Abstract
To explore the functional significance of cGMP-dependent protein kinase type I (cGKI) in the regulation of erythrocyte survival, gene-targeted mice lacking cGKI were compared with their control littermates. By the age of 10 weeks, cGKI-deficient mice exhibited pronounced anemia and splenomegaly. Compared with control mice, the cGKI mutants had significantly lower red blood cell count, packed cell volume, and hemoglobin concentration. Anemia was associated with a higher reticulocyte number and an increase of plasma erythropoietin concentration. The spleens of cGKI mutant mice were massively enlarged and contained a higher fraction of Ter119+ erythroid cells, whereas the relative proportion of leukocyte subpopulations was not changed. The Ter119+ cGKI-deficient splenocytes showed a marked increase in annexin V binding, pointing to phosphatidylserine (PS) exposure at the outer membrane leaflet, a hallmark of suicidal erythrocyte death or eryptosis. Compared with control erythrocytes, cGKI-deficient erythrocytes exhibited in vitro a higher cytosolic Ca2+ concentration, a known trigger of eryptosis, and showed increased PS exposure, which was paralleled by a faster clearance in vivo. Together, these results identify a role of cGKI as mediator of erythrocyte survival and extend the emerging concept that cGMP/cGKI signaling has an antiapoptotic/prosurvival function in a number of cell types in vivo.
Keywords: apoptosis, Ca2+ channels, phosphatidylserine, spleen
Nitric oxide (NO) has been shown to be a powerful regulator of cell survival (1, 2). Depending on the source or concentration of NO and the influence of additional regulators, NO may stimulate or inhibit apoptosis (3). NO exerts its effects in part through S-nitrosylation of target proteins. However, the effect of NO donors on Ca2+-induced phosphatidylserine (PS) exposure, a hallmark of apoptosis, could be mimicked by cGMP analogs (4), suggesting the involvement of soluble guanylyl cyclase, cGMP, and cGMP-dependent protein kinase type I (cGKI), a well known signaling cascade downstream of NO (5, 6).
Recent studies indicated that erythroid cells possess a functional NO/cGMP pathway (7–9), which may be involved in the regulation of eryptosis, the suicidal death of erythrocytes (10). Erythrocyte cGMP production might also be stimulated by NO generated in the endothelium. The cGKI can also be activated independent of cGMP in response to oxidative stress (11). Eryptosis may follow osmotic shock, energy depletion, and oxidative stress (10), which activate Ca2+-permeable cation channels (12). Subsequent Ca2+ entry leads to activation of Ca2+-sensitive K+ channels, exit of KCl with osmotically obliged water, and thus cell shrinkage (13). In addition, Ca2+ entry triggers Ca2+-sensitive scrambling of the cell membrane (14, 15) with subsequent exposure of PS at the erythrocyte surface (12). Cell membrane scrambling may further be triggered by ceramide (16) and activation of protein kinase C (17). PS-exposing erythrocytes bind to PS receptors (18) and are recognized, engulfed, and degraded by macrophages (19).
The present study was performed to explore the role of cGKI in the survival of circulating erythrocytes. To this end, gene-targeted mice lacking functional cGKI were compared with their control littermates.
Results
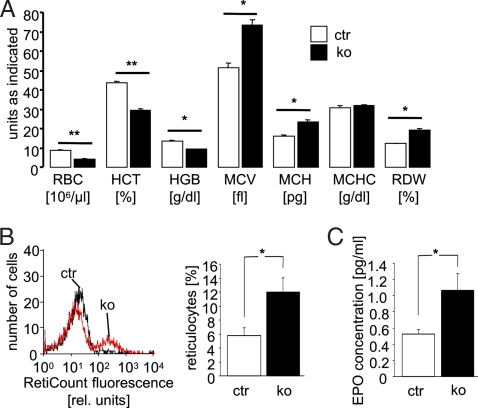
The analysis of peripheral blood showed significant erythrocyte abnormalities in conventional cGKI knockout (KO) mice as compared to litter-matched control (ctr) mice. The red blood cell (RBC) counts, hematocrit (HCT), and hemoglobin (HGB) concentration were significantly smaller in 10-week-old cGKI KO than in ctr mice (Fig. 1A). The difference was larger for erythrocyte number (≈52%) than for packed cell volume (≈33%) and HGB (≈31%). Accordingly, mean corpuscular volume (MCV) and mean corpuscular HGB content (MCH) of erythrocytes were higher in 10-week-old cGKI KO than in ctr mice, whereas the mean corpuscular HGB concentration (MCHC) was not altered.
Fig. 1.
Anemia in cGKI-deficient mice. Circulating blood of 10-week-old ctr (ctr, open bars) and cGKI KO (ko, black bars) mice was analyzed. (A) Counts of RBC, HCT, and HGB concentration and MCV, MCH, MCHC, and RDW. The data shown were obtained from a litter-matched group of mice (n = 3–4) and are representative of at least three experiments with independent groups of animals. (B) Representative histogram of Retic count fluorescence (Left) and reticulocyte number (Right, n = 8). The first and the second peaks correspond to cell populations with low and high staining intensity, respectively. (C) Plasma erythropoietin concentration (n = 4). * and ** indicate significant differences between genotypes with P < 0.05 and P < 0.01, respectively. The genotypes of the ctr mice were cGKI+/+ or cGKI+/L−.
