Abstract
Primitive erythroid cells (EryP) are the earliest differentiated cell type of the mammalian embryo. They appear in the yolk sac by embryonic day 7.5, begin to enter the embryonic circulation 2 days later and continue to mature in a stepwise and synchronous fashion. Like their adult counterparts, EryP enucleate. However, EryP circulate throughout the embryo for several days before the first enucleated forms can be identified in the blood. We have used transgenic mouse lines in which GFP marks EryP to investigate this seemingly long lag and have identified a previously unrecognized developmental niche for EryP maturation. After exiting the yolk sac, EryP begin to express cell adhesion proteins, including α4, α5, and β1 integrins, on their surface and migrate into the fetal liver (FL), where they interact with macrophages within erythroblastic islands. Binding of EryP to FL macrophages in vitro is stage-specific and partly depends on VCAM-1. The ability to tag and track EryP nuclei using a transgenic mouse line expressing an H2B-EGFP fusion allowed us to identify and characterize extruded EryP nuclei and to demonstrate that molecules such as α4, α5, and β1 integrins are redistributed onto the plasma membrane surrounding the extruding nucleus. FL macrophages engulf extruded EryP nuclei in cocultures and in the native FL in vivo. We conclude that EryP home to, complete their maturation, and enucleate within the FL, a tissue that is just developing as EryP begin to circulate. Our observations suggest a simple solution for a puzzling aspect of the development of the primitive erythroid lineage.
Keywords: enucleation, mouse embryo, primitive erythropoiesis, fetal liver, macrophage
Primitive erythroid cells (EryP) are the first to differentiate in the postimplantation embryo and are detectable within the blood islands of the yolk sac by embryonic day 7.5 (E7.5) (1–3). EryP enter the circulation around E9.5 as a synchronous cohort and continue to mature in a stepwise, developmentally regulated fashion (4). Surprisingly, the terminal event in EryP differentiation, enucleation, is not detected for another 3 days (E12.5) (4, 5); it is largely completed by E15.5 (4).
To tag and track EryP within the mouse embryo, particularly after midgestation, when large numbers of definitive erythroid cells (EryD) are present in the blood, we used a transgenic mouse line in which GFP is expressed in EryP (4, 6). We found that circulating EryP expressed a subset of cell adhesion molecules, including integrins, on their surface beginning around E12.5 and then appeared to redistribute some of these proteins, notably α4, α5, and β1 integrins, from the membrane of the nascent EryP reticulocyte to that surrounding the expelled nucleus (4). On the basis of these observations, we proposed that at least a subset of EryP may home to the fetal liver (FL) (4).
The FL is a major site for the development of EryD (7), which mature within erythroblastic islands (EBIs). EBIs, first identified in bone marrow and later in FL and spleen, are morphologically distinct 3D structures comprising a central macrophage surrounded by EryD at various stages of maturation (for a review, see ref. 8). Scanning EM studies have revealed macrophage extensions that surround peripheral erythroblasts, providing intimate membrane contact between these cells. The central macrophages of the EBI are thought to function as nurse cells during erythropoiesis and to provide an essential scavenger function, engulfing nuclei expelled from the maturing erythroblasts (reviewed in refs. 8–10).
Here, we report that the FL provides a previously unrecognized developmental niche for the maturation and enucleation of EryP. As early as E10.5, EryP are found in the FL, within EBIs, and continue to accumulate there through E14.5. One day later, nearly all EryP have enucleated and reentered the circulation. Integrins α4, α5, and β1 are dramatically up-regulated on the surface of EryP that have entered the FL, reflected in greatly enhanced binding to FL macrophages (FLM) in vitro. Blocking of vascular cell adhesion molecule-1 (VCAM-1), a counterreceptor for very late antigen-4 (VLA-4 or α4β1 integrin) expressed on macrophages, abrogates this adhesion. To examine EryP enucleation at higher resolution, we generated a transgenic mouse line in which the nuclei of EryP are marked by a histone H2B-EGFP fusion protein. Extruded EryP nuclei were detected in large numbers in the FL, even at a time when reticulocytes are first detected in the blood. Our findings suggest that EryP enucleation begins earlier than previously believed, and that the resulting reticulocytes may not be released into the circulation immediately. Adhesion molecules (α4, α5, and β1 integrins) were greatly enriched on the surface of the extruded nuclei, demonstrating unequivocally that these proteins are selectively partitioned away from the body of the developing reticulocyte. FLM engulf extruded EryP nuclei in cocultures and in the FL in vivo. These findings suggest a simple explanation for the puzzling lag between the initial formation of EryP in the yolk sac and the appearance in the bloodstream of enucleated EryP and provide important insights into the cellular events involved in the development of this lineage.
