Abstract
Half a century has passed since the hydrogen-bonded secondary structures of polypeptides and proteins were first recognized. An extraordinary wealth of conformational information is now available on peptides and proteins, which are formed of α-amino acid residues. More recently, the discovery of well-folded structures in oligopeptides containing β-amino acids has focused a great deal of current interest on the conformational properties of peptides constructed from higher homologues (ω) of α-amino acids. This review examines the nature of intramolecularly hydrogen-bonded conformations of hybrid peptides formed by amino acid residues, with a varying number of backbone atoms. The β-turn, a ubiquitous structural feature formed by two residue (αα) segments in proteins and peptides, is stabilized by a 10-atom (C10) intramolecular 4→1 hydrogen bond. Hybrid turns may be classified by comparison with their αα counterparts. The available crystallographic information on hydrogen-bonded hybrid turns is surveyed in this review. Several recent examples demonstrate that individual ω-amino acid residues and hybrid dipeptide segments may be incorporated into the regular structures of α-peptides. Examples of both peptide helices and hairpins are presented. The present review explores the relationships between folded conformations in hybrid sequences and their counterparts in all α-residue sequences. The use of stereochemically constrained ω-residues promises to expand the range of peptide design strategies to include ω-amino acids. This approach is exemplified by well-folded structures like the C12 (αγ) and C14 (γγ) helices formed in short peptides containing multiply substituted γ-residues. The achiral γ-residue gabapentin is a readily accessible building block in the design of peptides containing γ-amino acids. The construction of globular polypeptide structures using diverse hybrid sequences appears to be a realistic possibility.
Keywords: hydrogen bonds, peptide conformation, hybrid peptides, ω-amino acids, backbone-homologated amino acids
1. Introduction
A little over 60 years ago, Maurice Huggins reviewed the hydrogen-bonded structures of polypeptide chains, at a time when no detailed structural information was available on oligopeptides and proteins (Huggins 1942, 1943). Attempts to rationalize limited X-ray diffraction data in terms of regular hydrogen-bonded polypeptide structures were summarized by Bragg et al. (1950) in a paper that concluded that ‘there appears a real simplicity of chain structures in myoglobin, which will perhaps be shown by other favourably built proteins… There is hope that the study of such proteins may lead to a reliable determination of structure’. This expectation has been amply fulfilled in the last half century with the result that almost every feature of polypeptide chain folding is now firmly established by experimental studies. An interesting feature of the Bragg, Kendrew and Perutz paper is the consideration of intramolecularly hydrogen-bonded structures, which were later shown to be stereochemically and energetically unacceptable. Pauling's remarkable insights led to the correct formulation of the hydrogen-bonded helical structures of polypeptides (Pauling et al. 1951; Pauling & Corey 1952). One of the last attempts to propose alternative hydrogen-bonded structures was made by Huggins in 1952, when he suggested that a 11-membered hydrogen-bonded ring could be considered as an alternative to Pauling's α-helix, which is characterized by a 13-membered hydrogen bond (Huggins 1952a,b). This was quickly dismissed by Pauling, since the peptide units would have to deviate substantially from planarity to accommodate the 11-atom hydrogen-bonded ring (Pauling & Corey 1952). Current interest in the hydrogen-bonded structures of polypeptides chains stems from the realization that the repertoire of polypeptide chains can be vastly expanded when backbone-homologated amino acid residues are introduced (Gellman 1998; Cheng et al. 2001; Hill et al. 2001; Gademann et al. 2003; Lelias & Seebach 2004; Roy & Balaram 2004; Seebach et al. 2004, 2006; Kimmerlin & Seebach 2005). This overview considers recent research on hydrogen-bonded structures of peptides containing β-, γ- and δ-amino acids which have provided several examples of novel hydrogen-bonded patterns, hitherto unknown in all α-polypeptide structures.
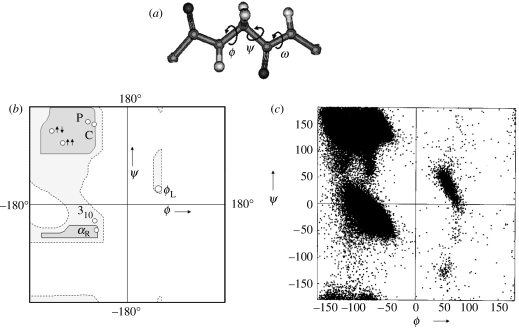
Naturally occurring polypeptide chains found in proteins are heteropolymers of α-amino acid residues linked together by peptide bonds. The stereochemistry of the polypeptide chain is best described by analysing the various orientations of two planar peptide units linked together by a central tetrahedral carbon atom. The torsional degrees of freedom about the N–Cα and the Cα–C′ bonds permit the chain to sample conformational space. Several stable conformations corresponding to energy minima are characterized by specific values of ϕ and ψ at each residue (Ramachandran & Sasisekharan 1968, figure 1). The nature of polypeptide chain folding is determined by short-range non-bonded interactions and intramolecular hydrogen bond formation between acceptor CO and donor NH groups. Over the course of a little more than half a century, the principles governing the folding of polypeptide chains and the nature of the stable secondary structures have been well established. In proteins, sequence variability generated by the incorporation of 20 different genetically coded α-amino acids, which differ in the substituent at Cα, is the key to structural and functional diversity.
Figure 1.
(a) A system of two linked peptide units defining the backbone torsion angles at an α-amino acid residue in a polypeptide chain. (b) Ramachandran map showing sterically allowed regions for l-alanine. The regions corresponding to the important secondary structures are marked αR (right-handed α-helix), αL (left-handed α-helix), 310 (310-helix), two upward arrows (parallel β-sheet), left up, right down arrows (antiparallel β-sheet), PII (polyproline II), C(collagen triple helix). (c) Scatter plot of 104 718 non-Gly residues in protein crystal structures (less than or equal to 2.0 Å) showing the distribution of the backbone dihedral angles ϕ and ψ (Singh 2001).
β-Amino acids and higher backbone-homologated (ω) residues are found in nature as constituents of peptide metabolites produced by micro-organisms. Poly-γ-d- glutamate first reported from Bacillus anthracis (Hanby & Rydon 1946; Rydon 1964) has subsequently been shown to be produced by several species of bacillus (Ashiuchi & Misono 2002). Much of the early work on homopolypeptides of ω-amino acids was stimulated by the interest in the structures of nylons (Glickson & Applequist 1971; Lovinger 1978; Fernandez-Santin et al. 1984; Munoz-Guerra et al. 1985; Puiggali & Munoz Guerra 1986; Lopez-Carrasquero et al. 1995; Bella et al. 1992; Navas et al. 1995; Aleman et al. 1997). More recently, the characterization of well-defined helical conformations in homo-oligomeric β-peptide sequences (Appella et al. 1996, 1997; Seebach et al. 1996a,b, 1997; 1998a–c; Banerjee and Balaram 1997; Seebach & Matthews 1997b) has resulted in a sudden surge of interest in the conformational properties of peptides containing ω-amino acids. Hybrid polypeptides, heteropolymers composed of diverse ω-amino acids, are of special interest in attempts to mimic all α-peptide backbones, using sequences containing non-protein amino acids (Karle et al. 1997). This review surveys the conformational properties of hybrid peptides containing α-, β-, γ- and δ-amino acids as observed in crystal structures and advances the use of backbone torsion angles as convenient parameters for description of conformational variability.
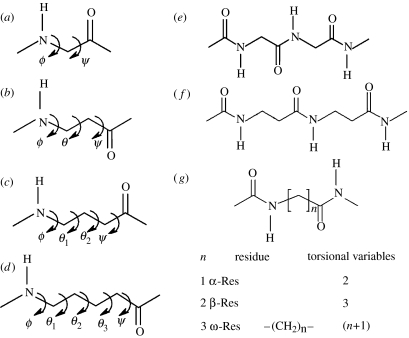
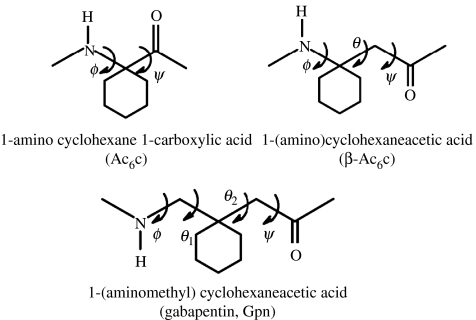
For unwary readers, a note of caution regarding nomenclature may be in order. For β and higher ω-amino acid residues, the backbone atoms are designated as Cα, Cβ, Cγ, etc. beginning from the carboxyl end of the residue. For β-residues, the backbone torsion angles are ϕ, θ and ψ and for γ- and higher residues the backbone torsion angles (θn) follow the order θ1, θ2, …, θn in the reverse direction, i.e. θ1 is towards the N-terminus while the θn is towards the C-terminus side of the residue. The residue designations α, β, γ must also be carefully distinguished from the use of the same Greek letters to denote well-established structural features in proteins and all α-polypeptides such as the α-helix, β-sheet, β-turns and γ-turns. Curiously, the α-turn is a feature involving three successive α-amino acids residues (ααα), while the β-turn involves two (αα) amino acid residues and the γ-turn involves one (α) amino acid residue. A convenient way out of this confusing situation does not appear to be feasible at this time, since usage of the various turns is well entrenched in the literature.
2. Background to conformational analysis
Figure 1 shows the Ramachandran map for the l-alanine residue. The stereochemically allowed regions of conformational space are mapped on to the ϕ, ψ plane, with disallowed regions being characterized by unacceptably close approach of non-bonded atoms (Ramachandran et al. 1963). The validity of the Ramachandran map has been established by the observation that individual amino acid residue conformations, determined from protein crystal structures, cluster in the ‘allowed regions’. The contact criterion, which simply states that non-bonded atoms cannot approach one another at distances less than the sum of their van der Waals' radii, has been found to be an excellent filter for distinguishing stereochemically allowed and disallowed conformations. The use of backbone torsion angles as variable parameters permits the analysis of a three-dimensional problem in two-dimensional, torsion angle space. Regular α-polypeptide structures, like the Pauling 3.613 (α)-helix, 310-helix, parallel and antiparallel β-sheets and polyproline structures are ideally represented by a single point in ϕ, ψ space. The use of dihedral angles as descriptors of local conformations at individual residues has permitted systematic analysis of backbone structural features in a large body of experimentally determined protein structures.
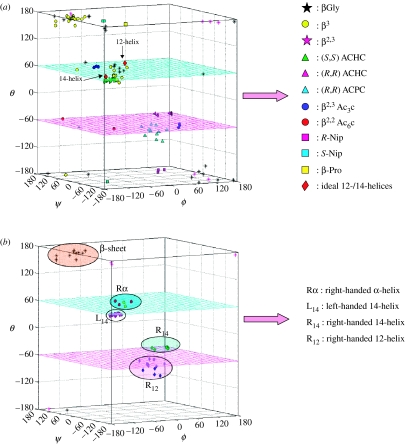
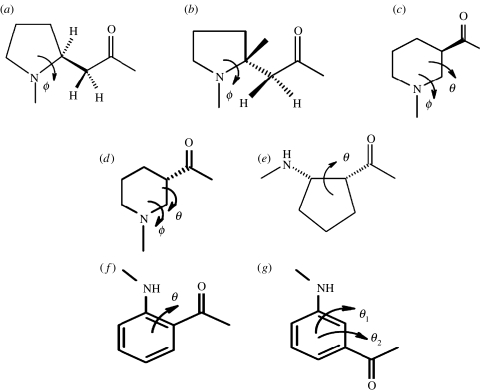
For β- and the higher γ-residues, the number of torsional variables increases as a consequence of introduction of additional carbon atoms into the backbone. The conformation of β-residues can be described using three torsional variables ϕ, θ and ψ. Figure 2 summarizes the nomenclature used for describing backbone torsion angles in ω-residues. Three stable minima corresponding to θ values of ±60° (gauche) and 180° (trans) are anticipated for β-residues. While homologated polypeptide backbones were examined structurally in the 1960s and 1970s (Banerjee & Balaram 1997), the observation of well-defined helical structures in oligopeptides by the groups of Seebach and Gellman in 1996, has led to a considerable resurgence of interest in the conformational properties of the higher homologues of α-amino acids. The characterization of the 12-helix [(M) 2.51 or (P) 2.51] (Appella et al. 1997, 1999a,c; Seebach & Matthews 1997a), which can be formally considered as an expanded version of the 310-helical structure of the α-polypeptides, the mixed 10/12-helix (Seebach & Matthews 1997b; Seebach et al. 1997, 1998a) and the C14-helix [(M) 31 or (P) 31] (Appella et al. 1996, 1999a,b; Seebach et al. 1996a,b, 1997a, 1998a), which has reversed hydrogen-bond directionality, provided the first evidence for the considerable potential of β- and higher ω-amino acid residues in expanding the repertoire of polypeptide chain conformations. For β- and higher ω-amino acids, torsion angle space becomes multidimensional as the number of rotatable backbone bonds increase, with the consequence that a simple graphical, Ramachandran-type representation of stereochemically allowed conformations is no longer possible. Figure 3 illustrates the distribution of the observed β-residue conformations, thus far established from the crystal structures of peptides, in three-dimensional ϕ, θ, ψ space. In considering the conformation of hybrid peptides, it is therefore convenient to use well-established, folded structures of all α-peptide backbones as the starting point. The conformational problem then reduces to questions on the effect of inserting additional backbone atoms into canonical α-peptide structures. This approach is illustrated below with specific examples.
