Abstract
Most natural proteins performing sophisticated tasks contain multiple domains where an active site is located at the domain interface. Comparative structural analyses suggest that major leaps in protein function occur through gene recombination events that connect two or more protein domains to generate a new active site, frequently occurring at the newly created domain interface. However, such functional leaps by combination of unrelated domains have not been directly demonstrated. Here we show that highly specific and complex protein functions can be generated by joining a low-affinity peptide-binding domain with a functionally inert second domain and subsequently optimizing the domain interface. These directed evolution processes dramatically enhanced both affinity and specificity to a level unattainable with a single domain, corresponding to >500-fold and >2,000-fold increases of affinity and specificity, respectively. An x-ray crystal structure revealed that the resulting “affinity clamp” had clamshell architecture as designed, with large additional binding surface contributed by the second domain. The affinity clamps having a single-nanomolar dissociation constant outperformed a monoclonal antibody in immunochemical applications. This work establishes evolutionary paths from isolated domains with primitive function to multidomain proteins with sophisticated function and introduces a new protein-engineering concept that allows for the generation of highly functional affinity reagents to a predefined target. The prevalence and variety of natural interaction domains suggest that numerous new functions can be designed by using directed domain interface evolution.
Keywords: affinity reagent, epitope, molecular evolution, protein design, PDZ domain
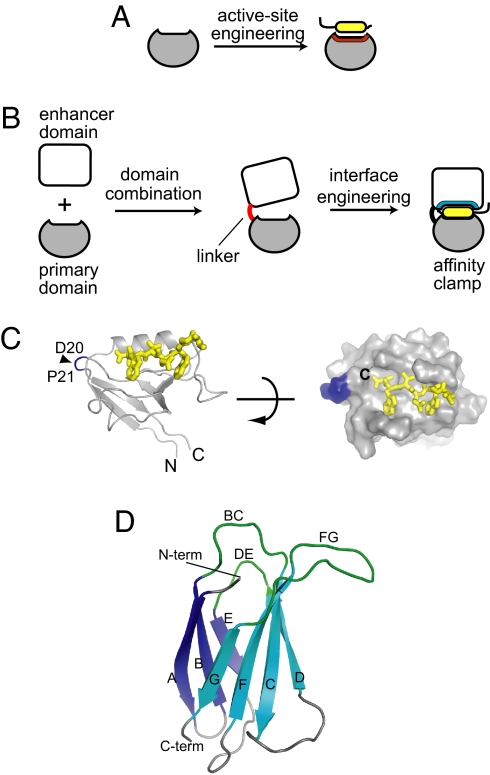
Directed evolution-based protein engineering creates new protein functions by exploiting processes that occur during natural evolution of proteins. Protein evolution progresses via point mutations, duplication, and recombination of genes under selective pressure. The processes of gene duplication and subsequent sequence divergence (1) have been successfully recapitulated in directed evolution and computational protein design (2, 3) where preexisting active sites within natural protein scaffolds are altered to produce new functions (Fig. 1A) (4–7). However, this type of functional evolution generally is incremental and requires a starting scaffold that is already predisposed to the desired type of function (6, 8, 9).
Fig. 1.
The concept of directed domain interface evolution and building blocks used in this work. (A and B) Comparison of domain interface engineering with conventional protein engineering. In the conventional engineering that mimics gene duplication and sequence divergence (A), the interface predefined in the starting scaffold is altered/refined, which tends to produce incremental changes in function. In contrast, domain interface engineering that mimics gene combination and sequence divergence (B) produces a new functional site at the interface between two domains, which can result in a major leap in protein function. (C) The structure of the Erbin PDZ bound to a peptide (PDB entry 1MFG). The N and C termini are indicated. The positions for the new termini of the circularly permutated PDZ (cpPDZ) are shown with a triangle and residue numbers. Right shows the surface of the PDZ domain with the peptide as a stick model, illustrating the shallow binding pocket. (D) The structure of FN3 (PDB entry 1FNF). The loops that are diversified to construct combinatorial libraries are labeled. The termini are also labeled. Note that the N terminus and the recognition loops are located on the same side of the FN3 protein.
Concatenations of protein domains due to gene recombination have long been considered the driving force for major leaps in protein function (10–13). Autonomously folded single domains of ≈100 residues usually possess an active site for primitive binding function with low specificity (14), whereas multidomain proteins such as enzymes often form an active site for sophisticated function at the interface between domains (11, 13). Enzymes such as NAD-dependent dehydrogenases consist of a combination of a common nucleotide-binding domain and a unique substrate-binding domain (11). Individual domains of eukaryotic, multidomain proteins are often encoded in an exon. Together, these observations of modern proteins support a plausible evolutionary path for the generation of a new active site for sophisticated function at the interface between two newly combined, evolutionally unrelated domains. However, such a path has not been directly demonstrated or exploited in the design of new protein functions.
