Abstract
In addition to its role in the storage of fat, adipose tissue acts as an endocrine organ, and it contains a functional renin-angiotensin system (RAS). Angiotensin-converting enzyme (ACE) plays a key role in the RAS by converting angiotensin I to the bioactive peptide angiotensin II (Ang II). In the present study, the effect of targeting the RAS in body energy homeostasis and glucose tolerance was determined in homozygous mice in which the gene for ACE had been deleted (ACE−/−) and compared with wild-type littermates. Compared with wild-type littermates, ACE−/− mice had lower body weight and a lower proportion of body fat, especially in the abdomen. ACE−/− mice had greater fed-state total energy expenditure (TEE) and resting energy expenditure (REE) than wild-type littermates. There were pronounced increases in gene expression of enzymes related to lipolysis and fatty acid oxidation (lipoprotein lipase, carnitine palmitoyl transferase, long-chain acetyl CoA dehydrogenase) in the liver of ACE−/− mice and also lower plasma leptin. In contrast, no differences were detected in daily food intake, activity, fed-state plasma lipids, or proportion of fat excreted in fecal matter. In conclusion, the reduction in ACE activity is associated with a decreased accumulation of body fat, especially in abdominal fat depots. The decreased body fat in ACE−/− mice is independent of food intake and appears to be due to a high energy expenditure related to increased metabolism of fatty acids in the liver, with the additional effect of increased glucose tolerance.
Keywords: fatty acid metabolism, obesity, ACE knockout mice, glucose tolerance
The renin-angiotensin system (RAS) is important in both cardiovascular and body fluid homeostasis (1–3) and has recently been implicated in obesity and energy balance (see ref. 4 for a review). All of the components of the RAS are present in adipose tissue and evidence suggests that this RAS is fully functional and can contribute to the accumulation of fat and to obesity (5–7). Recent studies showed that transgenic mice lacking the precursor peptide, angiotensinogen (AGT), have impaired weight gain and adipose tissue development (8), whereas mice with an overabundance of AGT in adipose tissue have markedly increased fat mass (9).
Overexpression of genes associated with the RAS has been reported in human visceral adipose tissue in overweight subjects (10), and polymorphisms of the angiotensin-converting enzyme (ACE) gene have been linked to the incidence of obesity and alterations of body mass index (11, 12). ACE plays a key role in the RAS in that it converts angiotensin I to the bioactive peptide angiotensin II (Ang II). Ang II has been identified as a trophic factor in the differentiation of preadipocytes to mature adipocytes (13). There is evidence that administration of ACE inhibitors reduces body weight gain in spontaneously hypertensive rats (14), obese Zucker rats (15), and humans (16). However, some studies have shown that the activity of the RAS is inversely related to the gain of body weight. For example, infusion of Ang II caused a reduction in food intake and body weight in rats (17–19) and sheep (20). Overall, it is still unclear how the RAS influences energy homeostasis to induce changes in body weight gain and adipose tissue growth.
In this study, we investigated animals with a genetic deletion of ACE (21). As a result of the deletion of this gene in somatic cells and testis, the mice have reductions of Ang II of 70% in plasma and between 85–97% in tissues (22). Characterization of their phenotype has shown a reduced blood pressure, fertility, and hematocrit (21, 23) and decreased ability to concentrate urine (24). They have not been studied in regard to energy homeostasis, although it is known that they have a lower body weight compared with wild-type mice (21). The aim of our study was to determine how the absence of ACE influences body weight by measuring all aspects of their energy balance compared with wild-type littermates. Specifically, we tested for differences in energy intake as food, excretion in feces, storage as adipose tissue and expenditure in relation to locomotor activity. The expression of key genes involved in lipid metabolism in the liver, fat, and muscle tissues was also measured.
One of the risk factors associated with obesity is decreased insulin sensitivity and there is evidence that Ang II may be involved in insulin signaling as improved glucose metabolism and insulin sensitivity has been demonstrated in both rats (15) and humans (25) treated with RAS antagonists. As a further important marker of energy metabolism, we tested for differences in insulin sensitivity in the ACE−/− mice by comparing their response to an oral glucose challenge with that of wild-type littermates.
Results
Food, Water Intake, and Fecal Fat Content.
Daily food intake in ACE−/− mice was not different from wild-type mice either as a raw figure or adjusted for body weight. Water consumption of the ACE−/− mice was more than double that of the wild-type mice (see Table 1; n = 14 per group). There was no difference between ACE−/− and wild-type mice in the proportion of fat excreted in the fecal matter (n = 7 per group).
Table 1.
Food intake (FI), water intake (WI), fecal fat, and body composition of ACE+/+ and ACE−/− mice
| Appetite and body composition | ACE+/+ | ACE−/− |
|---|---|---|
| FI, g (n = 14) | 3.5 ± 0.2 | 3.0 ± 0.2 |
| FI, g/body weight, g (n = 14) | 0.11 ± 0.01 | 0.13 ± 0.01 |
| WI, ml (n = 14) | 4.2 ± 0.2 | 9.8 ± 0.5* |
| Fat in dry feces, % (n = 7) | 4.7 ± 0.2 | 4.9 ± 0.1 |
| Digestability, % (n = 7) | 94.1 ± 0.3 | 94.5 ± 0.2 |
| Body fat, g (n = 7) | 5.5 ± 0.7 | 2.3 ± 0.2* |
| Fat-free mass, g (n = 7) | 26.4 ± 0.6 | 24.9 ± 1.0 |
| Bone density, g/cm2 (n = 7) | 0.078 ± 0.001 | 0.076 ± 0.001 |
Values are mean ± SEM. ∗, P < 0.05 (ACE−/− vs. ACE+/+).