Results
Primitive Erythroblasts Accumulate Transiently in the FL.
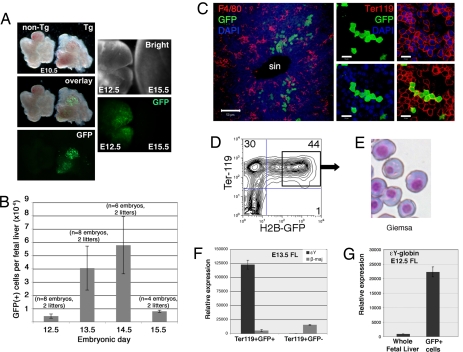
Our previous discovery that cell adhesion molecules are up-regulated on the surface of circulating EryP/GFP(+) cells (4) led us to consider the possibility that EryP home to a fetal tissue such as the liver, where they continue to mature. Indeed, we observed green fluorescence in the FLs of ε-globin::KGFP transgenic embryos, in which expression of the GFP reporter is targeted to EryP from a human embryonic (ε) globin promoter (see legend, Fig. 1), from E10.5 through E14.5 (Fig. 1A and data not shown). By E15.5, when the majority of EryP (>95%) have enucleated (4), GFP was no longer detectable (Fig. 1A). FACS analysis revealed a peak in the numbers of EryP/GFP(+) cells approximately E13.5–14.5, with a sharp reduction by E15.5 (Fig. 1B). Immunostaining of FL sections for the erythroid marker Ter119 or the macrophage marker F4/80 revealed that EryP/GFP(+) cells were present within the parenchyma of the FL in close association with macrophages (Fig. 1C). These observations suggest that EryP collect within and possibly home to the FL, a prominent site of definitive hematopoietic differentiation from around midgestation until the time of birth.
Fig. 1.
Transient accumulation of EryP within the FL. (A) Brightfield and fluorescent images of whole FLs from E10.5 (Left), E12.5 (left side of Right), and E15.5 (right side of Right) ε-globin::KGFP transgenic embryos. The transgene contained a minimal human embryonic ε-globin promoter and 3′ splice and polyadenylation signals, a truncated μLCR, and a GFP reporter (4, 6). GFP is expressed within the entire EryP cell. (B) Quantitation of GFP(+) cells per FL at different developmental stages. The numbers of embryos and litters examined are indicated. (C) (Left) Immunostaining of 20-μm cryosection from E14.5 FL. F4/80 was used as a macrophage marker. sin, sinusoid. (Scale bar, 50 μm.) (Right) High-magnification view of E14.5 FL section. Ter119 was used as a marker for erythroid cells. At this stage, both EryP [green, Ter119(+)] and EryD [red only, Ter119(+)] are seen. (Scale bars, 10 μm.) (D) Sorted Ter119(+)GFP(+) cells from ε-globin::H2B-EGFP FL show the characteristic morphology of EryP (Giemsa stain, E), express εY- (but not βmaj-) globin at high levels (F) and are greatly enriched for εY-globin RNA compared with total FL (G). Ter119(+)GFP(−) cells (EryD) express βmaj- but not εY-globin (F).
To tag, track, and FACS-purify EryP nuclei, we created a “second generation” transgenic mouse line, ε-globin::H2B-EGFP, which expresses a histone H2B-EGFP fusion protein that labels chromatin at all phases of the cell cycle (see refs. 11 and 12). The transgene contained the same regulatory elements used for the ε-globin::KGFP line (see legend, Fig. 1). To confirm that GFP(+) cells from the ε-globin::H2B-EGFP embryos are EryP, we analyzed their morphology and globin gene expression. FACS-sorted Ter119(+)GFP(+) cells from FL (Fig. 1D) and blood (data not shown) displayed the characteristic morphology (Fig. 1E) and large size (scatter data, data not shown) of EryP, express embryonic εY- (but not adult βmaj-) globin at high levels (Fig. 1F), and compared with total FL are greatly enriched for endogenous εY-globin RNA (Fig. 1G). In contrast, the Ter119(+)GFP(−) cells (EryD) expressed βmaj- but not εY-globin (Fig. 1F). Therefore, GFP detected in ε-globin::H2B-EGFP FLs reflects the transient accumulation of EryP within this tissue.