Here we present directed evolution of protein function using a new protein-engineering concept, termed “directed domain interface evolution,” which is inspired by natural protein evolution through domain combination. This method combines a natural interaction domain (referred to as “primary domain”) (14) with another unrelated domain (“enhancer domain”), followed by combinatorial optimization of the enhancer domain surface in a process that mimics natural sequence divergence under selective pressure (Fig. 1B). The resulting two-domain protein possesses an active site that is distinctly different from those of either starting domain. This method is distinct from concatenation of multiple interaction domains in the rewiring studies of signaling networks where individual domains retain their respective functions (15). It also differs from homologous recombination and DNA shuffling applied to members within a protein family (16, 17). Here we demonstrate the impact of directed domain interface evolution by using it to address two major challenges in the design of protein-based affinity reagents (18): development of high-affinity reagents for short peptide motifs and targeting affinity reagents to a predefined epitope. We collectively term this class of engineered molecules “affinity clamps.”
Results and Discussion
Affinity Clamp Design.
In our design, the chosen primary domain is the Erbin PDZ domain (96 residues), one of the best characterized protein domains known (19). PDZ domains are small globular domains that primarily bind to C-terminal sequences in target proteins with low affinity. Erbin-PDZ binds with a low-micromolar dissociation constant (Kd) to the C termini of p120-related catenins [δ-catenin and Armadillo repeat gene deleted in Velo-cardio-facial syndrome (ARVCF)] (20) using a shallow cleft for peptide binding (Fig. 1C), a commonly observed mode of binding among interaction domains. For our enhancer domain, we chose the 10th fibronectin type III domain of human fibronectin (FN3; 91 residues). FN3 is a robust scaffold for producing antibody-like binding proteins with three loops located at one end of the molecule that can be extensively diversified to create a repertoire of binding surfaces (Fig. 1D) (21, 22). No known natural proteins contain both PDZ and FN3 domains, and the naive FN3 scaffold used here has no significant affinity to the PDZ targets.
We first sought to address the topological challenge in constructing the clamshell architecture necessary for the formation of the domain interface surrounding the active site [Fig. 1B and supporting information (SI) Fig. S1]. As is common among interaction domains (14), the N and C termini of Erbin-PDZ are located on the opposite side of the peptide-binding site (Fig. 1C). Consequently, simply connecting FN3 at a PDZ terminus would not orient FN3 in such a way that its recognition loops are proximal to the peptide-binding cleft of the PDZ domain. Thus, new N and C termini were created in the PDZ domain by circular permutation, with Pro21 as the new N terminus and Asp20 as the new C terminus (residue numbering is according to PDB entry 1N7T; Fig. 1C and Fig. S1). This structural alteration mildly affected the PDZ function with an ≈10-fold reduction in the affinity toward the ARVCF peptide. In nature, such circular permutation can occur as a result of gene duplication, and so it is evolutionarily accessible and relevant (23). Indeed, the Htr family of PDZ domains has a topology similar to the circularly permutated erbin PDZ domain (24).
The C terminus of the circularly permutated PDZ (hereafter termed cpPDZ) and the N terminus of FN3 were connected with a five-residue linker (GGSGG). The resulting two-domain protein is termed cpPDZFN. As expected, this domain combination did not significantly affect the peptide-binding function of the PDZ domain (Table 1; note that the affinity decrease seen in cpPDZFN relative to PDZ in Table 1 is due to circular permutation). We then constructed a combinatorial phage-display library of ≈109 independent sequences in which three surface loops of FN3 were diversified (Table 1). After three rounds of library sorting using an eight-residue peptide corresponding to the C-terminal sequence of ARVCF, two clones exhibiting high affinity to the ARVCF peptide were identified (termed ePDZ-a and ePDZ-b, respectively; “e” stands for “enhanced”; Table 1).
Table 1.
Library design and binding parameters of affinity clamps
| Protein | BC loop (25–30)* | DE loop (52–55)* | FG loop (75–83)* | kon,† M−1S−1 (ARVCF) | koff,† S−1 (ARVCF) |
Kd, nM |
IC50,† nM (ARVCF) | Affinity enhancement‡ | Specificity index§ | |
|---|---|---|---|---|---|---|---|---|---|---|
| ARVCF | δ-catenin | |||||||||
| Library¶ | X4–8 VX | (SY)(GSY) (S/Y)2 | X8–14 | — | — | — | — | — | — | — |
| PDZ | — | — | — | ND | ND | 2,200 | 6,300 | 3,000‖ | — | 3 |
| cpPDZFN | SSSSVS | GSKS | SSSSSSSSS | ND | ND | 24,800 | >25,000 | ND | 1 | ND |
| ePDZ-a | SYYGVS | YSSS | YSDYYGSHHY | 2.9 × 105 | 1.5 × 10−2 | 56 ± 5 | 429 | 40 | 520 | 8 |
| ePDZ-b | YYDSHVS | GSKS | HYNYHYYS | 1.9 × 105 | 1.1 × 10−2 | 56 ± 6 | >25,000 | 59 | 520 | >446 |
| ePDZ-b1 | YRELPVS | GSKS | HYNYHYYS | 7.3 × 104 | <3.7 × 10−4 | <5 | >25,000 | ND | >4,960 | >5,000 |
| ePDZ-b2 | FTDLPVS | GSKS | HYNYHYYS | 7.0 × 104 | <2.9 × 10−4 | <4 | >25,000 | ND | >6,200 | >6,250 |
ND, not determined.