Body Weight, Body Composition, and Visualization of Regional Fat Mass.
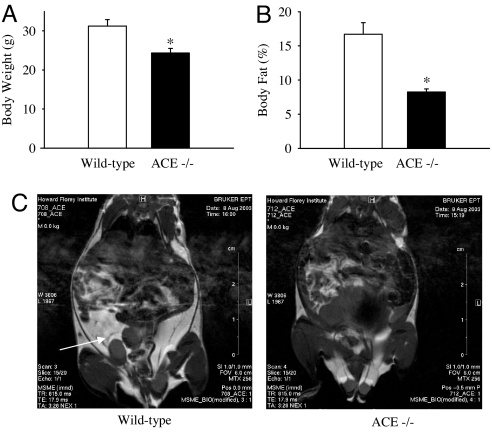
In comparison with wild-type littermates, ACE−/− mice weighed ≈20% less (P < 0.01; Fig. 1A; n = 14 per group) and had ≈50% less body fat (P < 0.001; Fig. 1B; n = 7 per group). There was no difference between the fat-free mass or bone mineral density (BMD) of ACE−/− mice and wild-type mice (see Table 1; n = 7 per group). Visual comparison of MRI images of wild-type and ACE−/− mice demonstrated that the mass of adipose tissue was considerably less in the latter group (Fig. 1C). This effect was most noticeable in abdominal fat mass, as indicated by the arrow.
Fig. 1.
ACE deficiency altered body weight, fat mass, and distribution. (A and B) The ACE−/− mice (open bars) had significantly lower body weight (A) and proportion of body fat (B) than ACE+/+ mice (filled bars). The values are mean ± SEM (n = 14 per group); ∗, P < 0.05. (C) Proton density-weighted axial MRI images across the body of ACE+/+ and ACE−/− mice. Bright, white areas denote fat. The white arrowhead indicates the larger android fat stores in ACE+/+ mice.
Glucose Tolerance Testing.
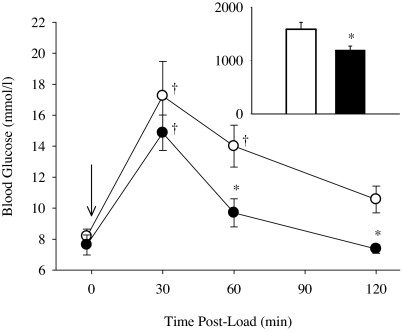
An oral glucose load resulted in a rapid increase in blood glucose in both ACE−/− and wild-type mice (Fig. 2). Glucose was cleared more rapidly in ACE−/−, and plasma glucose returned close to the baseline level after 60 and 120 min. In contrast, plasma glucose remained elevated at these times in the wild-type mice. The area under the curve of the graph for plasma glucose vs. time (Fig. 2) was significantly less in the ACE−/− mice compared with the wild-type mice.
Fig. 2.
After glucose load, ACE−/− mice had faster clearance than ACE+/+ mice, blood glucose was significantly lower at 60 and 120 min, with a reduced AUC. n = 6 per group. ∗, P < 0.05 vs. ACE+/+ mice; †, P < 0.05 vs. baseline.
Indirect Calorimetry.
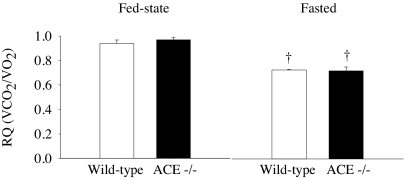
Calorimetric measurements were conducted during the fed state and after overnight fasting. The respiratory quotient (RQ) of ACE−/− mice did not differ from wild-type littermates during either condition (Fig. 3). The RQ of both groups was lower in the fasted state (both P < 0.01).
Fig. 3.
The RQ of ACE−/− mice did not differ from ACE+/+ mice either under fed-state conditions or during fasting. Fasting resulted in a significantly lower RQ in both ACE−/− and ACE+/+ mice. n = 6 per group. †, P < 0.05 vs. baseline.
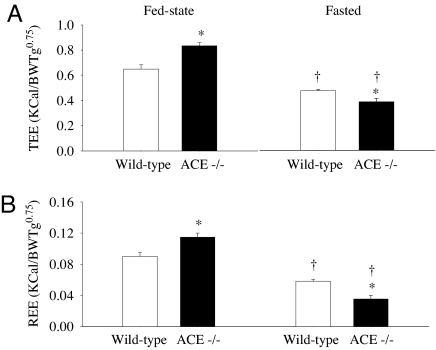
During the fed state, ACE−/− mice had higher TEE (Fig. 4A) and REE (Fig. 4B) compared with ACE+/+ mice. Fasting resulted in a reduction in TEE of >50% in ACE−/− mice, whereas this reduction was smaller (≈25%) when measured in the wild-type littermates (Fig. 4A). After overnight fasting, the reductions in REE (Fig. 4B) were even greater for both ACE−/− (≈70%) and wild-type mice (≈35%).