Figure 2.
The nomenclature used for describing the backbone torsion angles in (a) α-residues, (b) β-residues, (c) γ-residues, (d) δ-residues, (e) all α-polypeptide chain, (f) all β-chain and (g) all ω-chain.
Figure 3.
(a) Distribution of the observed β-residues, established from crystal structures of peptides, in ϕ–θ–ψ space. (b) Locations for ordered secondary structures for β-residues in ϕ–θ–ψ space (Roy 2005).
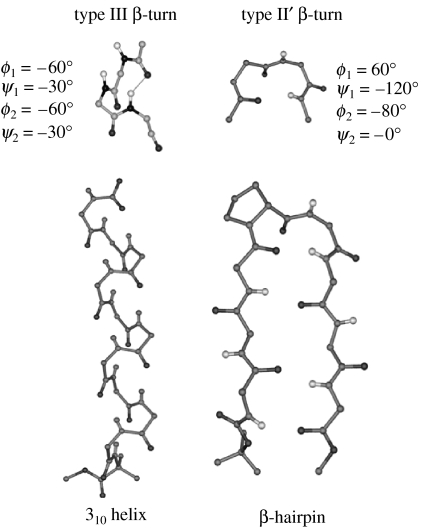
3. Reverse turns
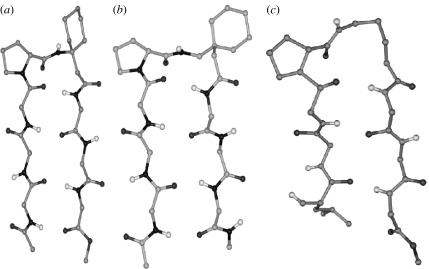
Figure 4 schematically illustrates three common turn structures which facilitate polypeptide chain reversal in all α-residue sequences (Rose et al. 1985). The C7 or γ-turn conformation is generated by the formation of a single seven-atom intramolecularly hydrogen-bonded ring and is characterized by the torsion angles ϕ≈−70°, ψ≈+70° or ϕ ≈+70°, ψ≈−70° at residue i+1 (Mathews 1972; Milner-White & Poet 1986, 1987; Milner-White et al. 1988). The C7 structure is effectively determined by the conformational preferences at a single residue. The C10 (β-turn) structure is a two-residue feature defined by torsion angles ϕ(i+1), ψ(i+1), ϕ(i+2) and ψ(i+2). β-Turns are structural elements widely found in proteins and peptides. They have been classified into distinct groups defined by the torsion angles at the two central residues. β-Turns are generally stabilized by a 4→1 hydrogen bond between CO(i) and NH(i+3) (Wilmot & Thornton 1988). The C13 or α-turn structure is determined by the conformational parameters at three successive α-residues and is characterized by a 5→1 hydrogen bond, between CO(i) and NH(i+4) (Nataraj et al. 1995; Pavone et al. 1996). Specific families in both C10- and C13-turn types can be successively repeated generating regular polypeptide helices. Notably, repetition of the type III β-turn motif results in a 310-helix (Donohue 1953; Toniolo & Benedetti 1991), while a repetitive set of α-turns results in the 3.613 (α)-helix. In all α-residue polypeptides, the largest diameter helix that may be considered in which side chains protrude outwards is the 4.416 (π) helix, in which the individual turn units are stabilized by a 6→1 hydrogen bond between the CO (i) and NH (i+5) (Ramachandran & Sasisekharan 1968). In proteins, the α-helix is the most widely observed secondary structure, its stability undoubtedly the consequence of optimum packing in the interior of the helical structure. For larger helices in this family, non-bonded interactions are far from optimal. The 310-helix is observed only in short stretches in proteins, but has been well characterized in synthetic peptides (Prasad & Balaram 1984; Karle & Balaram 1990). It may be noted that the β-helical structure observed in proteins is a large-diameter cylindrical structure. This is formed by winding an extended β-strand around a helix axis with parallel β-sheet hydrogen bonds between helical turns (Ienger et al. 2006).
Figure 4.
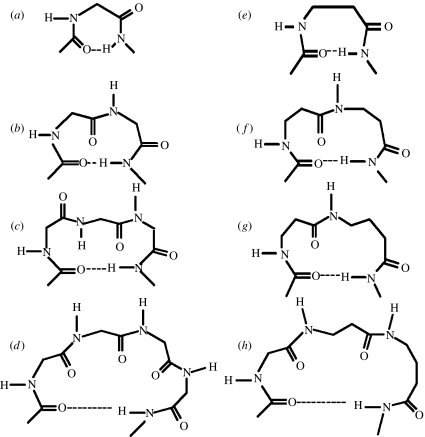
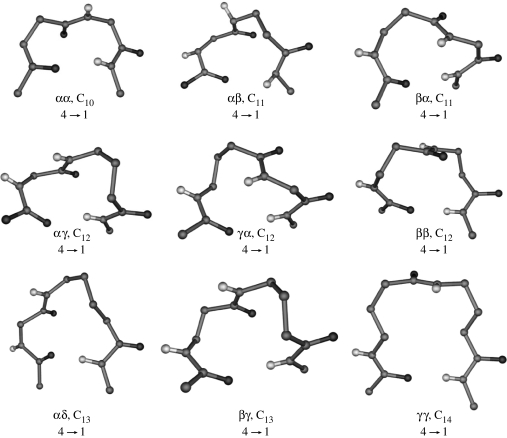
Schematic illustration of turns in all α-residue segments (a) γ-turn (C7), (b) β-turn (C10), (c) α-turn (C13), (d) π-turn (C16), (e) single-residue C8 hydrogen-bonded ring (expanded γ-turn), (f) two-residue ββ C12 hydrogen-bonded ring (expansion of β-turns), (g) two-residue βγ C13 hydrogen-bonded ring (α-turn analogue) and (h) three-residue αβγ C16 hydrogen-bonded ring (π-turn analogue).
Figure 4 also illustrates analogous structures generated by inserting one, two or three atoms into the polypeptide backbone. The C8 conformation of a single β-residue may be considered as an expansion of the γ-turn. γ-turns are widely observed in the proteins but are generally not characterized in small peptides in the solid-state. A recent example of two consequtive C7 hydrogen bonds is seen in the crystal structure of the dipeptide Piv-l–Pro-l-c3–Dip–NHMe (c3-Dip=2, 2-diphenyl-1-aminocyclopropanecarboxylic acid) (Jimenez et al. 2004). A novel C8 ribbon structure has been established in the tetrapeptide Boc-(1-(aminomethyl)cyclopropanecarboxylic acid)4OMe (Abele et al. 1999). A ββ segment generated by two contiguous β-residues results in a C12-turn, which is an expansion of the C10 (β-turn) structure of an αα segment. A βγ segment mimics the C13 (α)-turn formed by three contiguous α-residues. In all these cases, the hydrogen-bond directionality is maintained, with the acceptor CO group lying towards the N-terminus side, while the donor NH group is at the C-terminus end.
Reversal of hydrogen-bond directionality in isolated turns is generally not observed in all α-polypeptide structures, although the C8-turn involving a cis peptide bond and a hydrogen bond between NH(i) and CO(i+1) has been considered in short peptides in order to rationalize spectroscopic data (Pulla Rao et al. 1979). Structures with reversed hydrogen-bond directionality are stereochemically feasible in oligopeptides containing higher ω-amino acids, maintaining planarity of the peptide bond. A dramatic example is the C14-helix formed in the all β-peptide Boc-(trans-ACHC)6CO2CH2Ph (Appella et al. 1996). A C10 hydrogen bond with a reversed hydrogen-bond directionality has also been characterized in the tripeptide Boc-(1-(aminomethyl)cyclohexanecarboxylic acid)3OMe (Seebach et al. 1998c; Abele et al. 1999).
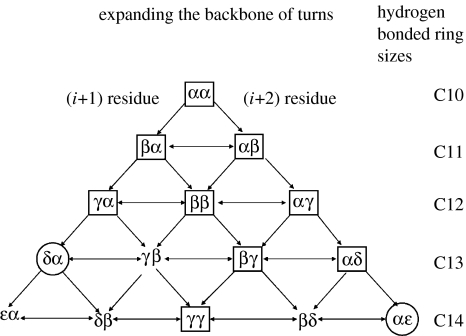
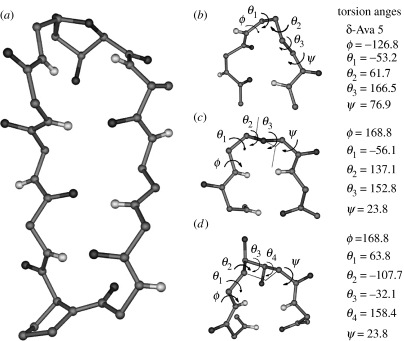
In considering reverse turns in hybrid αω sequences, it is useful to note that an α-residue contributes three atoms to the polypeptide backbone, while β-, γ- and δ-residues contribute 4, 5 and 6 atoms, respectively. Thus a δ-amino acid residue may be formally viewed as mimetic of a Gly–Gly segment in which the central peptide unit has been replaced by a CH2–CH2 group. Similarly, a βγ dipeptide segment which contains nine backbone atoms is formally related to an all α-tripeptide unit (figure 5, Banerjee et al. 1996). Figure 6 summarizes the hydrogen-bonded ring sizes that can be generated, in principle, from hybrid dipeptide sequences. In all cases, the directionality is assumed to be the same as that of the all α-polypeptide structures. Several recently determined peptide crystal structures provide examples of expanded hydrogen-bonded structures. Figure 7 shows examples of turn structures characterized by X-ray diffraction. In some cases, the hydrogen-bonded turns constitute part of a well-developed secondary structure in model peptides. Thus far, turn types containing up to 14 atoms in the hydrogen-bonded ring have been reported. C11- and C12-turns may be considered as backbone expanded analogues of αα C10-turns (β-turns), while the C14-turn may be considered as a one atom expanded analogue of the ααα C13-turn (α-turn).
Figure 5.
Comparison of the formally equivalent backbone segments (a) Gly–Gly and δ-Ava segment (number of backbone atoms is 6) and (b) Gly–Gly–Gly and β-Gly γ-Abu segment (number of backbone atoms is 9; Karle et al. 1997).
Figure 6.
Hydrogen-bonded ring sizes that can be generated in principle from hybrid dipeptide sequences, the hydrogen-bond directionality being the same as in normal all α-polypeptide structures. Turns encircled with rectangles have been characterized in single crystals by X-ray diffraction. Turns established only by NMR are indicated with circles.
Figure 7.
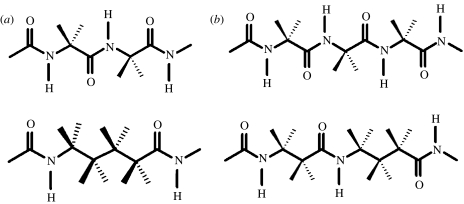
Examples of turn structures (normal hydrogen-bond direction) characterized by X-ray diffraction, αα, Boc–Leu–Phe–Val-dPro–Gly Leu–Phe–Val–OMe (Karle et al. 1996); αβ, Boc–Aib–Aib–βGly NHMe (Pavone et al. 1992); βα, Boc–Val–Ala–Phe–Aib–βVal–βPhe–Aib–Val–Ala–Phe–Aib–OMe (Roy et al. 2004); αγ, Boc–Aib–Gpn–Aib–Gpn–OMe (Ananda et al. 2005); γα, Boc–Aib–Gpn–Aib–Gpn–OMe (Ananda et al. 2005); ββ, Boc–(trans ACPC)8-CO2CH2Ph (Appella et al. 1999c); αδ, Boc–Leu–Val–Val–dPro–δAva–Leu–Val–Val–OMe (Rai et al. in press); βγ, Boc–Leu–Aib–Val–βGly γAbu–Leu–Aib–Val–OMe (Karle et al. 1997); γγ, Boc–γ-ResA–γ-ResB–NHMe (γ-ResA=2 benzyl, 4 methyl-γ-aminoisobutyric acid, γ-ResB=4 isopropyl, 2 methyl γ aminoisobutyric acid; Brenner & Seebach 2001).