*The residue numbering is according to that in Koide et al. (21).
†The kon and koff are shown for the ARVCF peptide. IC50 for the ARVCF peptide was determined by using competitive phage ELISA.
‡The affinity enhancement is defined as the ratio of the Kd of the parent protein (cpPDZFN) for ARVCF binding to that of an affinity clamp.
§The specificity index is defined as the ratio of the Kd for δ -catenin to the Kd for ARVCF.
¶The combinatorial library was constructed by diversifying the BC, DE, and FG loops of FN3. X denotes an amino acid mixture consisting of 40% Tyr, 20% Ser, 10% Gly, and 5% each of A, D, H, L, N and R.
‖Taken from Zhang et al. (42).
Affinity and Specificity of Affinity Clamps.
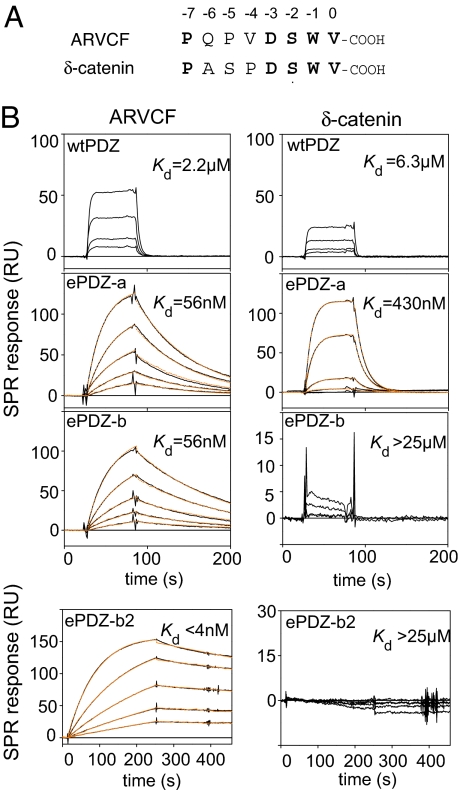
The two ePDZ clones were then expressed as free proteins in Escherichia coli. They were produced at high levels and highly soluble. Their binding kinetics measured by surface plasmon resonance (SPR) showed that they bound to the ARVCF peptide with Kd values of ≈60 nM, a 500 times higher affinity than that of the naive cpPDZ-FN3 fusion protein (Fig. 2B and Table 1). A cycle of affinity maturation of ePDZ-b produced second-generation affinity clamps with Kd values in the single-nanomolar range and dissociation half-lives of nearly 1 h (termed ePDZ-b1 and ePDZ-b2, respectively; Fig. 2B and Table 1). These values are comparable to those found for antibody–antigen interactions. Importantly, the affinity enhancement of >6,000-fold relative to cpPDZ (>500-fold relative to wild-type PDZ) (Table 1) by the affinity clamp strategy is far superior to the enhancement achieved by simple optimization of the peptide-binding interface of another PDZ domain alone (25), demonstrating the capacity of directed domain interface evolution to acquire function that is otherwise unattainable by manipulating only the primary domain.
Fig. 2.
Target binding properties of affinity clamps. (A) Amino acid sequences of the target peptides. Residue numbers are according to the convention for PDZ ligands. Identical residues between the two peptides are shown in bold. (B) SPR sensorgrams for the interaction of the ARVCF peptide (Left) and the δ-catenin peptide (Right) to wild-type PDZ, ePDZ-a, ePDZ-b, and ePDZ-b2. The target peptides were fused to SUMO for SPR measurements. Sensorgrams with 0, 10, 20, 40, 80, and 160 nM peptide are in black, and the global fittings of the 1:1 Langmuir binding model are in orange. Kd values are also shown. Kd values for wild-type PDZ were determined from equilibrium analyses using a broader range of target concentration than shown here.