EryPs in the FL Up-Regulate Cell Adhesion Molecule Expression and Interact with Macrophages in EBIs.
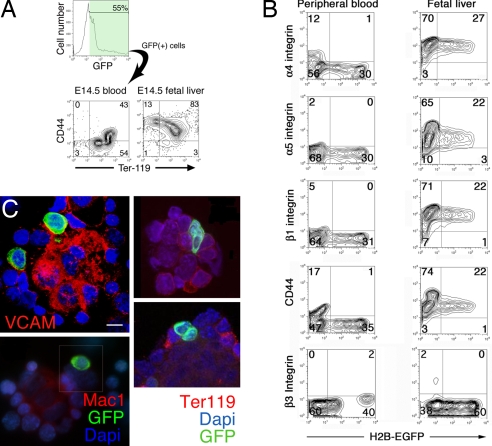
Trafficking of circulating cells to tissues requires up-regulation of surface adhesion molecule expression. We asked whether the progressive up-regulation of cell adhesion molecules detected on EryP in the blood (4) continued after these cells entered the FL. α4, α5, and β1 integrins and CD44 were all dramatically up-regulated on EryP/GFP(+) cells in the FL compared with those in the circulation (Fig. 2 A and B). Adhesion protein expression on FL-EryP was not promiscuous, however, because integrins β3 (Fig. 2B) and α2, αV, and β2 (data not shown) were not detected on EryP/GFP(+) in peripheral blood (PB-EryP) or FL. To determine whether EryP/GFP(+) cells are present within EBIs in the FL, native EBIs were isolated on coverslips [supporting information (SI) Fig. S1]. Coverslips were stained with antibodies against VCAM-1 or Mac1 to identify macrophages or for Ter119 to highlight erythroid cells (Fig. 2C). EryP/GFP(+) cells were clearly identified within structures resembling EBIs and containing a central macrophage. EBIs contained both EryP/GFP(+) cells and EryD, which do not express the transgene but stain for Ter119.
Fig. 2.
EryPs in the FL up-regulate cell adhesion molecule expression and interact with macrophages in EBIs. (A) EryP/GFP(+) cells were gated by FACS and evaluated for CD44 expression. CD44 was present at much higher (10- to 100-fold) levels on the Ter119(+)GFP(+) EryP in FL than on those in PB. (B) FACS analysis of adhesion molecules (4) on E14.5 ε-globin::H2B-EGFP EryP from blood and FL. α4, α5, and β1 integrins, like CD44, were expressed at considerably higher levels on FL vs. PB EryP. Comparable results were obtained using both transgenic (H2B-EGFP, nuclear, and KGFP, cytoplasmic and nuclear) lines. (C) Immunostaining analysis of native EBIs from ε-globin::KGFP transgenic FL (E14.5). Four examples are shown. (Left) VCAM-1 and Mac1 antibodies were used to detect macrophages. (Right), EBIs stained for Ter119 (erythroid). DAPI was used to stain nuclei. (Scale bar, 10 μm.)
The Ability of EryP to Bind to FLMs Is Developmentally Regulated and Depends on VCAM-1.
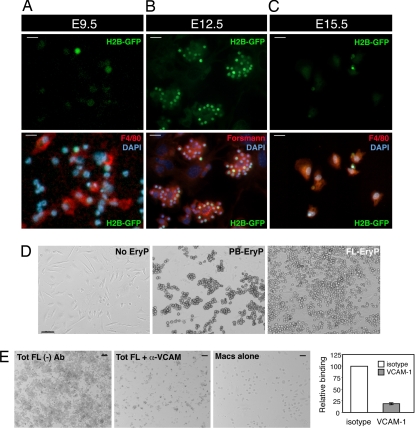
To dissect the molecular mechanisms underlying EryP maturation and enucleation, we established an EBI reconstitution assay in which EryP and macrophages are recombined and analyzed for binding. PB-EryP showed stage-dependent adhesion to FLMs (Fig. 3 A–C). E12.5–E14.5 PB-EryP bound rapidly (<45 min) to FLMs and formed large rosettes containing up to 20–30 EryP per macrophage (Fig. 3B and data not shown). Few rosettes formed when FLMs were combined with E9.5 EryP [which do not express the adhesion molecules we have examined to date (4), Fig. 3A] or E15.5 EryP (nearly all of which have enucleated) (Fig. 3C). The rosettes that did form from E9.5 or E15.5 EryP contained fewer than five EryP per macrophage. Therefore, circulating EryP can interact with FLMs during a distinct developmental window. It is worth noting that only nucleated EryP were found bound to macrophages; we have previously reported that the surface antigen phenotype of nucleated and enucleated EryP is distinct (4). Consistent with their high-level expression of cell adhesion molecules, the macrophage-binding capacity of FL-GFP(+)/EryP cells was significantly greater than that of PB-EryP (Fig. 3D).
