. Indeed, one of the best documented cases of climate-induced extinctions, that of high-elevation tropical frogs (36, 37), illustrates the complexity of impacts and the potential limitations in all of these strategies.
Ultimately, organisms with the greatest risk of species extinction from rapid climate change are those with a low tolerance for warming, limited acclimation ability, and reduced dispersal. Most terrestrial organisms having these characteristics are tropical and many of these organisms are occupying disappearing climate regimes (38). This conclusion is troubling because it places the greatest biological risks of climate change in the tropics where biodiversity is greatest.
Methods
Climate Data.
Baseline climate conditions for the late 20th century (1950–2000) were obtained from the Climatic Research Unit CL 2.0 high-resolution (10′) global monthly surface air temperatures and diurnal temperature ranges (19) (Fig. S2). For 21st century climate, we added spatial and temporal patterns of warming using climate model projections from the fourth assessment report of the Intergovernmental Panel on Climate Change (ref. 6 and Fig. S2B). The absolute magnitude of warming, ΔT, depends on the future time horizon, emissions scenario, and physical climate model. The latitudinal gradient of warming, however, which is our main concern, is qualitatively independent of all of these: a poleward intensification of warming emerges in every model, emissions scenario, and time period. Here, we have used monthly temperature anomalies (relative to a control) from 2070–2100 in a simulation from the Geophysical Fluid Dynamics Laboratory model CM2.1 (20) that is forced by the A2 greenhouse gas emissions scenario. Climate warming in each month was added to the observed temperature distribution to eliminate any biases from model errors in simulating the baseline (i.e., 20th century) climate. The diurnal temperature range was assumed to retain its observed seasonal and geographic pattern into the next century. The spatial resolution of our results is limited by that of the global climate model but is appropriate for present purposes.
Site-Specific Performance.
To predict physiological consequences of climate change, we must first estimate a thermal performance curve, P(T), at each location for each taxonomic group. For insects, we also use the term fitness curve because the ordinate is derived from intrinsic population growth rates, a direct measure of Darwinian fitness. For all taxa, these curves are asymmetric, and we use a Gaussian function to describe the rise in performance up to the optimal temperature, Topt, and a quadratic decline to zero performance at CTmax and higher temperatures (Fig. 1) (14):
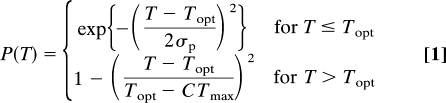 |
This formulation of performance conforms to both observed and theoretical considerations (14, 26). Because an organism's critical thermal minimum (CTmin) does not enter directly into this formulation (the Gaussian performance at cold temperatures never strictly reaches zero), we adopt an operational definition of CTmin as the temperature 4σP below Topt, (i.e., CTmin = Topt − 4σP), where performance reaches a low value (P ≈ 1.8%). We then define the asymmetry, α, of a performance curve as the ratio of its width on the warm and cold sides of Topt, so that α = (CTmax − Topt)/(Topt − CTmin) = (CTmax − Topt)/4σp.
The fitness curves of insects are derived by fitting data on observed intrinsic rates of population growth at multiple temperatures to the above equation (14), yielding least-squares estimates of all three parameters [σp, Topt, and CTmax (the temperature at which populations are unable to grow); see Dataset S1 for data). To ensure physiologically realistic fitness curves, the cost function for the least squares fit additionally penalized solutions whose asymmetry deviated from a specified value. Because the true asymmetry is unknown, we used a range of target values of α from 2–8, thus obtaining a family of plausible-fit curves. For each species, we took the average of those solutions that satisfied two objective criteria: the rms error in growth rates was <10% of the mean value, and the asymmetry remained between 1 and 10. Most of the insect species (38 of 46) yielded at least one fitness curve meeting these criteria, and of the 8 species that did not, only one would have yielded a thermal impact outside the main latitudinal trend (Fig. 1).
Global Performance.
To extrapolate the set of insect performance curves from individual locations to the global scale, we use empirical relationships between the estimated thermal performance parameters obtained above, and the seasonal climate variability where each species was studied. Seasonality is chosen as the predictor variable because its global distribution is well known (Fig. S2) and it has long been considered a mechanistic driver of variations in the thermal performance of organisms (9, 10).