Fig. 4.
Under fed-state conditions, ACE−/− mice had a higher TEE (A) and REE (B) compared with ACE+/+. Fasting resulted in lower TEE and REE in both ACE−/− and ACE+/+ mice. When fasted ACE−/− mice had significantly lower TEE and REE than ACE+/+ mice. n = 6 per group. ∗, P < 0.05 vs. ACE+/+ mice; †, P < 0.05 vs. baseline.
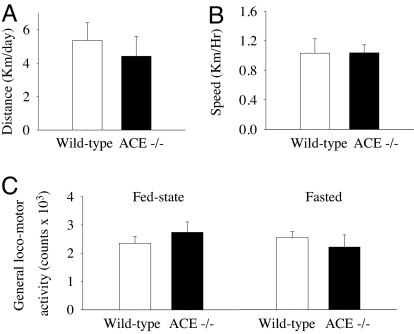
General Locomotor Activity and Running Wheel Activity.
Running wheel activity (distance traveled and average speed; Fig. 5A) and general locomotor activity (Fig. 5B) were not different between ACE−/− and wild-type mice. General locomotor activity was not altered by fasting.
Fig. 5.
Running wheel distance (n = 5 per group) (A), average speed (n = 5 per group) (B), and general locomotor activity (n = 6 per group) (C) were not affected in ACE−/− mice. General locomotor activity was unaffected by fasting.
Plasma Composition and Hormone Levels.
No differences were observed in the basal plasma concentration of glucose, triglyceride (TG), or total cholesterol (Table 2). Plasma leptin levels were substantially lower in ACE−/− mice compared with their wild-type littermates (Table 2).
Table 2.
Fed-state plasma biochemistry, hematocrit, and leptin of ACE+/+ and ACE−/− mice
| Plasma biochemistry | ACE+/+ | ACE−/− |
|---|---|---|
| Triglyceride, mmol/liter (n = 7) | 0.85 ± 0.26 | 0.47 ± 0.06 |
| Cholesterol, mmol/liter (n = 7) | 1.68 ± 0.14 | 1.97 ± 0.11 |
| Glucose, mmol/liter (n = 7) | 14.31 ± 1.72 | 10.81 ± 0.87 |
| Leptin, μg/ml (n = 6) | 8.3 ± 2.0 | 1.3 ± 0.3* |
Values are mean ± SEM. ∗, P < 0.05 (ACE−/− vs. ACE+/+).
Gene Expression.
No differences were detected in adipose tissue gene expression between the wild-type and ACE−/− mice for all measured transcripts, including lipoprotein lipase (LPL), hormone-sensitive lipase (HSL), fatty acid synthase (FAS), peroxisome proliferator activated receptor γ (PPARγ), PPARγ coactivator-1 (PGC-1α), and uncoupling protein 1 (UCP-1). Similarly, skeletal muscle expression of the genes for carnitine palmitoyl transferase 1 (CPT-1), LPL, PPARγ, and PGC-1α was also unaffected. However, the ACE−/− mice exhibited 400-fold less expression of UCP-1, suggesting a lack of residual adipocytes within the skeletal muscle. There were considerable alterations in liver gene expression, with the ACE−/− mice having higher levels (2- to 4-fold) of the transcripts for key genes involved in fatty acid metabolism LPL, long-chain acyl-CoA dehydrogenase (LCAD), CPT-1, and PGC-1α (see Table 3).
Table 3.
Fold-increase (FI) in expression of genes involved in lipid metabolism in the livers of ACE−/− mice compared with ACE+/+ mice
| Gene | FI | Role in fat metabolism |
|---|---|---|
| Lipoprotein lipase | 4.4* | Lipolysis |
| PPARγ coactivator-1 | 2.8* | Induction of fat metabolism |
| Carnitine palmitoyl transferase | 2.3* | Fatty acid oxidation |
| Long chain acyl-CoA dehydrogenase | 2.1* | Fatty acid oxidation |
| Hormone sensitive lipase | 1.4 | Lipolysis |
| Fatty acid synthase | 1.0 | Fat storage |
*, P < 0.05 (ACE−/− vs. ACE+/+).
Discussion
This study demonstrates that mice with a deletion of the ACE gene have 50–60% less body fat, accounting for 60–70% of the body weight difference, between ACE−/− mice and their wild-type littermates. It is shown that the lower body fat in ACE−/− mice is primarily due to increased energy expenditure and is not related to differences in food intake or energy digestibility. The increase in energy expenditure was independent of locomotor activity and appears to be mediated by increased fatty acid oxidation in the liver. Furthermore, this study demonstrates that glucose clearance is improved in ACE−/− mice, consistent with reports that drugs that inhibit Ang II protect against the development of insulin resistance (15, 25).
The finding that there was no difference in food intake or faecal fat output between ACE−/− and wild-type mice is consistent with the work of Massiera et al. (8) in AGT-deficient mice. Similar findings on appetite and body composition have been observed in animals chronically treated with an ACE inhibitor (26). Thus, the decreased body fat observed in Ang II-deplete models is independent of energy intake or digestive efficiency. The large increase in water intake in the ACE−/− mice is a well known feature of both of these mouse models, which have an impaired ability to concentrate urine due to abnormal renal development (27, 28).