In theory, anemia could have resulted from decreased erythrocyte formation, which should be reflected by a decreased number of reticulocytes. However, the reticulocyte number was higher in cGKI KO than in ctr mice (Fig. 1B). Consistent with reticulocytosis, the RBC distribution width (RDW) was increased in cGKI KO mice (Fig. 1A). Thus, the anemia could not be explained by decreased formation of erythrocytes. The increased reticulocyte number in cGKI KO mice could have resulted from enhanced stimulation of erythropoiesis by erythropoietin. As illustrated in Fig. 1C, the plasma erythropoietin concentration was indeed ≈2-fold higher in cGKI KO than in ctr mice. Increased reticulocyte and erythropoietin levels could be indicative of hemolytic anemia in cGKI KO mice. To explore this possibility, the plasma concentration of haptoglobin was determined. The haptoglobin concentration in anemic cGKI KO mice was similar to ctr mice (in mg/ml, cGKI KO 0.11 ± 0.02, n = 8; ctr 0.12 ± 0.03, n = 11), excluding hemolytic anemia. Moreover, iron deficiency due to chronic bleeding or impaired iron absorption is unlikely, because plasma transferrin levels were not significantly different between genotypes (in mg/ml, cGKI KO 1.17 ± 0.05, n = 8; ctr 1.37 ± 0.08, n = 7), and the erythrocytes of cGKI KO mice were not microcytic (Fig. 1A).
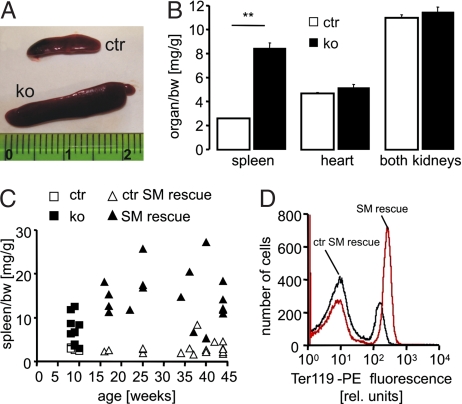
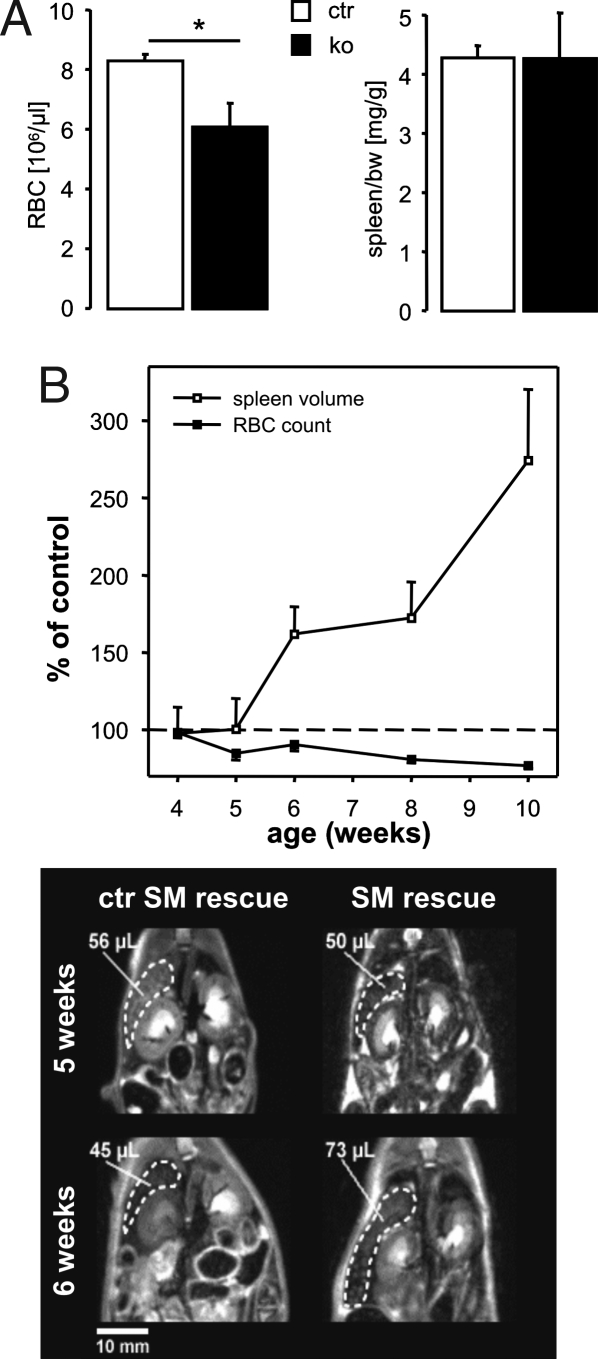
Anemia of cGKI-deficient mice was associated with severe splenomegaly. Based on the organ/body weight (bw) ratio, 10-week-old cGKI KO mice had ≈3-fold larger spleens than ctr mice, whereas the size of the heart and kidneys was normal (Fig. 2 A and B). Analysis of individual animals revealed that splenomegaly was evident in most cGKI KO mice aged 8–10 weeks (Fig. 2C). It is important to note that conventional cGKI KO mice have a severe smooth muscle (SM) phenotype, which causes premature death of ≈50% of the mutant animals by 6 weeks of age, presumably due to gastrointestinal dysfunction (20, 21). In line with their increased MCV (Fig. 1A), 10-week-old cGKI KO animals tended to have lower plasma levels of vitamin B12 (in ng/dl, cGKI KO 1,770 ± 318, n = 3; ctr 2,718 ± 252, n = 5, P = 0.08) and folic acid (in ng/dl, cGKI KO 6,981 ± 1,001, n = 3; ctr 9,373 ± 848, n = 5, P = 0.13), but the differences did not reach statistical significance. Vitamin B12 and folic acid deficiency of conventional cGKI KO mice could be due to gastrointestinal malabsorption. To estimate the potential contribution of SM dysfunction and poor health status to anemia and splenomegaly of cGKI KO mice, another cGKI-deficient mouse model, the so-called cGKI SM rescue mouse (22), was analyzed. In cGKI SM rescue mice, expression of cGKI has been restored selectively in SM cells but not in other cell types, resulting in the rescue of SM dysfunction and a dramatic extension of lifespan to >1 year (22). Thus, cGKI SM rescue mice are a useful genetic model to study the role of cGKI in non-SM cells of relatively “healthy” mice. Similar to conventional cGKI KO mice, 10-week-old cGKI SM rescue mice suffered from anemia and splenomegaly [supporting information (SI) Fig. S1]. Their hematological phenotype was similar to that of conventional cGKI KO mice. However, as compared with litter-matched ctr mice (referred to as “ctr SM rescue”), their MCV and MCH were only mildly increased, and their RDW was not altered (Fig. S1). “Older” cGKI SM rescue mice (16- to 45-week-old) were also anemic (RBC in 106/μl, SM rescue 7.3 ± 0.3, n = 19; ctr SM rescue 9.8 ± 0.2, n = 29; P < 0.001) and showed massive splenomegaly at all ages analyzed (Fig. 2C). Their plasma levels of vitamin B12 (in ng/dl, SM rescue 2,215 ± 244, n = 4; ctr SM rescue 2,573 ± 101, n = 6) and folic acid (in ng/dl, SM rescue 15,826 ± 2,477, n = 4; ctr SM rescue 15,620 ± 1,341, n = 6), however, were not decreased. Thus, anemia and splenomegaly of the SM rescue mice were not due to SM dysfunction, potential malabsorption, or poor health.
