Abstract
Controlled proteolytic degradation of specialized junctional structures, corneodesmosomes, by epidermal proteases is an essential process for physiological desquamation of the skin. Corneodesmosin (CDSN) is an extracellular component of corneodesmosomes and, although considerable debate still exists, genetic studies have suggested that the CDSN gene in the major psoriasis-susceptibility locus (PSORS1) may be responsible for susceptibility to psoriasis, a human skin disorder characterized by excessive growth and aberrant differentiation of keratinocytes. CDSN is also expressed in the inner root sheath of hair follicles, and a heterozygous nonsense mutation of the CDSN gene in humans is associated with scalp-specific hair loss of poorly defined etiology. Here, we have investigated the pathogenetic roles of CDSN loss of function in the development of skin diseases by generating a mouse strain with targeted deletion of the Cdsn gene. Cdsn-deficient mouse skin showed detachment of the stratum corneum from the underlying granular layer and/or detachment within the upper granular layers due to the disrupted integrity of the corneodesmosomes. When grafted onto immunodeficient mice, Cdsn-deficient skin showed rapid hair loss together with epidermal abnormalities resembling psoriasis. These results underscore the essential roles of CDSN in hair physiology and suggest functional relevance of CDSN gene polymorphisms to psoriasis susceptibility.
Keywords: corneodesmosome, hypotrichosis simplex of the scalp, keratinocyte, psoriasis
The desmosome has a characteristic ultrastructural appearance, in which the cell membrane of two adjacent cells forms a symmetrical junction with a central intercellular space of ≈30 nm containing a densely staining line (1, 2). Corneodesmosomes, the modified desmosomes of the uppermost layers of the epidermis, play an important role in corneocyte cohesion, and controlled proteolytic degradation of corneodesmosomes by epidermal proteases is an essential process for physiological desquamation of the skin. The major transmembrane corneodesmosomal proteins are desmoglein 1 (Dsg1) and desmocollin 1, and heterophilic interactions between these two glycoproteins constitute desmosome-mediated cell–cell adhesion (3–5). Another desmosomal component with a less well defined function is corneodesmosin (CDSN) (6, 7). CDSN, originally identified as the S gene located 160 kb telomeric of HLA-C (8), encodes a late differentiation epidermal glycoprotein and constitutes an extracellular component of corneodesmosomes (6, 9). CDSN is located in the corneodesmosomal core and is covalently linked to the cornified envelope of corneocytes.
Although considerable debate still exists, genetic studies in human subjects have suggested that polymorphisms of the CDSN gene are associated with susceptibility to psoriasis (10–12), a chronic inflammatory disorder of the skin characterized by excessive growth and aberrant differentiation of keratinocytes (13). Histological characteristics of psoriasis include hyperplasia of the epidermis (acanthosis), infiltration of leukocytes into the epidermis, and dilation of blood vessels. Although the underlying cause of the disease is still largely unknown, genetic association and linkage studies have indicated that the most important genomic region in psoriasis susceptibility is PSORS1, near HLA-C (14–18). Three strongly psoriasis-associated susceptibility alleles have been identified within PSORS1. These include CDSN*TTC, HLA-Cw6, and HCR*WWCC. Strong linkage disequilibrium between these three alleles has made it difficult to distinguish their individual genetic effects (11, 15, 17, 19), and no previous functional studies have addressed the exact roles of CDSN in the pathogenesis of psoriasis per se.
In addition to its possible association with psoriasis susceptibility, a heterozygous nonsense mutation of the CDSN gene is associated with hypotrichosis simplex of the scalp (HSS; OMIM 146520) (20), an autosomal dominant form of isolated alopecia. Notably, abnormal proteolytic cleavage of CDSN has been observed in individuals with HSS, and a dominant negative interaction between the mutant and wild-type CDSN protein may account for loss of cohesion in the inner root sheath (IRS) of the hair follicle. In view of the delayed onset of alopecia in HSS and the fact that lost hair is not regenerated, it is assumed that the abnormal CDSN aggregates are toxic to the hair follicle cells, thereby contributing to the hair loss (20); however, the possibility of a simple gene-dosage effect due to a heterozygous mutation of the CDSN gene in the pathogenesis of HSS has not been formally excluded.
Here, we have investigated the pathogenetic roles of CDSN loss-of-function in the development of skin diseases by generating a mouse strain with targeted deletion of the Cdsn gene. The results underscore the essential roles of CDSN in hair physiology and suggest functional relevance of CDSN gene polymorphisms to psoriasis susceptibility.