4. Conformational diversity of peptide turns
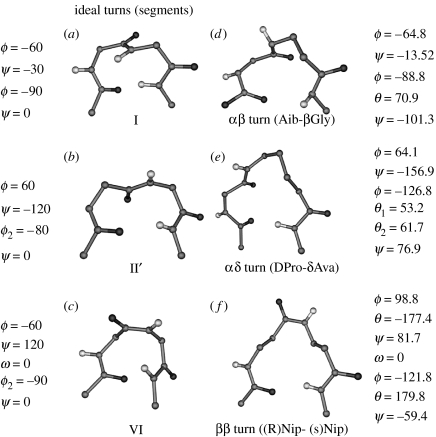
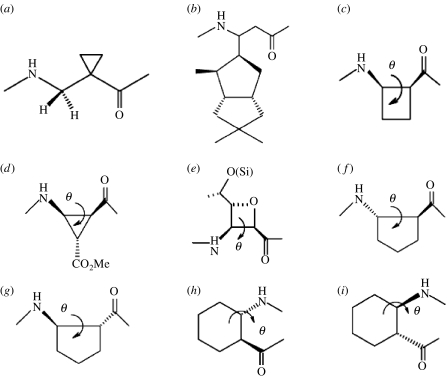
In the case of α-peptides, the two-residue β-turn or β-bend is the most widely distributed and best characterized structure (Venkatachalam 1968; Chandrasekaran et al. 1973; Lewis et al. 1973; Richardson 1981; Wilmot & Thornton 1988). A 4→1 C10 hydrogen bond is a characteristic feature of this family of two-residue (αα) turns. Six distinct turn types I/I′, II/II′ and III/III′, which lead to sharp reversal of chain direction, have been extensively discussed in the literature. In these cases, all the three peptide bonds involved in the turn adopt trans geometry. Turn classification is based on the torsion angles ϕ, ψ at the corner residue, designated as the (i+1) and (i+2) positions of the turn (Type I: ϕ(i+1)∼−30°, ψ(i+1)∼−60°, ϕ(i+2)∼−90°, ψ(i+2)∼0°; Type II: ϕ(i+1)∼−60°, ψ(i+1)∼120°, ϕ(i+2)∼80°, ψ(i+2)∼0°; Type III: ϕ(i+1)∼−60°, ψ(i+1)∼−30°, ϕ(i+2)∼−60°, ψ(i+2)∼−30°). The prime turns I′, II′, III′ have the signs of all the torsion angles reversed. The type I and the type III turns are very closely related to each other and may be considered as a single family. Type II turns differ from type I turns in the orientation of the central peptide unit, which is flipped by about 180° resulting in a concerted change of ψ(i+1) and ϕ(i+2) (Gunasekaran et al. 1998). A unique class of β-turns involving a central cis peptide bond, the type VI turn, is also encountered in proteins and peptides. Figure 8 shows a view of the type I, type II and type VI β-turns.
Figure 8.
(a) Type I β-turns, (b) type II′ β-turns, (c) type VI β-turns and crystallographically characterized examples of homologated turns containing similar conformational features, (d) αβ-turn in Boc–Aib–Aib–β-Gly NHMe (Pavone et al. 1992), (e) αδ-turn in Boc–Leu–Val–Val–dPro–δAva–Leu–Val–Val–OMe (Rai et al. in press) and (f) ββ-turn Boc–β-Ala–(R)-Nip–(S)-Nip–β-Ala–NHMe (Chung et al. 2000). In the case of αβ- and αδ-turns, the ϕ, ψ values at residues (i+1) are similar to those at (i+1) position of type I and type II′ turns. The ββ-turn is an example of a structure with a cis central peptide bond which can be formally viewed as homologation of both (i+1) and (i+2) residues of a type VI β-turn.
By analogy with the all α-peptide structures, conformational diversity is also anticipated in the case of the homologated turns obtained in hybrid sequences. Classification of the experimentally determined hydrogen-bonded hybrid structures may be readily achieved by comparison with their α-peptide counterparts. Figure 8 shows examples of homologated turns, which are formally analogous to the canonical β-turn structures in αα sequences. It is clear that although hybrid turns may contain the same number of atoms as their α-peptide counterparts, the precise turn type may vary. It must be emphasized that in the case of the αα, C10-structures, type I/III turns nucleate helices, type II turns are generally isolated features, while type I′/II′ turns facilitate registered antiparallel hairpin formation (Venkatraman et al. 2001; Aravinda et al. 2003). As noted earlier, heterogeneity of turn types will undoubtedly also occur in the case of hybrid turns. In the history of the development of α-peptide stereochemistry, an initial theoretical analysis of all the possible intramolecularly hydrogen-bonded structures for a system of three linked peptide units by C. M. Venkatachalam in G. N. Ramachandran's laboratory in Madras (Venkatachalam 1968), played an important role in identifying the diverse nature of the β-turn types. A similar systematic investigation in the case of hybrid turns is merited.
Tables 1 and 2 list the torsion angles and hydrogen-bond parameters observed in the crystallographically determined turn structures involving ω-amino acid residues. In considering hybrid turns, we have chosen segments which may be a part of larger secondary structures. It should be emphasized that the repetition of specific turn types along a sequence results in the generation of helical polypeptide structures. In table 1, the analogous α-peptide turn type is indicated. This has been obtained by examining the superposition of the crystallographically characterized hybrid turns with the parent α-peptide turns.
Table 1.
Torsion angle parameters observed in crystallographically determined turn structures involving ω-amino acid residues. βVal, β valine; βPhe, β phenylalanine; Aib, α-aminoisobutyric acid; ACPC, trans-2-aminocyclopentanecarboxylic acid; β-Gly, β glycine; Dec, (2S,4S,6S)-2-amino-6-hydroxy-4-methyl-8-odxe-ocanoic acid; MHA, (4S, 2E)-4-methylhex-2-enoic acid; DPDA, (2S)-N,N-dimethylpropane-l,2-diamine; Me Pro, Methyl proline; HyLeu, hydroxyleucine; DAP, diamino pimelic acid; β-Res1, 8-aminocyclooct-4-enecarboxylic acid; Gpn, Gabapentin, (1-(aminomethyl) cyclohexaneacetic acid); γAbu, γ-aminoisobutyric acid; γ-Res Z, 2, 2 di-fluoro,3-oxo,4-methyl γ-aminoisobutyric acid; γ-ResX, 3-methoxy, 4-methyl γ-aminoisobutyric acid; γ-ResY, 4-methyl, 2–3 eneyl γ-aminoisobutyric acid; β-Res A, 2-benzyl, 3-(thio phenyl)methyl β-glycine; β-Res B, 3-ethyl,2-methyl β-glycine; Nip, nipecotic acid; δAva, δ aminovaleric acid; γ-Res A, 2-benzyl, 4-methyl γ-aminoisobutyric acid; γ-Res B, 4-isopropyl, 2-methyl γ-aminoisobutyric acid; γ- Res C, 2-, 3-, 4-trimethyl, γ-aminoisobutyric acid and γ-Res D, 2-isopropyl, 3-,4- dimethyl γ-aminoisobutyric acid.
| peptide/reference | turn type | residue | torsion angles (deg.) | analogous α-turn | ||||
|---|---|---|---|---|---|---|---|---|
| ϕ | θ1 | θ2 | θ3 | ψ | ||||
| αβ-turns (C11) | ||||||||
| Boc-Val-Alu-Phe-Aib-βVal-βPhe-Aib-Val-Alu-Phe-Aib-OMe/Roy et al. (2004) | βα | β-Phe 6 | −88 | 80 | — | — | −118 | type III |
| Aib 7 | −55 | — | — | — | −49 | |||
| Boc-(Aib-trans ACPC)4-OBz/Schmitt et al. (2005) | βαa | trans ACPC | −95.8 | 94.5 | — | — | −89.9 | type III |
| Aib | −53.5 | — | — | −39.9 | ||||
| Boc-β-Gly-Aib-Leu-Aib-OMe/Banerjee et al. (2003) | βα | βGly1 | −103.8 | 83.7 | — | — | − 84.7 | type III |
| Aib 2 | −56.1 | — | −44.6 | |||||
| Boc-(trans ACPC-trans ACPC-Phe)2OMe/Schmitt et al. (2006) | βα | trans ACPC 2 | −67.5 | 105.8 | — | — | −129.8 | type III |
| Phe 3 | −69.9 | — | — | — | −23.1 | |||
| Boc-(trans ACPC-Aib-Aib)2-OMe/Schmitt et al. (2006) | βαb | trans ACPC | −89.2 | 89.2 | — | — | −94.3 | type III |
| Aib | −54.6 | — | — | −32.3 | ||||
| Boc-(trans ACPC- transACPC-Phe)2OMe/Schmitt et al. (2006) | αβ | Phe 3 | −69.9 | — | — | — | −23.1 | type III |
| trans ACPC 4 | −112.5 | 74.9 | — | — | 163.0 | |||
| Boc-Aib-Aib-βGly-NHMe/Pavone et al. (1992) | αβc | Aib 2 | −64.8 | — | — | — | −13.52 | type III |
| βGly 3 | −88.8 | 70.9 | — | — | −101.3 | |||
| Boc-(Aib-trans ACPC)4-OBz/Schmitt et al. (2005) | αβa | Aib | −53.9 | — | — | — | −41.2 | type III |
| trans ACPC | −95.8 | 94.5 | — | — | −89.9 | |||
| MHA-MePro-Dec-HyLeu-Leu-Leu-Aib-Aib-βGly-DPDA, Cerrini et al. (1989) | αβ | Aib 8 | −66.8 | — | — | — | −49.2 | type III |
| βGly 9 | −103.3 | 79.7 | — | — | −78.5 | |||
| Boc-(trans ACPC-Aib-Aib)2-OMe/Schmitt, et al. (2006) | αβ | Aib3 | −52.0 | — | — | — | −40.0 | type III |
| trans ACPC 4 | −95.0 | 95.8 | — | — | −85.1 | |||
| Boc-Aib-βRes1-Aib-OMe/Tanaka et al. (2001) | αβ | Mol. A | variant tupe | |||||
| Aib | 46.26 | — | — | — | −135.7 | |||
| β-Res | −72.79 | 72.9 | — | — | 83.78 | |||
| Mol.B | ||||||||
| Aib | −47.38 | — | — | — | 130.2 | |||
| β-Res | 84.64 | 69.14 | — | — | −193.71 | |||
| αγ-turns (C12) | ||||||||
| Boc-Aib-Gpn-Aib-Gpn-OMe/Ananda et al. (2005) | αγ | Aib 1 | −59.8 | — | — | — | −37.8 | — |
| Gpn 2 | −126.8 | 52.1 | 63.8 | −107.9 | ||||
| Boc-Aib-Gpn-Aib-Gpn-OMe/Ananda et al. (2005) | γα | Gpn 2 | −126.8 | 52.1 | 63.8 | — | −107.9 | — |
| Aib 3 | −51.5 | — | — | — | −48.8 | |||
| Boc-Leu-Aib-Val-βGly-γAbu-Leu-Aib-Val-OMe/Karle et al. (1997) | γα | γAbu5 | −108 | 58 | 66 | — | −169 | — |
| Leu 6 | −86 | — | — | — | −18 | |||
| Boc-Leu-Aib-Val-βGly-γAbu-Leu-Aib-Val-Ala-Leu-Aib-OMe/Karle et al. (1997) | γα | γAbu5 | −121 | 62 | 67 | — | −169 | — |
| Leu 6 | −57 | — | — | — | −37 | |||
| Boc-Val-Ile-γResZ-Val-Ile-OMe/Wolfe et al. (1998) | γα | γ-ResZ | −101.2 | 65.2 | 62.6 | — | −140.5 | |
| Leu | 90.0 | — | — | — | −28.5 | — | ||
| Boc-γ-Abu-Aib-Ala-Aib-OMe/Maji et al. (2002) | γα | γAbu1 | 92.7 | −69.7 | −65.8 | — | 155.7 | — |
| Leu 2 | 58.4 | — | — | — | 26.1 | |||
| Boc-γ-ResX-DPro-Gly-γ-ResY-OMe/Hagihara et al. (1992) | γα | MolA | ||||||
| γ-ResX | −98.7 | 66.2 | 66.63 | — | −149.3 | |||
| Pro | −64.2 | — | — | — | −14.2 | — | ||
| MolB | ||||||||
| γ-ResX | −100.8 | 58.8 | 65.11 | — | −160.1 | — | ||
| Pro | −69.9 | — | — | — | −14.8 | |||
| Boc-γ-Abu-Aib-Ala-OMe/Maji et al. (2002) | γα | MolA | ||||||
| γAbu1 | −109.2 | 65.2 | 62.6 | — | −140.4 | |||
| Aib2 | −54.5 | — | — | — | −37.0 | — | ||
| MolB | ||||||||
| γAbu1 | 107.5 | −62.3 | −65.1 | — | 138.4 | — | ||
| Aib2 | 58.35 | — | — | — | 36.36 | |||
| ββ-turns (C12) | ||||||||
| Boc-(trans-ACPC)8-CO2CH2Ph/Appella et al. (1999c) | ββd | trans ACPC | 97.9 | 96.4 | — | — | 106.3 | — |
| trans ACPC | 97.9 | 96.4 | — | — | 106.3 | |||
| Boc-(trans ACPC-trans ACPC-Phe)2 OMe/Schmitt et al. (2006) | ββ | trans ACPC1 | −122.5 | 77.9 | — | — | −112.0 | — |
| trans ACPC2 | −67.5 | 105.8 | — | — | −129.8 | |||
| Boc-(trans ACPC- trans ACPC-Phe)2OMe/Schmitt et al. (2006) | ββ | trans ACPC4 | −112.5 | 74.9 | — | — | −78.8 | — |
| trans ACPC5 | −92.2 | 90.1 | — | — | −98.9 | |||
| Piv-β-Res A-Nip1-Nip2-β-Res B-NHMe/Chung et al. (1998) | ββ | Nip 1 | 115.7 | −175.2 | — | — | 81.8 | — |
| Nip 2 | −117.4 | −176.6 | — | −71.2 | ||||
| Boc-B-Ala-(R)-Nip-(S)- Nip-β-Ala-NHMe/Chung et al. (2000) | ββe | Nip 1 | 98.8 | −177.4 | — | — | 81.7 | — |
| Nip 2 | −121.8 | 179.3 | — | −59.4 | ||||
| βγ-turns (C13) | ||||||||
| Boc-Leu-Aib-Val-βGly-γAbu-Leu-Aib-Val-OMe/Karle et al. (1997) | βγ | βGly4 | −103 | 78 | — | — | −107 | — |
| γAbu5 | −121 | 63 | 57 | — | −121 | |||
| αδ-turns (C13) | ||||||||
| Boc-Leu-Val-Val-DPro-δAva-Leu-Val-Val-OMe/Rai et al. (in press) | αδ | DPro 4 | 64.1 | — | — | — | −156.9 | — |
| δAva5 | −126.8 | 53.2 | 61.7 | 166.5 | 76.9 | |||
| γγ-turns (C14) | ||||||||
| Boc-γ-Res A-γ-Res B-NHMe/Brenner & Seebach (2001) | γγ | γ-Res1 | 157.9 | −167 | 67.8 | — | −113.6 | — |
| γ-Res2 | −121.8 | 59.5 | −177.8 | — | 149.6 | |||
| Ac-γ-Res A-γ-Res B-NHMe/Brenner & Seebach (2001) | γγe | γ-Res1 | 148.1 | −169.4 | 73.4 | — | −112.6 | — |
| γ-Res2 | −119.9 | 60.4 | 176.7 | — | 147.1 | |||
| Boc-(γ-ResC-γ-ResD-)2OBz/Seebach et al. (2001a) | γγe | γ-Res3 | 132.4 | −69.9 | −56.9 | — | 138.9 | — |
| γ-Res4 | 153.5 | −70.4 | −56.3 | — | 124.9 | |||
Torsion angle values for Aib residue in the αβ-turn is averaged over Aib residues 1, 3 and 5 and in the βα turn is averaged over the Aib residues 3, 5 and 7. Torsion angles of trans ACPC residue is averaged over residues 2, 4 and 6.
