Abstract
Indoleamine 2,3 dioxygenase (IDO) has emerged as an important mediator of immune tolerance via inhibition of Th1 responses. However, the role of IDO in antigen-induced tolerance or allergic inflammation in the airways that is regulated by Th2 responses has not been elucidated. By using IDO−/− mice, we found no impairment of airway tolerance, but, surprisingly, absence of IDO provided significant relief from establishment of allergic airways disease, as evident from attenuated Th2 cytokine production, airway inflammation, mucus secretion, airway hyperresponsiveness, and serum ovalbumin-specific IgE. Myeloid dendritic cells isolated from lung-draining lymph nodes of mice immunized for either Th1 or Th2 response revealed fewer mature dendritic cells in the lymph nodes of IDO−/− mice. However, the net functional impact of IDO deficiency on antigen-induced responses was more remarkable in the Th2 model than in the Th1 model. Collectively, these data suggest that IDO is not required for the induction of immune tolerance in the airways but plays a role in promoting Th2-mediated allergic airway inflammation via unique effects on lung dendritic cells.
Keywords: tryptophan, DC, airways, asthma
Indoleamine 2,3 dioxygenase (IDO) is an enzyme that mediates tryptophan catabolism into its metabolites including kynurenine or quinolinic acid (1–3). IDO protein is widely expressed in a variety of cell types including B cells, macrophages, eosinophils, dendritic cells (DCs), endothelial cells, and many types of tumor cells (2, 4–9). Because products of tryptophan metabolism are required for normal cell growth, depletion of IDO adversely impacts cell function (10). In the last decade, IDO has emerged as an important negative regulator in the immune system primarily because of its function in DCs, which play a quintessential role in antigen presentation to T cells (11). Several studies have implicated IDO in tumor-associated immune tolerance, and IDO has been associated with inhibition of Th1-mediated disease states (7, 11–15). Although IDO has been generally associated with Th1 inhibition, Trp metabolites were shown to induce Th2-like regulatory cells that suppressed Th1 pathology in experimental autoimmune encephalomyelitis, an experimental model for multiple sclerosis (14). Despite suggestion of differential effects of IDO on Th1 versus Th2 cells in these reports, no study has sufficiently explored this concept.
There is little doubt that IDO plays a role in immune suppression and induction of T cell anergy. However, it has not been fully evaluated whether IDO-mediated immune suppression is pervasive under all circumstances. We investigated the role of IDO in established models of antigen-induced airway tolerance and inflammation by using IDO-deficient mice. We found that lack of IDO does not impair induction of immune tolerance induced by repeated antigen inhalation. On the contrary, IDO deficiency significantly attenuated the Th2 phenotype in the lungs in response to allergen provocation. When WT and IDO−/− mice were immunized via the lung to develop either Th1 or Th2 response, fewer mature DCs were found in the lymph nodes (LNs) of the IDO−/− mice in both models. Despite the similarity with respect to DC maturation in both Th1 and Th2 models, the functional readout in response to antigen such as antigen-specific Ig response was significantly attenuated in IDO−/− mice in the Th2 but not in the Th1 model. Antigen-induced cytokine response, airway eosinophilia, and airway hyperresponsiveness were also significantly lower in the IDO−/− mice in the Th2 model. We propose, therefore, that IDO expression in lung dendritic cells promotes Th2-type immune responses in the airways.
Results
IDO Deficiency Does Not Impair Immune Tolerance Induction, but IDO May Be Important for Th2-Mediated Airway Inflammation.
Given that IDO has been widely linked to development of immune tolerance, particularly in cancer (16), we investigated whether it also has a role in immune tolerance in the lungs in response to an allergen. We used an established protocol in which tolerance is induced by repeated exposure to aerosolized ovalbumin (OVA), used by us and others previously (17–19). Surprisingly, we found that IDO−/− mice also developed robust immune tolerance to OVA similar to BALB/c WT controls upon antigen challenge, as assessed by examination of airway inflammation and OVA-specific IgE production [see supporting information (SI) Fig. S1 and SI Materials and Methods]. Contrary to expectation, overall, the Th2 response was lower in the IDO−/− mice in this acute model of allergic airway inflammation in which mice were sensitized via the i.p. route with OVA/alum and challenged by aerosolized antigen (Fig. S1).
IDO−/− Mice Subjected to a Chronic Asthma Model Displayed Blunted Th2-Mediated Airway Inflammation and Airway Hyperresponsiveness.