Results
Targeted Deletion of the Murine Cdsn Gene Results in Stratum Corneum Detachment Due to Defective Desmosome Formation.
To gain insight into the roles of CDSN in the pathogenesis of skin disorders, we generated Cdsn-null mutant mice by replacing a 3′ portion of Cdsn exon 1 together with the whole of exon 2, which contains the entire coding region of Cdsn, with a LacZ-neor fusion gene [supporting information (SI) Fig. S1A]. Correct targeting of the Cdsn locus in ES cells was confirmed by Southern blot analysis (Fig. S1B). After establishing germ-line transmission, we performed heterozygous crossing to obtain offspring homozygous for Cdsn deficiency. We obtained no adult Cdsn-deficient mice, although at embryonic day (E)18.5, the numbers of Cdsn-deficient embryos approximated those expected for Mendelian inheritance (data not shown). Protein extracted from total skin of Cdsn-deficient mice at E18.5 showed no Cdsn expression, as assessed by Western blot analysis (Fig. S1C).
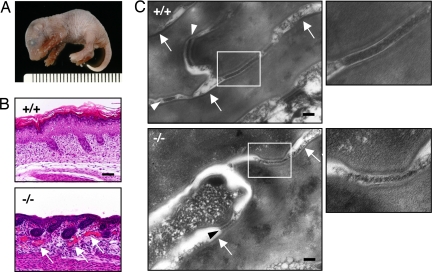
Cdsn-deficient mice at E18.5 were viable and appeared grossly normal, suggesting that Cdsn is dispensable for embryonic development until full term. However, Cdsn deficiency proved to be lethal within several hours after birth; death was associated with defect in the skin, predominantly at sites of trauma and friction (Fig. 1A). Because Cdsn expression is confined to the skin (ref. 8 and unpublished observation) and because histological evaluation on newborn Cdsn-deficient mice showed no pathological changes in major organs (i.e., brain, heart, lung, liver, stomach, small and large intestines, pancreas, kidney, thymus, spleen, and muscle) (data not shown), we speculate that mortality in Cdsn-deficient mice is due to dehydration caused by the defective skin barrier function after birth (see below), a situation similar to that seen in Spink5-deficient mice (21) (see Discussion).
Fig. 1.
Cdsn deficiency results in stratum corneum detachment due to defective desmosome formation. (A) Macroscopic appearance of formalin-fixed Cdsn-deficient newborn mouse with skin erosions on the limbs. (B) Loss of upper epidermis due to detachment of the stratum corneum from the underlying granular layer and/or within the upper granular layers in Cdsn-deficient mouse epidermis. Dilation of capillaries beneath the affected epidermis was also noted (arrows). (Scale bar: 100 μm.) (C) Electron density of each corneodesmosome from Cdsn-deficient mice (Lower Left, arrowhead) was markedly lower than that in wild-type mice (Upper Left, arrowheads); areas included in Fig. S2B are outlined. In contrast, formation of cornified cell envelopes (arrows) was indistinguishable between wild-type and Cdsn-deficient mice. (Right) Higher magnification of the outlined areas shown in Left. (Scale bars: 100 nm).
Histological evaluation of H&E-stained newborn Cdsn-deficient mouse epidermis demonstrated the loss of upper epidermis due to detachment of the stratum corneum from the underlying granular layer and/or within the upper granular layers (Fig. 1B). In addition, dilation of capillaries beneath the affected epidermis was noted (see arrows in Fig. 1B). Transmission electron microscopy analysis of epidermis from Cdsn-deficient mice showed detachment of the stratum corneum from the underlying granular layer and/or detachment within the upper granular layers (Fig. S2A), which was consistent with histological changes observed with H&E staining. Of note, the electron density of corneodesmosomes from Cdsn-deficient mice was extremely low compared with that of wild-type mice (Fig. 1C Left, arrowheads, and enlarged in Right). Furthermore, desmosomal structures were very few and were confined to the lower stratum corneum in Cdsn-deficient mice (unpublished observation). These results suggest that Cdsn is required for formation of normal corneodesmosomal structures necessary for epidermal integrity. In contrast, the formation of cornified cell envelopes was indistinguishable between newborn wild-type and Cdsn-deficient mice (Fig. 1C Left, arrows). Although upper epidermis was largely lost from the E18.5 Cdsn-deficient mouse skin because of the defective epidermal integrity, immunohistochemical analysis showed indistinguishable levels of protein expression of keratin (K)1, K5, K6, involucrin, filaggrin, loricrin, and Dsg1 from the residual epidermis (Fig. S3).