At the collection site of each insect species, mean habitat temperature (Thab) and seasonality (σt) were extracted from high-resolution climate data (see Climate Data) as the mean and standard deviation of monthly surface air temperatures, respectively (25). Consistent with theoretical expectations (9, 10), seasonality is a strong predictor of warming tolerance (WT = CTmax − Thab; Table S1) and also of thermal safety margins (TSM = Topt − Thab; Table S1). These correlations simply reference the position of performance curves to local climate conditions, and are used with the same global climatology (Fig. S2) to compute empirically interpolated maps of both CTmax and Topt. The remaining free parameter, σp, is assigned by noting that the asymmetry of insect performance curves exhibits no large-scale pattern. We therefore use a constant value of α = 3 that is typical of insects. A range of performance asymmetry values and deviations from constancy was also investigated, but the quantitative influence on the predicted impact was found to be small and restricted to high latitudes, where current climate temperatures are much cooler than Topt for most species (see SI Methods). The similarity between point estimates of impact based on actual performance curves and the global maps derived in this model (see Fig. 3A) confirms that the patterns of WT and TSM are indeed the primary variables governing thermal impacts and are well captured by linear relationships to seasonality.
The global model developed for insects can now be extended to three other taxonomic groups: frogs, lizards, and turtles. For these vertebrate taxa, measured critical thermal limits have been published for multiple related species at locations spanning large climate gradients (see Fig. S5 and Table S1), but empirical thermal performance curves are generally unavailable. Using the same procedure as for insects, we compute warming tolerance and its linear correlation to seasonality at the site where each species was collected. We find strong positive relationships between seasonality and warming tolerance that are highly consistent across all taxa (Fig. S5).
Because estimates of Topt were not available for these taxa, we have provisionally ascribed the relationship between thermal safety margin and seasonality for insects to these taxa as well. Although this assumption introduces some uncertainty into our prediction of increasing high-latitude performance, it does not affect our conclusion that the most deleterious impacts will be in the tropics. The strong latitudinal gradient in warming tolerance permits no realistic pattern of thermal safety margins that could reverse the overall latitudinal trend in impact (see SI Methods and Fig. S6).
Supplementary Material
Acknowledgments.
This manuscript was much improved by the suggestions of three anonymous reviewers. This work was supported by a Program on Climate Change fellowship (C.A.D.), by National Science Foundation (NSF) Grant IOB-0416843 (to R.B.H.), and by NSF predoctoral fellowships (K.S.S. and D.C.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709472105/DCSupplemental.
References
- 1.Sala OE, et al. Biodiversity: Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 2.Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C, Yohe G A. Globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 4.Root TL, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 5.Davis AJ, Jenkinson LS, Lawton JH, Shorrocks B, Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998;391:783–786. doi: 10.1038/35842. [DOI] [PubMed] [Google Scholar]
- 6.IPCC. Working Group I Contribution to the Fourth Assessment Report of the IPCC. Cambridge, UK: Cambridge Univ Press; 2007. Climate Change 2007: The Physical Science Basis. [Google Scholar]
- 7.Bernardo J, Ossola RJ, Spotilla JR, Crandall KA. Interspecies physiological variation as a tool for cross-species assessments of global warming-induced endangerment: Validation of an intrinsic determinant of macroecological and phylogeographic structure. Biol Lett. 2007;3:695–698. doi: 10.1098/rsbl.2007.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calosi P, Bilton DT, Spicer JI. Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol Lett. 2007;4:99–102. doi: 10.1098/rsbl.2007.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janzen DH. Why mountain passes are higher in tropics. Am Nat. 1967;101:233–249. [Google Scholar]
- 10.Ghalambor C, Huey RB, Martin PR, Tewksbury JJ, Wang G. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr Comp Biol. 2006;46:5–17. doi: 10.1093/icb/icj003. [DOI] [PubMed] [Google Scholar]
- 11.Addo-Bediako A, Chown SL, Gaston KJ. Thermal tolerance, climatic variability and latitude. Proc R Soc London Ser B Biol Sci. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pörtner HO, et al. Trade-offs in thermal adaptation: The need for a molecular to ecological integration. Physiol Biochem Zool. 2006;79:295–313. doi: 10.1086/499986. [DOI] [PubMed] [Google Scholar]
