Abstract
Glucagon-like peptide-1 (GLP-1) is a polypeptide hormone secreted from enteroendocrine L cells and potentiates glucose-dependent insulin secretion in pancreatic β cells. Recently the GLP-1 receptor (GLP-1 R) has been a focus for new anti-diabetic therapy with the introduction of GLP-1 analogues and DPP-IV inhibitors, and this has stimulated additional interest in the mechanisms of GLP-1 signaling. Here we identify a mechanism for GLP-1 action, showing that the scaffold protein β-arrestin-1 mediates the effects of GLP-1 to stimulate cAMP production and insulin secretion in β cells. Using a coimmunoprecipitation technique, we also found a physical association between the GLP-1 R and β-arrestin-1 in cultured INS-1 pancreatic β cells. β-Arrestin-1 knockdown broadly attenuated GLP-1 signaling, causing decreased ERK and CREB activation and IRS-2 expression as well as reduced cAMP levels and impaired insulin secretion. However, β-arrestin-1 knockdown did not affect GLP-1 R surface expression and ligand-induced GLP-1 R internalization/desensitization. Taken together, these studies indicate that β-arrestin-1 plays a role in GLP-1 signaling leading to insulin secretion, defining a previously undescribed mechanism for GLP-1 action.
Keywords: CREB, IRS-2
Glucagon-like peptide-1 (GLP-1) is a polypeptide hormone produced mainly in enteroendocrine L cells of the gut. GLP-1 is secreted in a nutrient-dependent manner and potentiates glucose-dependent insulin secretion in pancreatic β cells and inhibits glucagon secretion from α cells. Chronic administration of GLP-1 also promotes insulin synthesis, β cell proliferation, and neogenesis (1–3). Recent drug discovery has focused on GLP-1 action because of its therapeutic utility in the treatment of type 2 diabetes. GLP-1 analogues and small molecule compounds that inhibit GLP-1 degrading enzyme DPP-IV are all effective at improving glycemic profiles and β cell performance (4, 5). Thus, a thorough understanding of GLP-1's cellular actions assumes greater importance.
The GLP-1 receptor (GLP-1 R) is a member of the seven-transmembrane family of G protein-coupled receptors (7TMRs) (6). A large body of literature exists on many aspects of 7TMR signaling and function, and it is now recognized that β-arrestin-1 is an important adaptor protein for several 7TMRs and functions in the process of transmitting receptor-mediated downstream signals, receptor internalization, and receptor desensitization (7, 8). β-Arrestin-1 can also function as an adaptor/signaling protein in other receptor systems, including the IGF-1 receptor (9, 10), the TNF-α receptor (11), and others (7, 8, 12–16). Given the widespread functions of β-arrestin-1, particularly in relationship to 7TMRs, we hypothesized that β-arrestin-1 could play a role in GLP-1 R action. In the current work, we tested this proposition in a variety of ways and found that β-arrestin-1 coassociates with the GLP-1 R and plays a role in GLP-1 signaling events that stimulate cAMP production, phosphoprotein generation, and insulin secretion in the pancreatic β cell line INS-1 cells. These results establish a role for β-arrestin-1 and provide further insight into the cellular mechanisms of GLP-1 action.
Results
β-Arrestin-1 Associates with the GLP-1 Receptor.
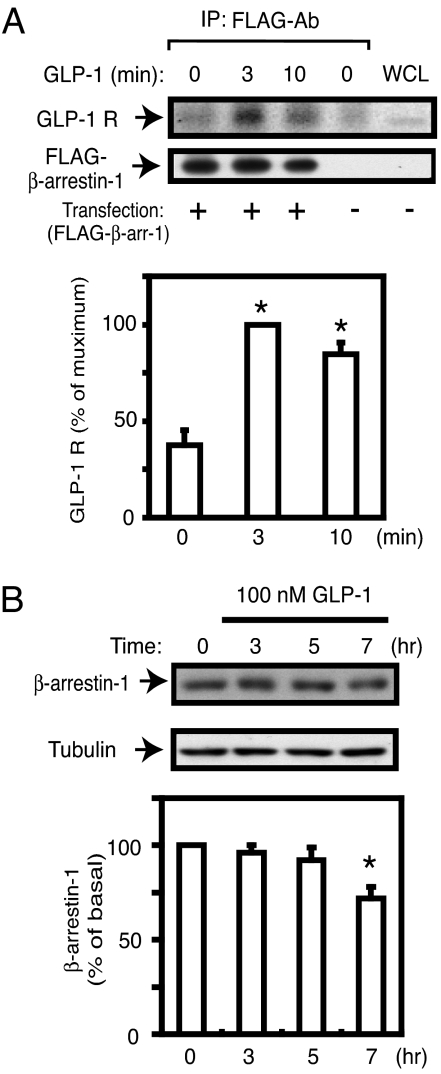
The GLP-1 receptor (GLP-1 R) is a member of the 7TMR family (1, 2, 6), and β-arrestin-1 has diverse functions as an adaptor molecule for several classes of receptor types (7, 8, 10). To determine whether there is an association between β-arrestin-1 and the GLP-1 R in a β cell model, we conducted coimmunoprecipitation experiments in FLAG-tagged β-arrestin-1-expressing INS-1 cells. As shown in Fig. 1A, an interaction between the GLP-1 R and β-arrestin-1 was strongly enhanced in an agonist (GLP-1)-dependent manner.