Essential Roles of Cdsn in Normal Hair Growth.
In addition to the upper spinous and granular layers of the epidermis, the Cdsn gene is expressed in the IRS of hair follicles, which is clearly demonstrated by X-Gal staining of skin from LacZ gene-inserted Cdsn-deficient mice (Fig. S4A). In situ hybridization analysis of human skin also showed CDSN gene expression in the IRS (Fig. S4 B and C), and immunohistochemical analysis demonstrated CDSN expression from all three layers of IRS, including the innermost cuticle layer (Fig. S4 D and E). Because of a heterozygous nonsense mutation of the CDSN gene in patients with HSS, together with delayed onset of alopecia, toxicity of aggregates formed by a dominant-negative interaction between the mutant and wild-type CDSN protein to the hair follicle cells is considered to be responsible for the pathogenesis of the disease (20). Supporting this notion, mice heterozygous for the Cdsn-null mutation showed no hair or skin phenotypes during an observation period of up to 1 year (unpublished observation), thus clearly ruling out a simple gene-dosage effect due to a heterozygous mutation of the CDSN gene in the pathogenesis of HSS.
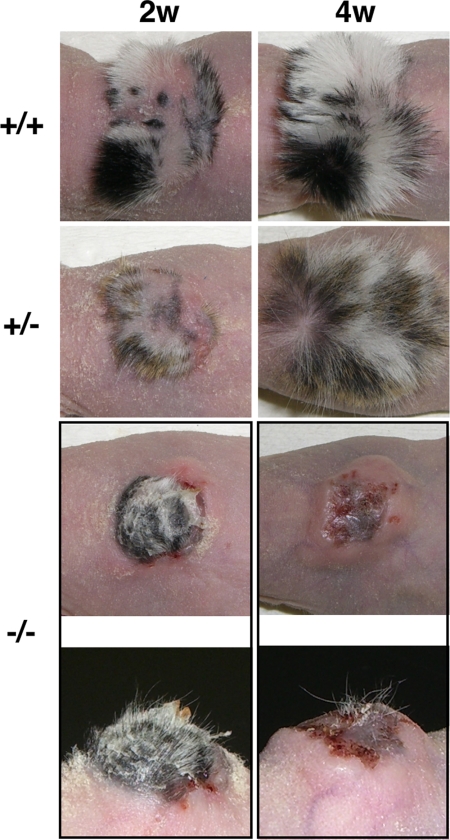
To further investigate the roles of Cdsn in hair and skin physiology by overcoming the neonatal lethality of Cdsn deficiency in mice, we grafted whole skin obtained from E18.5 Cdsn-deficient embryos onto the backs of nude mice. Two weeks after skin grafting, both wild-type and heterozygous Cdsn-deficient grafts showed hair growth, which became more prominent thereafter (Fig. 2). In contrast, homozygous Cdsn-deficient grafts showed some hair growth only initially, and each individual hair was thin and slightly curly (Fig. 2 Left, bottommost). After two further weeks, hair growth ceased and was followed by hair loss, resulting in bald skin or a residual skin graft scar (Fig. 2 Right, bottommost). Thus, in contrast to the delayed onset of alopecia in HSS patients, Cdsn-deficient skin showed rapid hair loss.
Fig. 2.
Abnormal hair growth from grafted Cdsn-deficient mouse skin. Embryonic skin obtained from the trunks of wild-type mice (uppermost row), heterozygous Cdsn-deficient mice (second row from top), and homozygous Cdsn-deficient mice (third row from top) was grafted onto the backs of nude mice. Gross appearance of the grafted skins 2 weeks (Left) and 4 weeks (Right) after transplantation. Thin and slightly curly hairs from skin of homozygous Cdsn-deficient mice at 2 and 4 weeks after transplantation are shown from different angles bottom most row. Representative results of one mouse for each group examined (wild-type, n = 2; heterozygous Cdsn-deficient, n = 4; homozygous Cdsn-deficient, n = 3) are shown.