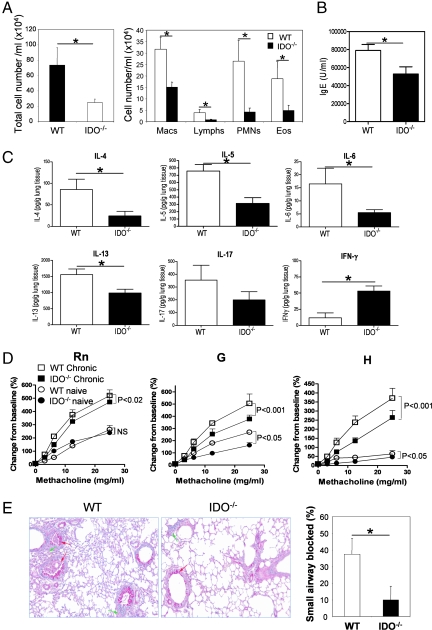
To pursue the apparent reduction in Th2-type airway inflammation under the condition of IDO deficiency, we asked whether the reduced Th2 response in the IDO−/− mice would be more significant in a chronic model of airway inflammation. The rationale for this investigation was the fact that an important feature of asthma is episodic exacerbation in response to allergen exposure, in which it is believed that resident Th2 cells are reactivated by antigen presented by local DCs in the airways or in the parenchyma (20). As in the acute model, animals were sensitized by i.p. immunization with OVA/alum, which, overall, yields a strong Th2 cytokine response, and once recruited to the lungs in response to inhaled antigen, the Th2 cells, via effector functions, establish disease phenotype, as shown previously by us (21, 22). Our goal was to give the animals the most optimal chance to prime for Th2 and then investigate whether the Th2-induced responses would be less well sustained upon stimulation by IDO-deficient DCs in the lung. To that end, animals were challenged by OVA periodically over the course of ≈6 weeks after sensitization. Under these conditions, IDO−/− animals showed a dramatic reduction in essentially all parameters commonly used to assess allergic airway inflammation. Bronchoalveolar lavage fluid cellularity and eosinophilia (Fig. 1A), OVA-specific IgE (Fig. 1B), and Th2 cytokine expression in the lung tissue (Fig. 1C) were all significantly lower in the IDO−/− mice as compared with that in WT controls. Interestingly, expression of the Th1 cytokine, IFN-γ, was elevated in the lung tissue of knockout animals in agreement with the trend observed in the acute model. In addition, all measures of lung mechanics were significantly lower in the IDO-deficient animals (Fig. 1D). IDO−/− mice were significantly less responsive in terms of Rn (P < 0.02), but in particular for G (P < 0.001) and H (P < 0.001). These results would predict that disruption of the IDO gene reduces airway narrowing and closure in the larger as well as smaller airways as compared with that occurring in the WT mice and was consistent with the histological findings (Fig. 1E). The IDO−/− animals displayed a dramatic reduction in the amount of mucus plugging the airways (Fig. 1E), which is one of the most deleterious sequelae in fatal human asthma (23, 24). Thus, the diminished Th2 cell-mediated airway inflammation in the IDO−/− mice observed in the acute model was significantly accentuated in the chronic model.
Fig. 1.
IDO−/− mice show attenuated Th2-type airways inflammation in chronic experimental asthma. Animals were subjected to a model of chronic airway inflammation consisting of periodic exposure to OVA over an extended period. (A) Total (Left) and differential (Right) cell counts. Macs, macrophages; Lymphs, lymph nodes; PMNs, polymorphonuclear leukocytes; Eos, eosinophils. (B) OVA-specific serum IgE as determined by ELISA. Data shown are mean ± SD. (C) Cytokine in lung homogenates as determined by multiplex assay. Data shown are mean ± SD. (D) AHR in response to inhaled MCh challenge (n = 6 mice per group). Both WT and IDO−/− mice showed increased AHR in OVA-challenged mice in response to inhaled MCh in all indices of lung function as compared with naive MCh-challenged mice. Rn, G, and H were all lower in OVA-challenged IDO−/− mice with respect to similarly challenged WT mice, and the difference reached statistical significance as indicated. (E) Production and secretion of mucus in lung tissue by periodic acid-Schiff reagent staining. Arrows denote inflammation (green) and mucus production (red). Magnification, ×100. Assessment of mucus plugging of the airways of mice from the two groups was based on the evaluation of both proximal and distal airways (n = 6–7 mice per group). Values are mean ± SD. ∗, P < 0.05. Data shown are representative of two independent experiments.
Reduced Antigen-Induced T Cell Expansion in Lung-Draining Lymph Nodes and Reduced Th2 Cytokine Production in Lungs of IDO−/− Mice.