Directed domain interface evolution was also highly effective in enhancing binding specificity. The homologous peptide targets ARVCF and δ-catenin (Fig. 2A) provide a stringent test for the binding specificity of affinity clamps. Whereas the parent PDZ domain and ePDZ-a did not greatly discriminate the two peptides, the ePDZ-b family bound very weakly to δ-catenin (Kd > 25 μM), discriminating the two targets by as much as 6,000-fold (Fig. 2B and Table 1). Interestingly, the affinity of ePDZ-b1 and -b2 toward the δ-catenin peptide was weaker than that of the parent PDZ domain, suggesting that the enhancer domain can not only enhance the affinity toward a cognate target but also reduce the binding affinity of the primary domain, probably by competing against a noncognate target.
In the absence of the attached PDZ domain, the FN3 variants of these affinity clamps showed no detectable binding to the ARVCF peptide (data not shown). These results indicate that the FN3 domain of the affinity clamps recognize their target only when it is connected to the primary domain.
The X-Ray Crystal Structure of an Affinity Clamp.
To determine whether the observed enhancements in affinity and specificity were due to successful construction of the designed architecture, we then characterized the structure of an affinity clamp. The NMR spectrum of ePDZ-a showed excellent dispersion, indicative of a well structured protein. An addition of the ARVCF peptide caused significant changes in the spectrum, consistent with the presence of a large binding interface (Fig. S2).
The x-ray crystal structure of the ePDZ-a/ARVCF peptide complex at a 1.8-Å resolution revealed clamshell architecture as designed (Fig. 3A; statistics are given in Table S1), and the peptide was deeply buried at the domain interface (Fig. 3B). The circular permutation did not significantly alter the overall fold of the PDZ domain (Fig. 3D), and consequently the peptide–PDZ interactions were well conserved. Likewise, the FN3 structure was well maintained except for the mutated loops.
Fig. 3.
The x-ray crystal structure of ePDZ-a in complex with the ARVCF peptide. (A) Ribbon representations of the overall structure. The cpPDZ and FN3 portions and the peptide are shown in gray, cyan, and yellow, respectively. Missing residues for the linker segment are indicated with dashed lines. (B) Clamping of the peptide by ePDZ-a. Only the region in the dashed box in A is shown. The surfaces originating from the cpPDZ and FN3 portions are shown in gray and cyan, respectively, and the peptide is shown as a yellow stick model. (C) Interactions of the FN3 loops with the cpPDZ/peptide complex. The three FN3 loops (BC, DE, and FG loops) are shown as sticks in blue, cyan, and red, respectively. The surface of the PDZ portion is shown in gray, and the peptide is shown as yellow spheres. In Lower, the surfaces of the PDZ and peptide portions in contact with the BC, DE, and FG loops (within 5 Å are shown in blue, cyan, and red, respectively, and those in contact with both BC and FG loops are in magenta. The peptide surfaces without FN3 contact are shown in yellow, and the green line encloses the bound peptide. (D) Superposition of wild-type PDZ (PDB entry 1MFG, green) and cpPDZ (gray). The original and new termini are indicated. The rmsd for the equivalent 97 Cα atoms was 0.54 Å. The structures are in the same orientation as in A.
The interface structure rationalized the dramatic affinity enhancement. The affinity clamp buried 69% (844 Å2) of the solvent-accessible surface area of the peptide, a 3.5-fold increase with respect to that by the PDZ domain only. The three mutated loops of the FN3 domain all interacted with the peptide and/or the PDZ domain, with a binding interface diagonally covering the surfaces of both cpPDZ and the peptide (Fig. 3C), reminiscent of the mode of recognition of the MHC/peptide complex by the T cell receptor (26). The newly introduced contacts mediated by the FN3 loops are predominantly hydrophobic, with only three hydrogen bonds between FN3 and the peptide. Details of the interactions are shown in Fig. S3. The shape complementarity between FN3 and the cpPDZ/peptide complex was unusually high with a shape correlation (Sc) value (27) of 0.79. Thus, the significantly enlarged interface with tight packing provides the enhanced affinity of ePDZ-a to the target peptide.
Affinity Clamps as Antibody Alternatives.
The affinity clamps are excellent tools in common immunochemical analyses. The affinity clamps are robust and stable proteins. They remained monomeric and retained full activity after incubation at 50°C for 2 h (Fig. 4 A and B). Both ePDZ-b1 and ePDZ-b2 in the form of an alkaline phosphatase fusion protein outperformed a monoclonal antibody that was raised against a C-terminal region of ARVCF (28) in Western blotting for ARVCF expressed in mammalian cells (Fig. 4C). They specifically precipitated a protein tagged with the ARVCF peptide from E. coli lysate with nearly stoichiometric efficiency, whereas naive cpPDZ did not precipitate an appreciable amount of the target (Fig. 4D). These results demonstrate the effectiveness of affinity clamps as an antibody alternative. Because affinity clamps are recombinant proteins, they offer additional favorable attributes including the “monoclonal” nature, the ease of production, storage and distribution, the immediate access to amino acid and DNA sequences, and the ease of reformatting into various fusion proteins.