Microscopic analysis of hair follicles from grafted Cdsn-deficient skin revealed abnormal cystic dilatation of follicular infundibulum and/or cystic dilatation of infundibulum filled with keratinized cells (Fig. S5). In addition, infundibular epithelium was thickened in grafted Cdsn-deficient skin compared with that from grafted control skin; together, these findings might be associated with the rapid hair loss in the absence of Cdsn in the IRS. In contrast, hair-follicle expression of Dsg4, of which gene mutation is responsible for an inherited hypotrichosis (1, 22), was indistinguishable between the grafted skin from control mice and that from Cdsn-deficient mice (data not shown).
Psoriasis-Like Skin Abnormalities in Grafted Skin from Cdsn-Deficient Mice.
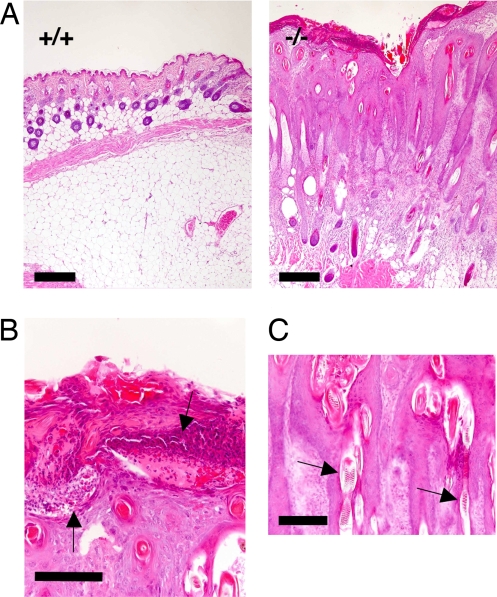
To assess the pathological relevance of Cdsn dysfunction to the development of skin abnormalities seen in psoriasis, we evaluated sections of the Cdsn-deficient skin 4 weeks after grafting onto nude mice. Histologically, there was a marked contrast between the grafted skin from control mice and that from Cdsn-deficient mice (Fig. 3A). Changes in the grafts from Cdsn-deficient mice included marked acanthosis, hyperkeratosis, parakeratosis, loss of the granular layer, severe dermal infiltration of inflammatory cells, and proliferation and dilation of capillaries, all of which resembled those seen in psoriatic lesions (13). A notable feature was neutrophilic abscesses beneath the stratum corneum (Fig. 3B), again reminiscent of psoriasis. The configuration of the hair follicles, which engulfed aberrant hair shafts (Fig. 3C), and keratin materials, which frequently formed cysts (Fig. S5), mimicked the elongated rete ridges seen in psoriasis.
Fig. 3.
Psoriasis-like skin abnormalities in grafted skin from Cdsn-deficient mice. (A) H&E staining of skin from control mice (Left) and Cdsn-deficient mice (Right) 4 weeks after grafting onto nude mice. Hyperkeratosis, parakeratosis, and marked acanthosis with structures similar to elongated rete ridges were observed in grafted Cdsn-deficient mouse skin; also note that sebaceous glands are rarely evident. (Scale bars: 300 μm.) (B) Subcorneal and intracorneal pustules with neutrophil infiltration (arrows). (Scale bar: 100 μm.) (C) Aberrant hair shafts engulfed in rete ridge-like structures (arrows). (Scale bar: 200 μm.)
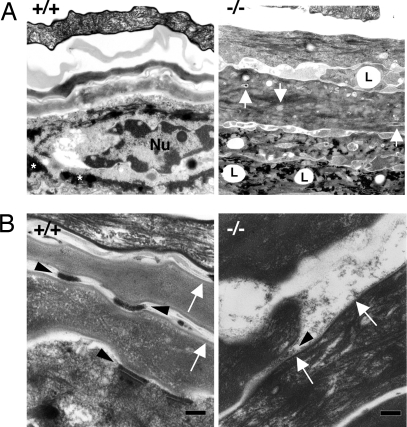
Analysis by transmission electron microscopy also supported the similarity of grafted Cdsn-deficient mouse skin and human psoriatic lesional skin (23). Many abnormal cellular structures, including lipid droplets and remnants of nuclei and organelles, were observed in the stratum corneum of grafts from Cdsn-deficient mice (Fig. 4A Right). In addition, keratohyalin granules were reduced in grafted Cdsn-deficient mouse skin, which is consistent with the disappearance of the granular layers observed by light microscopy (Fig. 3B). Higher magnification also confirmed that the electron density of each corneodesmosome from grafted Cdsn-deficient mouse skin (Fig. 4B Right, arrowhead) was extremely low compared with that from grafted wild-type mouse skin (Fig. 4B Left, arrowheads). Furthermore, formation of cornified cell envelopes was defective in the grafted Cdsn-deficient mouse skin (Fig. 4B Right, arrows), which is also a feature seen in psoriatic lesions. Because cornified cell envelopes were intact in untreated E18.5 Cdsn-deficient mouse skin (Fig. 1C), additional factors such as continuous augmented environmental stimuli due to the defective barrier function of the skin in the absence of normal corneodesmosomes may contribute to the accelerated desquamation (absence of normal granular layer formation) and subsequent development of psoriasis-like skin lesions. Interestingly, Stat3 (signal transduction and activators of transcription 3) activation in epidermal keratinocytes has been associated with the development of psoriatic lesions (24, 25) (Fig. S6A). Immunohistochemistry with anti-Stat3 antibody revealed that Stat3 was partially translocated in the nuclei of keratinocytes from Cdsn-deficient skin grafts, whereas no such changes were noted in control skin grafts (Fig. S6B), suggesting that Stat3 in keratinocytes from grafted Cdsn-deficient skin is activated, thereby also contributing to the psoriatic changes observed in this grafted tissue. Taken together, Cdsn has a significant impact on the proliferative and differentiative programs of epidermal keratinocytes, whose abnormality plays a role in the pathogenetic features of psoriasis.