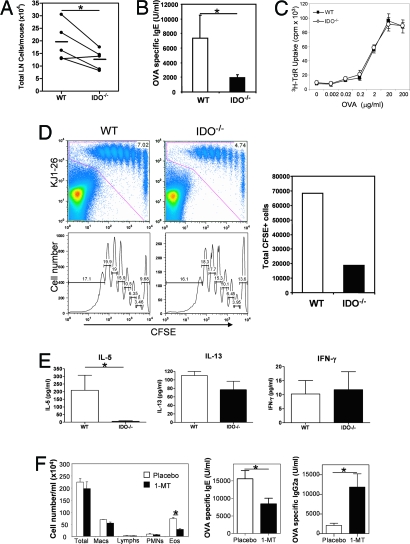
To explore the mechanism underlying reduced Th2-type airway inflammation in the IDO−/− mice, we analyzed antigen-induced T cell expansion and allergic immune response by using OVA in combination with the mucosal adjuvant cholera toxin (CT), a model established previously by us (25). The goal was to immunize via the respiratory tract, which would cause lung DCs to prime T cells in lung-draining LNs. This allowed us to investigate T cell responses and inflammation elicited by antigen-loaded local DCs. Mice were given three consecutive daily intranasal treatments of OVA plus CT to initiate Th2 responses. Antigen-induced T cell expansion in the LN, as determined by total cell counts, was consistently lower in IDO−/− mice as compared with that in wild-type controls (Fig. 2A), which was in line with the reduced levels of OVA-specific IgE in sera from the immunized IDO−/− mice (Fig. 2B). When equal numbers of total LN cell populations were cultured with OVA in vitro to elicit a recall response, [3H]thymidine incorporation was similar between cultures from IDO−/− and wild-type animals (Fig. 2C). These results suggested that, once a T cell was induced to differentiate, the primed T cells from IDO-deficient mice on a per-cell basis were as capable of responding to antigen as cells from wild-type mice. Thus, the reduced Th2-type response in IDO−/− mice was more likely due to defective priming in the lung microenvironment resulting in the generation of fewer Th2-type cells.
Fig. 2.
Reduced T cell expansion in lung-draining lymph nodes and reduced Th2 cytokine production in lung tissue in IDO−/− mice. (A) IDO−/− and WT animals received OVA/CT for three consecutive days via the intranasal route. Five days after the last treatment, lung-draining LN cells were isolated. Total LN cell counts representative of five independent experiments are shown. The lines connect age-matched animals for each individual experiment. The horizontal bars indicate the mean total cell count for the five experiments. ∗, P < 0.05. (B) OVA-specific serum IgE was determined by ELISA. Data shown are mean ± SD. ∗, P < 0.05. (C) Total LN cells were incubated with OVA, and proliferation was assessed by [3H]thymidine incorporation. Data shown are representative of two independent experiments. (D) DO11.10 TCR transgenic CD4+ T cells were labeled with CFSE and adoptively transferred intravenously into naive wild-type or IDO−/− animals. The mice received OVA/CT for two consecutive days. CFSE dilution in transferred T cells expressing the clonotypic TCR, as recognized by KJ1–26 mAb, was evaluated 1 day later by flow cytometry. The data show the equivalent percentage of proliferation in each generation of cells derived from WT and IDO−/− mice. However, the total count of CFSE-labeled cells, as shown in the right, was lower in the lymph nodes of IDO-deficient mice. (E) Cytokine levels in lung tissue of the same animals at the early time point after transfer. All data shown are mean ± SD. ∗, P < 0.05. Data shown are representative of three independent experiments. (F) Inhibition of allergic response upon treatment with 1-MT. Pellets containing 1-MT (releasing 10 mg/day) or placebo were implanted under the dorsal skin in BALB/c mice 1 day before sensitization. OVA/CT was instilled on three successive days as described in (A). On day 5 after the last instillation, the animals were challenged by aerosolized 1% OVA for seven consecutive days. 24 h after the last aerosol treatment, lungs were lavaged, and blood was collected. The results shown are mean cell number per ml of bronchoalveolar lavage (BAL) fluid per mouse ± SD. OVA-specific IgE and IgG2a levels in the sera were measured by ELISA. Data shown are means of Ig levels in four mice per group ± SD and are representative of two independent experiments. Macs, macrophages; Lymphs, lymph nodes; PMNs, polymorphonuclear leukocytes; Eos, eosinophils.
To further examine in vivo whether the reduction of Th2 dominant cell expansion in IDO−/− mice was due to priming deficiencies in the lung, CD4+ T cells purified from DO11.10 T cell antigen receptor (TCR) mice were labeled with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) and adoptively transferred into naive animals followed by administration of OVA plus CT. Here, DCs in the lungs would be expected to take up OVA and migrate to lung-draining lymph nodes and present the processed antigens to the transferred CD4+ T cells (26). Exposure to OVA/CT through the airways resulted in fewer CFSE+ cells in the lung-draining lymph nodes of IDO−/− animals, suggesting that fewer effector T cells were being generated/maintained in the IDO−/− mice (Fig. 2D). Cytokine analysis showed significantly less IL-5 level in the lung tissue of the IDO−/− mice, which confirmed that IDO deficiency impaired Th2 development in the lung (Fig. 2E). At this early time point, as expected, IL-13 levels were low, and the difference did not reach statistical significance between the two groups of mice.