Fig. 1.
Interaction between GLP-1 receptor and β-arrestin-1. (A) FLAG-tagged β-arrestin-1 (FLAG-βarr-1)-expressing INS-1 cells were starved with KRBH buffer containing 2.5 mM glucose for 120 min, then stimulated with GLP-1 (100 nM) for 3 min or 10 min and incubated with DSP cross-linker for 1 h on ice. The resulting cell lysates were immunoprecipitated (IP) with anti-FLAG antibody, and IP samples were analyzed by Western blotting with GLP-1 receptor antibody (GLP-1 R) or FLAG antibody, as described in SI Materials and Methods. A representative image from three independent experiments is shown. The scanned bar graph is shown as percentage maximum of GLP-1 R blots, normalized by FLAG-βarr-1 blots. WCL, whole-cell lysates. *, P < 0.05 vs. basal. (B) INS-1 cells cultured in RPMI medium 1640 were treated with 100 nM GLP-1 for different times as indicated, and cell lysates were analyzed by Western blotting with β-arrestin-1 antibody or β-tubulin antibody as an internal control. A representative image from four independent experiments is shown. The bar graph is shown as percentage of basal β-arrestin-1 contents normalized by β-tubulin blots. *, P < 0.05 vs. basal.
It is known that β-arrestin-1 is degraded after ligand stimulation in several receptor signaling systems (14, 17–19), and, to determine whether this was the case in our model, we measured β-arrestin-1 protein content after treatment of INS-1 cells with 100 nM GLP-1. As shown in Fig. 1B, GLP-1 had a time-dependent effect to decrease cellular β-arrestin-1 levels, and β-arrestin-1 was decreased by ≈30% after 7 h of GLP-1 treatment.
Effect of β-Arrestin-1 Knockdown on GLP-1 Signaling.
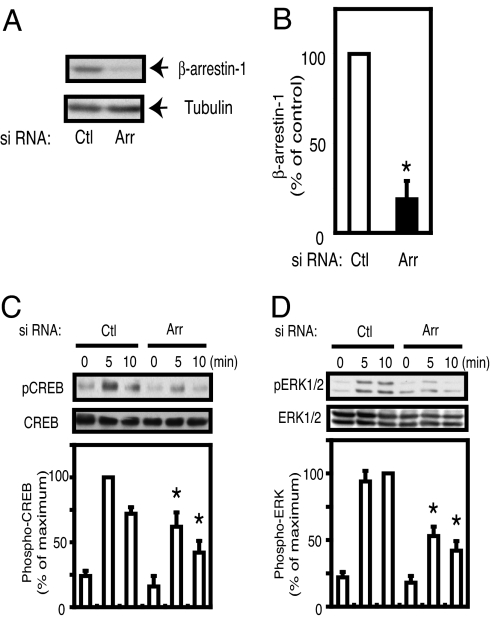
The data in Fig. 1 strongly suggest that the GLP-1 signaling system directly couples into β-arrestin-1. Therefore, we used INS-1 cells to focus on the potential functional role of β-arrestin-1 in GLP-1 action. To end this, we induced β-arrestin-1 knockdown in INS-1 cells using RNAi. As shown in Fig. 2 A and B, 48 h after electroporation of siRNA, we observed an ≈80% decrease in β-arrestin-1 protein expression in β-arrestin-1 knockdown cells, compared with cells transduced with a control scrambled siRNA. GLP-1 stimulation activates numerous signaling cascades (1–3), and we assessed the role of β-arrestin-1 in GLP-1-induced CREB and ERK1/2 activation using phosphospecific CREB and ERK1/2 antibodies. As seen in Fig. 2 C and D, GLP-1 stimulation led to rapid phosphorylation of both of CREB and ERK1/2 in control cells. In contrast, GLP-1-stimulated CREB and ERK1/2 phosphorylation was markedly attenuated in β-arrestin-1 knockdown cells.
Fig. 2.
Effect of β-arrestin-1 knockdown on GLP-1 signaling. (A and B) INS-1 cells were electroporated with scrambled siRNA (Ctl) or β-arrestin-1 siRNA (Arr). Forty-eight hours after electroporation, immunoblotting was performed with anti-β-arrestin-1 antibody or β-tubulin antibody as an internal control (A). The scanned bar graph of β-arrestin-1 blots is shown as percentage of control, normalized by β-tubulin blots from three independent experiments (B). *, P < 0.05 vs. control. (C and D) Forty-eight hours after electroporation with β-arrestin-1 siRNA (Arr) or scrambled control siRNA (Ctl), INS-1 cells were starved with KRBH buffer containing 2.5 mM glucose without serum for 120 min, then stimulated with GLP-1 (100 nM) for 5 min or 10 min, and cell lysates were analyzed by Western blotting with anti-phospho CREB antibody (pCREB), anti-CREB antibody (C), anti-phospho ERK1/2 antibody (pERK1/2), or anti-ERK1/2 antibody (D). The bar graphs are shown as percentage maximum of scrambled siRNA-transduced cells from three independent experiments. *, P < 0.05 vs. control on each time point.