Torsion angle values for trans ACPC is averaged over residues 1 and 4, while Aib is averaged over residues 2 and 5.
Achiral peptide.
Torsion angles averaged over the 7 trans ACPC residues of the peptide from the N-terminus. C-terminal residue has been excluded as the helix frays at the C-terminus.
Torsion angles averaged over the residues in the two independent molecules in the crystal.
Table 2.
Hydrogen-bond parameters observed in crystallographically determined turn structures involving ω-amino acid residues. (Abbreviations are the same as given in table legend 1.)
| peptide sequence | turn type | turn residues | donor | acceptor | hydrogen bond parameters | |||
|---|---|---|---|---|---|---|---|---|
| O⋯H (Å) | N⋯O (Å) | C=O⋯H (deg.) | C=O⋯N (deg.) | |||||
| 11-membered hydrogen bonds | ||||||||
| Boc-(Aib- trans ACPC)3-OBz/Schmitt et al. (2005) | βα | trans ACPC2 Aib3 | trans ACPC4 NH | Aib 1 C=O | 2.03 | 2.84 | 137.5 | 145.1 |
| βα | trans ACPC4 Aib5 | trans ACPC6 NH | Aib 3 C=O | 2.00 | 2.85 | 132.9 | 136.3 | |
| βα | trans ACPC6 Aib7 | trans ACPC8 NH | Aib 5 C=O | 1.94 | 2.79 | 138.0 | 143.6 | |
| Boc-Val-Ala-Phe-Aib-βVal-βPhe-Aib-Val-Ala-Phe-Aib-OMe/Roy et al. (2004) | βα | β Phe6 Aib 7 | Val 8 NH | β Val 5 C=O | 2.57 | 3.05 | 122.5 | 137.0 |
| Boc-β-Gly-Aib-Leu-Aib-OMe/Banerjee et al. (2003) | βα | βGly1 Aib2 | Leu 3 NH | Boc 0 C=O | 2.20 | 3.02 | 131.8 | 137.1 |
| Boc-(trans ACPC-trans ACPC-Phe)2OMe/Schmitt et al. (2006) | βα | trans ACPC2 Phe 3 | trans ACPC4 NH | trans ACPC1 C=O | 2.17 | 2.93 | 150.7 | 160.8 |
| Boc-(trans ACPC-Aib-Aib)2-OMe/Schmitt et al. (2006) | βα | trans ACPC1 Aib2 | Aib 3 NH | Boc 0 C=O | 2.08 | 2.89 | 141.5 | 148.0 |
| βα | trans ACPC4 Aib5 | Aib 6 NH | Aib 3 C=O | 2.22 | 3.06 | 134.7 | 141.5 | |
| Boc-(Aib-trans ACPC)3-OBz/Schmitt et al. (2005) | αβ | Aib1 trans ACPC2 | Aib3 NH | Boc 0 C=O | 2.10 | 2.93 | 141.0 | 145.0 |
| αβ | Aib3 trans ACPC4 | Aib5 NH | trans ACPC2 C=O | 2.00 | 2.88 | 144.3 | 146.8 | |
| αβ | Aib5 trans ACPC6 | Aib7 NH | trans ACPC4 C=O | 2.03 | 2.91 | 150.4 | 149.7 | |
| Boc-(trans ACPC-Aib-Aib)2-OMe/Schmitt et al. (2006) | αβ | Aib3 trans ACPC4 | Aib2 NH | Aib5 C=O | 2.07 | 2.95 | 138.8 | 139.9 |
| Boc-Aib-Aib-βGly-NHMe/Pavone et al. (1992) | αβ | Aib 2 βGly 3 | NHMe NH | Aib1 C=O | 2.24 | 3.07 | 150.7 | 143.7 |
| MHA-MePro-Dec-HyLeu-Leu-Leu-Aib-Aib-βGly-DPDA/Cerrini et al. (1989) | αβ | Aib 8 βGly 9 | NHMe NH | Aib 7 C=O | 2.03 | 3.01 | 96.5 | 114.2 |
| Boc-Aib-βRes1-Aib-OMe/Tanaka et al. (2001) | αβ | Aib 1 βRes2 | Aib3 NH | Boc0 C=O | 1.99 | 2.94 | 158.5 | 163.1 |
| 2.07 | 3.08 | 165.8 | 164.4 | |||||
| 12-membered hydrogen bonds | ||||||||
| Boc-Aib-Gpn-Aib-Gpn-OMe/Ananda et al. (2005) | αγ | Aib1 Gpn2 | Aib3 NH | Boc0 C=O | 2.11 | 2.93 | 140.9 | 144.6 |
| γα | Gpn2 Aib3 | Gpn4 NH | Aib1 C=O | 2.19 | 3.05 | 138.0 | 139.8 | |
| Boc-Leu-Aib-Val-βGly-γAbu-Leu-Aib-Val-OMe/Karle et al. (1997) | γα | γAbu5 Leu6 | Aib7 NH | βGly4 C=O | 2.03 | 2.8 | 155.2 | 165.0 |
| Boc-Leu-U-V-βG-γAbu-Leu-Aib-Val-Ala-Leu-Aib-OMe/Karle et al. (1997) | γα | γAbu5 Leu6 | Aib7 NH | βGly4 C=O | 1.88 | 2.8 | 144.8 | 148.0 |
| Boc-γ-Abu-Aib-Ala-OMe/Maji et al. (2002) | γα | γ-Abu1 | Ala 3 | Boc 0 | 2.0 | 2.8 | 153.2 | 162.0 |
| Aib2 | NH | C=O | 2.1 | 2.9 | 155.1 | 163.0 | ||
| Boc-γ-Abu-Aib-Ala-Aib-OMe/Maji et al. (2002) | γα | γ-Abu1 Aib2 | Ala 3 NH | Boc 0 C=O | 2.2 | 3.1 | 151.4 | 159.7 |
| 12-membered hydrogen bonds | ||||||||
| Boc-(trans-ACPC)8-CO2CH2Ph/Appella et al. (1999c) | ββ | trans ACPC1 trans ACPC2 | trans ACPC 3 NH | Boc 0 C=O | 2.16 | 2.93 | 170.3 | 175.1 |
| ββ | trans ACPC2 trans ACPC3 | trans ACPC 4 NH | trans ACPC 1 C=O | 2.03 | 2.83 | 155.7 | 148.3 | |
| ββ | trans ACPC3 trans ACPC4 | trans ACPC 5 NH | trans ACPC 2 C=O | 2.02 | 2.83 | 152.8 | 149.7 | |
| ββ | trans ACPC4 trans ACPC5 | trans ACPC 6 NH | trans ACPC 3 C=O | 2.03 | 2.88 | 148.3 | 147.3 | |
| ββ | trans ACPC5 trans ACPC6 | trans ACPC 7 NH | trans ACPC4 C=O | 2.14 | 2.87 | 159.3 | 154.7 | |
| ββ | trans ACPC6 trans ACPC7 | trans ACPC 8 NH | trans ACPC5 C=O | 2.02 | 2.87 | 151.0 | 145.9 | |
| Boc-(trans ACPC-trans ACPC-Phe)2 OMe/Schmitt et al. (2006) | ββ | trans ACPC1 trans ACPC2 | Phe3 NH | Boc 0 C=O | 2.07 | 2.94 | 143.9 | 142.5 |
| ββ | trans ACPC4 trans ACPC5 | Phe6 NH | Phe3 C=O | 2.07 | 2.93 | 141.0 | 137.2 | |
| Boc-B-Ala-(R)-Nip-(S)- Nip-β-Ala-NHMe/Chung et al. (2000) | ββ | R-Nip2 S- Nip3 | B-Ala4 NH | B-Ala1 C=O | 2.11 | 2.92 | 153.6 | 161.5 |
| 13/14-membered hydrogen bonds | ||||||||
| Boc-Leu-Aib-Val-βGly-γAbu-Leu-Aib-Val-OMe/Karle et al. (1997) | βγ | βGly4 γAbu5 | Leu (6) NH | Val (3) C=O | 2.05 | 2.93 | 142.1 | 141.9 |
| Boc-Leu-Val-Val-DPro-δAva-Leu-Val-Val-OMe/Rai et al. (in press) | αδ | DPro4 δAva5 | Leu (6) NH | Val (3) C=O | 2.06 | 3.03 | 151.8 | 147.3 |
| Boc-(γ-Res C-γ-Res D)2 OBz/Seebach et al. (2001a) | γγ-helix | γ-ResC γ-Res D | γ-Res C NH | Boc (0) C=O | 2.09 | 2.89 | 154.4 | 170.4 |
| Boc-γ-Res A-γ-Res B-NHMe/Brenner & Seebach (2001) | γγ-hairpin | γ-ResA γ-Res B | NHMe NH | Boc (0) C=O | 2.04 | 3.24 | 150.6 | 166.2 |
Figure 9 illustrates examples of the superpositions of equivalent atoms of observed αβ/βα-turns with their α-peptide type I and type II turn counterparts. It is clearly evident that the overwhelming majority of C11-turns in table 1 corresponds to a one-atom expansion of the backbone of a helix promoting α-peptide turn. The only example of a variant β-turn analogue is the case of the αβ C11-turns observed in the tripeptide Boc–Aib–8-aminocyclooct-4-enecarboxylic acid–Aib–OMe (Tanaka et al. 2001). Interestingly, this achiral peptide crystal contains two independent molecules in the asymmetric unit, with Aib adopting an unusual, semiextended conformation in both the cases, compatible with its occurrence at the (i+1) position of a type II β-turn analogue. In comparing the αβ/βα-turns with their αα counterparts, the ϕ, ψ value of the α-residue appears to be indicative of the nature of the β-turn type.
Figure 9.
Superpositions of nine equivalent atoms (only Cβ of the β-residue is omitted) of observed αβ/βα-turns with their α-peptide type I and type II turn counterparts (ideal structures). Superposition of (a) type I β-turn and αβ (1) turn of Boc-(Aib-trans ACPC)4OBz, (b) type II β-turn and αβ (1) turn of Boc-(Aib-trans ACPC)4OBz, (c) type I β-turn and βα (1) turn of Boc-(Aib-trans ACPC)4OBz, (d) type II β-turn and βα (1) turn of Boc-(Aib-trans ACPC)4OBz, (e) type I β-turn and αβ-turn of molecule B of Boc–Aib–β-Res–Aib–OMe and (f) type II β-turn and αβ-turn of molecule B of Boc–Aib–β-Res–Aib–OMe (β-Res in E and F=8-aminocyclooct-4-ene carboxylic acid).