To rule out the possibility that our observations related to some developmental defect associated with IDO deficiency from birth, we used a complementary approach of inhibiting IDO with the specific inhibitor 1-methyl-d-tryptophan (1-MT) in WT mice during the course of the OVA/CT model. As shown in Fig. 2F, 1-MT instillation in mice caused a significant reduction in both OVA-specific IgE levels and lung eosinophilia as compared with placebo treatment.
Reduced Expression of Key Costimulatory Molecules on IDO-Deficient Lung DCs upon Antigenic Stimulation and Reduced Th2 Response.
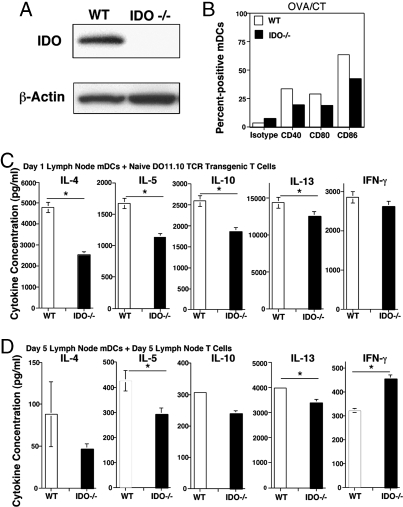
Lung DCs have been shown to be critical for the presentation of airway-delivered antigen and, in turn, T cell activation (26, 27). Our next goal was to characterize the effect of IDO deficiency on lung myeloide DC (mDC) phenotype and function. We first confirmed IDO protein expression in lung DCs by immunoblotting techniques, and, as shown in Fig. 3A, IDO protein was readily detected in extracts made from lung mDCs isolated from WT but not IDO−/− mice.
Fig. 3.
Fewer mature mDCs in lung-draining lymph nodes of immunized IDO−/− mice and a corresponding decrease in the ability to stimulate Th2 cytokine production. (A) IDO expression in purified lung DCs from WT and IDO−/− mice as determined by immunoblotting. Expression of β-actin was monitored as a loading control. (B) mDCs from lung-draining LNs were partially purified before analysis by flow cytometry. The mDCs were identified by expression of CD11c and high-level MHC class II expression, as described previously (25), and were then assessed for expression of the indicated costimulatory molecules. (C) Based on expression of CD11c and MHC class II, lung-draining LN mDCs were purified by cell sorting 1 day after the last of three daily administrations of OVA/CT. The sorted mDCs were cultured with CD4+CD25− T cells from naive DO11.10 TCR transgenic mice in the presence of specific OVA peptide for 5 days, at which time cytokines secreted in the culture supernatant were assessed by multiplex assay. (D) mDCs from lung-draining LNs were similarly purified 5 days after the last of three daily administrations of OVA/CT and were cocultured with CD4+ T cells purified from the same LN with whole OVA protein (100 μg/ml). Cytokines secreted in the culture supernatants after 4 days were assessed by multiplex assay. ∗, P < 0.05. The experiment was repeated, yielding similar results.
To draw a functional comparison between WT and IDO-deficient DCs, mice were immunized with OVA/CT, as described for data shown in Fig. 2 and, 1 day after the last immunization, mDCs were isolated from the lung-draining LNs. As shown in Fig. 3B, the percentage of mDCs expressing the costimulatory molecules CD40, CD80, or CD86 that are associated with DC maturation was lower when cells were isolated from IDO-deficient mice. When the total numbers were calculated, the difference was even more striking and was also evident when animals were immunized with OVA and CpG oligodeoxynucleotide (ODN) to induce a Th1 response (see Fig. S2). We inspected the ability of the cells from both groups to stimulate cytokine production from CD4+ T cells isolated from naive DO11.10 TCR transgenic mice. In this in vitro setting, in direct one-to-one comparison, the mDCs from IDO−/− mice were less potent than those from WT animals in stimulating Th2 cytokine production, but the difference was less with respect to IFN-γ production (Fig. 3C). Next, we allowed priming of naive CD4+ T cells in the lung-draining LNs to proceed for 5 days after three successive OVA/CT administrations and then examined whether the mDCs in the LNs of IDO−/− mice continued to be less potent in stimulating a recall response in the primed T cells. It was clear that mDCs from IDO−/− mice induced less Th2 cytokine production from primed T cells (Fig. 3D). It should be noted that this difference was evident despite equalizing for mDC numbers and CD4+ T cells in the in vitro coculture.
Reduced Priming Under IDO-Deficient Conditions with Lower Antigen-Specific Immune Response in Th2- but Not Th1-Biased Models.
