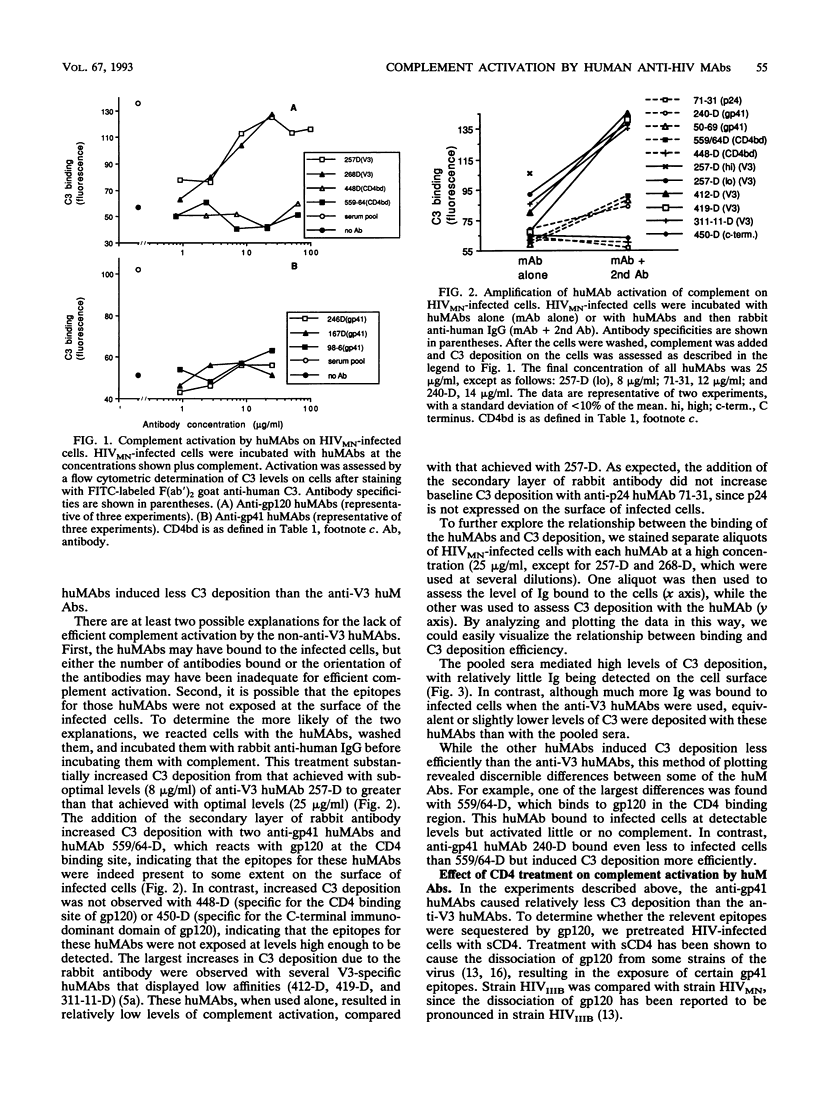
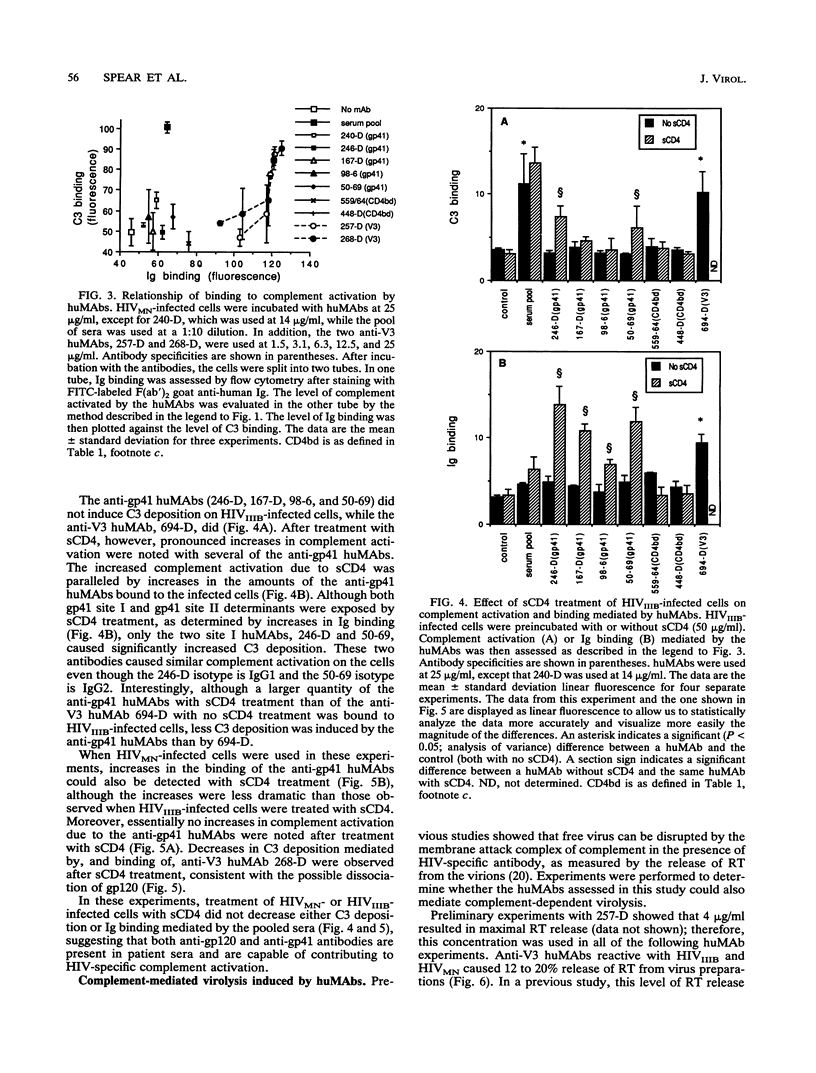
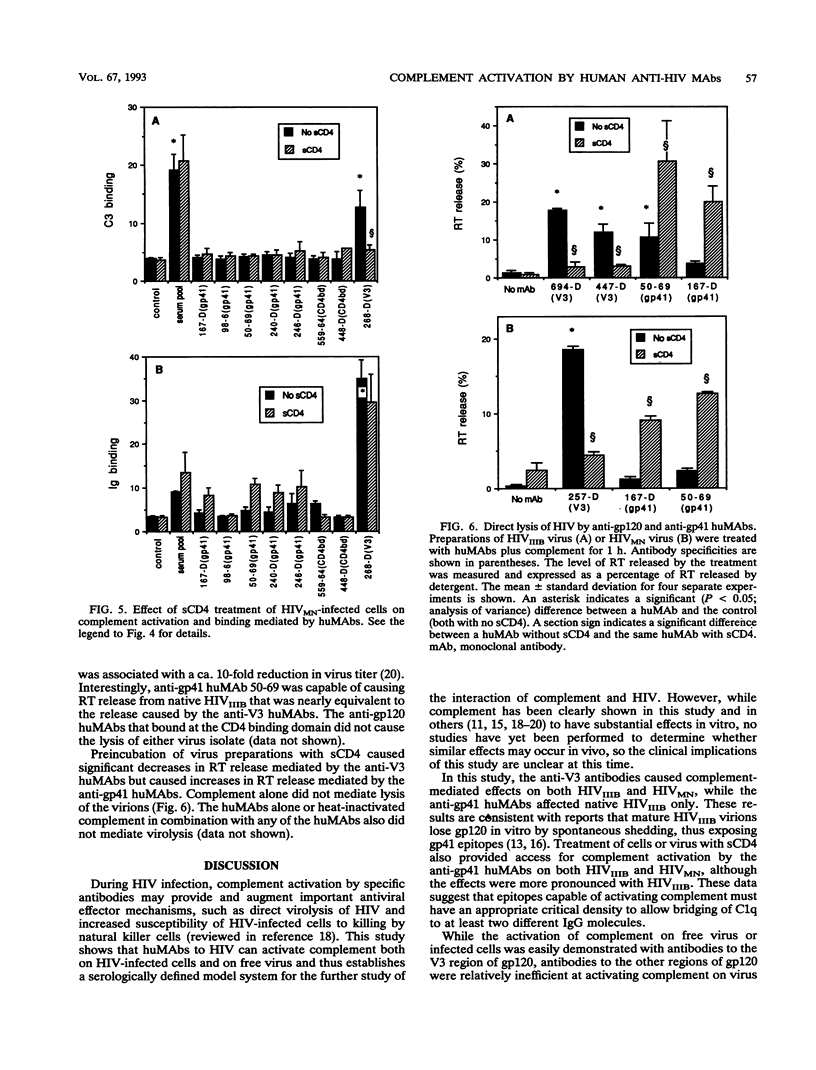
Abstract
It has been shown that the incubation of human immunodeficiency virus (HIV) with polyclonal antibodies from HIV-infected persons and complement results in complement-mediated neutralization due, at least in part, to virolysis. The current study was performed to determine whether any of a panel of 16 human monoclonal antibodies to HIV could activate complement and, if so, which determinants of the HIV envelope could serve as targets for antibody-dependent complement-mediated effects. Human monoclonal antibodies directed to the third variable region (V3 region) of HIVMN gp120 induced C3 deposition on infected cells and virolysis of free virus. Antibodies to two other sites on HIVMN gp120 and two sites on gp41 induced few or no complement-mediated effects. Similarly, only anti-V3 antibodies efficiently caused complement-mediated effects on the HIVIIIB isolate. In general, the level of C3 deposition on infected cells paralleled the relative level of bound monoclonal antibodies. As expected, pooled polyclonal antibodies from infected persons were much more efficient than monoclonal antibodies inducing C3 deposition per unit of bound immunoglobulin. Treatment of virus or infected cells with soluble CD4 resulted in increases in anti-gp41 antibody-mediated virolysis and C3 deposition but decreases in anti-V3 antibody-mediated virolysis and C3 deposition. In general, virolysis of HIV was more sensitive as an indicator of complement-mediated effects than infected-cell surface C3 deposition, suggesting the absence of or reduced expression of functional complement control proteins on the surface of free virus. Thus, this study shows that human monoclonal antibodies to the V3 region of gp120 are most efficient in causing virolysis of free virus and C3 deposition on infected cells. Elution of gp120 with soluble CD4 exposes epitopes on gp41 that can also bind antibody, resulting in virolysis and C3 deposition. These findings establish a serologically defined model system for the further study of the interaction of complement and HIV.