- 13.Wilson EO. The Diversity of Life. Cambridge, MA: Harvard Univ Press; 1992. [Google Scholar]
- 14.Huey RB, Stevenson RD. Integrating thermal physiology and ecology of cctotherms: Discussion of approaches. Am Zool. 1979;19:357–366. [Google Scholar]
- 15.Frazier MR, Huey RB, Berrigan D. Thermodynamics constrains the evolution of insect population growth rates: “Warmer is better”. Am Nat. 2006;168:512–520. doi: 10.1086/506977. [DOI] [PubMed] [Google Scholar]
- 16.Brattstrom BH. Thermal acclimation in anuran amphibians as a function of latitude and altitude. Comp Biochem Physiol. 1968;24:93–111. doi: 10.1016/0010-406x(68)90961-4. [DOI] [PubMed] [Google Scholar]
- 17.Hutchison VH, Vinegar A, Kosh RJ. Critical thermal maxima in turtles. Herpetologica. 1966;22:32–41. [Google Scholar]
- 18.van Berkum FH. Latitudinal patterns of the thermal sensitivity of sprint speed in lizards. Am Nat. 1988;132:327–343. [Google Scholar]
- 19.New M, Lister D, Hulme M, Makin I. A high-resolution data set of surface climate over global land areas. Clim Res. 2002;21:1–25. [Google Scholar]
- 20.Delworth TL, et al. GFDL's CM2 global coupled climate models. Part I: Formulation and simulation characteristics. J Clim. 2006;19:643–674. [Google Scholar]
- 21.Pörtner HO, Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 2007;315:95–97. doi: 10.1126/science.1135471. [DOI] [PubMed] [Google Scholar]
- 22.Somero GN. Thermal physiology and vertical zonation of intertidal animals: Optima, limits, and costs of living. Integr Comp Biol. 2002;42:780–789. doi: 10.1093/icb/42.4.780. [DOI] [PubMed] [Google Scholar]
- 23.Stillman JH. Acclimation capacity underlies susceptibility to climate change. Science. 2003;301:65. doi: 10.1126/science.1083073. [DOI] [PubMed] [Google Scholar]
- 24.Feder ME. Environmental variability and thermal acclimation in Neotropical and temperate zone salamanders. Physiol Zool. 1978;51:7–16. [Google Scholar]
- 25.Burger R, Lynch M. Evolution and extinction in a changing environment: A quantitative-genetic approach. Evolution (Lawrence, Kans) 1995;49:151–163. doi: 10.1111/j.1558-5646.1995.tb05967.x. [DOI] [PubMed] [Google Scholar]
- 26.Huey RB, Kingsolver JG. Evolution of resistance to high-temperature in ectotherms. Am Nat. 1993;142:S21–S46. [Google Scholar]
- 27.Balanyá J, Oller JM, Huey RB, Gilchrist GW, Serra L. Global genetic change tracks global climate warming in Drosophila subobscura. Science. 2006;313:1773–1775. doi: 10.1126/science.1131002. [DOI] [PubMed] [Google Scholar]
- 28.Kearney M, Porter WP. Mapping the fundamental niche: Physiology, climate, and the distribution of a nocturnal lizard. Ecology. 2004;85:3119–3131. [Google Scholar]
- 29.Crozier L, Dwyer G. Combining population-dynamic and ecophysiological models to predict climate-induced insect range shifts. Am Nat. 2006;167:853–866. doi: 10.1086/504848. [DOI] [PubMed] [Google Scholar]
- 30.Martin PR, McKay JK. Latitudinal variation in genetic divergence of populations and the potential for future speciation. Evolution (Lawrence, Kans) 2004;58:938–945. doi: 10.1111/j.0014-3820.2004.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 31.Williams SE, Bolitho EE, Fox S. Climate change in Australian tropical rainforests: An impending environmental catastrophe. Proc R Soc London Ser B Biol Sci. 2003;270:1887–1892. doi: 10.1098/rspb.2003.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann AA, Weeks AR. Climatic selection on genes and traits after a 100 year-old invasion: A critical look at the temperate-tropical clines in Drosophila melanogaster from eastern Australia. Genetica. 2007;129:133–147. doi: 10.1007/s10709-006-9010-z. [DOI] [PubMed] [Google Scholar]
- 33.Pertoldi C, Bach LA. Evolutionary aspects of climate-induced changes and the need for multidisciplinarity. J Therm Biol. 2007;32:118–124. [Google Scholar]
- 34.Davis MB, Shaw RG. Range shifts and adaptive responses to Quaternary climate change. Science. 2001;292:673–679. doi: 10.1126/science.292.5517.673. [DOI] [PubMed] [Google Scholar]
- 35.Dynesius M, Jansson R. Evolutionary consequences of changes in species' geographical distributions driven by Milankovitch climate oscillations. Proc Natl Acad Sci USA. 2000;97:9115–9120. doi: 10.1073/pnas.97.16.9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pounds JA, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 37.Whitfield SM, et al. Amphibian and reptile declines over 35 years at La Selva, Costa Rica. Proc Natl Acad Sci USA. 2007;104:8352–8356. doi: 10.1073/pnas.0611256104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams JW, Jackson ST, Kutzbacht JE. Projected distributions of novel and disappearing climates by 2100 AD. Proc Natl Acad Sci USA. 2007;104:5738–5742. doi: 10.1073/pnas.0606292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.