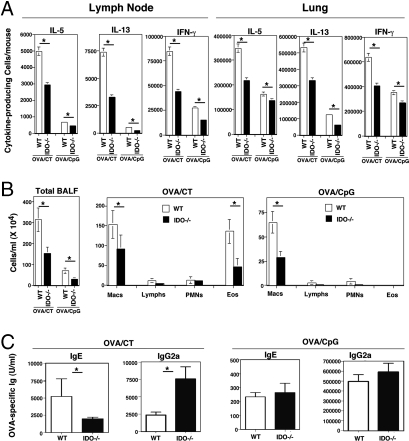
We next examined whether there were differences in the number of total cells, CD4+ T cells, and mDCs in the LNs as well as the lung after allowing for priming in immunized WT and IDO-deficient mice. Mice were immunized intranasally with either OVA/CT or OVA/CpG ODN to induce Th2 or Th1 responses, respectively, and cells were isolated on day 5 after the last of three successive immunizations. As shown in Fig. S3A, the numbers of total, CD4+ T cells, and mDCs were lower in the LNs of IDO-deficient mice as compared with those in WTs regardless of the model. When cells from LNs were restimulated to determine priming efficiency by enzyme-linked immunosorbent spot (ELISPOT) technique, fewer cytokine-producing cells were identified in the LNs of IDO−/− mice as compared with that in WT mice with numbers adjusted for total CD4+ T cells recovered (Fig. 4A). We next examined the ability of equal numbers of mDCs isolated from the lungs of the immunized WT and IDO−/− mice to stimulate a recall response in the primed T cells from the LNs of the mice by using equal numbers of LN cells. A lower response was detected when mDCs from IDO-deficient mice were used except in the case of IL-13 and IFN-γ (see Fig. S3B). The more appreciable difference in numbers of IL-13-secreting cells between WT and IDO−/− mice shown in Fig. 4A as compared with almost similar cytokine secretion profiles in the recall assay in the two groups suggested that deficiency in priming contributed to the attenuated Th2 response in IDO−/− mice. Thus, the more important question was the actual status of cell numbers in the lung compartment where, after priming, effector cells would accumulate and be stimulated by local DCs. Interestingly, here, the difference in total and CD4+ T cells numbers between WT and IDO−/− mice was still observed when cells were isolated from OVA/CT-immunized mice but was less remarkable in the case of OVA/CpG-immunized mice (Fig. S3A). When lung cells were examined by the ELISPOT technique, the difference in Th2 cytokine-producing cells between WT and IDO−/− mice could be better appreciated in the OVA/CT model as compared with the difference in IFN-γ-producing cells in the OVA/CpG ODN model (Fig. 4A). Of note, in contrast to the OVA/CT combination, the OVA/CpG combination induced much less inflammation both in the LN and in the lung (also see Fig. 4B). Although spot-forming units for IL-5 or -13 were detected in the lungs of OVA/CpG-immunized mice (Fig. 4A), realistically, these cells produced very low levels of these cytokines as compared with corresponding cells from OVA/CT-immunized mice as shown (see Fig. S3B). However, the CD4+ T cells isolated from OVA/CpG ODN-immunized mice did secrete high levels of IFN-γ, demonstrating the strong Th1 bias of this model.
Fig. 4.
IDO deficiency impacts antigen-induced Th2 responses more than Th1 responses. Wild-type and IDO −/− mice received three daily intranasal administrations of OVA/CT or OVA/CpG. (A) Lungs and lung-draining lymph nodes were harvested from four or five animals per group 5 days after the last intranasal treatment. (B and C) The remaining animals were rested for 3 days, given an intranasal boost with OVA/CT or CpG, rested an additional 2 days, and then challenged with aerosolized OVA for ten consecutive days. (A) Immediately after isolation, the LN and lung T cells were subjected to ELISPOT assay after brief stimulation with phorbol 12-myristate 13-acetate (25 ng/ml) and ionomycin (500 ng/ml). The results are expressed as the number of cytokine-expressing cells per mouse based on the number of CD4+ T cells recovered (see Fig. S4). (B) One day after the last challenge with aerosolized OVA, BAL was performed to determine the total number of cells in the BAL fluid (Left), and differential cell counts were performed (Center and Right). Macs, macrophages; Lymphs, lymph nodes; PMNs, polymorphonuclear leukocytes; Eos, eosinophils. (C) OVA-specific serum IgE and IgG2a concentrations were determined by ELISA by using sera from blood collected 1 day after the immunization plus challenge protocol. Data shown are means of Ig levels in four mice per group ± SD.∗, P < 0.05. Shown is an experiment representative of two.