Fig. 2.
Splenomegaly associated with increased erythroid cell mass in cGKI-deficient mice. (A) Spleens and (B) organ/bw ratios of 10-week-old ctr (open bars) and KO (black bars) mice (**, P < 0.01). The data shown were obtained from a litter-matched group of mice (n = 3–4) and are representative of at least three experiments with independent groups of animals. (C) Spleen/bw ratios of individual mice at various ages. The diagram includes conventional KO mice (black boxes) and their ctr littermates (open boxes) and cGKI SM rescue mice (black triangles) and their ctrs (ctr SM rescue, open triangles). (D) Representative flow-cytometric quantification of Ter119+ spleen cells isolated from a 42-week-old cGKI SM rescue mouse and a litter-matched ctr mouse. The first and the second peaks correspond to cell populations with low and high staining intensity, respectively. The genotypes of ctr mice were cGKI+/+ or cGKI+/L−. The genotypes of ctr SM rescue mice were cGKI+/L−;SM-Iα+/− or cGKI+/L−;SM-Iβ+/−.
Further experiments were performed to elucidate characteristics of the cells that apparently accumulated in the enlarged spleens of cGKI-deficient mice. Freshly prepared splenocytes were stained with an antibody against Ter119, a marker of late-stage murine erythroid cells and mature erythrocytes (23) and with annexin V to examine the externalization of PS indicative of suicidal cell death. Flow-cytometric analysis showed that cGKI SM rescue mice had, proportionally, ≈96% more Ter119+ splenocytes than ctr SM rescue mice (Fig. 2D, Table 1). When corrected for the increased spleen size in cGKI mutants, this reflects a ≈6-fold increase in spleen erythroid cell mass. Annexin V binding indicated that Ter119+ splenocytes of cGKI-deficient animals contained, proportionally, twice as many PS-exposing cells than ctr mice (percentage of PS-exposing/total erythroid cells, SM rescue ≈20%, ctr SM rescue ≈9%), whereas nonerythroid cells showed no significant difference in the level of annexin V-labeled cells between genotypes (Table 1). Immunophenotyping for marker proteins revealed that the relative proportion of splenic CD41+ megakaryocytes (24), CD4+ T cells, CD8+ T cells, and B220+ B cells, and the proliferative activity of the splenocytes in the absence and presence of LPS were similar in ctr and cGKI SM rescue animals (Table 1). Moreover, the analysis of splenocytes for intracellular and secreted cytokines did not reveal altered levels of proinflammatory (IL-2, IL-17, IFN-γ, TNF) or antiinflammatory (IL-4, IL-10) mediators in the spleens of cGKI mutants compared with ctrs (data not shown). Similar results were obtained with spleens of 10-week-old conventional cGKI KO mice; they contained approximately twice as many eryptotic erythroid cells as their litter-matched ctrs (percentage of PS-exposing/total erythroid cells, KO 20 ± 3%, n = 3; ctr 8 ± 2%, n = 4; P < 0.05), whereas the nonerythroid cells of cGKI KO mice showed no significant abnormalities according to the parameters listed in Table 1 (data not shown). Thus, increased spleen size of the cGKI-deficient mice was not due to an increase in nonerythroid cells including lymphocytes but was caused, at least in large part, by an increased number of eryptotic erythrocytes.
Table 1.
Cellular makeup of spleens from cGKI SM rescue mice
| Parameter | Ctr SM rescue | SM rescue |
|---|---|---|
| Erythroid cells, % (Ter119+/total splenocytes) | 28 ± 3.6 | 55 ± 6.5* |
| Apoptotic erythroid cells, % (Ter119+ and annexin V+/total splenocytes) | 2.5 ± 0.4 | 11 ± 0.7* |
| Apoptotic nonerythroid cells, % (Ter119- and annexin V+/total splenocytes) | 27 ± 6.5 | 20 ± 3.5 |
| CD41+ megakaryocytes, % | 10 ± 4.0 | 7.9 ± 2.0 |
| CD4+ T cells, % | 31 ± 5.8 | 24 ± 4.9 |
| CD8+ T cells, % | 9.1 ± 1.4 | 6.1 ± 1.4 |
| B220+ B cells, % | 35 ± 4.1 | 30 ± 6.1 |
| 3H-thymidine incorporation | ||
| Basal, cpm | 448 ± 95 | 377 ± 109 |
| LPS (1 μg/ml), cpm | 6,563 ± 1688 | 4,781 ± 1,854 |
All data are means ± SEM (n = 3–5 mice). 34- to 45-week-old cGKI SM rescue mice and their control littermates (genotype: cGKI+/L−;SM-Iα +/−, or cGKI+/L−;SM-Iβ +/−) were analysed. Statistical test results are reported as P value by t test. *, P < 0.05 vs. ctr SM rescue.