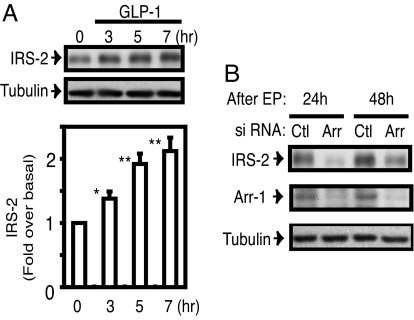
Because CREB and ERK1/2 modulate transcription and IRS-2 is a known CREB target gene (20), we assessed the effect of GLP-1 on IRS-2 in INS-1 cells. GLP-1 stimulation led to a time-dependent increase in IRS-2 protein level in INS-1 cells (Fig. 3A), consistent with a previous report (21). We found that IRS-2 expression level was remarkably down-regulated in β-arrestin-1 knockdown cells at both 24 h and 48 h after transduction of β-arrestin-1 siRNA (Fig. 3B). IRS-2 is the major IRS protein in pancreatic β cells and serves as an important regulator of β cell growth and function (22–25), suggesting that β-arrestin-1 plays a role in GLP-1 action, such as insulin secretion from β cells.
Fig. 3.
Induction of IRS-2 expression by GLP-1 treatment and the effect of β-arrestin-1 knockdown on IRS-2 expression. (A) INS-1 cells cultured in RPMI medium 1640 were treated with 100 nM GLP-1 for different times as indicated, and cell lysates were analyzed by immunoblotting with IRS-2 antibody or β-tubulin antibody as an internal control. The scanned bar graph is shown as fold over basal of IRS-2 contents normalized by β-tubulin blots from three independent experiments. *, P < 0.05; **, P < 0.01 vs. basal. (B) Twenty-four and 48 h after electroporation with β-arrestin-1 siRNA (Arr) or scrambled control siRNA (Ctl), cell lysates were analyzed by immunoblotting with IRS-2 antibody, β-arrestin-1 antibody (Arr-1), or β-tubulin antibody. A representative image is displayed from three independent experiments.
Knockdown of β-Arrestin-1 Inhibits GLP-1-Stimulated Insulin Secretion.
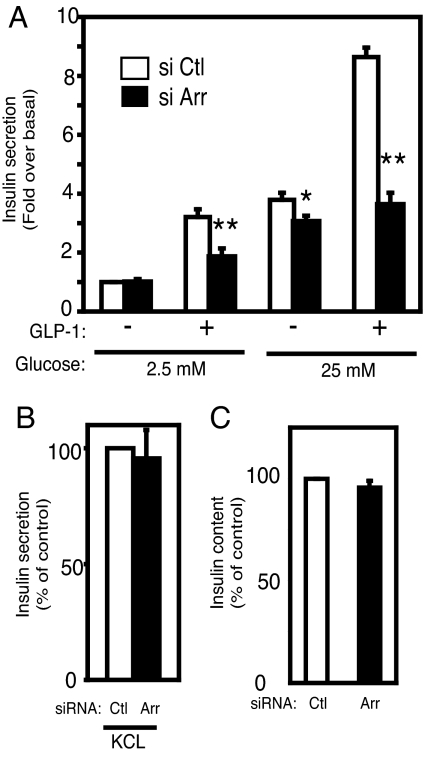
To further assess the role of β-arrestin-1 in GLP-1-induced insulin secretion, we evaluated GLP-1-induced insulin secretion in β-arrestin-1 knockdown INS-1 cells. INS-1 cells were incubated with GLP-1 (100 nM) in 2.5 mM glucose (low-glucose) or 25 mM glucose (high-glucose) conditions for 120 min. As shown in Fig. 4A, no significant difference in insulin secretion between control cells and β-arrestin-1 knockdown cells was observed without GLP-1 under the low-glucose condition. Addition of GLP-1 led to an ≈3-fold increase in insulin secretion in control INS-1 cells. This effect was inhibited ≈40% by β-arrestin-1 knockdown. Because GLP-1 potentiates insulin secretion in a glucose-dependent manner, we also evaluated the effect of GLP-1 at 25 mM glucose. As shown in Fig. 4A, GLP-1-induced insulin secretion at high glucose was strikingly increased in control cells, and this effect was inhibited by ≈60% in β-arrestin-1 knockdown cells. Interestingly, basal insulin secretion at 25 mM glucose was moderately inhibited ≈18% in β-arrestin-1 knockdown cells compared with control cells. However, total cellular insulin content was not affected by β-arrestin-1 knockdown (Fig. 4C), suggesting that the effects on insulin secretion were not attributed to a decrease in insulin availability. We also evaluated GLP-1-induced insulin secretion at several GLP-1 concentrations (1 nM, 10 nM, and 100 nM) for different incubation times (30 min, 60 min, and 120 min) in control and β-arrestin-1 knockdown cells. As shown in supporting information (SI) Fig. S1, GLP-1 led to an increase in insulin secretion in a time- and dose-dependent manner, and these effects were inhibited in β-arrestin-1 knockdown cells compared with control cells. Next we measured KCl (60 mM)-induced insulin secretion, because high K+-induced depolarization evokes insulin release by stimulating the distal steps of vesicule exocytosis from a primed readily releasable pool of insulin granules (26). As shown in Fig. 4B, depletion of β-arrestin-1 had no effect on KCl-induced insulin secretion. Taken together, these results suggest that β-arrestin-1 depletion attenuates an early step of GLP-1-mediated insulin secretion but does not affect distal events such as the exocytotic machinery.