Fig. 4.
Psoriasis-like skin phenotypes in grafted Cdsn-deficient mouse skin. (A) Many abnormal cellular structures including lipid droplets (L) and remnants of nuclei and organelles (arrows) were observed in the stratum corneum of grafts from Cdsn-deficient mice (Right). Keratohyalin granules (*, Left) were reduced in grafted skin from Cdsn-deficient mice compared with those in grafted skin from wild-type mice. Nu, nucleus. (B) Electron density of each corneodesmosome in grafted skin from Cdsn-deficient mice (Right, arrowhead) was markedly lower than that in grafted skin from wild-type mice (Left, arrowheads). Formation of cornified cell envelopes was also defective in grafted skin from Cdsn-deficient mice (Right, arrows).
Discussion
There is a longstanding debate whether CDSN gene polymorphisms are significantly associated with susceptibility to psoriasis (11, 15, 17). Although the major psoriasis-susceptibility locus of PSORS1 has been narrowed down to an ≈300-kb region containing HLA-C and CDSN, extensive variation, together with the existence of particularly strong extended haplotypes in this region, has hampered high-resolution analysis (14, 18, 19). Homozygous complete loss-of-function Cdsn gene mutation analyzed in mice in the present study and the more subtle changes in CDSN function observed in the skin of human subjects with CDSN gene polymorphisms may not recapitulate the same pathological features of CDSN dysfunction. Indeed, mRNAs transcribed from a CDSN risk haplotype presented a rather increased stability (26). Furthermore, psoriasis-like skin lesions had developed in Cdsn-deficient grafts without involvement of acquired immunity; Cdsn-deficient skin was grafted onto immunodeficient nude mice in our study. Our results seem to be inconsistent with the widely accepted notion that psoriasis is a T cell-mediated dermatosis (27, 28). However, we speculate that intrinsic defect in CDSN function may result in the altered keratinocyte differentiation program associated with Stat3 activation, which is predisposed to the full development of psoriatic lesions when activated T cells are present in that setting. In this regard, we believe that it is best to consider psoriasis a genetically programmed disease of dysregulated inflammation, which is triggered by environmental stimuli (27). Alternatively, inflammatory changes in the epidermis caused by still-unknown mechanisms damage the desmosomal structures containing CDSN, which may contribute to the development of psoriatic pathology (25, 28). Although a recent study of families suggested that HLA-C, not CDSN, may be the major disease-associated allele at the psoriasis-susceptibility locus that is responsible for early onset disease in white populations (15), this study was not case controlled. It may be that studies focusing on broader disease categories in broader populations may be necessary to define the role of CDSN.