An inspection of the results summarized in table 1 reveals that crystallographically characterized examples of αβ (C11), αγ, ββ (C12), βγ, αδ (C13) and γγ (C14) are available. In the cases of hydrogen-bonded ring sizes of 12 atoms or greater, direct analogies with corresponding all α-amino acid turns are not immediately apparent. Within each category, families of turns distinguished by specific values of backbone torsion angles may be identified. For example, two distinct groups are observed in the case of the ββ C12-turn in which constrained β-residues are forced to adopt a θ value of approximately +90° while in the other θ is approximately 180°. The hydrogen-bond parameters in table 2 do not reveal any significant difference between the various types of hydrogen-bonded turns. Hydrogen-bond optimization thus appears to have been achieved in all cases.
5. Hydrogen-bonded turns as components of secondary structures
The following features emerge from the extensive literature on protein and peptide structures. Two-residue αα-turns in proteins may be classified into three distinct types as follows.
Helix promoting turns in which successive turn formation occurs and there is a shared residue between any pair of contiguous turns. For example, repetitive type III turns give rise to the 310-helix (note that the polypeptide 310 and the α-helical structures lie very close to one another in conformational space and are separated by relatively low barriers to interconversion).
Hairpin promoting turns in which a central two residue β-turn facilitates sharp chain reversal resulting in the formation of registered antiparallel strands which are held together by cross-strand hydrogen bonds. Analysis of protein structures in a large body of evidence accumulated from designed peptide structures establish that the prime turns I′/III′ and II′ promote hairpin formation when positioned centrally in sequences made up of l-amino acids.
Isolated turns which do not lie within secondary structures but permit a change of polypeptide chain direction, a property essential for the formation of globular compact structures. While all turn types can, in principle, occur as isolated structural features, attention may be drawn to the type II β-turn structure which cannot promote conventional helix formation and also does not readily permit registry of antiparallel strands. Figure 10 illustrates the nucleation of helices and hairpins by local turn formation in the α-polypeptide structures.
Figure 10.
Nucleation of helices and hairpins by local turn formation in α-polypeptide structures. Turns shown are idealized structures. The helix is a view of pBrBz-(Aib)11-OtBu (Gesman et al. 2003). The average ϕ, ψ values for the 310-helix are −52.4 and −31.1°.The hairpin is from the structure of Boc–Leu–Val–Val–dPro–Gly–Leu–Val–Val–OMe (Karle et al. 1996). The ϕ, ψ values at the turn residues are: ϕ(i+1)=62.5°, ψ(i+1)=−132.4°, ϕ(i+2)=−80.7° and ψ(i+2)=−3.4°. Side-chain atoms of the residues and protecting groups in the helix and hairpin have been deleted for clarity.
By analogy with the α-polypeptide structures, hybrid turns must also fall into distinct categories capable of generating hybrid helices or isolated chain reversals. Examination of available crystal structures of ω-amino acids containing peptides provides glimpses into the structural possibilities for creating new folded polypeptides. Hybrid turns have been characterized within the context of regular secondary structures. For example, insertion of two contiguous β-residues into the centre of a potentially helical host α-peptide in the sequence Boc–Val–Alu–Phe–Aib,βVal–βPhe–Aib–Val–Alu–Phe–Aib–OMe (Roy et al. 2004, figure 11), results in the formation of a C11 βα-turn between the CO of βVal 5 and NH of the Val 8 (table 1). Successive C11 H-bonded turns are observed in the alternating hybrid sequence Boc-(Aib- trans ACPC)4OBz (ACPC=trans-2-aminocyclopentanecarboxylic acid; Schmitt et al. 2005, figure 11). This structure corresponds to a C11-helix, which may be considered as an expanded version of the 310-helix formed by the insertion of one backbone atom at every alternate residue (table 1). The insertion of a βγ hybrid sequence into a host α-peptide helical sequence in the peptide, Boc–Leu–Aib–Val–βGly γAbu–Leu–Aib–Val–OMe and Boc–Leu–Aib–Val–βGly γAbu–Leu–Aib–Val–Alu–Leu–Aib–OMe (βGly=β-glycine, γAbu=γ-aminoisobutyric acid), results in the formation of C12-turns within the context of a helical structure (table 1, Karle et al. 1997). The C12-helix formed in the case of all β-peptide Boc-(trans ACPC)8-CO2CH2Ph, is also a 310-helix analogue with homologation at each position along the chain (table 1, figure 11, Appella et al. 1999c). Regular C12-helical structures can also be generated by (αγ)n sequences as illustrated by the crystal structures of Boc–Aib–Gpn–Aib–Gpn–OMe (Gpn=1-(aminomethyl)cyclohexane acetic acid; Ananda et al. 2005, table 1, figure 11). A βγ segment forming a C13 hydrogen bond has been characterized in the crystals of Boc–Leu–Aib–Val–βGly γAbu–Leu–Aib–Val–OMe and Boc–Leu–Aib–Val–βGly γAbu–Leu–Aib–Val–Alu–Leu–Aib–OMe (Karle et al. 1997, table 1).This hydrogen-bonded turn is formally equivalent to a three-residue ααα C13-turn. Seebach et al. (2001a) have established an incipient 2.614 (14-helix) in a protected tetrapeptide formed from 2,3,4-trisubstituted γ-amino acids (figure 11, Seebach et al. 2001a).The formation of the two consequtive C14 hydrogen-bonded turn is undoubtedly promoted by the substitution pattern along the backbone (R, R, R) which promotes adoption of the gauche (+)–gauche (+) conformations about the Cβ–Cγ (θ1) and Cα–Cβ(θ2) bonds of the γ-residue. Recent modelling studies suggest that the construction of regular hybrid helices varying in the nature of the repeating hydrogen-bonded turn are indeed stereochemically and energetically favourable (Ananda et al. 2005; Baldauf et al. 2006a,b). While crystallographic characterization of hybrid helices has been restricted to relatively short peptides it is clear that crystal structures of longer hybrid sequences will undoubtedly be forthcoming.
Figure 11.
Helices containing ω-amino acids. (a) Contiguous ββ segment inserted into a potential helical peptide made up of all α-amino acids (Boc–Val–Ala–Phe–Aib–βVal–βPhe–Aib–Val–Ala–Phe–Aib–OMe, Roy et al. 2004), (b) C11-helix formed in Boc-(Aib-trans-ACPC)4OBz (Schmitt et al. 2005), (c) C12-helix formed in Boc-(trans ACPC)8CO2CH2Ph (Appella et al. 1999c), (d) C12-helix in Boc–Aib–Gpn–Aib–Gpn–OMe (Ananda et al. 2005) and (e) C14-helix in Boc-(γ-ResC-γ-ResD−)2OBz. (γ-Res C=2,3,4-trimethyl, γ-aminoisobutyric acid; γ-Res D=2-isopropyl,3,4-dimethyl γ-aminoisobutyric acid; Seebach et al. 2001a). Side-chain atoms of the residues and protecting groups have been deleted for clarity.
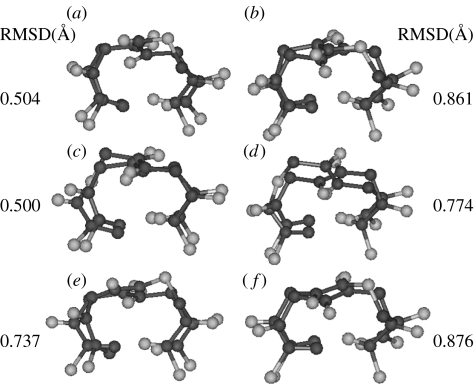
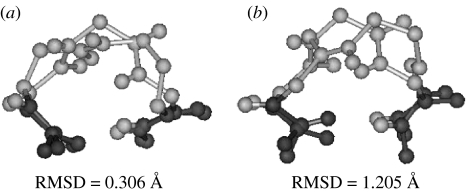
In the creation of hybrid structures which mimic the regular α-polypeptide conformation, the additional atoms inserted into the backbone can sometimes be accommodated as protrusions. In such cases, the terminal backbone atoms involved in providing the scaffold for the hydrogen bond remain roughly at the same locations. A superposition of the all α-residue turn with a hybrid peptide analogue results in a good superposition of eight backbone atoms in the N- and the C-terminal ends of the turns. Figure 12 illustrates this point. It is noteworthy that the βγ-turn observed in peptide Boc–Leu–Aib–Val–βGly γAbu–Leu–Aib–Val–Ala–Leu–Aib–OMe superposes very well (RMSD=0.306 Å) with the α-helical ααα-turn established in the model peptide Boc–Leu–Aib–Val–Ala–Leu–Aib–Val–Ala–Leu–Aib–OMe (Aravinda et al. 2004). In contrast the αδ-turn observed in the hairpin peptide Boc–Leu–Val–Val–dPro–δAva–Leu–Val–Val–OMe, which contains the same number of backbone atoms as the α-helical turn superposes poorly (RMSD=1.205 Å). Clearly of the two crystallographically characterized C13-turns, the βγ example corresponds to an analogy of the α-peptide helical turn, while the αδ example mimics the hairpin forming prime turns observed in αα sequence.
Figure 12.
Superposition of an all α-residue turn with hybrid peptide analogue. Four backbone atoms in the N(Cαi, Ci, Oi, N(i+1)) and C (C(i+2), O(i+2), N(i+3), Cα(i+3)) terminal ends of the turns are superposed from crystal structures. Superposition of (a) βγ (C13)-turn observed in peptide Boc–Leu–Aib–Val–βGly γAbu–Leu–Aib–Val–Alu–Leu–Aib–OMe (Karle et al. 1997) with ααα-turn established in the model α-helical peptide Boc–Leu–Aib–Val–Ala–Leu–Aib–Val–Ala–Leu–Aib–OMe (Aravinda et al. 2004) and (b) αδ-turn observed in the peptide Boc–Leu–Val–Val–dPro–δAva–Leu–Val–Val–OMe (Rai et al. in press) with an ααα (C13)-turn established in a model α-helical peptide Boc–Leu–Aib–Val–Ala–Leu–Aib–Val–Ala–Leu–Aib–OMe (Aravinda et al. 2004).
Hybrid turns can also facilitate antiparallel β-hairpin formation. Solution NMR studies have established β-hairpin structures in the sequences Boc–Leu–Val–Val–dPro–βPhe–Leu–Val–Val–OMe (Gopi et al. 2002), Boc–Leu–Phe–Val–dPro–βAc6c–Leu–Phe–Val–OMe and Boc–Leu–Phe–Val–dPro–Gpn–Leu–Phe–Val–OMe (Rai et al. in press). In these cases, the turns formed are of C11 (αβ)- or the C12 (αγ)-type with dPro at (i+1) position. The only crystallographically characterized β-hairpin with a central hybrid turn is in Boc–Leu–Val–Val–dPro–δAva–Leu–Val–Val–OMe (figure 13, table 1, Rai et al. in press). The hairpin forming hybrid turns may be considered as Type II′ β-turn homologues.
Figure 13.
Hybrid turns facilitate antiparallel sheet formation. Hairpin containing central αω segments (a) αβ-turn in Boc–Leu–Phe–Val–DPro-βAc6c-Leu–Phe–Val–OMe (Rai et al. in press), (b) αγ-turn in Boc–Leu–Phe–Val–DPro–Gpn–Leu–Phe–Val–OMe (Rai et al. in press), (c) αδ-turn in Boc–Leu–Val–Val–DPro–δAva–Leu–Val–Val–OMe (Rai et al. in press). (a) and (b) are NMR derived structures while (c) is determined by X-ray diffraction. Side-chains atoms of the residues in the hairpins and the protecting groups have been deleted for clarity.
6. Stereochemically constrained ω-amino acids in peptide design
The design of well defined conformations in short α-peptide sequences has been greatly facilitated by the use of stereochemically constrained amino acid residues. Local restrictions on the backbone conformational angles ϕ and ψ serve to nucleate specific structural features which in turn promote secondary structure formation. While a large number of modified residues have been used, this approach is best exemplified by α-aminoisobutyryl residue (Aib) which promotes helix nucleation and dPro residues which promotes prime turn formation resulting in the generation of hairpin structures (Venkatraman et al. 2001; Aravinda et al. 2003). Extension of this approach to the ω-amino acids is clearly warranted since the number of backbone torsional variables increase with the number of backbone atoms.
Figures 14 and 15 illustrate the structures of several β-amino acid residues in which rotation about the backbone torsion angles is restricted. For example, the Gellman group has successfully demonstrated structure formation in short β-peptides using the cyclic β-residues ACHC (trans-2-aminocyclohexanecarboxylic acid; Appella et al. 1996, 1999b) and ACPC (trans-2-aminocyclopentanecarboxylic acid; Appella et al. 1997, 1999c). In these cases, the constraints of cyclization restrict the values of θ to gauche conformations, which promote backbone folding. Nipecotic acid provides an example where the value of θ (approx. 180°) is restricted by cyclization to a value close to that required for an extended geometry (Chung et al. 1998, 2000). By analogy with the α-amino acids it is clear that substitution at backbone positions serves to restrict the range of accessible conformations. Substituent effects can be most readily understood in the case of α-amino acids by comparing the Ramachandran allowed regions for Gly, l-ala, Aib (Aravinda et al. 2003.). There is a progressive restriction in the accessible region of ϕ, ψ space upon backbone substitution. Similarly in β- and the higher ω-amino acid residues rotational restrictions about the flanking single bonds may be anticipated upon substitution at a specific position. The extensive literature on structure formation in peptides containing multiply substituted β- and γ-residues clearly illustrates the role of substituent effects (Hanessian et al. 1998; Rueping et al. 2002; Seebach et al. 2004). For example, Seebach and co-workers have established the effectiveness of α-, β-, γ-trisubstituted γ-amino acids in promoting folded structures in short sequences (Brenner & Seebach 2001; Seebach et al. 2001a). Figure 16 illustrates in interesting example of a hairpin conformation containing a δ-amino acid (sugar amino acid) at the (i+2) position of the β-turn (Grotenberg et al. 2004).