Fig. 3.
Developmentally regulated,VCAM-1-dependent adhesion of EryP to FLM. (A–C) Heterologous EBIs were reconstituted from wild-type E14.5 macrophages and FACS-sorted EryP from PB of E9.5 (A), E12.5 (B), and E15.5 (C) ε-globin-H2B::EGFP embryos. E12.5 EryP adhered strongly to FLMs. Few if any EryP isolated at E9.5 (A) or E15.5 (C) adhered to FLMs. Note the larger size of E9.5 EryP nuclei (A) compared with E12.5 (B) or E15.5 (C) nuclei, which are highly condensed. (Scale bars, 20 μm.) Comparable results were obtained by using ε-globin::KGFP embryos. (D) Consistent with their higher expression of cell adhesion molecules, the ability of FL-EryP to bind to macrophages was much greater than that of PB-EryP. “No EryP” shows macrophages alone. (Scale bar, 50 μm.) (E) Adhesion of EryP to FLMs is mediated by VCAM-1. FLM and FL- or PB-EryP were incubated in medium containing blocking antibody for 45 min at 37°C. Coverslips containing adherent macrophages and EryP were then rinsed and prepared for microscopic analysis. (Scale bar, 50 μm.)
Integrins α4 and β1 (VLA-4) are strongly expressed on EryP within the FL (Fig. 2B). The counterreceptor for VLA-4 is VCAM-1, which we detected on FLMs by immunostaining of native EBIs (Fig. 2C) and by FACS analysis of FL cells (data not shown). We found that formation of EryP clusters on FLMs depended partly on VCAM-1 (Fig. 3E). A blocking antibody against VCAM-1 reduced adhesion of cells from total FL (Fig. 3E) or PB-EryP (data not shown) by 75–80%. The experiments of Figs. 1–3 suggest that additional maturation steps, beyond those previously described for circulating EryP (4), occur in the FL.
Redistribution of Cell Adhesion Molecules onto Extruding EryP Nuclei.
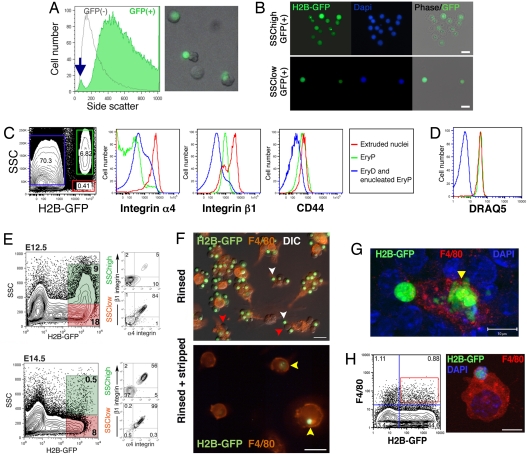
Our previous studies pointed to a selective reorganization of antigens such as α4 integrin from the nascent EryP reticulocyte to the plasma membrane surrounding the expelled nucleus (4). Scatter analysis of PB-EryP/GFP(+) cells from E12.5-E14.5 ε-globin::H2B-EGFP embryos revealed the presence of a rare subpopulation of GFP(+) cells (≈0.5–3%) with very low side scatter (SSC; Fig. 4A, blue arrow) and forward scatter (FSC; data not shown). These properties are indicative of the low granularity and small size expected for free nuclei. The more prominent GFP(+) SSChigh population contained large (high FSC), granular (high SSC) cells, as expected for EryP. In wet preps of GFP(+) cells from dispersed ε-globin::H2B-EGFP transgenic FL, we occasionally identified cells that appeared to be in the process of enucleating (Fig. 4A Inset) or structures that resembled isolated nuclei (data not shown). To determine whether the GFP(+)SSClow population contains extruded EryP nuclei, we sorted cells from E14.5 ε-globin::H2B-EGFP and examined them by fluorescence microscopy. DAPI exclusion of unfixed samples confirmed that the GFP(+)SSClow population does not contain dying cells. Fig. 4B shows images of cells from both GFP(+)SSClow and GFP(+)SSChigh populations. As we expected, the GFP(+)SSChigh population contained nucleated cells with a clearly identifiable cytoplasm and outer membrane, whereas the GFP(+)SSClow population comprised nuclear structures surrounded by only a narrow rim of cytoplasm (Fig. 4B). The GFP(+)SSChigh and GFP(+)SSClow populations both stained with DAPI (Fig. 4B) and with DRAQ5 (Fig. 4D), confirming their DNA content.