CDSN undergoes proteolysis predominantly by the serine proteinases SCCE (stratum corneum chymotryptic enzyme/KLK7) (29) and SCTE (stratum corneum tryptic enzyme/KLK5) (30), and this process is considered to be essential for destabilization of corneodesmosomes during physiological desquamation (6, 7). SPINK5 (serine protease inhibitor Kazal-type 5) (also known as lympho-epithelial Kazal-type related inhibitor, LEKTI) is a serine protease inhibitor that can antagonize both SCCE and SCTE. The essential role of SPINK5 in skin physiology is underscored by the fact that mutations in the SPINK5 gene cause Netherton syndrome (OMIM 256500) (31), a severe autosomal recessive genodermatosis. Indeed, inactivation of the mouse Spink5 gene by either insertional mutation (32) or targeted deletion (21, 33) results in desmosomal dissociation and stratum corneum detachment, mimicking the Netherton syndrome phenotypes. However, the molecular mechanisms underlying the abnormal desquamation in Spink5-deficient mice are not clear; abnormal degradation of Dsg1 (21), filaggrin (21, 33), or Cdsn (32) due to epidermal protease hyperactivity in the absence of Spink5 has been suggested. Based on the phenotypic similarity between Cdsn- and Spink5-deficient mice (i.e., desmosomal dissociation and stratum corneum detachment, as described above), we speculate that the most critical process accounting for the altered desquamation in Spink5-deficient mice is dysregulated Cdsn homeostasis. Thus, tightly regulated proteolytic processing of Cdsn by many factors, one of which is Spink5, is critical for the maintenance of normal epidermal structure and function.
In addition to the upper spinous and granular layers of the epidermis, Cdsn is expressed in the IRS of hair follicles (Fig. S4), and a heterozygous nonsense mutation of the CDSN gene in humans is associated with HSS (20). Toxicity of aggregates formed by a dominant negative interaction between the mutant and wild-type CDSN protein to the hair follicle cells is considered to be responsible for the pathogenesis of the disease (20). Consistent with this idea, we observed no hair phenotypes in mice heterozygous for the Cdsn-null mutation, ruling out a simple gene–dosage effect. Furthermore, the difference in the course of hair loss (i.e., in contrast to the delayed onset of alopecia in HSS patients, Cdsn-deficient skin showed rapid hair loss when grafted onto immunodeficient mice, probably due to the defective holding of hair shafts by Cdsn-deficient IRS; Fig. S5) further supports the idea that toxicity of the CDSN aggregate to hair follicles, rather than partial loss of function of the CDSN gene, is responsible for the pathogenesis of HSS. Interestingly, patients with HSS manifest no desmosomal splitting or epidermal differentiation abnormality other than alopecia (20). Given that complete loss of Cdsn function resulted in the altered desquamation as demonstrated in the present study, we speculate that a sufficient amount of intact CDSN protein still remains after dominant negative interaction between the mutant and wild-type CDSN protein in HSS, at least in the epidermis. Immunohistochemical analysis demonstrating the presence of CDSN aggregates in ridges of the superficial dermis, but not in the epidermis, from patients with HSS (20) additionally suggests that CDSN aggregates in the epidermis are probably degraded by epidermal proteases in a physiological manner.
In conclusion, we have demonstrated essential roles of Cdsn in hair physiology and have suggested functional relevance of CDSN gene polymorphisms to psoriasis susceptibility. Integration of genetic studies with functional analyses of the genes involved promises to clarify many aspects of the pathogenesis of psoriasis, and establishment of mouse models allowing insight from both perspectives should facilitate therapeutic approaches for this intractable skin disorder.
Materials and Methods
Mice.
Cdsn-deficient mice (RIKEN; accession no. CDB0481K) were generated by gene targeting. Briefly, the targeting vector was constructed by inserting a LacZ-neomycin resistance fusion gene (LacZ-neor) into exon 1 of the genomic Cdsn locus. The targeting vector was introduced into TT2 embryonic stem cells, and the homologous recombinant clones were first identified by PCR and confirmed by Southern blot analysis (34). After the targeted cells had been injected into morula-stage embryos, the resulting chimeric male mice were mated with C57BL/6 females (CLEA Japan) to establish germ-line transmission. BALB/cA Jcl-nu mice (BALB/cnu/nu mice) were purchased from CLEA Japan. The mice were maintained under pathogen-free conditions, and the protocols used in this study were in accordance with the Guidelines for Animal Experimentation of Tokushima University School of Medicine and were conducted with the approval of the RIKEN Kobe Animal Experiment Committee.
Histological Analysis.
Formalin-fixed tissue sections were subjected to H&E staining according to a standard protocol. Electron microscopical analysis was performed as described previously (35). Briefly, skin samples were excised and minced into 2-mm-square pieces and fixed at 4°C in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). Samples were placed in 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h and then in 0.2% ruthenium tetroxide with 0.25% potassium ferrocyanide for 4 h. After dehydration through a graded series of ethanol concentrations, the tissue was equilibrated in propylene oxide and embedded in Spurr's resin. Ultrathin sections were double-stained with uranyl acetate and lead citrate and examined with a JEM-100S (JEOL) electron microscope.