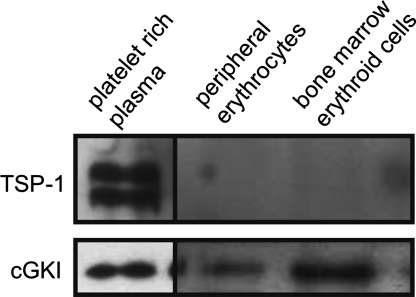
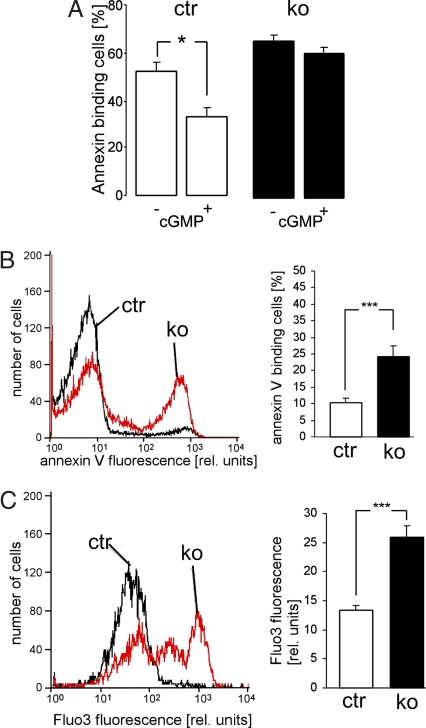
The accumulation of erythrocytes in the spleens of cGKI-deficient animals could be linked to their higher sensitivity to eryptosis. Therefore, we explored whether cGKI signaling plays a role in eryptosis. Western blot analysis showed that cGKI protein is present in both erythroid cells from the bone marrow and in peripheral erythrocytes (Fig. 3). To exclude a potential contamination of the erythroid cells with platelets containing high levels of cGKI (6, 25), the same membranes were stained for the platelet marker thrombospondin-1 (TSP-1). Although TSP-1 was readily detected in platelet-rich plasma, it was not present in erythroid cell preparations used for Western analysis, thus, excluding a contamination with platelets (Fig. 3). Further evidence for cGKI activity in erythrocytes came from functional analysis of erythrocytes isolated from ctr and cGKI KO mice. Eryptosis was monitored by flow cytometry via annexin V-binding to PS-exposing cells. The membrane-permeable cGMP analog, 8-Br-cGMP, suppressed ionomycin-induced eryptosis in ctr but not in cGKI KO erythrocytes indicating that activation of the cGMP/cGKI signaling pathway protects from erythrocyte death (Fig. 4A). Accordingly, erythrocytes from cGKI KO mice showed a significantly higher percentage of PS-exposing erythrocytes than ctr mice after a 48-h incubation in Ringer solution at 37°C (Fig. 4B). Externalization of PS is stimulated by increased cytosolic Ca2+ concentration (12). Ca2+ imaging with Fluo-3 showed that the cytosolic Ca2+ concentration was indeed significantly higher in cGKI KO erythrocytes than in ctr cells (Fig. 4C). Similar results were obtained with erythrocytes isolated from cGKI SM rescue mice, i.e., these erythrocytes displayed significantly enhanced cytosolic Ca2+ and eryptosis as compared with the respective ctr erythrocytes (Fig. S2). These data suggested that cGKI inhibits erythrocyte death in vitro by reducing the cytosolic Ca2+ concentration, which in turn limits the exposure of PS at the cell surface. To investigate whether cGKI deficiency also affected mechanical properties of the erythrocytes, cGKI KO and ctr erythrocytes were exposed to shear stress, and the resulting cell elongation as a measure of erythrocyte flexibility was determined by using laser defractometry. The cGKI KO erythrocytes were slightly but significantly more flexible than ctr cells. For instance, the percentage elongation at a shear stress of 3.1 Pa in a high-viscosity solution (24.4 mPa · sec) was 24 ± 0.4 in cGKI KO and 19 ± 0.3 in ctr erythrocytes (P < 0.05), and the percentage elongation at a shear stress of 2.6 Pa in a low viscosity solution (10.4 mPa · sec) was 17 ± 0.2 in cGKI KO and 14 ± 0.4 in ctr erythrocytes (P < 0.05). Because deformability of cGKI-deficient erythrocytes was slightly improved, their mechanical properties could not account for their accumulation in the spleens of cGKI mutant mice. Furthermore, the osmotic resistance of cGKI-deficient erythrocytes was not altered (Fig. S3).
Fig. 3.
Expression of cGKI in murine erythroid cells. Western blot analysis of cGKI expression in erythroid cells from a 26-week-old wild-type mouse. Protein extracts of platelet-rich plasma (5 μg) and of Ter119+ erythroid cells isolated from peripheral blood (30 μg) or bone marrow (30 μg) were stained with an antiserum raised against cGKI (Lower) or thrombospondin-1 (TSP-1, Upper).
Fig. 4.
Inrceased eryptosis and intracellular Ca2+ level in cGKI-deficient (ko, black bars) erythrocytes as compared to ctr (ctr, open bars) erythrocytes. (A) Effect of cGMP on eryptosis. PS exposure of ctr and KO erythrocytes was determined by annexin V binding after a 30-min incubation with the Ca2+-ionophore ionomycin (0.1 μM) at 37°C. Before addition of ionomycin, cells were preincubated for 30 min with the guanylyl cyclase inhibitor methylene blue (20 μM) followed by additional 60 min in the absence (−) or presence (+) of 1 mM 8-bromo-cGMP (n = 11–12; *, P < 0.05). (B) Surface exposure of PS as determined by annexin V binding and (C) measurement of intracellular Ca2+ by Fluo-3 fluorescence in ctr and KO erythrocytes after incubation in Ringer solution at 37°C for 48 h. Left shows a representative flow-cytometric histogram, and Right shows the statistical analysis (n = 12; ***, P < 0.001). The first and the second peaks correspond to cell populations with low and high staining intensity, respectively. Erythrocytes were isolated from 10-week-old cGKI KO mice and their litter-matched ctrs. The genotypes of the ctr mice were cGKI+/+ or cGKI+/L−.