To investigate the alteration in energy homeostasis further, energy expenditure and locomotor activity were measured simultaneously and in combination with fed and fasted states. In the fed state, ACE−/− had increased total and resting energy expenditure compared with the wild-type littermates, whereas general locomotor activity was not different. Similarly, running wheel activity in both the light and dark phases was not different. This finding is in contrast to AGT−/− mice, which had higher locomotor activity yet an unchanged energy expenditure relative to wild-type mice (8). ACE−/− mice reduced their energy expenditure after an overnight fast. This result is of interest, because it demonstrates their reduced energy storage as fat.
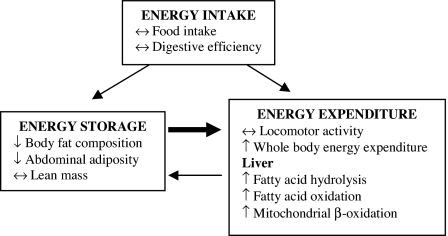
In regard to the possibility that the differences in body fat and energy expenditure were due to differences in fat metabolism, we measured increases in the expression of key genes involved in hepatic lipid metabolism (LPL, LCAD, and CPT-1) in ACE−/− mice. LPL increases hydrolysis of triglycerides into free fatty acids, whereas CPT-1 transfers the fatty acids into the mitochondria where they are metabolized (29). LCAD is an important enzyme that catalyzes breakdown and β-oxidation of fatty acids in both mitochondria and peroxisomes. There was also increased expression of PGC-1α, a key transcriptional regulator of metabolic pathways that has also been implicated in mitochondrial and peroxisomal biogenesis (30). The quantification of mRNA transcripts is generally accepted as a reliable marker of increased hepatic protein or enzyme activity, and previous studies have correlated the expression of hepatic enzymes with fatty acid oxidation in animal models of obesity (31, 32). This study shows that changes in hepatic fatty acid metabolism might be related to the reduction in fat mass in the ACE−/− mice. The results are summarized by the scheme in Fig. 6, which shows how deficiency in ACE alters the relationship among energy intake, storage, and expenditure to reduce body fat mass.
Fig. 6.
A scheme of energy balance in ACE−/− mice, where energy intake as food is normal, but the equilibrium between energy storage and utilization is in favor of the latter. The increases in whole-body energy expenditure (measured by indirect calorimetry) and hepatic fatty acid oxidation dissipate ingested calories, reducing energy storage in adipose tissue and lowering body fat mass.
The current observations on the fat-reducing effects of ACE deficiency complement a number of previous studies, which have shown a direct link between the RAS and adiposity. Evidence from cultured adipocytes suggests that Ang II can inhibit lipolysis directly and increase the activity of fatty acid synthase (33), mediated by the AT1-receptor (34). Consistent with this evidence, AGT−/− mice have a reduced plasma TG and total cholesterol level compared with the wild-type mice, and these changes are associated with a decrease in fatty acid synthase activity in epididymal fat pads (8).
Other studies have investigated the effect of Ang II on adipose tissue perfusion and lipolysis. Whereas i.v. infusion of Ang II at both sub- and pressor doses had little effect on whole body lipolytic rates (35), interstitial infusion of Ang II into s.c. fat over the abdomen reduced lipolysis in both lean and obese human subjects (36). In aged animals, chronic treatment with angiotensin antagonists preserved renal mitochondrial oxidative capacity (37). These data suggest that local effects of the RAS in adipose tissue are important in local lipid metabolism, whereas our study showed that the influence of the RAS extends to hepatic fatty acid oxidation and whole-body energy utilization. In contrast, Cassis et al. (38) have shown that systemic infusion of rats with Ang II for 2 weeks caused an initial reduction, followed by a prolonged increase in whole-body oxygen consumption, that was accompanied by decreases in appetite and body weight.
Although the majority of studies have focused on the systemic influences of the RAS on metabolism, there is also evidence that brain angiotensin may also have a determining influence. Rats with a deficiency in brain angiotensinogen that is induced by an angiotensinogen-antisense gene coupled to a glial cell-specific promoter were found to have reduced body fat composition, improved glucose tolerance, low blood pressure, and increased appetite compared with wild-type rats (39, 40). Because both AGT−/− and ACE−/− mice have the respective gene deleted from all somatic cells, it remains possible that the role of angiotensin in the brain may have a determining influence on metabolism, appetite, and body composition.
Importantly, comparison of axial MRI images of ACE−/− and wild-type mice demonstrated that the influence of ACE deficiency was on the accumulation of android fat, particularly in the visceral region. Android fat deposition has been implicated as a factor in insulin resistance and diabetes (41, 42). This is consistent with our finding that ACE−/− mice have a faster glucose clearance after glucose load, indicating improved insulin sensitivity relative to wild-type mice. There was also a tendency for fed-state plasma glucose and triglyceride levels to be lower in ACE−/− mice. Although this difference was not statistically significant, it suggests a favorable effect of ACE inhibition on glucose and lipid metabolism.