Fig. 4.
Stability and applications of affinity clamps. (A) Gel-filtration chromatograms of ePDZ-a before (dashed line) and after (solid line) heat treatment (2 h at 50°C). Gel filtration was performed by using a Superdex75 column (Amersham Biosciences) in PBS (pH 7.4). (B) The SPR sensorgrams of ePDZ-a before (dashed line) and after (solid line) the same heat treatment as in A. (C) Western blotting of wild-type ARVCF in mammalian cell lysates. Lysates of an MDCK cell line stably expressing human ARVCF (denoted as +) (41) and the parent cell line (−) were detected with ePDZ-b2 fused with alkaline phosphatase. mAb indicates a positive control with anti-ARVCF monoclonal antibody 4B1 (41). Left shows Coomassie brilliant blue staining, and Right shows Western blotting. (D) Pull-down (immunoprecipitation) of SUMO tagged with the ARVCF peptide from E. coli lysate by affinity clamps. The lysate was mixed with an affinity clamp immobilized to streptavidin magnetic beads. SDS/PAGE of the input (I), unbound (U), wash (W), and bound (B) fractions visualized with Coomassie brilliant blue staining are shown. “Beads” indicates a control experiment without an immobilized affinity clamp. “cpPDZFN” indicates a control experiment using cpPDZ fused to the unmodified FN3 scaffold. The position of the captured target is marked with the triangle for ePDZ-b2 (lane B), and the equivalent position is also marked for cpPDZFN. “Binder” indicates the position of ePDZ-b2 and cpPDZFN, and “SA” indicates the position of streptavidin.
Conclusions
Our results demonstrate evolutionarily accessible paths where major functional leaps are produced by domain combination followed by interface optimization. They imply that distinct and sophisticated functions can emerge from combinations of a limited number of primordial domains.
Directed domain interface evolution considerably expands the scope of protein engineering. It provides a rational guideline for producing high-performance affinity reagents targeted to a predefined target motif. These unique features offer a clear advantage over conventional antibodies and antibody mimics whose epitopes cannot be defined a priori. Clearly, binding specificity of affinity clamps can be significantly altered by replacing the primary domain or introducing mutations in the primary domain. There exist diverse families of interaction domains that possess characteristics similar to the PDZ domain used in this work (14). Therefore, we anticipate that affinity clamps with novel functions can be readily produced by using directed domain interface evolution. Such affinity clamps will be effective tools in many fields including proteomics, cell biology, and drug discovery.
Experimental Procedures
Preparation of Peptide Targets.
Target peptides corresponding to the C-terminal eight residues of ARVCF and δ-catenin (NH2-PQPVDSWV-COOH and NH2-PASPDSWV-COOH, respectively) were prepared as C-terminal fusion to yeast SUMO (29) and cloned in the pHFT2 vector. The pHFT2 vector is a derivative of pHFT1 that contains a His10 tag instead of a His6 tag (30). The proteins were expressed in BL21(DE3) and purified by Ni affinity chromatography using standard methods described previously (21). The SUMO-peptide fusion proteins were cleaved with SUMO-hydrolase (29), and the cleaved peptide was purified by reversed-phase HPLC.
The ARVCF peptide was synthesized by using standard Fmoc protocols and purified by reversed-phase HPLC. The peptide was biotinylated by using sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate (EZ-Link Sulfo-NHS-SS-Biotin; Pierce) following the protocols provided by the manufacturer and purified by using reversed-phase HPLC. The purity and identity of the peptide were verified by liquid chromatography/mass spectrometry (LC/MS).
Construction of Phage Display Vectors and Combinatorial Libraries.
The locations of the new PDZ termini were chosen based on effects of Gly4 insertion mutagenesis. The phage display vectors contained the DsbA signal sequence (31), the circularly permutated Erbin PDZ domain, and the FN3 domain (22), in which the PDZ and FN3 domains were connected with a GGSGG linker. A stabilization mutation, D7N (32), was also introduced to FN3. Combinatorial libraries in which the BC, DE, and FG loops of FN3 were diversified were constructed by using Kunkel mutagenesis as previously described (22, 33, 34). Phage particles were prepared in the absence of isopropyl β-d-1-thiogalactopyranoside.
Library Sorting.
A combinatorial library of ≈109 independent clones was sorted with the biotinylated ARVCF peptide as the target. Phage display sorting was performed by using streptavidin-coated magnetic beads (Streptavidin MagneSphere Paramagnetic Particles; Promega) and a Thermo Kingfisher instrument as previously described (35). A total of four rounds of sorting of the initial library were performed by using decreasing target concentrations of 100, 20, 4, and 1 nM. The amino acid sequences of affinity clamp clones were deduced by DNA sequencing.