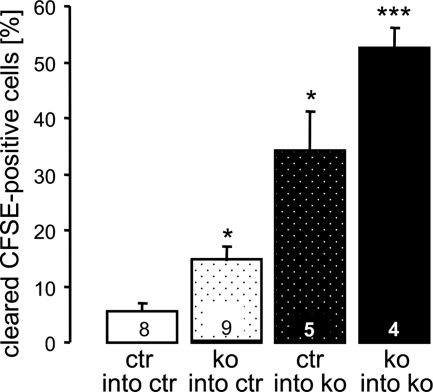
The above experiments suggested that cGKI deficiency promotes the death of erythrocytes, which might accumulate in the spleens of the mutant animals, thus leading to splenomegaly. To determine their in vivo survival, erythrocytes were isolated from ctr or cGKI KO donor mice, labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE), and subsequently injected into the tail vein of ctr or cGKI KO mice. The clearance of CFSE-labeled erythrocytes from the circulation, an indirect indicator of erythrocyte death, was determined 2 days after reinjection. In line with the accelerated in vitro eryptosis of cGKI-deficient erythrocytes, ≈14% of cGKI-deficient erythrocytes disappeared from the peripheral blood of ctr mice as compared with only ≈5% of ctr erythrocytes (Fig. 5). CFSE-labeled ctr erythrocytes were cleared more rapidly in KO than in ctr mice, most likely because of their retention in the enlarged spleens of the recipient mice (≈34% of the initial cells were cleared 2 days after injection). Clearance of erythrocytes was even faster when KO cells were injected into cGKI KO mice (≈53% of the initial cells were cleared 2 days after injection) (Fig. 5). Together, these data indicated that anemia of cGKI-deficient mice was not only due to an erythrocyte-autonomous component, i.e., increased eryptosis, but was potentiated by the development of enlarged spleens retaining more erythrocytes than normal spleens. Along those lines, anemia but not splenomegaly was detectable in 3- to 4-week-old cGKI KO mice (Fig. 6A), whereas 10-week-old KO mice were both anemic and had enlarged spleens (Figs. 1A and 2 A–C). Note that MCV was not increased in 3- to 4-week-old cGKI KO mice (data not shown), although they were already anemic. Cause and effect of the observed phenotypes were further resolved in a time course study with cohorts of cGKI SM rescue and litter-matched ctr mice. Animals were monitored from 4 to 10 weeks of age for RBC count and by MRI for spleen volume. As shown in Fig. 6B, by 5 weeks of age, the cGKI SM rescue mice became anemic but still had a normal spleen size. Thus, anemia appeared to precede splenomegaly in cGKI-deficient mice.
Fig. 5.
In vivo clearance of CFSE-labeled erythrocytes. Erythrocytes were isolated from and injected into 10-week-old cGKI KO mice or their litter-matched ctrs. The configuration of each transfer experiment is indicated below each bar, and the number of injected animals (n) is indicated inside each bar. The percentage of cleared CFSE-labeled cells was determined 2 days after injection (*, P < 0.05 vs. ctr; ***, P < 0.001 vs. ctr). The genotypes of the ctr mice were cGKI+/+ or cGKI+/L−.
Fig. 6.
Anemia precedes splenomegaly in cGKI-deficient mice. (A) The number of RBC (n = 5) and the spleen/bw ratio (n = 10) were determined in 3- to 4-week-old ctr (ctr, open bars) and cGKI KO (ko, black bars) mice (*, P < 0.05). The genotypes of ctr mice were cGKI+/+ or cGKI+/L−. (B) Time course of the development of anemia and splenomegaly in cGKI SM rescue mice. Spleen volume was determined by noninvasive MRI. RBC count (black boxes), and spleen volume (open boxes) were longitudinally monitored from 4 to 10 weeks of age in four cGKI SM rescue mice and five litter-matched ctr mice (ctr SM rescue). For each experimental day, RBC counts and spleen volumes of the SM rescue mice were normalized to the respective mean value of the ctr animals, which was set to 100%. Note that 5-week-old SM rescue mice had already a significantly lower RBC count than ctr mice (P < 0.05) but a normal spleen volume. Lower shows representative MR images (coronal sections, top view) of individual ctr and SM rescue mice at 5 and 6 weeks of age. Spleens (broken lines) and corresponding volumes are indicated. The genotypes of the ctr SM rescue mice were cGKI+/L−;SM-Iα+/− or cGKI+/L−;SM-Iβ+/−.
Discussion
The present study shows that cGKI-deficient mice develop anemia and splenomegaly. The anemia is most likely not due to impaired formation but rather to shortened survival of erythrocytes. Increased levels of plasma erythropoietin and reticulocytes indicate that erythropoiesis is indeed activated in cGKI-deficient mice but eventually cannot compensate for the loss of erythrocytes resulting in anemia. Ter119+ erythroid cells with an increased level of PS exposure accumulate in the spleens of the mutant animals explaining the enlarged spleens and supporting a role of cGKI signaling in erythrocyte survival. Indeed, cGKI-deficient erythrocytes show a decreased survival both in vitro and in vivo. Mechanistically, the decreased survival of cGKI-deficient erythrocytes might result from an increased intracellular Ca2+ concentration triggering the exposure of PS at the erythrocyte surface followed by engulfment by splenic macrophages.
The cytosolic Ca2+ concentration is higher in circulating erythrocytes from cGKI-deficient as compared with ctr mice. It is well known that the NO/cGMP/cGKI pathway inhibits agonist-induced increases in cytosolic Ca2+ in vascular SM cells (20, 26). Many studies have suggested this pathway decreases the cytosolic Ca2+ concentration also in a variety of other cell types, and that the Ca2+ lowering effect might, at least in part, be due to inhibition of Ca2+ entry through Ca2+-permeable channels (27–29) or stimulation of the Ca2+-ATPase (30, 31). In line with their increased cytosolic Ca2+ concentration, a well known trigger of phospholipid scrambling (12), cGKI-deficient erythrocytes show increased surface exposure of PS, a hallmark of eryptosis (14, 15). The exposure of PS at the erythrocyte surface mediates binding to PS receptors of macrophages (18) followed by engulfment (19) and clearance from circulating blood (32). Indeed, cGKI-deficient erythrocytes disappear faster from the circulation than ctr erythrocytes. The mechanical properties of cGKI-deficient erythrocytes are not affected adversely and, therefore, cannot contribute to their accumulation in the spleen.