Figure 14.
Helix promoting β-amino acid residues. (a) 1-(Aminomethyl) cyclopropane carboxylic acid (Abele et al. 1999); (b) C-linked carbo-β-amino acid (Sharma et al. 2006); (c) cis-β-amino cyclobutanecarboxylic acid (Izquierdo et al. 2004); (d) cis-β-aminocycloproanecarboxylic acid (De Pol et al. 2004); (e) (2R,3S)-oxetane 3-amino-2-carboxylic acid (Claridge et al. 2001); (f) (S, S)-trans-ACPC (ACPC=trans-2-aminocyclopentanecarboxylic acid, θ=90°) (Appella et al. 1999c); (g) (R, R)-trans-ACPC (θ=−90°) (Appella et al. 1999c); (h) (S, S)-trans-ACHC (ACHC=trans-2-aminocyclohexanecarboxylic acid, θ=60°) (Appella et al. 1999b) and (i) (R, R)-trans-ACHC (θ=−60°) (Appella et al. 1999b).
Figure 15.
Constrained β-amino acids characterized in non-helical conformations. (a) (S)-(3-HProline (ϕ=−60°) (Seebach et al. 2004); (b) (R)-(3-HProline (ϕ=60°) (Seebach et al. 2004); (c) (R)-nipecotic acid (ϕ=100°–120 °, (=180 °) (Chung et al. 2001); (d) (S)-nipecotic acid (ϕ=−100° to −120°, θ=180°) (Chung et al. 2001); (e) (S, R)-trans-ACPC (Martinek et al. 2002); (f) 2-amino benzoic acid (θ=0°); (g) 3-amino benzoic acid (θ1=180°, θ2=180°) (Ramana Rao et al. 2003).
Figure 16.
(a) View of cyclo-(SAA–Val–Orn–Leu–DPhe–Pro–Val–Orn–Leu) (Grotenberg et al. 2004). (b) Central αδ-turn (C13) segment in Boc–Leu–Val–Val–DPro–δAva–Leu–Val–Val–OMe (Rai et al. in press).The sugar amino acid residue (SAA) 2,5-anhydro-6-azido-6 deoxy-4-o-pivaloyl-d-gluconic acid may be viewed in two ways. (c) As a δ-residue in which the α and the δ carbon atoms are covalently bridged by CHR–CHR segment. The oxygen atom occupies the γ position and (d) as an ϵ-amino acid residue disubstituted at the γ and δ positions with β and ϵ atoms covalently bridged by the heteroatom in the sugar. The side-chain atoms have been deleted for clarity.
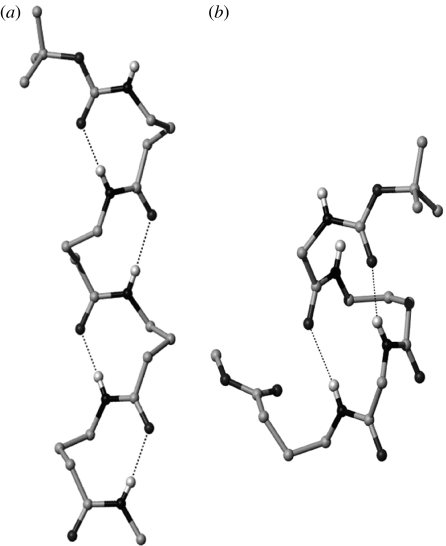
Ongoing work in this laboratory has focused on two achiral geminally disubstituted β- and γ-amino acids, β-Ac6c (1-aminocyclohexaneacetic acid) and Gpn (gabapentin, 1-(aminomethyl)cyclohexaneacetic acid; Ananda et al. 2003). These may be viewed as the homologues of the αα di-substituted residue Ac6c (1-aminocyclohexane-1-carboxylic acid; figure 17, Paul et al. 1986). Gabapentin is a widely used anti-epileptic agent (Chadwick et al. 1998; Sill 2006) which has also been advanced for the treatment of deep neuropathic pain (Rosenberg et al. 1997; Gilron & Flatters 2006). The availability of gabapentin as a bulk drug makes it an attractive starting point in the synthesis of designed hybrid peptides containing γ-residues. The geminal substituents at the central Cβ-atom restrict the values of θ1, θ2 to about ±60°. As a consequence, the gabapentin residue strongly promotes backbone folding with concomitant formation of intramolecular hydrogen bonds. Figure 18 illustrates two distinct structural motifs that have been characterized in Gpn peptides. In Gpn oligomers, the C9 ribbon (Vasudev et al. 2005) is formed which may be formally viewed as the expansion of the C7 ribbon or the 2.2helix in all α-polypeptides. In the alternating peptide Boc–Aib–Gpn–Aib–Gpn–OMe (Ananda et al. 2005), two consequtive C12-turns are formed constituting an incipient C12-helical (αγ)n structure. A large body of crystallographic observations on synthetic peptides carried out in this laboratory reveal that the Gpn residue is almost exclusively restricted to the gauche, gauche conformation about the Cα–Cβ and Cβ–Cγ bonds (P. G. Vasudev, N. Shamala & P. Balaram, unpublished result).
Figure 17.
Structures of Ac6c, β-Ac6c and Gpn.
Figure 18.
Two distinct structural motifs characterized in gabapentin peptides. (a) C9 ribbon (expansion of 2.27-helix) in Boc–Gpn–Gpn–Gpn–Gpn–NHMe (Vasudev et al. 2005) and (b) C12 helix (expansion of 310 (C10)-helix) in Boc–Aib–Gpn–Aib–Gpn–OMe (Ananda et al. 2005). Side-chain atoms of the residues have been deleted for clarity.
7. Conclusion
The growing body of structural studies on hybrid peptides containing α-, β-, γ- and δ-amino acid residues suggests that backbone modification of the backbone by the homologation of the chain vastly expands the repertoire of polypeptide structures. Contrary to original expectations, higher homologues of the α amino acid residues can be readily accommodated into well-folded secondary structures. Thus even unsubstituted β-, γ- and δ-residues have been shown to adopt folded conformations when incorporated into peptide sequences (Karle et al. 1997; Rai et al. in press). Undoubtedly the energy differences between the trans and the gauche forms of the homologues of α-amino acid residues is not large and can be readily offset by the compensating contributions of intramolecular hydrogen bonds. Diversity in peptide structures has traditionally being generated by varying the nature of the side chain in the α-amino acid residues. In hybrid peptides, an additional mode of generating molecular diversity becomes apparent. The enhanced stability of peptide bonds formed by ω-amino acids to proteolysis is an important consideration in the use of backbone-homologated amino acid residues in the design of peptide mimetics (Hintermann & Seebach 1997; Seebach et al. 1998b, 2001b; Frackenpohl et al. 2001; Porter et al. 2002, Gopi et al. 2003; Lelias & Seebach 2003; Hook et al. 2004; Schmitt et al. 2004). Conformational features in hybrid sequences can be established only in synthetic model peptides making the collection of structural data a slow process. This is in contrast to the case of α-polypeptides, where each structure determination of a naturally occurring protein provides an enormous wealth of conformational information. The propensity of higher homologues of α-amino acids to adopt folded conformations augurs well for the design of relatively long hybrid sequences, which may fold into globular structures, mimicking those formed in naturally occurring peptides/proteins. Clearly, the attributes of globularity and order can be achieved in polypeptides with non-α amino acid backbones. There is little doubt that many novel structural features remain to be established in hybrid peptide sequences. The availability of readily available, constrained ω-amino acids, of which gabapentin is an example, should greatly facilitate the task of rational design of predictably folded hybrid peptides.
Acknowledgments
The continued collaboration of Prof. N. Shamala and her group of the department of Physics, Indian Institute of Science and Dr I. L. Karle, Naval Research Laboratory, Washington, DC is gratefully acknowledged in the determination of the peptide crystal structures. This overview presents only a cursory survey of the vast body of work on peptides containing ω-amino acids and is not intended to be comprehensive. The insights provided from many uncited publications have contributed to the growth of the field. Work in this area in the authors' laboratory has been supported by a program granted in the area of Molecular Diversity and Design from the Department of Biochemistry, Government of India. S.C. acknowledges the grant of a Senior Research Fellowship from the Council of Scientific and Industrial Research, India.
References
- Abele S, Seiler P, Seebach D. Synthesis, crystal structures, and modelling of β-oligopeptides consisting of 1-(aminomethyl) cyclopropane-carboxylic acid: ribbon type arrangement of 8-membered hydrogen-bonded rings. Helv. Chim. Acta. 1999;82:1559–1571. doi:10.1002/(SICI)1522-2675(19991006)82:10<1559::AID-HLCA1559>3.0.CO;2-A [Google Scholar]
- Aleman C, Navas J.J, Munoz-Guerra S. Effect of the side group on the helix-forming tendency of α-alkyl-β-l-aspartyl residues. Biopolymers. 1997;41:721–729. doi:10.1002/(SICI)1097-0282(199706)41:7<721::AID-BIP2>3.0.CO;2-R [Google Scholar]
- Ananda K, Aravinda S, Vasudev P.G, Raja K.M.P, Sivaramakrishnan H, Nagarajan K, Shamala N, Balaram P. Stereochemistry of gabapentin and several derivatives: solid state conformations and solution equilibria. Curr. Sci. 2003;85:1002–1011. [Google Scholar]
- Ananda K, Vasudev P.G, Sengupta A, Raja K.M.P, Shamala N, Balaram P. Polypeptide helices in hybrid peptide sequences. J. Am. Chem. Soc. 2005;127:16 668–16 674. doi: 10.1021/ja055799z. doi:10.1021/ja055799z [DOI] [PubMed] [Google Scholar]
- Appella D.H, Christianson L.A, Karle I.L, Powell D.R, Gellman S.H. β-peptide foldamers: robust helix formation in a new family of β-amino acid oligomers. J. Am. Chem. Soc. 1996;118:13 071–13 072. doi:10.1021/ja963290l [Google Scholar]
- Appella D.H, Christianson L.A, Klein D.A, Powell D.R, Huang L, Barchi J.J, Gellman S.H. Residue based control of helix shape in β-peptide oligomers. Nature. 1997;387:381–384. doi: 10.1038/387381a0. doi:10.1038/387381a0 [DOI] [PubMed] [Google Scholar]
- Appella D.H, Barchi J.J, Durell S.R, Gellman S.H. Formation of short stable helices in aqueous solution by β-amino acid hexamers. J. Am. Chem. Soc. 1999a;121:2309–2310. doi:10.1021/ja983918n [Google Scholar]
- Appella D.H, Christianson L.A, Karle I.L, Powell D.R, Gellman S.H. Synthesis and characterization of trans-2-aminocyclohexanecarboxylic acid oligomers: an unnatural helical secondary structure and implications for β-peptide secondary structures. J. Am. Chem. Soc. 1999b;121:6206–6212. doi:10.1021/ja990748l [Google Scholar]
- Appella D.H, Christianson L.A, Klein D.A, Richards M.A, Powell D.R, Gellman S.H. Synthesis and structural characterization of helix forming β-peptides: trans-2-aminocyclopentanecarboxylic acid oligomers. J. Am. Chem. Soc. 1999c;121:7574–7581. doi:10.1021/ja991185g [Google Scholar]
- Aravinda S, Shamala N, Roy R.S, Balaram P. Non-protein amino acids in peptide design. Proc. Indian Acad. Sci. (Chem. Sci.) 2003;115:373–400. [Google Scholar]
- Aravinda S, Datta S, Shamala N, Balaram P. Hydrogen bond lengths in polypeptide helices: no evidence for short hydrogen-bonds. Angew. Chem. Int. Ed. 2004;43:6728–6731. doi: 10.1002/anie.200461127. doi:10.1002/anie.200461127 [DOI] [PubMed] [Google Scholar]
- Ashiuchi M, Misono H. Biochemistry and molecular genetics of poly-γ-glutamate synthesis. Appl. Microbiol. Biotechnol. 2002;59:9–14. doi: 10.1007/s00253-002-0984-x. doi:10.1007/s00253-002-0984-x [DOI] [PubMed] [Google Scholar]
- Baldauf C, Gunther R, Hofmann H.J. Theoretical prediction of basic helix types in α, β-hybrid peptides. Biopolymers. 2006a;84:408–413. doi: 10.1002/bip.20493. doi:10.1002/bip.20493 [DOI] [PubMed] [Google Scholar]