The second-generation library of ePDZ-b was constructed by diversifying either only the BC or FG loop of ePDZ-b. The resulting library was sorted twice as described above, followed by two rounds of off-rate selection performed as follows. Phages were first mixed with the biotinylated target, and, after 15 min of incubation, 1,000-fold excess of the nonbiotinylated form of the SUMO-ARVCF peptide fusion protein was added as a competitor. Phages were captured with streptavidin-coated magnetic beads, and the magnetic beads were incubated in the washing buffer [TBS containing 0.05% Tween (TBST)] with vigorous mixing for 3 minutes. After a total of three washing periods, phages were recovered.
Protein Purification.
The affinity clamp genes in the enriched pool of phage clones were transferred into the pHFT-2 expression vector, and individual clones were expressed as soluble proteins with a His10 tag. Proteins were purified with Ni affinity chromatography followed by a Sephacryl S-100 column in PBS [50 mM sodium phosphate containing 150 mM NaCl (pH 7.4)] to ensure that proteins were monomeric. For protein crystallization, the N-terminal His10 tag was cleaved with TEV protease and the cleaved protein was purified by passing through a Ni-Sepharose column. For NMR characterization, proteins were expressed in the M9 minimal media with 15NH4Cl as the sole nitrogen source.
Affinity Measurements.
SUMO fusion peptides were used to facilitate SPR detection. SPR measurements were performed in 20 mM Hepes (pH 7.4), 150 mM NaCl, and 0.005% Tween 20 on a BIAcore 2000 instrument. An affinity clamp was immobilized on a Ni-NTA chip to the level of approximate 300 RU, and the interaction with a SUMO-peptide fusion protein was monitored. The lack of interaction between SUMO and the affinity clamps was confirmed through competition experiments using the synthetic ARVCF peptide in the phage display format.
Competition Phage ELISA.
Biotinylated ARVCF peptide was immobilized in the wells of a Maxisorp plate (NUNC) that had been coated with neutravidin and blocked with BSA. Phages displaying an affinity clamp were preincubated for 45 min with serial dilutions of nonbiotinylated ARVCF peptide in 0.05% TBST and then captured in the wells containing the immobilized peptide. After incubating for 30 min, the plate was washed with TBST and incubated with horseradish peroxidase-conjugated anti-M13 antibody (Amersham) and detected with one-step Turbo-ELISA (Pierce).
X-Ray Crystallography.
The ePDZ-a/ARVCF peptide complex was crystallized in 1.0 M (NH4)2HPO4 containing 0.1 M Tris·HCl (pH 9) by using the hanging drop vapor diffusion method. Crystals were cryoprotected in the mother solution containing 20% glycerol and flash-frozen in liquid nitrogen. The x-ray data were collected at APS beamline 24-ID (Advanced Photon Source at Argonne National Laboratory). X-ray diffraction data were processed with HKL2000 (36). The structure was determined by molecular replacement with the program MOLREP in CCP4 (37). The structures of an FN3 mutant (PDB ID code 2OBG) and Erbin PDZ domain (PDB ID code 1MFG) were used as the search models. Refmac5 (38) was used for the structural refinement. Model building was carried out by using the program Coot (39). The structure of engineered loops, linker, and ARVCF peptide were built at this stage. Molecular graphics were generated by using Pymol (www.pymol.org). Data collection and refinement statistics are listed in Table S1.
Pull-Down Assay.
Affinity clamps were biotinylated as described above. A biotinylated affinity clamp was immobilized onto streptavidin-coated magnetic beads. After washing the beads with PBS (pH 7.4), E. coli BL21(DE3) cell lysate containing a trace amount of SUMO-ARVCF was added to the beads and incubated for 45 min. After further washing steps, the beads were resuspended in PBS and SDS/PAGE sample buffer. The captured proteins were analyzed by SDS/PAGE stained with Coomassie brilliant blue. A control experiment was carried out with the magnetic beads to which no affinity clamp had been added.
Western Blotting.
Alkaline phosphatase fusion proteins of affinity clamps were made by cloning the alkaline phosphatase gene (a gift of Brian Kay, University of Illinois, Chicago, IL) (40) to the 3′ of the affinity clamp gene in the phage display vector described above in such a way that the alkaline phosphatase replaces the phage pIII protein. The fusion proteins were expressed in XL1-Blue cells, and the periplasmic fraction containing AP-affinity clamp fusion proteins was used for detection.
The Madin–Darby canine kidney II (MDCK II) cell line and the MDCK II cell line stably transfected with a human ARVCF expression vector were kindly provided by Albert B. Reynolds (Vanderbilt University, Nashville, TN) and cultured as described (41). Cells were washed once with PBS at room temperature, then lysed in ice-cold lysis buffer (0.5% Nonidet P-40/50 mM Tris, pH 7.4/150 mM NaCl) containing protease inhibitor mixture. Lysates were cleared with centrifugation, and protein concentration was estimated with SDS/PAGE. Cell lysates were separated by SDS/PAGE using 4–20% Precise Gel (Pierce) and blotted to the Immobilon-FL membrane (Millipore). The membrane was probed with the alkaline phosphatase fusion protein described above using BCIP/NBT (5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium) Color Development Substrate (Promega). A monoclonal antibody for ARVCF (mAb 4B1; Santa Cruz Biotechnology) was used as a positive control, and its binding was detected with alkaline phosphatase-conjugated secondary antibody (Promega).