Fig. 4.
Redistribution of α4 and β1 integrins onto extruded nuclei from EryP. (A) Scatter analysis of the PB of E14.5 ε-globin::H2B-EGFP embryos revealed a small population (<0.5%) of GFP(+) cells with low granularity (SSC), suggestive of extruded nuclei. Fluorescent micrograph of wet prep of FACS sorted GFP(+) cells from a ε-globin::H2B-EGFP E14.5 FL. Cell (Center) appears to be in process of enucleation. (B) Fluorescent images of FACS-purified GFP(+) cells with low and high SSC. DAPI staining confirmed that both populations are nucleated. In contrast with the SSChigh cells, which by phase contrast were clearly surrounded by cytoplasm and a cell membrane, the cytoplasmic rim surrounding the SSClow nuclei was barely detectable. DAPI staining of nuclei coincided with expression of H2B-EGFP. (Scale bar, 10 μm.) (C) Ter119(+) cells from ε-globin::H2B-EGFP PB were gated and analyzed for expression of CD44, α4 and β1 integrin. Scatter analysis revealed GFP(+) cells with high (green box, EryP) and low (red box, extruded nuclei) SSC. The GFP(−) Ter119(+) population (blue box) contains enucleated EryP and EryD. Histograms show expression of α4 and β1 integrin and CD44 on each population. The expression of both integrins but not CD44 was much higher on the extruded nuclei (red) than on nucleated EryP (green). (D) FACS analysis of DRAQ5 stained Ter119(+) cells from PB. As expected, EryP and extruded nuclei, which contain DNA, stained with DRAQ5, whereas the enucleated EryP and EryD did not. Legend is the same as for C. (E) SSChighGFP(+) EryP and SSClowGFP(+) extruded nuclei were present in FL at E12.5 and E14.5. Surprisingly, in E12.5 FL, most (18/26 or ≈70%) EryP had already enucleated. By E14.5, very few nucleated EryP remained, and the numbers of extruded nuclei was also significantly smaller. At E12.5, very few SSChighGFP(+) expressed α4 or β1 integrins but by E14.5, the integrins were present on more than half of these cells. In contrast, large numbers of extruded SSClowGFP(+) nuclei displayed these adhesion molecules at high levels at both stages. (F) Imaging of extruded EryP nuclei and EryP-reticulocytes in EryP-macrophage cocultures. (Upper) Coculture after gentle rinsing of the coverslip to remove nonadherent cells. White arrowheads indicate free GFP(+) nuclei. Red arrowheads indicate EryP-reticulocytes (enucleated EryP). (Lower) Coculture after rinsing and stripping in PBS containing EDTA. Yellow arrowheads, engulfed nuclei (one of which is being degraded, Lower, right side). (Scale bars, 20 μm.) DIC, differential interference contrast. See also Fig. S3. (G) Macrophage engulfment of EryP nuclei in vivo. Merged rendered confocal image of cryosection (20 μm) of E14.5 ε-globin::H2B-EGFP FL, immunostained with F4/80 to identify FLMs. (For additional images, see Fig. S3.) The degrading nucleus is evident within the FLM (yellow triangle). (Scale bar, 10 μm.) (H) Immunofluorescence analysis of FACS-sorted F4/80(+)GFP(+) macrophages. Gate used for sorting is indicated by dashed box. Cytospin preparations from the sorted cells were stained for F4/80 to identify FLMs. A macrophage containing an EryP nucleus labeled with H2B-EGFP is shown. (For additional images, see Fig. S3). (Scale bar, 10 μm.)