- Baldauf C, Gunther R, Hofmann H.J. Helix formation in α, γ- and β, γ-hybrid peptides: theoretical insights into mimicry of α- and β-peptides. J. Org. Chem. 2006b;71:1200–1208. doi: 10.1021/jo052340e. doi:10.1021/jo052340e [DOI] [PubMed] [Google Scholar]
- Banerjee A, Balaram P. Stereochemistry of peptides and polypeptides containing omega amino acids. Curr. Sci. 1997;73:1067–1077. [Google Scholar]
- Banerjee A, Pramanik A, Bhattacharjya S, Balaram P. Omega amino acids in peptide design, incorporation into helices. Biopolymers. 1996;39:769–777. doi: 10.1002/(SICI)1097-0282(199612)39:6%3C769::AID-BIP4%3E3.0.CO;2-T. doi:10.1002/(SICI)1097-0282(199612)39:6<769::AID-BIP4>3.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- Banerjee A, Maji S.K, Drew M.G.B, Halder D, Bannerjee A. Supramolecular peptide helix from novel double turn containing peptide containing a β-amino acid. Tetrahedron Lett. 2003;44:699–702. doi:10.1016/S0040-4039(02)02675-8 [Google Scholar]
- Bella J, Alemann C, Fernandez-Santin J, Alegre C, Subirana J.A. Conformation of the helical polyamide poly(α-isobutyl l-aspartate) Macromolecules. 1992;25:5225–5230. doi:10.1021/ma00046a018 [Google Scholar]
- Bragg L, Kendrew J.C, Perutz M.F. Polypeptide chain configurations in crystalline proteins. Proc. R. Soc. A. 1950;203:321–357. [Google Scholar]
- Brenner M, Seebach D. Design, synthesis, NMR-solution and x-ray crystal structure of N-acyl-γ-dipeptide amides that form a βII′-type turn. Helv. Chim. Acta. 2001;84:2155–2166. doi:10.1002/1522-2675(20010815)84:8<2155::AID-HLCA2155>3.0.CO;2-8 [Google Scholar]
- Cerrini S, Lamba D, Scatturin A, Rossi C, Ughetto G. The crystal and molecular structure of the α-helical nonapeptide antibiotic leucinostatin A. Biopolymers. 1989;28:409–420. doi: 10.1002/bip.360280138. doi:10.1002/bip.360280138 [DOI] [PubMed] [Google Scholar]
- Chadwick D.W, Anhut H, Greiner M.J, Alexander J, Murray G.H, Garofalo E.A, Pierce M.W. A double- trial of gabapentin monotherapy for newly diagnosed partial seizures. International gabapentin monotherapy study group. 945–77. Neurology. 1998;51:1282–1288. doi: 10.1212/wnl.51.5.1282. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran R, Lakshminarayanan A.V, Pandya U.V, Ramachandran G.N. Conformation of the ll and ld hairpin bends with internal hydrogen-bonds in proteins and peptides. Biochim. Biophys. Acta. 1973;303:14–27. doi: 10.1016/0005-2795(73)90143-8. [DOI] [PubMed] [Google Scholar]
- Cheng R.P, Gellman S.H, DeGrado W.F. β-peptides: from structure to function. Chem. Rev. 2001;101:3219–3232. doi: 10.1021/cr000045i. doi:10.1021/cr000045i [DOI] [PubMed] [Google Scholar]
- Chung Y.J, Christianson L.A, Stranger H.E, Powell D.R, Gellman S.H. A β-peptide reverse turn that promotes hairpin formation. J. Am. Chem. Soc. 1998;120:10 555–10 556. doi:10.1021/ja982249a [Google Scholar]
- Chung Y.J, Huck B.R, Christianson L.A, Stranger H.E, Krauthauser S, Powell D.R, Gellman S.H. Stereochemical control of hairpin formation in β-peptides containing di-nipecotic acid reverse turn segments. J. Am. Chem. Soc. 2000;122:3995–4004. doi:10.1021/ja993416p [Google Scholar]
- Claridge T.D.W, Goodman J.M, Moreno A, Angus D, Barker S.F, Taillefumier C, Watterson M.P, Fleet G.W.J. 10-Helical conformations in oxytane β-aminoacid hexamers. Tetrahedron Lett. 2001;42:4251–4255. doi:10.1016/S0040-4039(01)00661-X [Google Scholar]
- De Pol S, Zorn C, Klein C.D, Zerbe O, Reiser O. Surprisingly stable helical conformations in α/β-peptides by incorporation of cis-β-aminocyclopropane carboxylic acids. Angew. Chem. Int. Ed. 2004;43:511–514. doi: 10.1002/anie.200352267. doi:10.1002/anie.200352267 [DOI] [PubMed] [Google Scholar]
- Donohue J. Hydrogen bonded helical conformations of polypeptide chain. Proc. Natl Acad. Sci. USA. 1953;39:470–478. doi: 10.1073/pnas.39.6.470. doi:10.1073/pnas.39.6.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Santin J.M, Aymani J, Rodriguez-Galan A, Munoz-Guerra S, Subirana J.A. A pseudo-α-helix from poly-(α-isobutyl-L-aspartate), a nylon-3-derivative. Nature. 1984;311:53–54. doi:10.1038/311053a0 [Google Scholar]
- Frackenpohl J, Arvidsson P.I, Schreiber J.V, Seebach D. The outstanding biological stability of β- and γ-peptides toward proteolytic enzymes: an in vitro investigation with fifteen peptidases. ChemBioChem. 2001;2:445–455. doi: 10.1002/1439-7633(20010601)2:6<445::aid-cbic445>3.3.co;2-i. doi:10.1002/1439-7633(20010601)2:6<445::AID-CBIC445>3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- Gademann K, Hane A, Rueping M, Jaun B, Seebach D. The fourth helical secondary structure of β-peptides: the (P)-28-helix of a β-hexapeptide consisting of (2R,3S)-3-amino-2-hydroxy acid residues. Angew. Chem. Int. Ed. Engl. 2003;42:1534–1537. doi: 10.1002/anie.200250290. doi:10.1002/anie.200250290 [DOI] [PubMed] [Google Scholar]
- Gellman S.H. Foldamers: a manifesto. Acc. Chem. Res. 1998;31:173–180. doi:10.1021/ar960298r [Google Scholar]
- Gesman R, Bruchner H, Petratos K. Three complete turns of 310 helix at atomic resolution: the crystal structure of Z-(Aib)11OMe. J. Pept. Sci. 2003;9:753–762. doi: 10.1002/psc.490. doi:10.1002/psc.490 [DOI] [PubMed] [Google Scholar]
- Gilron I, Flatters S.J. Gabapentin and pregabalin for the treatment of neuropathic pain: a review of laboratory and clinical evidence. Pain Res. Manag. 2006;11(Suppl A):16A–29A. [Google Scholar]
- Glickson J.D, Applequist J. Conformation of poly-beta-alanine in aqueous solution from proton magnetic resonance and deuterium exchange studies. J. Am. Chem. Soc. 1971;93:3276–3281. doi: 10.1021/ja00742a030. doi:10.1021/ja00742a030 [DOI] [PubMed] [Google Scholar]
- Gopi H.N, Roy R.S, Raghothama S, Karle I.L, Balaram P. β-hairpins generated from hybrid peptide sequences containing both α- and β-amino acids. Helv. Chim. Acta. 2002;85:3313–3330. doi:10.1002/1522-2675(200210)85:10<3313::AID-HLCA3313>3.0.CO;2-P [Google Scholar]
- Gopi H.N, Ravindra G, Pal P.P, Pattanaik P, Balaram H, Balaram P. Proteolytic stability of β-peptide bonds probed using quenched fluorescent substate incorporating a heamoglobin cleavage site. FEBS Lett. 2003;535:175–178. doi: 10.1016/s0014-5793(02)03885-1. doi:10.1016/S0014-5793(02)03885-1 [DOI] [PubMed] [Google Scholar]
- Grotenberg G.M, Timmer M.S.M, Llamas-Siaz A.L, Verdoes M, van der Marel G.A, van Raaij M.J, Overkleeft H.S, Overhand M. An unusual reverse turn structure adopted by a furanoid sugar amino acid incorporated in gramicidin S. J. Am. Chem. Soc. 2004;126:3444–3446. doi: 10.1021/ja0397254. doi:10.1021/ja0397254 [DOI] [PubMed] [Google Scholar]
- Gunasekaran K, Gomathi L, Ramakrishnan C, Chandrasekhar J, Balaram P. Conformational interconversions in peptide β-turns: analysis of turns in proteins and computational estimates of barriers. J. Mol. Biol. 1998;284:1505. doi: 10.1006/jmbi.1998.2154. doi:10.1006/jmbi.1998.2154 [DOI] [PubMed] [Google Scholar]
- Hagihara M, Anthony N.J, Stout T.J, Clardy J, Schreiber S.L. Vinylogous polypeptides: an alternative peptide backbone. J. Am. Chem. Soc. 1992;114:6568–6570. doi:10.1021/ja00042a052 [Google Scholar]
- Hanby W.E, Rydon H.N. The capsule substance of Bacillus anthracis. Biochem. J. 1946;40:297–309. [PubMed] [Google Scholar]
- Hanessian S, Luo X, Schaum R, Michnick S. Design of secondary structures in unnatural peptides: stable helical γ-tetra-, hexa-, and octapeptides and consequences of α-substitution. J. Am. Chem. Soc. 1998;120:8569–8570. doi:10.1021/ja9814671 [Google Scholar]
- Hill D.J, Mio M.J, Prince R.B, Hughes T.S, Moore J.S. A field guide to foldamers. Chem. Rev. 2001;101:3893–4011. doi: 10.1021/cr990120t. doi:10.1021/cr990120t [DOI] [PubMed] [Google Scholar]
- Hintermann T, Seebach D. The biological stability of β-peptides: no interactions between α- and β-peptidic structures. Chimia. 1997;51:244–247. [Google Scholar]
- Hook D, Gessier F, Noti C, Kast P, Seebach D. Probing the proteolytic stability of β-peptides containing α-fluoro- and α-hydroxy-β-amino acids. ChemBioChem. 2004;5:691–706. doi: 10.1002/cbic.200300827. doi:10.1002/cbic.200300827 [DOI] [PubMed] [Google Scholar]
- Huggins M.L. X-ray studies of the structure of compounds of biochemical interest. Annu. Rev. Biochem. 1942;11:27–50. doi:10.1146/annurev.bi.11.070142.000331 [Google Scholar]
- Huggins M.L. The structure of fibrous proteins. Chem. Rev. 1943;32:195. doi:10.1021/cr60102a002 [Google Scholar]
- Huggins M.L. Polypeptide helices in proteins. J. Am. Chem. Soc. 1952a;74:3963–3963. doi:10.1021/ja01135a529 [Google Scholar]
- Huggins M.L. Coordinates of the 11-atom ring polypeptide helix. J. Am. Chem. Soc. 1952b;74:3963. doi:10.1021/ja01135a530 [Google Scholar]
- Ienger P, Joshi N.V, Balaram P. Conformational and sequence signatures in β-helix proteins. Structure. 2006;14:529–542. doi: 10.1016/j.str.2005.11.021. doi:10.1016/j.str.2005.11.021 [DOI] [PubMed] [Google Scholar]
- Izquierdo S, Kogan M.J, Parella T, Moglioni A.G, Branchadell V, Giralt E, Ortuño R.M. Helical folding in a cyclobutane containing β-tetrapeptide. J. Org. Chem. 2004;69:5093–5099. doi: 10.1021/jo0497555. doi:10.1021/jo0497555 [DOI] [PubMed] [Google Scholar]
- Jimenez A.I, Ballano G, Cativiela C. First observation of two consecutive γ turns in a crystalline linear dipeptide. Angew. Chem. Int. Ed. 2004;396:399. doi: 10.1002/anie.200461230. doi:10.1002/anie.200461230 [DOI] [PubMed] [Google Scholar]
- Karle I.L, Balaram P. Structural characteristics of α-helical peptide molecules containing Aib residues. Biochemistry. 1990;29:6747–6756. doi: 10.1021/bi00481a001. doi:10.1021/bi00481a001 [DOI] [PubMed] [Google Scholar]
- Karle I.L, Awasthi S.K, Balaram P. A designed β-hairpin peptide in crystals. Proc. Natl Acad. Sci. USA. 1996;93:8189–8193. doi: 10.1073/pnas.93.16.8189. doi:10.1073/pnas.93.16.8189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle I.L, Pramanik A, Bannerjee A, Bhattacharya S, Balaram P. Omega amino acids in peptide design: crystal structures and solution conformations of a peptide helix containing a β-alanyl-γ-aminobutyryl segment. J. Am. Chem. Soc. 1997;119:9087–9095. doi:10.1021/ja970566w [Google Scholar]
- Kimmerlin T, Seebach D. 100 years of peptide synthesis: ligation methods for peptide and protein synthesis with applications to β-peptide assemblies. J. Pept. Res. 2005;65:229–260. doi: 10.1111/j.1399-3011.2005.00214.x. doi:10.1111/j.1399-3011.2005.00214.x [DOI] [PubMed] [Google Scholar]
- Lelias G, Seebach D. Synthesis, CD spectra, and enzymatic stability of β2-oligoazapeptides prepared from (S)-2-hydrazino carboxylic acids carrying the side chains of Val, Ala, and Leu. Helv. Chim. Acta. 2003;86:4152–4168. doi:10.1002/hlca.200390342 [Google Scholar]