Erythrocytes from cGKI KO animals are more rapidly cleared from circulating blood than erythrocytes from ctr mice after injection into either KO or ctr animals. These results disclose that the anemia is, at least in part, due to a property of KO erythrocytes. The accelerated death of the erythrocytes presumably leads to enlargement of the spleen, which in turn is expected to enhance the sequestration of circulating erythrocytes. Accordingly, erythrocytes from both KO and ctr animals are more rapidly cleared after injection into KO animals than into ctr animals. It is important to note that anemia is observed in cGKI mutant mice before development of splenomegaly (Fig. 6). Thus, anemia cannot be explained by splenomegaly alone, even though it presumably contributes to anemia in older animals. Beyond enhanced susceptibility to eryptosis and splenomegaly, vitamin B12 insufficiency could also contribute to the development of anemia in conventional cGKI KO mice. Their vitamin B12 deficiency most likely results from deranged intestinal motility and contributes to their increased erythrocyte volume. Interestingly, the cGKI SM rescue mice also develop anemia and splenomegaly but have normal vitamin B12 levels and only slightly increased cell volumes, which could result from decreased activation of KCl symport in the absence of cGKI. KCl symport is normally stimulated by cGK leading to cellular KCl loss and cell shrinkage (33).
The present results disclose a role of cGKI in the regulation of erythrocyte survival. cGMP and cGKI have been shown to counteract apoptosis of nucleated cells (4). The mechanism that suppresses eryptosis via cGKI and lowering of the cytosolic Ca2+ concentration may be similarly effective in the regulation of apoptotic death of nucleated cells. Although indirect effects, e.g., resulting from altered erythropoiesis or defects in nonerythroid cells of the cGKI mutants, cannot be completely ruled out, several findings suggest a direct effect of cGKI on the survival of mature erythrocytes. First, the eryptotic phenotype is observed in isolated mature erythrocytes, and KO erythrocytes are cleared faster from the circulation than ctr cells. Second, anemia and splenomegaly are similarly present in ”sick“ conventional cGKI KO mice and in relatively ”healthy“ cGKI SM rescue mice. Third, anemia appears to precede splenomegaly, excluding the possibility that development of a large spleen and the associated increased clearance of erythrocytes are the primary cause of anemia. Thus, we propose that the primary defect leading to anemia in cGKI-deficient mice is a higher susceptibility of mature erythrocytes to eryptosis, which results in accumulation of the apoptotic cells in the spleen and, thereby, in its enlargement and further retention of erythrocytes. In this way, a vicious cycle is generated in which anemia and splenomegaly potentiate each other to reach the level observed in the adult cGKI mouse mutants. Clearly, to unequivocally establish a causal relationship between erythrocyte cGKI, anemia, and splenomegaly, an erythrocyte-specific cGKI KO would be needed.
A major stimulator of cGKI is NO (4, 34, 35), which can be released or removed by erythrocytes (36–39). Oxygenated HGB takes up NO, whereas deoxygenated HGB releases NO (37, 40, 41). Most recently, NO donors have been shown to counteract eryptosis, an effect partially mimicked by dibutyryl-cGMP (42). Similar to what has been shown in nucleated cells (43, 44), NO may further be effective in erythrocytes through nitrosylation of enzymes necessary for induction of cell membrane scrambling (42). Because NO is released from deoxygenated erythrocytes (45–47), the antieryptotic effect of NO would be particularly important in hypoxic tissue. Impaired NO formation in erythrocytes has been implicated in the vasoconstriction and ischemia after transfusion (46, 47), pulmonary hypertension (39), and deranged microcirculation in sickle cell anemia (48). A decrease of NO-dependent activation of cGKI could participate in the pathophysiology of those conditions. Moreover, accelerated eryptosis participates in the pathophysiology of iron deficiency (32), hemolytic uremic syndrome (49), sepsis (50), malaria (51), Wilson's disease (52), thalassemia (53), glucose-phosphate dehydrogenase deficiency (53), and diabetes (54). At least in theory, stimulation of cGKI could reverse accelerated eryptosis in those diseases.
In conclusion, the present study demonstrates that lack of cGKI leads to an increased cytosolic Ca2+ concentration with subsequent breakdown of PS asymmetry, accelerated clearance of circulating erythrocytes and anemia despite counterregulation by erythropoietin release. These observations identify a function of cGKI signaling in the regulation of erythrocyte survival and support the emerging concept that pathways via NO, cGMP, and cGKI stimulate growth and survival in a number of cell types in vivo (55–58).
Materials and Methods
Experimental Animals and Cells.
Experiments were performed with 3- to 10-week-old conventional cGKI KO mice (21) carrying the L-null allele (cGKI KO mice, genotype: cGKIL−/L−) and with 4- to 45-week-old cGKI SM rescue mice (22), in which the expression of the cGKIα or cGKIβ isozyme was selectively restored in SM but not in other cell types of cGKIL−/L− mice (SM-Iα or SM-Iβ rescue mice, genotype: cGKIL−/L−;SM-Iα+/− or cGKIL−/L−;SM-Iβ+/−). As ctrs, litter- and gender-matched mice with the following genotypes were used: For cGKI KO mice, wild-type (cGKI+/+) mice and heterozygous cGKI (cGKI+/L−) mutants were used, collectively referred to as “ctr” in the text. For SM rescue mice, mice expressing endogenous cGKI and the respective SM-Iα or SM-Iβ transgene (SM-Iα or SM-Iβ ctr mice, genotype: cGKI+/L−;SM-Iα+/− or cGKI+/L−;SM-Iβ+/−) were used as ctrs, collectively referred to as “ctr SM rescue” in the text. The results did not significantly differ between male and female mice of the same genotype, between SM-Iα and -Iβ rescue mice, between cGKI+/+ and cGKI+/L− ctr mice, and between SM-Iα and -Iβ ctr mice. Therefore, data were pooled from both sexes, from SM-Iα and -Iβ rescue mice, from cGKI+/+ and cGKI+/L− ctr mice, and from SM-Iα and -Iβ ctr mice. All mice were on a 129/Sv genetic background. The targeted alleles were established in the R1 embryonic stem cell line (59), which was derived from a (129×1/SvJ × 129S1) F1 3.5-day blastocyst.