To test our hypothesis (4) that selected cell adhesion molecules are sorted onto the membrane surrounding the extruding nucleus, we stained blood cells from ε-globin::H2B-EGFP embryos with antibodies against α4, α5, and β1 integrins and CD44 and analyzed their expression by FACS. Expression of integrins α4 and β1 (Fig. 4C; α5, data not shown) but not CD44 was much higher on extruded nuclei (red) than on EryP (green), indicating redistribution of these molecules during enucleation. Enucleated EryP and EryD (blue) displayed even lower levels of the integrins. The presence of free nuclei in circulation was consistent with the possibility that EryP enucleate in the blood. To determine whether FL-EryP were the source of the extruded nuclei, we analyzed cells dispersed from FL. A large population of GFP(+)SSClow extruded nuclei was already present in FL at E12.5 and, surprisingly, most (≈70%) of the EryP in the FL at this stage had apparently already enucleated (Fig. 4E). By E14.5, very few nucleated EryP remained and the population containing extruded nuclei was also significantly smaller, presumably reflecting active phagocytosis and degradation by macrophages. At E12.5, few GFP(+)SSChigh expressed α4 or β1 integrin but by E14.5, these molecules were present on more than half of the cells within this population. In contrast, large numbers of the extruded GFP(+)SSClow nuclei displayed these adhesion molecules at high levels at both stages.
Macrophage Engulfment of Extruded EryP Nuclei in Vitro and in Vivo.
To determine whether macrophages play a role in enucleation, we cultured ε-globin::H2B-EGFP PB-EryP alone or with adherent FLMs for 24 h using several different protocols (SI Methods). Nonadherent and loosely adherent cells were collected, stained with DRAQ5 and analyzed by FACS (Fig. S2). Large (FSChighSSChigh) cells were gated and the numbers of enucleated EryP (DRAQ5negGFPneg) measured. Statistically significant enhancement of enucleation in macrophage-containing cultures was not detected under any of the conditions tested, for EryP at E10.5, E12.5, or E14.5. Nevertheless, immunofluorescence analysis of the EBIs formed during coculture revealed not only adherent EryP but also EryP reticulocytes (enucleated) and extruded nuclei (Fig. 4F). We have not detected adhesion of enucleated EryP to macrophages in binding assays; therefore, we infer that those reticulocytes bound to FLMs after 24 h must have enucleated during culture. After rinsing the coverslips and stripping off bound EryP, we could identify macrophages containing engulfed nuclei, some in the process of degradation (Fig. 4F). Immunostaining of FL sections (Fig. 4G and Fig. S3B) and sorted F4/80(+)GFP(+) FLMs (Fig. 4H and Fig. S3C) revealed that FLMs engulf extruded nuclei in vivo and in vitro. We have not observed EryP in association with F4/80(+) macrophages in fetal tissues other than liver.
Discussion
The FL Is a Previously Unrecognized Niche for Primitive Erythroid Development.
EryP progenitors form and expand in the yolk sac of the mammalian embryo over a 2-day period (E7.5–E9.5), enter the newly functional circulation and continue to mature in a synchronous, stepwise developmental progression (4) that terminates in enucleation (4, 5). EryP are detected in the blood throughout gestation and constitute a stable cell population that is present as late as 3 weeks after birth (4). The findings presented here suggest a simple and elegant solution to the puzzling question of why enucleation of EryP is not detected until days after their appearance: terminal maturation, including nuclear extrusion, occurs in the FL, which does not form until midgestation. EryP are not simply nomadic cells but apparently home to the FL.
Function of Adhesion Molecules in Primitive Erythroid Maturation in the FL.
After entering the circulation, EryP begin to up-regulate the expression of a variety of adhesion proteins, including α4, α5, and β1 integrins and CD44 (4). Concomitant with their migration into the FL, a further, dramatic increase in adhesion molecule expression occurs. Whether the latter changes are cell autonomous or are triggered by extrinsic signals such as cytokines, interactions with other cells, and/or the hypoxic milieu of the FL is not known. However, they are of functional significance, because the ability of circulating EryP to bind to FLMs is developmentally regulated and maximal around the time of rapid enucleation (4) and EryP within the FL are able to bind to macrophages far more strongly than their circulating counterparts. After enucleation, the ability of circulating EryP to adhere to macrophages is lost and their numbers in the FL decline.