The reduction in body fat mass was accompanied by a large reduction in circulating levels of the adipocytokine leptin. The relationship between body fat mass and the plasma leptin level is consistent with previous reports (43, 44), although it is noteworthy that AGT-deficient mice had similar levels of leptin to wild-type mice despite a greatly reduced fat mass (8). One notable difference between the two strains is that ACE−/− mice have significantly elevated levels of bradykinin (22). It is unlikely that bradykinin is involved in the effects on body weight and composition, because AGT−/− mice also display the decreased body weight phenotype. However, it is possible that the increased level of bradykinin could explain the improvement in glucose tolerance, because there is evidence in both human and animal models of the insulin-sensitizing effects of bradykinin (45, 46). Intracellular signaling in response to insulin involves activation of a tyrosine kinase-mediated signaling pathway that eventually results in increased cellular uptake of glucose via the GLUT-4 transporter (47). Bradykinin appears to enhance this pathway by increasing nitric oxide generation, which inhibits dephosphorylation of insulin receptor substrate-1, thereby prolonging insulin signaling (48). Ang 1-7, which is elevated in ACE−/− mice, has also been shown to potentiate the action of bradykinin, possibly by increasing arteriolar dilation and delivery of glucose to tissues. Ang 1-7 signals via the G protein coupled receptor Mas. Deletion of this receptor in FVB-n Mas knockout mice caused a phenotype that included increased insulin and leptin, a 50% increase in abdominal fat mass and decreased insulin-stimulated uptake of glucose into adipocyte (49). This suggests that any effect of increased Ang 1-7 signaling via the Mas receptor does not have a strong effect in ACE−/− animals.
There is also evidence that the improved glucose uptake may be directly related to the reduction in Ang II. It has been shown that inhibition of Ang II signaling by treatment with an AT1 receptor antagonist increases the expression of GLUT-4 receptors and improves glucose uptake in skeletal muscle of obese Zucker rats (50). Similarly, a recent paper has shown that chronic treatment with AT1 receptor antagonist protects against insulin resistance during aging in Fischer 344 rats (51).
In summary, our study demonstrated the effect of life-long ACE deficiency on body fat accumulation, energy expenditure, and glucose tolerance. The significant reduction of body fat in the ACE−/− compared with wild-type mice confirms the critical role of RAS on adipose tissue growth. The decrease of body fat in ACE−/− mice was independent of food intake and digestive efficiency and appears to be due to a higher energy expenditure related to increased fatty acid metabolism in the liver and independent of energy utilized for general locomotor activity. Although the increase in fatty acid oxidation may also mediate the increased glucose tolerance, it is also possible this effect is mediated by increased bradykinin in the ACE−/− mice.
Materials and Methods
Mice.
Male and female heterozygous ACE knockout mice (ACE+/−) were obtained from the laboratory of Pierre Meneton [Institut National de la Santé et de la Recherche Médicale (INSERM) U367, Paris]. They were maintained on a C57BL/6J background at the Howard Florey Institute. Heterozygous (ACE+/−) mice were bred to produce 14 wild-type (ACE+/+) and 14 homozygous ACE-null offspring (ACE−/−). Real-time PCR incorporating dual-labeled Taqman probe technology (Applied Biosystems) was used for genotyping of ACE−/− and ACE+/+ offspring. Mice were housed in individual plastic cages with sloping grill lids (Wiretainers). The animals were maintained on the same diet throughout the study (Barastoc Mouse Breeder Cubes; Barastoc Stockfeeds). Food pellets were available ad libitum on the sloping section of the lid, and there was free access to tap water. The mice were maintained on a 12-h light/dark cycle. Age-matched male ACE+/+ and ACE−/− mice pairs that were 12 months old and had been maintained in the same housing conditions were selected for the study. The amount of food and water consumed was monitored daily for 1 week. The animal procedures were approved by the Animal Ethics Committees of the Howard Florey Institute and La Trobe University.
Body Composition Analysis by Dual Energy X-ray Absorptiometry (DEXA).
Whole-body composition of ACE−/− (n = 7) and ACE+/+ (n = 7) mice was performed by DEXA, using a densitometric scanner (QDR 4500; Hologic) equipped with software developed for small animals (version 9.10). The animals were scanned while in a prone position under anesthesia induced by i.p. injection (0.02 ml/g body weight) of a mixture of ketamine (0.75 ml of 100 mg/ml Ketaplex; Apex Lab) and xylazine (0.25 ml of 20 mg/ml Rompun; Bayer).
Visualization of Adipose Tissue Distribution by MRI.
Regional body fat distribution was visualized by MRI. Images were acquired on a Bruker BIOSPEC 47/30 MRI scanner equipped with a horizontal 4.7 tesla Oxford magnet. Proton density weighted axial images with the following parameters: number of slices, 20; slice thickness, 1 mm; field of view (FOV), 6 cm; matrix size, 256 × 256; repetition time (TR), 815 ms; echo time (TE), 17.9 ms were acquired. Mice were anesthetized by placing them in an induction chamber with an exposure to an isoflurane (Abbott Australasia Pty. Ltd.) concentration of 5% in medical-grade air and subsequent reduction to the concentration of 2%.
Running Wheel Activity.
Animals (n = 5 per group) were allowed free access, for 14 days, to running wheels equipped with a speedometer (Sigma Sport BC700 calibrated for running wheel radius) fitted to the individual plastic cage with grill lid. The distance run (km) and speed (km/h) were measured daily over the final 10 days of testing. The mice were allowed free access to food and water.