Fig. 4.
Effects of β-arrestin-1 knockdown on insulin secretion. (A) INS-1 cells were electroporated with control siRNA (open bars, si Ctl) or β-arrestin-1 siRNA (filled bars, si Arr). Forty-eight hours after electroporation, INS-1 cells were incubated for 2 h with or without 100 nM GLP-1 in KRBH buffer containing 2.5 mM glucose or 25 mM glucose. Insulin content in the supernatants was measured by ELISA as described in Materials and Methods. Data represent fold over control under 2.5 mM glucose conditions without GLP-1. The mean ± SEM of five independent experiments is displayed. (B) INS-1 cells were electroporated with control siRNA (Ctl) or β-arrestin-1 siRNA (Arr). Forty-eight hours after electroporation, INS-1 cells were incubated for 2 h with 60 mM KCl in KRBH buffer containing 2.5 mM glucose. Data represent percentage of control. The mean ± SEM of three independent experiments is displayed. (C) INS-1 cells were electroporated with control siRNA (Ctl) or β-arrestin-1 siRNA (Arr). Forty-eight hours after electroporation, cells were lysed and intracellular insulin content was measured by ELISA. Data were normalized to total protein content and represent percentage of control. The mean ± SEM of five independent experiments is displayed. *, P < 0.05; **, P < 0.01 vs. control.
Reduction of GLP-1-Stimulated Insulin Secretion by β-Arrestin-1 Knockdown Is Mediated Through a cAMP-Dependent Pathway.
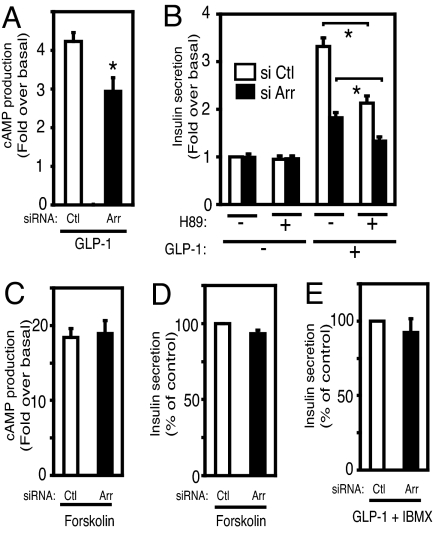
It has been shown that GLP-1-stimulated insulin secretion is mediated by PKA activation due to a GLP-1-induced increase in cAMP levels (1–3). To assess the potential role of β-arrestin-1 in this pathway, we next examined cAMP production by GLP-1 and the effect of the PKA inhibitor H89 in control and β-arrestin-1 knockdown cells. As shown in Fig. 5A, GLP-1 (100 nM) induced an ≈4-fold increase in cAMP production in control cells, and this effect was inhibited by ≈35% in β-arrestin-1 knockdown cells. Next we evaluated the effect of the PKA inhibitor H89 on GLP-1-stimulated insulin secretion. It has been reported that this PKA inhibitor only partially blocks GLP-1-stimulated insulin secretion, suggesting that Gs and cAMP can also signal to insulin secretion through PKA-independent pathways (1, 27–31). Consistent with these previous findings, we found that H89 inhibited GLP-1-induced insulin secretion by ≈35% in control cells and that β-arrestin-1 knockdown led to a further decrease (Fig. 5B). Furthermore, as shown in Fig. 5 C and D, forskolin-induced cAMP elevation and insulin secretion were comparable in both control and β-arrestin-1 knockdown cells, suggesting that β-arrestin-1 depletion does not affect adenylate cyclase activity itself. Interestingly, the majority of the reduction in GLP-1-induced insulin secretion in β-arrestin-1 knockdown cells was restored by IBMX treatment (Fig. 5E).
Fig. 5.