We propose that the alteration in macrophage-binding capacity is mediated, at least in part, by the partitioning of integrins α4, α5, and β1 onto the extruding nucleus. The resulting integrin-poor reticulocytes have now lost their counterreceptor for macrophage VCAM-1 and may more readily reenter the circulation. The redistribution of integrins during enucleation of EryP is likely preceded by modifications in their connections to components of the cytoskeleton (13). Partitioning of cell surface proteins is bidirectional: we have previously shown that Ter119 is present at higher levels on EryP reticulocytes than on nucleated EryP (4). We suggest that the mechanisms underlying enucleation in the primitive and definitive erythroid lineages are at least partly conserved. Selective partitioning of cell surface molecules on definitive erythroblasts (phosphatidylserine, β1 integrin, Ter119, and Emp) has been reported (13–16). Integrins α4 and α5 have also been detected on definitive erythroblasts in the FL (ref. 17 and our unpublished data), and we suspect that they are redistributed during EryD enucleation.
Although binding of EryP to FLMs in vitro depended on VCAM-1, little if any decrease in adhesion was observed when blocking antibodies against α4 or β1 integrins were used (data not shown). Similar results were obtained by others using a different α4 integrin blocking antibody, with only a modest inhibition of binding (18). We note that EryP may also interact with other components of the FL microenvironment (hepatoblasts, endothelial cells, extracellular matrix) during their maturation.
Monitoring Primitive Erythroblast Enucleation by Using a Histone H2B-EGFP Transgene Reporter.
The ε-globin::H2B-EGFP transgenic mouse line allowed us to identify and isolate newly extruded nuclei from PB and FL from E12.5 to E15.5. Similar structures were first described in the PB of hamster embryos (19) and have recently been reported in mouse embryos (18). The numbers of extruded EryP nuclei in circulation are much lower than in the FL, suggesting that a small fraction briefly escape engulfment by macrophages and enter the bloodstream. (We cannot exclude the possibility that some EryP enucleate while in circulation.) They presumably are phagocytosed later, when they circulate through FL or other tissues. That extruded EryP nuclei can be detected in the blood at all strongly suggests that they first become disconnected from EryP reticulocytes and are then engulfed by FLMs. We propose that adhesion molecules such as α4, α5, and β1 integrins create a sticky coating for the “shrink-wrapped” EryP nuclei and facilitate their phagocytosis. A model for the terminal steps in EryP maturation and enucleation is presented in Fig. S4.
Several lines of evidence suggest that macrophages are not essential for enucleation of definitive erythroblasts in vivo (9). In contrast with others (18), we have not found enhancement of EryP enucleation during coculture on FLMs. We believe our approach is rigorous, because we analyzed all of the cell populations in these experiments. Recently it was reported that proliferation of definitive erythroblasts is stimulated by coculture with macrophages (20). A possible role for macrophages in proliferation and/or later steps in the maturation of EryP maturation remains to be evaluated.
Materials and Methods
Transgenic Mouse Lines.
ε-globin::H2B-EGFP transgenic mice were generated by pronuclear injection of a construct analogous to ε-globin::KGFP (4, 6) at the Mount Sinai Mouse Genetics Shared Resource Facility and will be described elsewhere. Transgenic mice were maintained as hemizygotes or homozygotes on an ICR background; transgenic males were crossed with ICR females. All mice were bred at Mount Sinai School of Medicine according to institutional and American Veterinary Medical Association guidelines.
Supplementary Material
Acknowledgments.
We thank Dr. A.-K. Hadjantonakis (Memorial Sloan Kettering Institute, New York) for an H2B-EGFP fusion construct and for helpful discussions. We are grateful to Dr. M. A. Dyer for comments on the manuscript and to an anonymous reviewer for suggesting that we evaluate engulfment of extruded nuclei by macrophages in vivo. This work was supported by a postdoctoral fellowship from the Cooley's Anemia Foundation (J.I.) and by the National Institutes of Health (Grants RO1 DK52191, HL62248, and EB02209, to M.H.B.) and the Roche Foundation for Anemia Research (RoFAR, grant to M.H.B.). Transgenic mice were produced by the Mount Sinai Mouse Genetics Shared Research Facility (National Institutes of Health/National Cancer Institute Grant CA88302).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802032105/DCSupplemental.