Blood was retrieved either by puncture of the retroorbital venous plexus or, in case of killed mice, by puncture of the heart, and collected in heparin- or EDTA-coated tubes. Bone marrow was obtained by rinsing femur and tibia with PBS. Cells from the bone marrow and spleen were separated with Netwells (Corning) before further analysis.
Other detailed methods are provided in SI Materials and Methods.
Statistics.
Data are expressed as mean ± SEM, and statistical analysis was made by two-tailed unpaired t test. Significance was determined at P < 0.05.
Supplementary Material
Acknowledgments.
We thank Yasemin Colakoglu for excellent technical support, Peter Ruth (Department of Pharmacology, University of Tübingen), for the kind gift of the cGKI antiserum, and E. Schleicher and F. Baumgartner for measuring vitamin B12 and folic acid. This work was supported by the Deutsche Forschungsgemeinschaft, the Dr. Karl Kuhn-Stiftung, and the Volkswagen-Stiftung.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708940105/DCSupplemental.
References
- 1.Dimmeler S, Haendeler J, Zeiher AM. Regulation of endothelial cell apoptosis in atherothrombosis. Curr Opin Lipidol. 2002;13:531–536. doi: 10.1097/00041433-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Stamler JS. NO: an inhibitor of cell death. Cell Death Differ. 1999;6:937–942. doi: 10.1038/sj.cdd.4400578. [DOI] [PubMed] [Google Scholar]
- 3.Brune B. NO apoptosis or turning it ON? Cell Death Differ. 2003;10:864–869. doi: 10.1038/sj.cdd.4401261. [DOI] [PubMed] [Google Scholar]
- 4.Nagai-Kusuhara A, et al. cAMP-responsive element binding protein mediates a cGMP/protein kinase G-dependent anti-apoptotic signal induced by nitric oxide in retinal neuro-glial progenitor cells. Exp Eye Res. 2007;84:152–162. doi: 10.1016/j.exer.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86:1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- 7.Chen LY, Mehta JL. Evidence for the presence of L-arginine-nitric oxide pathway in human red blood cells: relevance in the effects of red blood cells on platelet function. J Cardiovasc Pharmacol. 1998;32:57–61. doi: 10.1097/00005344-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Ikuta T, Ausenda S, Cappellini MD. Mechanism for fetal globin gene expression: role of the soluble guanylate cyclase-cGMP-dependent protein kinase pathway. Proc Natl Acad Sci USA. 2001;98:1847–1852. doi: 10.1073/pnas.041599798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinbongard P, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–2951. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 10.Lang KS, et al. Mechanisms of suicidal erythrocyte death. Cell Physiol Biochem. 2005;15:195–202. doi: 10.1159/000086406. [DOI] [PubMed] [Google Scholar]
- 11.Burgoyne JR, et al. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 12.Lang KS, et al. Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ. 2003;10:249–256. doi: 10.1038/sj.cdd.4401144. [DOI] [PubMed] [Google Scholar]
- 13.Lang PA, et al. Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am J Physiol. 2003;285:C1553–C1560. doi: 10.1152/ajpcell.00186.2003. [DOI] [PubMed] [Google Scholar]
- 14.Bratosin D, et al. Programmed cell death in mature erythrocytes: a model for investigating death effector pathways operating in the absence of mitochondria. Cell Death Differ. 2001;8:1143–1156. doi: 10.1038/sj.cdd.4400946. [DOI] [PubMed] [Google Scholar]
- 15.Woon LA, Holland JW, Kable EP, Roufogalis BD. Ca2+ sensitivity of phospholipid scrambling in human red cell ghosts. Cell Calcium. 1999;25:313–320. doi: 10.1054/ceca.1999.0029. [DOI] [PubMed] [Google Scholar]
- 16.Lang KS, et al. Involvement of ceramide in hyperosmotic shock-induced death of erythrocytes. Cell Death Differ. 2004;11:231–243. doi: 10.1038/sj.cdd.4401311. [DOI] [PubMed] [Google Scholar]
- 17.Klarl BA, et al. Protein kinase C mediates erythrocyte “programmed cell death” following glucose depletion. Am J Physiol. 2006;290:C244–C253. doi: 10.1152/ajpcell.00283.2005. [DOI] [PubMed] [Google Scholar]
- 18.Fadok VA, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 19.Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci USA. 1998;95:3077–3081. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeifer A, et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wegener JW, et al. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res. 2002;90:18–20. doi: 10.1161/hh0102.103222. [DOI] [PubMed] [Google Scholar]
- 22.Weber S, et al. Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ Res. 2007;101:1096–1103. doi: 10.1161/CIRCRESAHA.107.154351. [DOI] [PubMed] [Google Scholar]
- 23.Kina T, et al. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol. 2000;109:280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 24.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 25.Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res. 2003;93:907–916. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- 26.Feil R, et al. Functional reconstitution of vascular smooth muscle cells with cGMP-dependent protein kinase I isoforms. Circ Res. 2002;90:1080–1086. doi: 10.1161/01.res.0000019586.95768.40. [DOI] [PubMed] [Google Scholar]
- 27.Ay B, et al. Cyclic nucleotide regulation of store-operated Ca2+ influx in airway smooth muscle. Am J Physiol. 2006;290:L278–L283. doi: 10.1152/ajplung.00188.2005. [DOI] [PubMed] [Google Scholar]
- 28.Gomes B, et al. The cGMP/protein kinase G pathway contributes to dihydropyridine-sensitive calcium response and cytokine production in TH2 lymphocytes. J Biol Chem. 2006;281:12421–12427. doi: 10.1074/jbc.M510653200. [DOI] [PubMed] [Google Scholar]