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd J. E., James K. B cell responses to HIV and the development of human monoclonal antibodies. Clin Exp Immunol. 1992 May;88(2):189–202. doi: 10.1111/j.1365-2249.1992.tb03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P. U., Mallal S. A., French M. A., Dawkins R. L. Major histocompatibility complex genes influence the outcome of HIV infection. Ancestral haplotypes with C4 null alleles explain diverse HLA associations. Hum Immunol. 1990 Dec;29(4):282–295. doi: 10.1016/0198-8859(90)90042-n. [DOI] [PubMed] [Google Scholar]
- Carini C., Perricone R., Fratazzi C., Fontana L., De Carolis C., D'Amelio R., Sirianni M. C., Aiuti F. Complement activation is associated with the presence of specific human immunodeficiency virus (HIV)-anti-HIV immune complexes in patients with acquired immunodeficiency syndrome-related complex or lymphoadenopathy syndrome. Scand J Immunol. 1989 Sep;30(3):347–353. doi: 10.1111/j.1365-3083.1989.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. H., Autran B., Jouvin M. H., Aubry J. P., Rozenbaum W., Banchereau J., Debré P., Revillard J. P., Kazatchkine M. Diminution des récepteurs érythrocytaires pour le fragment C3b du complément dans le syndrome d'immunodéficience acquise. Presse Med. 1988 Apr 23;17(15):727–730. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Gorny M. K., Gianakakos V., Sharpe S., Zolla-Pazner S. Generation of human monoclonal antibodies to human immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1624–1628. doi: 10.1073/pnas.86.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny M. K., Xu J. Y., Gianakakos V., Karwowska S., Williams C., Sheppard H. W., Hanson C. V., Zolla-Pazner S. Production of site-selected neutralizing human monoclonal antibodies against the third variable domain of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3238–3242. doi: 10.1073/pnas.88.