References
- 1.Wong PM, Chung SW, Eaves CJ, Chui DH. Ontogeny of the mouse hemopoietic system. Prog Clin Biol Res. 1985;193:17–28. [PubMed] [Google Scholar]
- 2.Belaoussoff M, Farrington SM, Baron MH. Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development. 1998;125:5009–5018. doi: 10.1242/dev.125.24.5009. [DOI] [PubMed] [Google Scholar]
- 3.Ferkowicz MJ, Yoder MC. Blood island formation: Longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Fraser ST, Isern J, Baron MH. Maturation and enucleation of primitive erythroblasts is accompanied by changes in cell surface antigen expression patterns during mouse embryogenesis. Blood. 2007;109:343–352. doi: 10.1182/blood-2006-03-006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingsley PD, Malik J, Fantauzzo KA, Palis J. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004;104:19–25. doi: 10.1182/blood-2003-12-4162. [DOI] [PubMed] [Google Scholar]
- 6.Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- 7.Koury MJ, Sawyer ST, Brandt SJ. New insights into erythropoiesis. Curr Opin Hematol. 2002;9:93–100. doi: 10.1097/00062752-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Chasis JA. Erythroblastic islands: Specialized microenvironmental niches for erythropoiesis. Curr Opin Hematol. 2006;13:137–141. doi: 10.1097/01.moh.0000219657.57915.30. [DOI] [PubMed] [Google Scholar]
- 9.Spike BT, Dibling BC, Macleod KF. Hypoxic stress underlies defects in erythroblast islands in the Rb-null mouse. Blood. 2007;110:2173–2181. doi: 10.1182/blood-2007-01-069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spike BT, Macleod KF. Effects of hypoxia on heterotypic macrophage interactions. Cell Cycle. 2007;6:2620–2624. doi: 10.4161/cc.6.21.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadjantonakis A-K, Papaioannou VE. Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol. 2004;4:33. doi: 10.1186/1472-6750-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser ST, et al. Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in es cells and mice. Genesis. 2005;42:162–171. doi: 10.1002/gene.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JC, et al. Mechanism of protein sorting during erythroblast enucleation: Role of cytoskeletal connectivity. Blood. 2004;103:1912–1919. doi: 10.1182/blood-2003-03-0928. [DOI] [PubMed] [Google Scholar]
- 14.Geiduschek JB, Singer SJ. Molecular changes in the membranes of mouse erythroid cells accompanying differentiation. Cell. 1979;16:149–163. doi: 10.1016/0092-8674(79)90196-x. [DOI] [PubMed] [Google Scholar]
- 15.Hanspal M, Smockova Y, Uong Q. Molecular identification and functional characterization of a novel protein that mediates the attachment of erythroblasts to macrophages. Blood. 1998;92:2940–2950. [PubMed] [Google Scholar]
- 16.Yoshida H, et al. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437:754–758. doi: 10.1038/nature03964. [DOI] [PubMed] [Google Scholar]
- 17.Eshghi S, Vogelezang MG, Hynes RO, Griffith LG, Lodish HF. Alpha4beta1 integrin and erythropoietin mediate temporally distinct steps in erythropoiesis: Integrins in red cell development. J Cell Biol. 2007;177:871–880. doi: 10.1083/jcb.200702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath KE, et al. Enucleation of primitive erythroid cells generates a transient population of “Pyrenocytes” In the mammalian fetus. Blood. 2008;111:2409–2417. doi: 10.1182/blood-2007-08-107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morioka K, Minamikawa-Tachino R. Temporal characteristics of the differentiation of embryonic erythroid cells in fetal peripheral blood of the Syrian hamster. Dev Growth Differ. 1993;35:569–582. doi: 10.1111/j.1440-169X.1993.00569.x. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes MM, Kopsombut P, Bondurant MC, Price JO, Koury MJ. Adherence to macrophages in EBIs enhances erythroblast proliferation and increases erythrocyte production by a different mechanism than erythropoietin. Blood. 2008;111:1700–1708. doi: 10.1182/blood-2007-06-098178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.