Reduction of GLP-1-stimulated insulin secretion by β-arrestin-1 knockdown is mediated through a cAMP-dependent pathway. (A and C) Forty-eight hours after electroporation of control siRNA (Ctl) or β-arrestin-1 siRNA (Arr), cells were incubated with or without 100 nM GLP-1 (A) or 10 μM forskolin (C) for 15 min in KRBH buffer containing 2.5 mM glucose and 500 μM IBMX. GLP-1-simulated cAMP production in β-arrestin-1 knockdown or control INS-1 cells was measured by enzyme immunoassay as described in Materials and Methods. Data were normalized to total protein content and represent fold over basal value without stimulation. The mean ± SEM of three independent experiments is displayed. (B) Forty-eight hours after electroporation of control siRNA (Ctl) or β-arrestin-1 siRNA (Arr), cells were incubated for 2 h with or without 100 nM GLP-1 and with or without 10 μM H89 as indicated in KRBH buffer containing 2.5 mM glucose. Insulin content in the supernatants was measured, and data represent fold over control at 2.5 mM glucose condition without GLP-1 and H89. The mean ± SEM of three independent experiments is displayed. (D and E) Forty-eight hours after electroporation of control siRNA (Ctl) or β-arrestin-1 siRNA (Arr), cells were incubated for 2 h with 10 μM forskolin (D) or 100 nM GLP-1 together with 500 μM IBMX (E) in KRBH buffer containing 2.5 mM glucose. Data represent percentage of control. The mean ± SEM of three independent experiments is displayed. *, P < 0.05.
β-Arrestin-1 Knockdown Does Not Affect Surface GLP-1 Receptor Number and GLP-1-Induced Receptor Internalization/Desensitization.
In many types of receptor systems, β-arrestin-1 is involved in ligand-induced receptor internalization and desensitization (7, 8). To assess this in our model, we measured cell-surface GLP-1 R expression levels by an 125I-GLP-1 binding assay with or without GLP-1 pretreatment. As shown in Fig. S2A, basal surface GLP-1 R expression levels were not changed by β-arrestin-1 knockdown, and total receptor expression was also the same between control and β-arrestin-1 knockdown cells as assessed by immunoblotting. Furthermore, 100 nM GLP-1 treatment for 60 min decreased surface receptor number by ≈50% in both control and β-arrestin-1 knockdown cells, suggesting that functional ligand-induced receptor internalization was intact in β-arrestin-1 knockdown cells. To evaluate this further with respect to functional aspects of desensitization, we treated INS-1 cells with 100 nM GLP-1 for 60 min. After a GLP-1-free 30-min wash period, cells were rechallenged with 100 nM GLP-1 and insulin secretion was measured over the next 120 min. As seen in Fig. S2B, GLP-1-induced insulin secretion in control and β-arrestin-1 knockdown cells was less than in nonpretreated cells, indicating that GLP-1 pretreatment led to desensitization of GLP-1's effects to enhance insulin secretion. However, the magnitude of desensitization was comparable in the control and β-arrestin-1 knockdown cells. These findings suggest that β-arrestin-1 depletion did not affect cell-surface GLP-1 R number or the overall process of desensitization.
Discussion
The GLP-1 receptor (GLP-1 R) is a seven-transmembrane receptor (7TMR) that is highly expressed in pancreatic β cells (1, 2, 6). After GLP-1 stimulation, the GLP-1 R can couple with the trimeric G protein Gαs, which results in activation of adenylate cyclase (AC), followed by elevation of cAMP levels (1–3, 32). This leads to cAMP-dependent activation of protein kinase A (PKA) and Epac 2, the two major regulators of insulin secretion in both pancreatic and cultured β cells (29–31). GLP-1 R activation also induces IRS-2 (21) and early gene expression through the activation of ERK1/2, protein kinase C, and phosphatidylinositol 3-kinase (PI3K), and these effects may promote β cell growth, differentiation, and maintenance (1, 2). Thus, the GLP-1 R stimulates many β cell signaling pathways, and, given the clinical importance of the GLP-1 R as a target for new anti-diabetes drugs such as GLP-1, its analogues, and DPP-IV inhibitors, a thorough understanding of the mechanism of GLP-1 action is of heightened importance.
β-Arrestin-1 is an adaptor protein for 7TMRs, as well as other receptor types, and it can mediate receptor desensitization and signaling to the mitogen-activated protein kinases ERK, JNK, and p38 as well as Akt, PI3K, and RhoA (7, 8). Therefore, in this study, we sought to determine whether β-arrestin-1 can interact with the GLP-1 R and whether it plays a functional role in GLP-1 signaling. We evaluated the role of β-arrestin-1 using INS-1 cells, a rat pancreatic β cell-derived line. Using a coimmunoprecipitation approach, we detected a direct physical association between the GLP-1 R and β-arrestin-1 in a GLP-1- and time-dependent manner (Fig. 1A). Furthermore, chronic GLP-1 treatment led to a decrease in β-arrestin-1 protein level (Fig. 1B), indicating that β-arrestin-1 is involved in GLP-1 signaling. These results are consistent with a recent report by Jorgensen et al. (33), which showed a positive BRET (bioluminescence resonance energy transfer)-mediated interaction between β-arrestin-1 and GLP-1 R in HEK293 cells, although these studies did not present any functional data regarding β-arrestin-1.