Indirect Calorimetry and General Locomotor Activity.
ACE−/− (n = 6) and ACE+/+ (n = 6) mice were placed in the calorimetry system cages for 36 h; the first 12 h was considered the acclimation phase, and data were analyzed only for the final 24 h. The system used was a custom-built, four-cage, open-circuit calorimetry system feeding. Drinking and general locomotor activity were also continuously measured (LabMaster; TSE Systems). The data analyzed were TEE, REE, RQ, and general locomotor activity. Animals were placed in this system twice, at least 1 week apart. On one occasion, they had ad libitum access to food, and on the other, they were fasted.
Analysis of Fecal Fat Content.
The lipid from 5 g of feces was extracted using 2:1 chloroform: methanol solution. The total lipid content was determined gravimetrically after extraction for 24 h at room temperature. The dry weight of the feces was determined on the lipid extracted residue. The total dry weight of feces was determined by adding the weight of the lipid extract to the dry weight of the fecal residue.
Glucose Tolerance Testing.
Animals were fasted overnight with ad libitum access to water. The following morning, mice were restrained, a small segment was cut from the tip of the tail, and blood was drawn. Blood samples were collected in microcuvettes (HemoCue), and fasting blood glucose was measured (HemoCue Glucose 201 blood glucose analyzer). A glucose load was injected (2g/kg body weight i.p.) and blood glucose was measured 30, 60, and 120 min after the injection.
Blood Analyses.
At the end of the experiment, mice were anesthetized by i.p. injection of the ketamine and xylazine mixture as described above and killed by cardiac puncture. The blood was collected into heparinized syringes and chilled on ice. Subsequently, the plasma was separated by centrifugation at 3,000 rpm for 15 min in a refrigerated centrifuge (Sorvall-RT7; Sorvall) and stored at −80°C until biochemical analysis. The plasma triglyceride and total cholesterol and glucose levels were measured by spectrophotometry according to the procedures described in commercially available kits (Beckman Coulter). Plasma leptin was measured as previously described (52).
Analysis of Gene Expression.
When the animals were killed, samples of liver, perirenal adipose, and quadriceps skeletal muscle tissue were snap-frozen in liquid nitrogen and stored at −80°C until quantitative analysis of RNA transcripts. RNA was extracted from ≈20 mg (wet weight) of liver and ≈40 mg adipose tissue by using the ToTALLY RNA Kit protocol and reagents (Ambion). RNA integrity and quantity were assessed on an Agilent Bioanalyzer 2100 with a RNA 6000 Nano LabChip-Kit (Agilent Technologies). Reverse transcription was performed using the AMV reverse transcriptase kit (A3500; Promega) protocols and reagents. To perform PCR, specific primers were designed for all genes by using Primer Express 2.0 software (Applied Biosystems) on SPECIFIC sequences obtained from GenBank and confirmed by BLAST sequence alignment determination. Real-time PCR was performed using the GeneAmp 7500 Sequence Detection System (Applied Biosystems), where target expression normalized to the amount of endogenous control (cyclophilin) relative to control value is given by 2-ΔΔCt (Applied Biosystems).
Statistical Analysis.
Data are reported as mean ± SEM in tables and figures with percentage differences also used in text. The differences between the two groups in respiratory quotient, total and resting energy expenditure, and locomotor activity were analyzed by two-way ANOVA. Glucose tolerance was analyzed by repeated-measures ANOVA. All other differences were analyzed by t test, and significance was accepted at P < 0.05 (Statistica 7.1; Statsoft).
Acknowledgments.
We thank W. Boon, M. Dashti, S. Kantor, A. Gibson, B. Gleeson, K. A. Carey, and T. Alexiou for their assistance in the study. R.S.W. was funded by National Health and Medical Research Council (Australia) Fellowship 217011. The support of grants from the Australian Research Council (DP0346830), the National Health and Medical Research Council (Australia) (350313), the Search Foundation, Robert J., Jr. and Helen C. Kleberg Foundation, and the G. Harold and Leila Y. Mathers Charitable Foundation is gratefully acknowledged.
Footnotes
The authors declare no conflict of interest.