- 29.Kwan HY, Huang Y, Yao X. Protein kinase C can inhibit TRPC3 channels indirectly via stimulating protein kinase G. J Cell Physiol. 2006;207:315–321. doi: 10.1002/jcp.20567. [DOI] [PubMed] [Google Scholar]
- 30.Dedkova EN, Blatter LA. Nitric oxide inhibits capacitative Ca2+ entry and enhances endoplasmic reticulum Ca2+ uptake in bovine vascular endothelial cells. J Physiol. 2002;539:77–91. doi: 10.1113/jphysiol.2001.013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rashatwar SS, Cornwell TL, Lincoln TM. Effects of 8-bromo-cGMP on Ca2+ levels in vascular smooth muscle cells: possible regulation of Ca2+-ATPase by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1987;84:5685–5689. doi: 10.1073/pnas.84.16.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kempe DS, et al. ) Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 2006;20:368–370. doi: 10.1096/fj.05-4872fje. [DOI] [PubMed] [Google Scholar]
- 33.Adragna NC, et al. KCl cotransport regulation and protein kinase G in cultured vascular smooth muscle cells. J Membr Biol. 2002;187:157–165. doi: 10.1007/s00232-001-0160-8. [DOI] [PubMed] [Google Scholar]
- 34.Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Iα attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem. 2006;281:38644–38652. doi: 10.1074/jbc.M606142200. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Billiar TR. The anti-apoptotic actions of nitric oxide in hepatocytes. Cell Death Differ. 1999;6:952–955. doi: 10.1038/sj.cdd.4400579. [DOI] [PubMed] [Google Scholar]
- 36.Crawford JH, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dejam A, et al. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grubina R, et al. Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy- and oxyhemoglobin. J Biol Chem. 2007;282:12916–12927. doi: 10.1074/jbc.M700546200. [DOI] [PubMed] [Google Scholar]
- 39.McMahon TJ, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 40.Power GG, et al. A novel method of measuring reduction of nitrite-induced methemoglobin applied to fetal and adult blood of humans and sheep. J Appl Physiol. 2007;103:1359–1365. doi: 10.1152/japplphysiol.00443.2007. [DOI] [PubMed] [Google Scholar]
- 41.Yang BC, Nichols WW, Mehta JL. Cardioprotective effects of red blood cells on ischemia and reperfusion injury in isolated rat heart: release of nitric oxide as a potential mechanism. J Cardiovasc Pharmacol Ther. 1996;1:297–306. doi: 10.1177/107424849600100405. [DOI] [PubMed] [Google Scholar]
- 42.Nicolay JP, et al. Inhibition of suicidal erythrocyte death by nitric oxide. Pflügers Arch. 2008;456:293–305. doi: 10.1007/s00424-007-0393-1. [DOI] [PubMed] [Google Scholar]
- 43.Benhar M, Stamler JS. A central role for S-nitrosylation in apoptosis. Nat Cell Biol. 2005;7:645–646. doi: 10.1038/ncb0705-645. [DOI] [PubMed] [Google Scholar]
- 44.Melino G, et al. S-nitrosylation regulates apoptosis. Nature. 1997;388:432–433. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]
- 45.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci USA. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diesen D, Stamler JS. S-nitrosylation and PEGylation of hemoglobin: toward a blood substitute that recapitulates blood. J Mol Cell Cardiol. 2007;42:921–923. doi: 10.1016/j.yjmcc.2007.03.739. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds JD, et al. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci USA. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci USA. 2005;102:2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang PA, et al. Suicidal death of erythrocytes in recurrent hemolytic uremic syndrome. J Mol Med. 2006;84:378–388. doi: 10.1007/s00109-006-0058-0. [DOI] [PubMed] [Google Scholar]
- 50.Kempe DS, et al. Suicidal erythrocyte death in sepsis. J Mol Med. 2007;85:273–281. doi: 10.1007/s00109-006-0123-8. [DOI] [PubMed] [Google Scholar]
- 51.Brand VB, et al. Dependence of Plasmodium falciparum in vitro growth on the cation permeability of the human host erythrocyte. Cell Physiol Biochem. 2003;13:347–356. doi: 10.1159/000075122. [DOI] [PubMed] [Google Scholar]
- 52.Lang PA, et al. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med. 2007;13:164–170. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- 53.Lang KS, et al. Enhanced erythrocyte apoptosis in sickle cell anemia, thalassemia and glucose-6-phosphate dehydrogenase deficiency. Cell Physiol Biochem. 2002;12:365–372. doi: 10.1159/000067907. [DOI] [PubMed] [Google Scholar]
- 54.Nicolay JP, et al. Stimulation of suicidal erythrocyte death by methylglyoxal. Cell Physiol Biochem. 2006;18:223–232. doi: 10.1159/000097669. [DOI] [PubMed] [Google Scholar]
- 55.Feil R, Feil S, Hofmann F. A heretical view on the role of NO and cGMP in vascular proliferative diseases. Trends Mol Med. 2005;11:71–75. doi: 10.1016/j.molmed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Fiedler B, et al. cGMP-dependent protein kinase type I inhibits TAB1–p38 mitogen-activated protein kinase apoptosis signaling in cardiac myocytes. J Biol Chem. 2006;281:32831–32840. doi: 10.1074/jbc.M603416200. [DOI] [PubMed] [Google Scholar]
- 57.Guo D, et al. A Rac-cGMP signaling pathway. Cell. 2007;128:341–355. doi: 10.1016/j.cell.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolfsgruber W, et al. A proatherogenic role for cGMP-dependent protein kinase in vascular smooth muscle cells. Proc Natl Acad Sci USA. 2003;100:13519–13524. doi: 10.1073/pnas.1936024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Rode JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.