We were curious to determine how the differences described above translated into functional antigen-specific response, such as Ig production, in the mice. Animals were sensitized with either OVA/CT or OVA/CpG ODN and repeatedly challenged with aerosolized antigen. OVA/CT immunization elicited a strong eosinophilic response, which was markedly less in the IDO−/− mice (Fig. 4B). The OVA/CpG immunization induced no eosinophilia, as was expected based on the inability of the primed CD4+ T cells to produce high levels of Th2 (IL-5) cytokines, as shown in Fig. S3B. Because IL-17, rather than IFN-γ, promotes lung neutrophilia (28), the neutrophilic response was also weak with not much IL-17 secretion from primed T cells derived from the OVA/CpG model (see Fig. S3B). Overall, there was an ≈10-fold increase in inflammation in OVA/CpG ODN-immunized mice as compared with baseline cell numbers in the BAL fluid obtained from naive or PBS-exposed mice, which was largely due to an increase in macrophage numbers. The macrophage numbers were lower in the BAL fluid derived from OVA/CpG ODN-immunized IDO−/− mice (Fig. 4B), which may have been because of the effects of IDO on expression of chemokine genes in epithelial cells. However, although as shown in Fig. 2B, OVA-specific IgE levels were lower in the sera of IDO−/− mice, the IgG2a levels were higher in these mice, and levels of both were similar in the sera of WT and IDO-deficient OVA/CpG ODN-immunized mice (Fig. 4D).
We also investigated whether there were differences in T regulatory cells in the lungs of immunized WT and IDO−/− mice in both models and did not see any difference in FoxP3-expressing cells (see Fig. S4). We were unable to detect biologically active TGF-β either in the BAL fluid or in the tissue in either WT or IDO−/− mice (data not shown). The tissue IL-10 levels were similar in WT and IDO−/− mice (see Fig. S3). The IL-10 response from primed T cells was also not stronger in the absence of IDO (Fig. 4B). Thus, IDO deficiency did not increase a T regulatory cell response that could account for the lower Th2 response in these mice. Also, an examination of thymic stromal-derived lymphopoietin (TSLP) expression by quantitative PCR (qPCR) techniques showed greater expression in the lungs of OVA/CT-immunized mice as compared with that in the lungs of OVA/CpG-immunized mice with not much difference in the presence or absence of IDO (see Fig. S5). Taken together, these results showed that IDO deficiency causes fewer mature mDCs to accumulate in the lung-draining LNs of mice whether they are subjected to a Th1 or a Th2 model. However, although the effect of IDO deficiency was translated into lower T cell response in the lung, the ensuing eosinophilic inflammatory response, and antigen-specific IgE levels in the Th2 model, this deficiency did not make a difference in the antigen-specific IgG2a response in the Th1 model.
Discussion
The role of IDO in the immune system has been extensively investigated in the last decade (2, 6). A key function of IDO that has emerged from these studies is immunosuppression resulting in acquired peripheral tolerance (16). However, the bulk of the literature on suppression of immune responses by IDO relates to Th1-mediated immune responses. In a recent study, Th2-mediated ocular inflammation was reduced by inhibition of IDO function with 1-MT (29). In mucosal immune responses, particularly in Th2-mediated disease states, the role of IDO has not been adequately studied. Our study did not reveal a role for IDO in antigen-induced immune tolerance in the airways but instead identified a role for IDO in promoting antigen-driven Th2 responses via effects on lung DCs. A finding in this study is a positive role for IDO in the accumulation of mature mDCs in the lung-draining lymph nodes of mice in response to antigen. Importantly, we show that this effect occurs in the lung simultaneously with previously described effects in the spleen (Fig. S1).
Given that the lung has at least two resident cell types, epithelial cells and mDCs, that constitutively express IDO protein, and two others, the pDC and the eosinophil, that can be recruited in larger numbers during infections or allergic inflammation, it appears that the function of IDO is stimulatory [from eosinophils (ref. 5 and our data)] or inhibitory (15, 30, 31) depending on the target cell, the stimulus, and the specific model. Thus, it is possible that epithelial or even mDC-derived IDO under normal conditions maintains homeostasis in the lung, which, when compromised by 1-MT, promotes inflammation, and when stimulated by CpG ODN, inhibits inflammation due to augmentation of homeostatic mechanisms. An important component of IDO-mediated homeostasis may be constitutive production of specific inhibitory Trp metabolites from lung epithelial cells in response to ambient inhaled particles that can be augmented in response to CpG ODN (30), akin to production of high levels of quinolinic acid in the brain by endotoxin, a TLR4 agonist (3). Recently, a downstream product of Trp metabolism, 3-hydroxyanthranilioc acid (3-HAA), was shown to inhibit experimental asthma (32). However, in an earlier study, 3-HAA was not detected in the lungs of either control mice or mice subjected to a lung infection model, although other Trp metabolites were detectable (33). Thus, 3-HAA may not be an endogenous regulator of Th2 responses and allergic airways disease.