Western Blot Analysis, Immunohistochemistry, and in Situ Hybridization.
Western blot analysis and immunohistochemistry were performed as described previously (24, 35). Anti-mouse CDSN polyclonal antibody was generated by immunizing rabbits with mouse Cdsn peptide (AAGPPVSEGKYFSS) or with human CDSN peptide (SNQRPCSSDIPDSP). X-Gal staining was performed with 1 mg/ml X-Gal dissolved in 100 mM phosphate buffer at pH 4.3, 3 mM K3Fe(CN)6, 3 mM K4Fe(CN)6, and 2 mM MgCl2. In situ hybridization for the detection of CDSN gene expression was performed as described previously (8).
Skin Graft.
Full-thickness samples of abdominal and dorsal skin were obtained en bloc from E18.5 embryos and grafted onto athymic nude mice as 1-cm squares, with a single piece of skin per recipient mouse. After 2 weeks under pathogen-free conditions, the threads were removed, and the grafted skin was exposed to air for 2 additional weeks. Skin samples for histological evaluations were taken at 4 weeks after initial skin grafting.
Supplementary Material
Acknowledgments.
We thank Drs. K. Toida, K. Ishimura, S. Anzai, and S. Itami for their variable suggestions for this work. This work was supported in part by a Grant for the 21st Century Center of Excellence Program of the Ministry of Education, Culture, Sports, Science and Technology (to M.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709345105/DCSupplemental.
References
- 1.McGrath JA, Wessagowit V. Human hair abnormalities resulting from inherited desmosome gene mutations. Keio J Med. 2005;54:72–79. doi: 10.2302/kjm.54.72. [DOI] [PubMed] [Google Scholar]
- 2.Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz MA, Owaribe K, Kartenbeck J, Franke WW. Desmosomes and hemidesmosomes: Constitutive molecular components. Ann Rev Cell Biol. 1990;6:461–491. doi: 10.1146/annurev.cb.06.110190.002333. [DOI] [PubMed] [Google Scholar]
- 4.Garrod DR, Merritt AJ, Nie Z. Desmosomal adhesion: Structural basis, molecular mechanism and regulation. Mol Membr Biol. 2002;19:81–94. doi: 10.1080/09687680210132476. [DOI] [PubMed] [Google Scholar]
- 5.Cheng X, Koch PJ. In vivo function of desmosomes. J Dermatol. 2004;31:171–187. doi: 10.1111/j.1346-8138.2004.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 6.Simon M, Montezin M, Guerrin M, Durieux JJ, Serre G. Characterization and purification of human corneodesmosin, an epidermal basic glycoprotein associated with corneocyte-specific modified desmosomes. J Biol Chem. 1997;272:31770–31776. doi: 10.1074/jbc.272.50.31770. [DOI] [PubMed] [Google Scholar]
- 7.Simon M, et al. Refined characterization of corneodesmosin proteolysis during terminal differentiation of human epidermis and its relationship to desquamation. J Biol Chem. 2001;276:20292–20299. doi: 10.1074/jbc.M100201200. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Chaplin DD. Identification in the HLA class I region of a gene expressed late in keratinocyte differentiation. Proc Natl Acad Sci USA. 1993;90:9470–9474. doi: 10.1073/pnas.90.20.9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonca N, et al. Corneodesmosin, a component of epidermal corneocyte desmosomes, displays homophilic adhesive properties. J Biol Chem. 2002;277:5024–5029. doi: 10.1074/jbc.M108438200. [DOI] [PubMed] [Google Scholar]
- 10.Allen MH, et al. A non-HLA gene within the MHC in psoriasis. Lancet. 1999;353:1589–1590. doi: 10.1016/S0140-6736(99)01618-9. [DOI] [PubMed] [Google Scholar]
- 11.Orru S, Giuressi E, Carcassi C, Casula M, Contu L. Mapping of the major psoriasis-susceptibility locus (PSORS1) in a 70-Kb interval around the corneodesmosin gene (CDSN) Am J Hum Genet. 2005;76:164–171. doi: 10.1086/426948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenisch S, et al. Corneodesmosin gene polymorphism demonstrates strong linkage disequilibrium with HLA and association with psoriasis vulgaris. Tissue Antigens. 1999;54:439–449. doi: 10.1034/j.1399-0039.1999.540501.x. [DOI] [PubMed] [Google Scholar]