Depletion of β-arrestin-1 caused a decrease in GLP-1-induced phosphorylation of CREB and ERK1/2 (Fig. 2 C and D), with a decrease in IRS-2 protein expression (Fig. 3B). We also confirmed these effects using different siRNA (Fig. S3 A and B). These molecules also serve as important factors for β cell proliferation, differentiation, and functional maintenance. These results raise the possibility that β-arrestin-1 signaling may play a role in β cell performance. To confirm and extend this idea, we used RNAi to show that β-arrestin-1 knockdown caused a marked decrease in GLP-1-stimulated insulin secretion in INS-1 cells at both low- and high-glucose concentrations (Fig. 4A and Figs. S1 and S3C). This indicates that β-arrestin-1 functionally participates in GLP-1 signaling to the insulin secretion system. Upon agonist binding, the GLP-1 receptor is coupled to the Gαs subunit with subsequent activation of adenylate cyclase, followed by elevation of cytosolic cAMP levels and stimulation of insulin secretion (1–3, 32). β-Arrestin-1 knockdown led to a marked decrease in cAMP elevation stimulated with GLP-1 (Fig. 5A) with a subsequent decrease in CREB phosphorylation (Fig. 2C). Moreover, the reduction of GLP-1-induced insulin secretion by β-arrestin-1 knockdown was restored by a 2-h treatment with IBMX, which causes a robust accumulation of cAMP by inhibiting phosphodiesterase (Fig. 5E). In addition, the effect of the adenylate cyclase stimulator forskolin to induce cAMP elevation was not affected by β-arrestin-1 knockdown (Fig. 5C). These results suggest that the dominant cause of the reduction in GLP-1-stimulated insulin secretion in β-arrestin-1 knockdown cells is mediated by down-regulating the Gs-cAMP pathway before adenylate cyclase itself.
Recent findings have indicated that, relevant to insulin secretion, there are at least two pathways induced by cAMP, a PKA-dependent pathway and a PKA-independent pathway including Epac, a cAMP-regulated guanine nucleotide exchange factor (1, 2, 31). Our data showed that the decrease in GLP-1-stimulated insulin secretion caused by the PKA inhibitor H89 was further reduced (≈30%) by β-arrestin-1 knockdown (Fig. 5B). This result suggests that β-arrestin-1 participates in both PKA-dependent and PKA-independent pathways. Based on these findings, we conclude that β-arrestin-1 plays an important role in GLP-1 signaling by regulating the Gs-cAMP pathway, which activates PKA and other target molecules to mediate insulin secretion.
Another possible mechanism for the reduction of GLP-1-induced insulin secretion by β-arrestin-1 knockdown may be mediated by the regulation of IRS-2. IRS-2 is the major IRS molecule in β cells and regulates the maintenance of β cell differentiation and performance (22–25). It has also been shown that IRS-2 can mediate GLP-1 R signaling (21). Previously, we reported that β-arrestin-1 can control IRS-1 degradation by competitive binding to the E3 ubiquitin ligase Mdm2 (34). It is also known that β-arrestin-1 can modulate gene expression through CREB and p300 (35) and that IRS-2 is a CREB target gene (20). Therefore, it is possible that β-arrestin-1 positively regulates IRS-2 expression by modulating IRS-2 degradation and/or gene expression. Although the precise mechanism of how β-arrestin-1 controls IRS-2 function remains to be fully defined, down-regulation of IRS-2 might affect β cell performance in our β-arrestin-1 knockdown model (Fig. 3B). Along these lines, high-glucose-induced insulin secretion was also moderately inhibited by β-arrestin-1 knockdown (Fig. 4A), and it is possible that the IRS-2 down-regulation may play a role in this process. Interestingly, it has been reported that GPCR 119, another Gs-coupled GPCR expressed in β cells, participates in glucose-induced insulin secretion (36), and it is possible that β-arrestin-1 may participate in glucose signaling to insulin secretion by other GPCRs in addition to GLP-1.
Although there are numerous reports showing a variety of actions of β-arrestins as signaling scaffolds, the traditional role of β-arrestins is as a mediator of receptor internalization and desensitization (7, 8). However, in our studies we did not observe any role for β-arrestin-1 in GLP-1 receptor trafficking (Fig. S2). Interestingly, it has been reported that GLP-1 receptor trafficking can be mediated through caveolin-1 (37). It is possible that β-arrestin-2, another ubiquitously expressed β-arrestin family member, has some role in this process. We found that β-arrestin-2 was expressed in INS-1 cells, but β-arrestin-2 expression levels were not changed in β-arrestin-1 knockdown cells, as assessed by quantitative PCR (Fig. S4). In addition, it has been shown that the GLP-2 receptor C terminus, which is necessary for interacting with β-arrestin-2, is dispensable for receptor endocytosis and ligand-induced desensitization (38). Thus, GLP-1 R endocytosis/trafficking appears to be independent of β-arrestins.