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges F., Hoffmann A., Oliveira de Araujo F., Hemmer R. Prolonged clinically asymptomatic evolution after HIV-1 infection is marked by the absence of complement C4 null alleles at the MHC. Clin Exp Immunol. 1992 May;88(2):237–242. doi: 10.1111/j.1365-2249.1992.tb03067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada Y., Lange M., McKinley G. F., Sonnabend J. A., Fonville T. W., Kanemitsu T., Tanaka M., Clark W. S. Hematologic correlates and the role of erythrocyte CR1 (C3b receptor) in the development of AIDS. AIDS Res. 1986 Summer;2(3):235–247. doi: 10.1089/aid.1.1986.2.235. [DOI] [PubMed] [Google Scholar]
- June R. A., Landay A. L., Stefanik K., Lint T. F., Spear G. T. Phenotypic analysis of complement receptor 2+ T lymphocytes: reduced expression on CD4+ cells in HIV-infected persons. Immunology. 1992 Jan;75(1):59–65. [PMC free article] [PubMed] [Google Scholar]
- June R. A., Schade S. Z., Bankowski M. J., Kuhns M., McNamara A., Lint T. F., Landay A. L., Spear G. T. Complement and antibody mediate enhancement of HIV infection by increasing virus binding and provirus formation. AIDS. 1991 Mar;5(3):269–274. doi: 10.1097/00002030-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Liou R. S., Rosen E. M., Fung M. S., Sun W. N., Sun C., Gordon W., Chang N. T., Chang T. W. A chimeric mouse-human antibody that retains specificity for HIV gp120 and mediates the lysis of HIV-infected cells. J Immunol. 1989 Dec 15;143(12):3967–3975. [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Huang Y. X., Ashkenazi A., Ho D. D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992 Jan;66(1):235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone R., Fontana L., de Carolis C., Carini C., Sirianni M. C., Aiuti F. Evidence for activation of complement in patients with AIDS related complex (ARC) and/or lymphoadenopathy syndrome (LAS). Clin Exp Immunol. 1987 Dec;70(3):500–507. [PMC free article] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Gorny M. K., Xu J. Y., Mitchell W. M., Zolla-Pazner S. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J Virol. 1991 Aug;65(8):4169–4176. doi: 10.1128/jvi.65.8.4169-4176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaldi G., Peakman M., McManus T., Davies E. T., Tee D. E., Vergani D. Activation of the complement system in human immunodeficiency virus infection: relevance of the classical pathway to pathogenesis and disease severity. J Infect Dis. 1990 Dec;162(6):1227–1232. doi: 10.1093/infdis/162.6.1227. [DOI] [PubMed] [Google Scholar]
- Spear G. T., Landay A. L., Sullivan B. L., Dittel B., Lint T. F. Activation of complement on the surface of cells infected by human immunodeficiency virus. J Immunol. 1990 Feb 15;144(4):1490–1496. [PubMed] [Google Scholar]
- Spear G. T., Sullivan B. L., Landay A. L., Lint T. F. Neutralization of human immunodeficiency virus type 1 by complement occurs by viral lysis. J Virol. 1990 Dec;64(12):5869–5873. doi: 10.1128/jvi.64.12.5869-5873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tausk F., Gigli I. The human C3b receptor: function and role in human diseases. J Invest Dermatol. 1990 Jun;94(6 Suppl):141S–145S. doi: 10.1111/1523-1747.ep12876125. [DOI] [PubMed] [Google Scholar]
- Till M. A., Zolla-Pazner S., Gorny M. K., Patton J. S., Uhr J. W., Vitetta E. S. Human immunodeficiency virus-infected T cells and monocytes are killed by monoclonal human anti-gp41 antibodies coupled to ricin A chain. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1987–1991. doi: 10.1073/pnas.86.6.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. S., Stanley S. D., Zolla-Pazner S., Gorny M. K., Shadduck P. P., Langlois A. J., Matthews T. J., Bolognesi D. P., Palker T. J., Weinhold K. J. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J Immunol. 1990 Nov 15;145(10):3276–3282. [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. Y., Gorny M. K., Palker T., Karwowska S., Zolla-Pazner S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J Virol. 1991 Sep;65(9):4832–4838. doi: 10.1128/jvi.65.9.4832-4838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