- 13.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 14.Nair RP, et al. Localization of psoriasis-susceptibility locus PSORS1 to a 60-kb interval telomeric to HLA-C. Am J Hum Genet. 2000;66:1833–1844. doi: 10.1086/302932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair RP, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78:827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trembath RC, et al. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum Mol Genet. 1997;6:813–820. doi: 10.1093/hmg/6.5.813. [DOI] [PubMed] [Google Scholar]
- 17.Asumalahti K, et al. Coding haplotype analysis supports HCR as the putative susceptibility gene for psoriasis at the MHC PSORS1 locus. Hum Mol Genet. 2002;11:589–597. doi: 10.1093/hmg/11.5.589. [DOI] [PubMed] [Google Scholar]
- 18.Veal CD, et al. Family-based analysis using a dense single-nucleotide polymorphism-based map defines genetic variation at PSORS1, the major psoriasis-susceptibility locus. Am J Hum Genet. 2002;71:554–564. doi: 10.1086/342289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elder JT. Fine mapping of the psoriasis susceptibility gene PSORS1: A reassessment of risk associated with a putative risk haplotype lacking HLA-Cw6. J Invest Dermatol. 2005;124:921–930. doi: 10.1111/j.0022-202X.2005.23729.x. [DOI] [PubMed] [Google Scholar]
- 20.Levy-Nissenbaum E, et al. Hypotrichosis simplex of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat Genet. 2003;34:151–153. doi: 10.1038/ng1163. [DOI] [PubMed] [Google Scholar]
- 21.Descargues P, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005;37:56–65. doi: 10.1038/ng1493. [DOI] [PubMed] [Google Scholar]
- 22.Kljuic A, et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: Evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell. 2003;113:249–260. doi: 10.1016/s0092-8674(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 23.Schaumburg-Lever G, Lever WF, Orfanos CE. Histopathology and electron microscopy. In: Roenigk HH Jr, Maibach HI, editors. Psoriasis. 2nd Ed. New York: Marcel Dekker; 1991. pp. 461–469. [Google Scholar]
- 24.Sano S, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 26.Capon F, et al. A synonymous SNP of the corneodesmosin gene leads to increased mRNA stability and demonstrates association with psoriasis across diverse ethnic groups. Hum Mol Genet. 2004;13:2361–2368. doi: 10.1093/hmg/ddh273. [DOI] [PubMed] [Google Scholar]
- 27.Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54:S67–S80. doi: 10.1016/j.jaad.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 28.Bos JD, De Rie MA. The pathogenesis of psoriasis: Immunological facts and speculations. Immunol Today. 1999;20:40–46. doi: 10.1016/s0167-5699(98)01381-4. [DOI] [PubMed] [Google Scholar]
- 29.Hansson L, et al. Cloning, expression, and characterization of stratum corneum chymotryptic enzyme. A skin-specific human serine proteinase. J Biol Chem. 1994;269:19420–19426. [PubMed] [Google Scholar]
- 30.Brattsand M, Egelrud T. Purification, molecular cloning, and expression of a human stratum corneum trypsin-like serine protease with possible function in desquamation. J Biol Chem. 1999;274:30033–30040. doi: 10.1074/jbc.274.42.30033. [DOI] [PubMed] [Google Scholar]
- 31.Chavanas S, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–142. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 32.Yang T, et al. Epidermal detachment, desmosomal dissociation, and destabilization of corneodesmosin in Spink5−/− mice. Genes Dev. 2004;18:2354–2358. doi: 10.1101/gad.1232104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hewett DR, et al. Lethal, neonatal ichthyosis with increased proteolytic processing of filaggrin in a mouse model of Netherton syndrome. Hum Mol Genet. 2005;14:335–346. doi: 10.1093/hmg/ddi030. [DOI] [PubMed] [Google Scholar]
- 34.Murata T, et al. Ang is a novel gene expressed in early neuroectoderm, but its null mutant exhibits no obvious phenotype. Gene Expr Patterns. 2004;5:171–178. doi: 10.1016/j.modgep.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Takagi S, et al. Alteration of the 4-sphingenine scaffolds of ceramides in keratinocyte-specific Arnt-deficient mice affects skin barrier function. J Clin Invest. 2003;112:1372–1382. doi: 10.1172/JCI18513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.