Taken together, our results illustrate a mechanism for GLP-1 action, showing that β-arrestin-1 couples to the GLP-1 R, mediating GLP-1 signaling to cAMP, CREB, ERK, and IRS-2. These effects place β-arrestin-1 as an important control point in the GLP-1 signaling pathway, which leads to enhanced glucose-stimulated insulin secretion and perhaps even β cell proliferation/differentiation. These studies may open up new therapeutic approaches to augmenting GLP-1-mediated insulin secretion.
Materials and Methods
Cell Culture.
Insulin-producing INS-1 cells were kind gift from Steven Chessler (University of California at San Diego). The cells were grown at 37°C and 5% CO2 in a humidified atmosphere. The culture medium was RPMI medium 1640 with 11.1 mM glucose supplemented with 10% FBS, 1 mM sodium pyruvate, 1 mM l-glutamine, and 50 μM mercaptoethanol.
siRNA Transduction.
For siRNA-mediated knockdown of β-arrestin-1 expression, nucleotides 449–467 (GenBank accession no. NM012910) of rat β-arrestin-1 cDNA (5′-GGCCTGCGGTGTGGATTAT-3′) was used as the target sequence, which was confirmed as having no homology to any other genes by BLAST search (National Center for Biotechnology Information, National Institutes of Health). The duplexes of siRNA of β-arrestin-1 and a negative control (scrambled sequence) were purchased from Dharmacon Research. Cells were transiently transfected with 2 nmol siRNA duplex by electroporation (GENE PULSER; Bio-Rad) as we previously described (11).
Insulin Secretion Assays.
INS-1 cells were grown in 24-well plates coated with polylysine (Sigma). Cells were washed with KRBH buffer (KRBH; 140 mM NaCl/3.6 mM KCl/0.5 mM NaH2PO4/0.5 mM MgSO4/1.5 mM CaCl2/2 mM NaHCO3/10 mM Hepes), then incubated with KRBH buffer containing 2.5 mM glucose for 30 min before the assay. Insulin secretion from INS-1 cells was measured in KRBH buffer containing 2.5 mM or 25 mM glucose with or without indicated stimulators and/or inhibitors at 37°C in a humidified incubator. The supernatants were collected, and intracellular insulin was extracted by acid ethanol method (15 mM HCl/75% ethanol). Insulin was detected by using the insulin enzyme immunoassay kit (Linco Research) according to the manufacturer's instructions. Final insulin concentrations per well were normalized by intracellular insulin content.
cAMP Measurement Assays.
INS-1 cells were grown in 24-well plates coated with polylysine (Sigma). Cells were washed with KRBH buffer, then incubated with KRBH buffer containing 2.5 mM glucose for 30 min before the assay. cAMP accumulation was measured over a period of 15 min in KRBH buffer containing 2.5 mM glucose, 500 μM IBMX, and indicated stimulators. Cells were lysed, and intracellular cAMP content was measured by using the cAMP enzyme immunoassay kit (Assay Designs) according to the manufacturer's instructions. Final cAMP concentrations per well were normalized by total protein as described previously (11, 12).
Statistical Analysis.
Densitometric quantification and normalization were performed by using NIH Image 1.63 software. The values are expressed as mean ± SE. Statistical significance was determined by using the two-tailed Student t test. A P value of <0.05 was considered significant.
Details.
Please see SI Materials and Methods for further details of other procedures used in this study.
Supplementary Material
Acknowledgments.
We are grateful to Dr. Steven Chessler (University of California at San Diego) for the gift of INS-1 cells and technical advice and Dr. Robert Lefkowitz (Howard Hughes Medical Institute, Duke University) for the gift of FLAG-tagged β-arrestin-1 expression vector. We thank Ms. Elizabeth J. Hansen for editorial assistance. This study was funded in part by National Institutes of Health Grants DK 033651 and DK 074868 (to J.M.O.) and by University of California Discovery Program Project bio03-10383 (BioStar) with matching funds from Pfizer Incorporated. N.S. is supported by the Manpei Suzuki Diabetic Foundation.
Footnotes
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710402105/DCSupplemental.