References
- 1.Dustan HP. Physiologic regulation of arterial blood pressure. Hypertension. 1982;4:62–67. doi: 10.1161/01.hyp.4.5_pt_2.iii62. [DOI] [PubMed] [Google Scholar]
- 2.Thurman JM, Schrier RW. Comparative effects of ACE inhibitors and ARBs on blood pressure and the kidney. Am J Med. 2003;114:588–598. doi: 10.1016/s0002-9343(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 3.Tarjan E, Denton DA, McBurnie MI, Weisinger RS. Water and Na intake of wild and New Zealand rabbits. Peptides. 1988;9:677–679. doi: 10.1016/0196-9781(88)90182-9. [DOI] [PubMed] [Google Scholar]
- 4.Weisinger RS, et al. The problem of obesity: Is there a role for antagonists of the renin-angiotensin system? Asia Pac J Clin Nutr. 2007;16(Suppl 1):359–367. [PubMed] [Google Scholar]
- 5.Hainault I, et al. Adipose tissue-specific increase in angiotensinogen expression and secretion in the obese Zucker rat. Am J Physiol Endocrinol Metab. 2002;282:E59–E66. doi: 10.1152/ajpendo.2002.282.1.E59. [DOI] [PubMed] [Google Scholar]
- 6.Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol. 2004;286:E891–E895. doi: 10.1152/ajpendo.00551.2003. [DOI] [PubMed] [Google Scholar]
- 7.Boustany CM, et al. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity. Am J Physiol. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 8.Massiera F, et al. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain. Endocrinology. 2001;142:5220–5225. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- 9.Massiera F, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 10.Giacchetti G, et al. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens. 2002;15:381–388. doi: 10.1016/s0895-7061(02)02257-4. [DOI] [PubMed] [Google Scholar]
- 11.Strazzullo P, et al. Genetic variation in the renin-angiotensin system and abdominal adiposity in men. Ann Intern Med. 2003;138:17–23. doi: 10.7326/0003-4819-138-1-200301070-00007. [DOI] [PubMed] [Google Scholar]
- 12.Kramer H, et al. ACE gene polymorphisms and obesity: An examination in 3 black populations. Obes Res. 2005;13:823–8288. doi: 10.1038/oby.2005.94. [DOI] [PubMed] [Google Scholar]
- 13.Saint-Marc P, Kozak LP, Ailhaud G, Darimont C, Negrel R. Angiotensin II as a trophic factor of white adipose tissue. Endocrinology. 2001;142:487–492. doi: 10.1210/endo.142.1.7883. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DJ, Duncan AM, Kladis A, Harrap SB. Converting enzyme inhibition and its withdrawal in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1995;26:426–436. doi: 10.1097/00005344-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Steen MS, et al. Interactions of exercise training and ACE inhibition on insulin action in obese Zucker rats. J Appl Physiol. 1999;86:2044–2051. doi: 10.1152/jappl.1999.86.6.2044. [DOI] [PubMed] [Google Scholar]
- 16.Enalapril in Hypertension Study Group (UK) Enalapril in essential hypertension: A comparative study with propranolol. Br J Clin Pharmacol. 1984;18:51–56. doi: 10.1111/j.1365-2125.1984.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassis LA, Marshall DE, Fettinger MJ, Rosenbluth B, Lodder RA. Mechanisms contributing to angiotensin II regulation of body weight. Am J Physiol. 1998;274:E867–E876. doi: 10.1152/ajpendo.1998.274.5.E867. [DOI] [PubMed] [Google Scholar]
- 18.Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor in rats. J Clin Invest. 1996;97:2509–2516. doi: 10.1172/JCI118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brink M, et al. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine IGF-I. Endocrinology. 2001;142:1489–1496. doi: 10.1210/endo.142.4.8082. [DOI] [PubMed] [Google Scholar]
- 20.Weisinger RS, Burns P. Role of brain angiotensin in thirst and Na appetite of rats. Protein Pept Lett. 1999;6:281–294. [Google Scholar]
- 21.Krege JH, et al. Male–female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 22.Campbell DJ, et al. Effect of reduced ACE gene expression and ACE inhibition on angiotensin and bradykinin levels in mice. Hypertension. 2004;43:854–859. doi: 10.1161/01.HYP.0000119190.06968.f1. [DOI] [PubMed] [Google Scholar]
- 23.Cole J, et al. Lack of angiotensin-facilitated erythropoiesis causes anemia in ACE-deficient mice. J Clin Invest. 2000;106:1391–1398. doi: 10.1172/JCI10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traynor T, et al. Tubulo-glomerular feedback in ACE-deficient mice. Am J Physiol. 1999;276:F751–F757. doi: 10.1152/ajprenal.1999.276.5.F751. [DOI] [PubMed] [Google Scholar]
- 25.Nagel JM, Tietz AB, Goke B, Parhofer KG. The effect of telmisartan on glucose and lipid metabolism in non-diabetic, insulin-resistant subjects. Metabolism. 2006;55:1149–1154. doi: 10.1016/j.metabol.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Carter CS, Onder G, Kritchevsky SB, Pahor M. Angiotensin-converting enzyme inhibition, body composition and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2003;60:1437–1446. doi: 10.1093/gerona/60.11.1437. [DOI] [PubMed] [Google Scholar]
- 27.Hilgers KF, Reddi V, Krege JH, Smithies O, Gomez RA. Aberrant renal vascular morphology in mice lacking ACE. Hypertension. 1997;29:216–221. doi: 10.1161/01.hyp.29.1.216. [DOI] [PubMed] [Google Scholar]