In our experiments, inhibition of IDO by 1-MT or lack of IDO in the Th2 model showed a tendency toward more Th1/IFN-γ-induced response. This was similar to increased cell proliferation observed in a previous study by using 1-MT and lung antigen-presenting cells (APCs) (15) and the reciprocal observation of inhibition of Th1 response/IFN-γ by Trp metabolites in an encephalomyelitis model (14). When CpG ODN was used to induce a Th1 response in our study, lack of IDO did not make a difference in the net antigen-specific IgG2a response, unlike the reproducible decrease in IgE response in the Th2 models. This observation needs to be reviewed in the context of the role of the cytokine balance (Th2 versus Th1) in driving the net Ig response as well as the role of Trp metabolites in inhibiting Th1 responses (11, 12, 14, 15). As reported previously, IL-4 (previously called BSF-1) and IFN-γ reciprocally regulate IgE and IgG2a responses (34). Thus, in the context of a lower IL-4/IFN-γ status in IDO−/− mice in the Th2 model, the dominant IgE response influenced by Th2 cytokines (IL-4/IL-13) was lower with a corresponding increase in the IgG2a response. Again, with a lower Th2 and similar Th1 response in the lung in the Th1 model in the IDO−/− mice, the net IgG2a response was actually slightly higher. Collectively, our explanation for why the Th1 response was not affected in IDO−/− mice is that the deficiency in mature mDCs because of lack of IDO was offset by the absence of inhibitory effects of specific Trp metabolites that have been shown to inhibit IFN-γ production (14). This was not observed in the case of Th2 cytokines because specific Trp metabolites may actually promote a Th2 response (5, 14).
An observation in our study is the effect of IDO on the accumulation of mature mDCs in the lung-draining LNs whether a Th1 (CpG ODN) or a Th2 adjuvant (CT) was used in conjunction with antigen. It is unknown whether IDO-induced mechanisms in lung DCs are important for their trafficking or survival, and more studies are needed to investigate this aspect of IDO function in depth.
Materials and Methods
Mice.
BALB/c ByJ mice were purchased from The Jackson Laboratory. IDO−/− mice, backcrossed onto to the BALB/c background, were originally generated at the Medical School of Georgia (35) and were subsequently bred and maintained in the Department of Laboratory Resources (DLAR) at the University of Pittsburgh. DO11.10 TCR transgenic mice were originally obtained from K. Murphy (Washington University School of Medicine, St. Louis) and were similarly maintained in the DLAR. All mice were used at 8–10 weeks of age. Maintenance and use of laboratory animals was approved by the Institutional Animal Care and Use Committee (IACUC).
Induction of Tolerance and Inflammation in the Airways and Assessment of Airway Inflammation and Serum Ig Levels.
A common protocol was used for airway tolerance induction that we have used previously (18). For further details, see SI Materials and Methods.
Cytokine Assays.
Cytokines were measured by ELISA (R&D Systems) or multiplex assays (Bio-Rad) and a Luminex analyzer according to each manufacturers' recommendations. For cytokine detection in total lung, the tissue was homogenized in 50 mM Tris·HCl, pH 7.5, containing 150 mM NaCl, 0.002% Tween 20, and protease inhibitor (Complete Mini, EDTA-free; Roche Applied Science). After centrifugation, supernatants were collected and stored at −80°C until assayed. BAL fluid and culture medium samples were similarly stored until assayed.
Antibodies and Flow Cytometry.
FACS analysis for surface molecules was performed as described previously (36). Flow cytometric analysis was performed on a FACSCalibur flow cytometer (BD Immunocytometry Systems), and data were analyzed by using FlowJo software (Tree Star).
The following antibodies were purchased from BD Pharmingen: hamster anti-mouse CD11c-APC, rat anti-mouse major histocompatibility antigen (MHC) II-FITC, rat anti-mouse CD40-phycoerythrin (PE), and rat anti-mouse. CD86-PE, rat IgG2a-PE, and rat IgG2b-FITC and hamster IgG1 λ-APC, also from BD Pharmingen, served as isotype controls. Rat anti-mouse OX40L-PE was purchased from eBioscience.
Measurement of Airway Hyperresponsiveness.
Airway responsiveness (AHR) to methacholine (MCh) was assessed as described previously by using the FlexiVent system (SCIREQ) (37, 38) (see SI Materials and Methods).
Isolation and Analysis of Dendritic Cells.
Lung tissue DCs were isolated as reported previously (39) and further sorted for low autofluorescence to minimize macrophage contamination. LN DCs were isolated as described previously (25).
Adoptive Transfer of CD4+ T Cells.
DO11.10 CD4+ T cells were isolated from spleen cells of DO11.10 mice by positive selection with anti-CD4 magnetic microbeads (Miltenyi Biotec) according to the manufacturer's instructions. The purified cells were labeled with CFSE by using a Vybrant CFDA SE cell tracer kit (Molecular Probes). Adoptive transfer was carried out with 5 × 106 labeled CD4+ T cells per animal delivered by i.v. injection. One day after adoptive transfer, mice received two consecutive daily treatments with OVA plus CT. One day after the last of these treatments, total lung-draining lymph node cells were assessed for dilution of CFSE by flow cytometry. Transferred T cells were identified by staining with anticlonotypic KJ1–26 antibody (BD Pharmingen).