References
- 1.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habener JF, Kemp DM. In: Diabetes Mellitus: A Fundamental and Clinical Text. 3rd ed. LeRoith D, Taylon SI, Olefsky JM, editors. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 99–113. [Google Scholar]
- 4.Drucker DJ, Nauck MA. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 5.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. J Am Med Assoc. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 6.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 8.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 9.Dalle S, Ricketts W, Imamura T, Vollenweider P, Olefsky JM. Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J Biol Chem. 2001;276:15688–15695. doi: 10.1074/jbc.M010884200. [DOI] [PubMed] [Google Scholar]
- 10.Hupfeld CJ, Olefsky JM. Regulation of receptor tyrosine kinase signaling by GRKs and beta-arrestins. Annu Rev Physiol. 2007;69:561–577. doi: 10.1146/annurev.physiol.69.022405.154626. [DOI] [PubMed] [Google Scholar]
- 11.Kawamata Y, et al. Tumor necrosis factor receptor-1 (TNF-R1) can function through a Galpha q/11/beta-arrestin-1 signaling complex. J Biol Chem. 2007;282:28549–28556. doi: 10.1074/jbc.M705869200. [DOI] [PubMed] [Google Scholar]
- 12.Hupfeld CJ, Dalle S, Olefsky JM. Beta-arrestin 1 down-regulation after insulin treatment is associated with supersensitization of beta 2 adrenergic receptor Galphas signaling in 3T3–L1 adipocytes. Proc Natl Acad Sci USA. 2003;100:161–166. doi: 10.1073/pnas.0235674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hupfeld CJ, Resnik JL, Ugi S, Olefsky JM. Insulin-induced beta-arrestin1 Ser-412 phosphorylation is a mechanism for desensitization of ERK activation by Galphai-coupled receptors. J Biol Chem. 2005;280:1016–1023. doi: 10.1074/jbc.M403674200. [DOI] [PubMed] [Google Scholar]
- 14.Dalle S, et al. Insulin induces heterologous desensitization of G-protein-coupled receptor and insulin-like growth factor I signaling by downregulating beta-arrestin-1. Mol Cell Biol. 2002;22:6272–6285. doi: 10.1128/MCB.22.17.6272-6285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imamura T, et al. beta-Arrestin-mediated recruitment of the Src family kinase Yes mediates endothelin-1-stimulated glucose transport. J Biol Chem. 2001;276:43663–43667. doi: 10.1074/jbc.M105364200. [DOI] [PubMed] [Google Scholar]
- 16.Imamura T, Ishibashi K, Dalle S, Ugi S, Olefsky JM. Endothelin-1-induced GLUT4 translocation is mediated via Galpha(q/11) protein and phosphatidylinositol 3-kinase in 3T3–L1 adipocytes. J Biol Chem. 1999;274:33691–33695. doi: 10.1074/jbc.274.47.33691. [DOI] [PubMed] [Google Scholar]
- 17.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 18.Shenoy SK, Lefkowitz RJ. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem. 2003;278:14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- 19.Shenoy SK, Lefkowitz RJ. Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J Biol Chem. 2005;280:15315–15324. doi: 10.1074/jbc.M412418200. [DOI] [PubMed] [Google Scholar]
- 20.Jhala US, et al. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park S, et al. Exendin-4 uses IRS2 signaling to mediate pancreatic beta cell growth and function. J Biol Chem. 2006;281:1159–1168. doi: 10.1074/jbc.M508307200. [DOI] [PubMed] [Google Scholar]
- 22.Withers DJ, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 24.Weir GC, Bonner-Weir S. A dominant role for glucose in beta cell compensation of insulin resistance. J Clin Invest. 2007;117:81–83. doi: 10.1172/JCI30862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terauchi YT, et al. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007;117:246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 27.Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1 protein translocation from the cytoplasm to the nucleus of pancreatic beta-cells by a cyclic adenosine monophosphate/protein kinase A-dependent mechanism. Endocrinology. 2001;142:1820–1827. doi: 10.1210/endo.142.5.8128. [DOI] [PubMed] [Google Scholar]
- 29.Kashima Y, et al. Critical role of cAMP-GEFII–Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276:46046–46053. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- 30.Ozaki N, et al. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 31.Holz GG. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorgensen R, Kubale V, Vrecl M, Schwartz TW, Elling CE. Oxyntomodulin differentially affects glucagon-like peptide-1 receptor beta-arrestin recruitment and signaling through Galpha(s) J Pharmacol Exp Ther. 2007;322:148–154. doi: 10.1124/jpet.107.120006. [DOI] [PubMed] [Google Scholar]
- 34.Usui I, et al. beta-arrestin-1 competitively inhibits insulin-induced ubiquitination and degradation of insulin receptor substrate 1. Mol Cell Biol. 2004;24:8929–8937. doi: 10.1128/MCB.24.20.8929-8937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang J, et al. A nuclear function of beta-arrestin1 in GPCR signaling: Regulation of histone acetylation and gene transcription. Cell. 2005;123:833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Chu ZL, et al. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148:2601–2609. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- 37.Syme CA, Zhang L, Bisello A. Caveolin-1 regulates cellular trafficking and function of the glucagon-like peptide 1 receptor. Mol Endocrinol. 2006;20:3400–3411. doi: 10.1210/me.2006-0178. [DOI] [PubMed] [Google Scholar]
- 38.Estall JL, Koehler JA, Yusta B, Drucker DJ. The glucagon-like peptide-2 receptor C terminus modulates beta-arrestin-2 association but is dispensable for ligand-induced desensitization, endocytosis, and G-protein-dependent effector activation. J Biol Chem. 2005;280:22124–22134. doi: 10.1074/jbc.M500078200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.