Supplementary Material
Acknowledgments.
We thank V. Tereshko, B. Pentelute, R. Jones, and W. Jun for assistance; A. Reynolds and B. Kay for reagents; and A. Kossiakoff and R. Gilbreth for discussion. This work was supported in part by National Institutes of Health Grants R01-GM072688 and R21-CA132700 and by the University of Chicago Cancer Research Center. The General Medicine and Cancer Institutes Collaborative Access Team (GM/CA CAT) has been funded in whole or in part with federal funds from the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Science (Y1-GM-1104). Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under Contract DE-AC02-06CH11357.
Footnotes
Conflict of interest statement: J.H., A.K., and S.K. are named as the inventors of a patent application on the technology described in this article.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2QBW).
This article contains supporting information online at www.pnas.org/cgi/content/full/0801097105/DCSupplemental.
References
- 1.Todd AE, Orengo CA, Thornton JM. Sequence and structural differences between enzyme and nonenzyme homologs. Structure. 2002;10:1435–1451. doi: 10.1016/s0969-2126(02)00861-4. [DOI] [PubMed] [Google Scholar]
- 2.Pluckthun A, Mayo SL. The design of evolution and the evolution of design. Curr Opin Struct Biol. 2007;17:451–453. [Google Scholar]
- 3.Kortemme T, Baker D. Computational design of protein-protein interactions. Curr Opin Chem Biol. 2004;8:91–97. doi: 10.1016/j.cbpa.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- 5.Binz HK, Amstutz P, Pluckthun A. Engineering novel binding proteins from nonimmunoglobulin domains. Nat Biotechnol. 2005;23:1257–1268. doi: 10.1038/nbt1127. [DOI] [PubMed] [Google Scholar]
- 6.Looger LL, Dwyer MA, Smith JJ, Hellinga HW. Computational design of receptor and sensor proteins with novel functions. Nature. 2003;423:185–190. doi: 10.1038/nature01556. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikuni Y, Ferrin TE, Keasling JD. Designed divergent evolution of enzyme function. Nature. 2006;440:1078–1082. doi: 10.1038/nature04607. [DOI] [PubMed] [Google Scholar]
- 8.Persson H, Lantto J, Ohlin M. A focused antibody library for improved hapten recognition. J Mol Biol. 2006;357:607–620. doi: 10.1016/j.jmb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 9.De Genst E, et al. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc Natl Acad Sci USA. 2006;103:4586–4591. doi: 10.1073/pnas.0505379103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bashton M, Chothia C. The generation of new protein functions by the combination of domains. Structure. 2007;15:85–99. doi: 10.1016/j.str.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Rossmann MG, Moras D, Olsen KW. Chemical and biological evolution of nucleotide-binding protein. Nature. 1974;250:194–199. doi: 10.1038/250194a0. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert W. Why genes in pieces? Nature. 1978;271:501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- 13.Blake CCF. Do genes-in-pieces imply proteins-in-pieces? Nature. 1978;273:267. [Google Scholar]
- 14.Pawson T, Nash P. Assembly of cell regulatory systems through protein interaction domains. Science. 2003;300:445–452. doi: 10.1126/science.1083653. [DOI] [PubMed] [Google Scholar]
- 15.Dueber JE, Yeh BJ, Chak K, Lim WA. Reprogramming control of an allosteric signaling switch through modular recombination. Science. 2003;301:1904–1908. doi: 10.1126/science.1085945. [DOI] [PubMed] [Google Scholar]
- 16.Carbone MN, Arnold FH. Engineering by homologous recombination: Exploring sequence and function within a conserved fold. Curr Opin Struct Biol. 2007;17:454–459. doi: 10.1016/j.sbi.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Kolkman JA, Stemmer WP. Directed evolution of proteins by exon shuffling. Nat Biotechnol. 2001;19:423–428. doi: 10.1038/88084. [DOI] [PubMed] [Google Scholar]
- 18.Haab BB, et al. A reagent resource to identify proteins and peptides of interest for the cancer community: A workshop report. Mol Cell Proteomics. 2006;5:1996–2007. doi: 10.1074/mcp.T600020-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Skelton NJ, et al. Origins of PDZ domain ligand specificity. Structure determination and mutagenesis of the Erbin PDZ domain. J Biol Chem. 2003;278:7645–7654. doi: 10.1074/jbc.M209751200. [DOI] [PubMed] [Google Scholar]