Statistical Analysis.
Comparisons of means ± SD were carried out by using Student's t test. In the case of AHR measurements, two-way ANOVA of entire curves generated in the experiments was carried out. Statistical significance was associated with P values <0.05.
Supplementary Material
Acknowledgments.
We thank B. Dixon–McCarthy, D. Ganiear, M. Paglia, and M. Yarlagadda for technical assistance, M. Arora and Y. Zhang for assistance with multiplex cytokine assays, and N. Krishnamoorthy for assistance with dendritic cell work. This work was supported by Grants HL 077430, HL 084932, and AI048927 (to A.R.), and HL 060207 (to P.R.) from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708809105/DCSupplemental.
References
- 1.Mellor AL, et al. Tryptophan catabolism and T cell responses. Adv Exp Med Biol. 2003;527:27–35. doi: 10.1007/978-1-4615-0135-0_3. [DOI] [PubMed] [Google Scholar]
- 2.Mellor AL, Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 3.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 4.Beutelspacher SC, et al. Expression of indoleamine 2,3-dioxygenase (IDO) by endothelial cells: implications for the control of alloresponses. Am J Transplant. 2006;6:1320–1330. doi: 10.1111/j.1600-6143.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- 5.Odemuyiwa SO, et al. Cutting edge: Human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 6.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 8.Fallarino F, et al. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14:65–68. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 9.Orabona C, et al. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood. 2005;107:2846–2854. doi: 10.1182/blood-2005-10-4077. [DOI] [PubMed] [Google Scholar]
- 10.Mellor A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem Biophys Res Commun. 2005;338:20–24. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]
- 11.Munn DH. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol. 2006;18:220–225. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Kwidzinski E, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 13.Terness P, Chuang JJ, Opelz G. The immunoregulatory role of IDO-producing human dendritic cells revisited. Trends Immunol. 2006;27:68–73. doi: 10.1016/j.it.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Platten M, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 15.Swanson KA, Zheng Y, Heidler KM, Mizobuchi T, Wilkes DS. CDllc+ cells modulate pulmonary immune responses by production of indoleamine 2,3-dioxygenase. Am J Respir Cell Mol Biol. 2004;30:311–318. doi: 10.1165/rcmb.2003-0268OC. [DOI] [PubMed] [Google Scholar]
- 16.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 18.Ostroukhova M, et al. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostroukhova M, et al. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huh JC, et al. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J Exp Med. 2003;198:19–30. doi: 10.1084/jem.20021328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das J, et al. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 22.Zhang DH, et al. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity. 1999;11:473–482. doi: 10.1016/s1074-7613(00)80122-3. [DOI] [PubMed] [Google Scholar]
- 23.Carroll NG, Mutavdzic S, James AL. Increased mast cells and neutrophils in submucosal glands and mucus plugging in patients with asthma. Thorax. 2002;57:677–682. doi: 10.1136/thorax.57.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Marco R, et al. Prognostic factors of asthma severity: A 9-year international prospective cohort study. J Allergy Clin Immunol. 2006;117:1249–1256. doi: 10.1016/j.jaci.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Oriss TB, et al. Dynamics of dendritic cell phenotype and interactions with CD4+ T cells in airway inflammation and tolerance. J Immunol. 2005;174:854–863. doi: 10.4049/jimmunol.174.2.854. [DOI] [PubMed] [Google Scholar]
- 26.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stumbles PA, et al. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, et al. Indoleamine 2,3-dioxygenase (IDO) is involved in promoting the development of anterior chamber-associated immune deviation. Immunol Lett. 2006;107:140–147. doi: 10.1016/j.imlet.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi T, et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Invest. 2004;114:270–279. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grohmann U, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi T, et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci USA. 2007;104:18619–18624. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci USA. 1990;87:2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 35.Baban B, et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61:67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Williams MS, Spain LM. Patterns of expression, membrane localization, and effects of ectopic expression suggest a function for MS4a4B, a CD20 homolog in Th1 T cells. Blood. 2006;107:2400–2408. doi: 10.1182/blood-2005-08-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irvin CG, Bates JH. Measuring the lung function in the mouse: the challenge of size. Respir Res. 2003;4:4. doi: 10.1186/rr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol. 2003;94:1297–1306. doi: 10.1152/japplphysiol.00706.2002. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, et al. Distinct responses of lung and spleen dendritic cells to the TLR9 agonist CpG oligodeoxynucleotide. J Immunol. 2006;177:2373–2383. doi: 10.4049/jimmunol.177.4.2373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.