- 28.Kihara M, et al. Genetic deficiency of angiotensinogen produces an impaired urine concentrating ability in mice. Kidney Int. 1998;53:548–555. doi: 10.1046/j.1523-1755.1998.00801.x. [DOI] [PubMed] [Google Scholar]
- 29.Louet JF, Hayhurst G, Gonzalez FJ, Girard J, Decaux JF. The coactivator PGC-1 is involved in the regulation of the liver carnitine palmitoyl transferase I gene. J Biol Chem. 2002;277:37991–38000. doi: 10.1074/jbc.M205087200. [DOI] [PubMed] [Google Scholar]
- 30.Puigserver P, Spiegelman BM. PGC-1α: Transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 31.Brix AE, et al. Evaluation of liver fatty acid oxidation in the leptin-deficient mouse. Mol Genet Metab. 2002;75:219–226. doi: 10.1006/mgme.2002.3298. [DOI] [PubMed] [Google Scholar]
- 32.Ji H, Friedman MI. Reduced capacity for fatty acid oxidation in rats with inherited susceptibility to diet-induced obesity. Metabolism. 2007;56:1124–1130. doi: 10.1016/j.metabol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones BH, Standridge MK, Moustaid N. Angiotensin increases lipogenesis in 3T3–L1 and human adipose cells. Endocrinology. 1997;138:1512–1519. doi: 10.1210/endo.138.4.5038. [DOI] [PubMed] [Google Scholar]
- 34.Goossens G, Blaak EE, Aren P, Saren WH, van Baak MA. Angiotensin II: A hormone that affects lipid metabolism in adipose tissue. Int J Obes. 2007;31:382–384. doi: 10.1038/sj.ijo.0803388. [DOI] [PubMed] [Google Scholar]
- 35.Townsend RR. The effects of angiotensin II on lipolysis in humans. Metabolism. 2001;50:468–472. doi: 10.1053/meta.2001.21021. [DOI] [PubMed] [Google Scholar]
- 36.Boschmann M, Ringel J, Klaus S, Sharma AM. Metabolic and hemodynamic responses of adipose tissue to angiotensin II. Obes Res. 2001;9:486–491. doi: 10.1038/oby.2001.63. [DOI] [PubMed] [Google Scholar]
- 37.de Cavanagh EM, et al. Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J. 2003;17:1096–1098. doi: 10.1096/fj.02-0063fje. [DOI] [PubMed] [Google Scholar]
- 38.Cassis LA, Helton M, English V, Burke G. Angiotensin II regulates oxygen consumption. Am J Physiol. 2002;282:R445–R453. doi: 10.1152/ajpregu.00261.2001. [DOI] [PubMed] [Google Scholar]
- 39.Kasper SO, et al. Growth, metabolism and blood pressure during aging in transgenic rats with altered renin-angiotensin systems. Physiol Genom. 2005;23:311–317. doi: 10.1152/physiolgenomics.00163.2005. [DOI] [PubMed] [Google Scholar]
- 40.Kasper SO, Ferrario CM, Ganten D, Diz DI. Rats with low brain angiotensinogen do not exhibit insulin resistance during aging. Endocrine J. 2006;30:167–174. doi: 10.1385/ENDO:30:2:167. [DOI] [PubMed] [Google Scholar]
- 41.Kissebah A, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 42.Rimm A, Hartz AJ, Fischer ME. A weight shape index for assessing risk of disease. J Clin Epidemiol. 1988;41:459–465. doi: 10.1016/0895-4356(88)90047-9. [DOI] [PubMed] [Google Scholar]
- 43.Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M. Plasma leptin and insulin relationships in obese and nonobese humans. Diabetes. 1996;45:695–698. doi: 10.2337/diab.45.5.695. [DOI] [PubMed] [Google Scholar]
- 44.Ahren B, Mansson S, Gingerich RL, Havel PJ. Regulation of plasma leptin in mice. Am J Physiol. 1997;273:R113–R120. doi: 10.1152/ajpregu.1997.273.1.R113. [DOI] [PubMed] [Google Scholar]
- 45.McCarty MF. ACE inhibition may decrease diabetes risk by boosting the impact of bradykinin on adipocytes. Med Hypotheses. 2003;60:779–783. doi: 10.1016/s0306-9877(02)00234-7. [DOI] [PubMed] [Google Scholar]
- 46.Henriksen EJ, Jacob S, Augustin HJ, Dietze GJ. Glucose transport: Effects of ACE inhibitors and bradykinin antagonism. Diabetes. 1996;45(Suppl 1):S125–S128. doi: 10.2337/diab.45.1.s125. [DOI] [PubMed] [Google Scholar]
- 47.Uehara M, et al. Effect on insulin sensitivity of ACE inhibitors with or without a sulphydryl group. Diabetologia. 1994;37:300–307. doi: 10.1007/BF00398058. [DOI] [PubMed] [Google Scholar]
- 48.Beard KM, Lu H, Ho K, Fantus IG. Bradykinin augments insulin-stimulated glucose transport in rat adipocytes. Diabetes. 2006;55:2678–2687. doi: 10.2337/db05-1538. [DOI] [PubMed] [Google Scholar]
- 49.Santos SH, et al. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes. 2007;57:340–347. doi: 10.2337/db07-0953. [DOI] [PubMed] [Google Scholar]
- 50.Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M. Selective angiotensin II receptor antagonism reduces insulin resistance in obese Zucker rats. Hypertension. 2001;38:884–890. doi: 10.1161/hy1101.092970. [DOI] [PubMed] [Google Scholar]
- 51.Gilliam-Davis S, et al. Long-term AT1 receptor blockade improves metabolic function in Fischer 344 rats. Am J Physiol. 2007;293:H1327–H1333. doi: 10.1152/ajpheart.00457.2007. [DOI] [PubMed] [Google Scholar]
- 52.Velkoska E, Morris MJ, Burns P, Weisinger RS. Leptin reduces food intake but does not alter weight regain following food deprivation in the rat. Int J Obes Relat Metab Disord. 2003;27:48–54. doi: 10.1038/sj.ijo.0802193. [DOI] [PubMed] [Google Scholar]