- 20.Laura RP, et al. The Erbin PDZ domain binds with high affinity and specificity to the carboxyl termini of delta-catenin and ARVCF. J Biol Chem. 2002;277:12906–12914. doi: 10.1074/jbc.M200818200. [DOI] [PubMed] [Google Scholar]
- 21.Koide A, Bailey CW, Huang X, Koide S. The fibronectin type III domain as a scaffold for novel binding proteins. J Mol Biol. 1998;284:1141–1151. doi: 10.1006/jmbi.1998.2238. [DOI] [PubMed] [Google Scholar]
- 22.Koide A, Gilbreth RN, Esaki K, Tereshko V, Koide S. High-affinity single-domain binding proteins with a binary-code interface. Proc Natl Acad Sci USA. 2007;104:6632–6637. doi: 10.1073/pnas.0700149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponting CP, Russell RB. Swaposins: Circular permutations within genes encoding saposin homologues. Trends Biochem Sci. 1995;20:179–180. doi: 10.1016/s0968-0004(00)89003-9. [DOI] [PubMed] [Google Scholar]
- 24.Krojer T, Garrido-Franco M, Huber R, Ehrmann M, Clausen T. Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature. 2002;416:455–459. doi: 10.1038/416455a. [DOI] [PubMed] [Google Scholar]
- 25.Ferrer M, et al. Directed evolution of PDZ variants to generate high-affinity detection reagents. Protein Eng Des Sel. 2005;18:165–173. doi: 10.1093/protein/gzi018. [DOI] [PubMed] [Google Scholar]
- 26.Garcia KC, et al. An alphabeta T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. [PubMed] [Google Scholar]
- 27.Lawrence MC, Colman PM. Shape complementarity at protein/protein interfaces. J Mol Biol. 1993;234:946–950. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- 28.Hogrefe HH, Mullinax RL, Lovejoy AE, Hay BH, Sorge JA. A bacteriophage lambda vector for the cloning and expression of immunoglobulin Fab fragments on the surface of filamentous phage. Gene. 1993;128:119–126. doi: 10.1016/0378-1119(93)90162-v. [DOI] [PubMed] [Google Scholar]
- 29.Malakhov MP, et al. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J Struct Funct Genomics. 2004;5:75–86. doi: 10.1023/B:JSFG.0000029237.70316.52. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Koide A, Nettle KW, Greene GL, Koide S. Conformation-specific affinity purification of proteins using engineered binding proteins: Application to the estrogen receptor. Protein Expression Purif. 2006;47:348–354. doi: 10.1016/j.pep.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Steiner D, Forrer P, Stumpp MT, Pluckthun A. Signal sequences directing cotranslational translocation expand the range of proteins amenable to phage display. Nat Biotechnol. 2006;24:823–831. doi: 10.1038/nbt1218. [DOI] [PubMed] [Google Scholar]
- 32.Koide A, Jordan MR, Horner SR, Batori V, Koide S. Stabilization of a fibronectin type III domain by the removal of unfavorable electrostatic interactions on the protein surface. Biochemistry. 2001;40:10326–10333. doi: 10.1021/bi010916y. [DOI] [PubMed] [Google Scholar]
- 33.Koide A, Koide S. Monobodies: Antibody mimics based on the scaffold of the fibronectin type III domain. Methods Mol Biol. 2007;352:95–109. doi: 10.1385/1-59745-187-8:95. [DOI] [PubMed] [Google Scholar]
- 34.Sidhu SS, Lowman HB, Cunningham BC, Wells JA. Phage display for selection of novel binding peptides. Methods Enzymol. 2000;328:333–363. doi: 10.1016/s0076-6879(00)28406-1. [DOI] [PubMed] [Google Scholar]
- 35.Fellouse FA, et al. High-throughput generation of synthetic antibodies from highly functional minimalist phage-displayed libraries. J Mol Biol. 2007;373:924–940. doi: 10.1016/j.jmb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 37.Collaborative Computational Project. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 38.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 39.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 40.Han Z, Karatan E, Scholle MD, McCafferty J, Kay BK. Accelerated screening of phage-display output with alkaline phosphatase fusions. Comb Chem High Throughput Screen. 2004;7:55–62. doi: 10.2174/138620704772884823. [DOI] [PubMed] [Google Scholar]
- 41.Mariner DJ, Wang J, Reynolds AB. ARVCF localizes to the nucleus and adherens junction and is mutually exclusive with p120(ctn) in E-cadherin complexes. J Cell Sci. 2000;113:1481–1490. doi: 10.1242/jcs.113.8.1481. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, et al. Convergent and divergent ligand specificity among PDZ domains of the LAP and zonula occludens (ZO) families. J Biol Chem. 2006;281:22299–22311. doi: 10.1074/jbc.M602902200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.