- Lelias G, Seebach D. β2 amino acid syntheses, occurence in natural products, and components of β-peptides 1, 2. Biopolymers. 2004;76:206–243. doi: 10.1002/bip.20088. doi:10.1002/bip.20088 [DOI] [PubMed] [Google Scholar]
- Lopez-Carrasquero F, Aleman C, Munoz-Guerra S. Conformational analysis of helical poly(β-l-aspartate)s by IR dichroism. Biopolymers. 1995;36:263–271. doi: 10.1002/bip.360360302. doi:10.1002/bip.360360302 [DOI] [PubMed] [Google Scholar]
- Lovinger A.J. Crystallographic factors affecting the structure of polymeric spherulites. II. X-ray diffraction analysis of directionally solidified polyamides and general conclusions. J. Appl. Phys. 1978;49:5014–5028. doi:10.1063/1.324448 [Google Scholar]
- Lewis P.N, Momany F.A, Scheraga H.A. Chain reversals in proteins. Biochim. Biophys. Acta. 1973;303:211–229. doi: 10.1016/0005-2795(73)90350-4. [DOI] [PubMed] [Google Scholar]
- Maji S.K, Bannerjee R, Velumurugan D, Razak A, Fun H.K, Banerjee A. Peptide design using ω-amino acids: unusual turn structures nucleated by an N-terminal single γ-aminoisobutyric acid residue in short model peptides. J. Org. Chem. 2002;67:633–639. doi: 10.1021/jo010314k. doi:10.1021/jo010314k [DOI] [PubMed] [Google Scholar]
- Martinek T.A, Toth G.K, Vass E, Hollosi M, Fulop F. Cis-2-aminocyclopentanecarboxylic acid oligomers adopt a sheetlike structure: switch from helix to nonpolar strand. Angew. Chem. Int. Ed. 2002;41:1718–1721. doi: 10.1002/1521-3773(20020517)41:10<1718::aid-anie1718>3.0.co;2-2. doi:10.1002/1521-3773(20020517)41:10<1718::AID-ANIE1718>3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- Mathews B.W. The γ-turn. Evidence for a new folded conformation in proteins. Macromolecules. 1972;5:818–819. doi:10.1021/ma60030a031 [Google Scholar]
- Milner-White E.J, Poet R. Four classes of beta-hairpins in proteins. Biochem. J. 1986;240:289–292. doi: 10.1042/bj2400289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-White E.J, Poet R. Loops, bulges, turns and hairpins in proteins. Trends Biochem. Sci. 1987;12:189–192. doi:10.1016/0968-0004(87)90091-0 [Google Scholar]
- Milner-White E.J, Ross B.M, Ismail R, Belhadj-Mostefa K, Poet R. One type of gamma turn, than the other gives rise to chain reversal in proteins. J. Mol. Biol. 1988;204:777–782. doi: 10.1016/0022-2836(88)90368-3. doi:10.1016/0022-2836(88)90368-3 [DOI] [PubMed] [Google Scholar]
- Munoz-Guerra S, Fernandez Santin J.M, Rodriguez-Galan A, Subirana J.A. Structural studies on the polymorphism of nylon-3. J. Polym. Sci. 1985;23:733–742. doi:10.1002/pol.1985.180230409 [Google Scholar]
- Nataraj D.V, Srinivasan N, Sowdhamini R, Ramakrishnan C. Turns in protein structure. Curr. Sci. 1995;69:434–437. [Google Scholar]
- Navas J.J, Aleman C, Lopez-Carrasquero F, Munoz-Guerra S. Analysis of the helical conformations in poly(beta-l-aspartate)s: poly(alpha-N-butyl beta-l-aspartate) and poly[alpha(2-methoxyethyl)beta-l-aspartate] Macromolecules. 1995;28:4487–4494. doi:10.1021/ma00117a017 [Google Scholar]
- Pavone L, et al. β-Alanine and β-bends. X-ray diffraction structures of three linear oligopeptides. J. Chem. Soc. Perkin Trans. 1992;8:1233. [Google Scholar]
- Pavone V, Gaeta G, Lombardi A, Nastri F, Maglio O, Isernia C, Saviano M. Discovering protein secondary structures: classification and description of isolated α-turns. Biopolymers. 1996;38:723–732. doi: 10.1002/(SICI)1097-0282(199606)38:6%3C705::AID-BIP3%3E3.0.CO;2-V. doi:10.1002/bip.360360606 [DOI] [PubMed] [Google Scholar]
- Paul P.K.C, Sukumar M, Bardi M, Piazzesi A.M, Valle G, Toniolo C, Balaram P. Stereochemically constrained peptides. Theoretical studies on the conformations of peptides containing 1-aminocyclohexanecarboxlic acid. J. Am. Chem. Soc. 1986;108:6363–6370. doi:10.1021/ja00280a038 [Google Scholar]
- Pauling L, Corey R.B. The planarity of the amide group in polypeptide. J. Am. Chem. Soc. 1952;74:3964–3964. doi:10.1021/ja01135a531 [Google Scholar]
- Pauling L, Corey R.B, Branson H.R. The structure of proteins; two hydrogen-bonded helical configuration of polypeptide chain. Proc. Natl Acad. Sci. USA. 1951;37:205–211. doi: 10.1073/pnas.37.4.205. doi:10.1073/pnas.37.4.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter E.A, Weisblum B, Gellman S.H. Mimicry of host-defense peptides by unnatural oligomers: antimicrobial β-peptides. J. Am. Chem. Soc. 2002;7324:7330. doi: 10.1021/ja0260871. doi:10.1021/ja0260871 [DOI] [PubMed] [Google Scholar]
- Prasad B.V.V, Balaram P. The stereochemistry of α-aminoisobutyric acid containing peptides. CRC Crit. Rev. Revs. Biochem. 1984;16:307–347. doi: 10.3109/10409238409108718. [DOI] [PubMed] [Google Scholar]
- Puiggali J, Munoz Guerra S. Extended-chain and three-fold helical forms of poly(glycyl-β-alanine) Macromolecules. 1986;19:1119–1124. doi:10.1021/ma00158a031 [Google Scholar]
- Pulla Rao Ch, Nagaraj R, Rao C.N.R, Balaram P. Infrared studies on conformation of synthetic alamethicin fragments and model peptides containing α-aminoisobutyric acid. FEBS Lett. 1979;100:244–248. doi: 10.1021/bi00544a004. doi:10.1016/0014-5793(79)80343-9 [DOI] [PubMed] [Google Scholar]
- Rai, R., Vasudev, P., Ananda, K., Raghothama, S., Shamala, N., Karle, I. L. & Balaram, P. In press. Hybrid peptides: expanding the β-turn in peptide hairpins by insertion of β-, γ- and δ-residues. Chem. Eur. J [DOI] [PubMed]
- Ramachandran G.N, Sasisekharan V. Conformation of polypeptides and proteins. Adv. Protein Chem. 1968;23:283–438. doi: 10.1016/s0065-3233(08)60402-7. [DOI] [PubMed] [Google Scholar]
- Ramachandran G.N, Ramakrishnan C, Sasisekharan V. Stereochemistry of polypeptide chain configurations. J. Mol. Biol. 1963;7:95–99. doi: 10.1016/s0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- Ramana Rao M.H.V, Kiran Kumar S, Kunwar A.C. Formation of β-hairpins in l-Pro-Gly containing peptides facilitated by 3-amino benzoic acid. Tetrahedron Lett. 2003;44:7369–7372. doi:10.1016/S0040-4039(03)01132-8 [Google Scholar]
- Richardson J.S. The anatomy and taxonomy of protein structure. Adv. Protein Chem. 1981;34:164–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Rose G.D, Gierasch L.M, Smith J.A. Turns in peptides and proteins. Adv. Protein Chem. 1985;37:1–109. doi: 10.1016/s0065-3233(08)60063-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg J.M, Harrell C, Ristic H, Werner R.A, de Rosayro A.M. The effect of gabapentin on neuropathic pain. Clin. J. Pain. 1997;13:251–255. doi: 10.1097/00002508-199709000-00011. doi:10.1097/00002508-199709000-00011 [DOI] [PubMed] [Google Scholar]
- Roy, R. S. 2005 Conformational analysis of designed alpha-omega hybrid peptides. Ph.D. thesis, Indian Institute of Science, Bangalore, India.
- Roy R.S, Balaram P. Conformational properties of hybrid peptides containing α-and ω-amino acids. J. Pept. Res. 2004;61:274–289. doi: 10.1111/j.1399-3011.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- Roy R.S, Karle I.L, Raghothama S, Balaram P. Alpha, beta hybrid peptides: a polypeptide helix with central unit containing two consequtive β-amino acid residues. Proc. Natl Acad. Sci. USA. 2004;101:16 478–16 482. doi: 10.1073/pnas.0407557101. doi:10.1073/pnas.0407557101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueping M, Schreiber J.V, Lelias G, Jaun B, Seebach D. Mixed β2/β3-hexapeptides and β2/β3-nonapeptides folding to (P)-helices with alternating twelve and ten membered hydrogen-bonded rings. Helv. Chim. Acta. 2002;85:2577–2593. doi:10.1002/1522-2675(200209)85:9<2577::AID-HLCA2577>3.0.CO;2-D [Google Scholar]
- Rydon D.N. Polypeptides. Part X. The optical rotatory dispersion of poly γ-glutamic acid. J. Chem. Soc. 1964:1328–1333. doi:10.1039/jr9640001328 [Google Scholar]
- Schmitt M.A, Weisblum B, Gellman S.H. Unexpected relationships between structure and function in x, β-peptides: antimicrobial foldamers with heterogeneous backbones. J. Am. Chem. Soc. 2004;126:6848–6849. doi: 10.1021/ja048546z. doi:10.1021/ja048546z [DOI] [PubMed] [Google Scholar]
- Schmitt M.A, Choi S.H, Guzei I.A, Gellman S.H. Residue requirements for helical folding in short α/β-peptides: crystallographic characterization of the 11-helix in an optimized sequence. J. Am. Chem. Soc. 2005;127:13 130–13 131. doi: 10.1021/ja0536163. doi:10.1021/ja0536163 [DOI] [PubMed] [Google Scholar]
- Schmitt M.A, Choi S.H, Guzei I.A, Gellman S.H. New helical foldamers: heterogeneous backbones with 1:2 and 2:1 α:β amino acid residue patterns. J. Am. Chem. Soc. 2006;128:4538–4539. doi: 10.1021/ja060281w. doi:10.1021/ja060281w [DOI] [PubMed] [Google Scholar]
- Seebach D, Matthews J.L. β-peptides: a surprise at every turn. Chem. Commun. 1997a:2017. [Google Scholar]
- Seebach D, Matthews J.L. Mixed β-peptides: a unique helical secondary structure in solution. Chem. Commun. 1997b:2015–2022. doi:10.1039/a704933a [Google Scholar]
- Seebach D, Ciceri P.E, Overhand M, Jaun B, Rigo D, Oberer L, Hommel U, Amstutz R, Widmer H. Probing the helical secondary structure of short-chain β-peptides. Helv. Chim. Acta. 1996a;79:2043–2066. doi:10.1002/hlca.19960790802 [Google Scholar]
- Seebach D, Overhand M, Kühnle F.N.M, Martinoni B, Oberer L, Hommel U, Widmer H. β-peptides: synthesis by Arndt–Eistert homologation with concomitant peptide coupling. Structure determination by NMR and CD spectroscopy and by X-ray crystallography. Helical secondary structure of a β-hexapeptide in solution and its stability towards pepsin. Helv. Chim. Acta. 1996b;79:913–941. doi:10.1002/hlca.19960790402 [Google Scholar]
- Seebach D, Gademann K, Schreiber J.V, Matthews J.L, Hintermann T, Jaun B, Oberer L, Hommel U, Widmer H. Mixed’ β-peptides: a unique helical secondary structure in solution. Preliminary communication. Helv. Chim. Acta. 1997;80:2033–2038. doi:10.1002/hlca.19970800703 [Google Scholar]
- Seebach D, et al. β2- and β3-peptides with proteinaceous side chains: synthesis and solution structures of constitutional isomers, a novel helical secondary structure and the influence of solvation and hydrophobic interactions on folding. Helv. Chim. Acta. 1998a;81:932–981. doi:10.1002/hlca.19980810513 [Google Scholar]
- Seebach D, et al. Biological and pharmacokinetic studies with beta -peptides. Chimia. 1998b;52:734–739. [Google Scholar]
- Seebach D, Abele S, Sifferlen T, Hänggi M, Gruner S, Seiler P. Preparation and structure of β-peptides consisting of geminally disubstituted β2,2-and β3,3-amino acids: a turn motif for β-peptides. Helv. Chim. Acta. 1998c;81:2218–2243. doi:10.1002/(SICI)1522-2675(19981216)81:12<2218::AID-HLCA2218>3.0.CO;2-0 [Google Scholar]
- Seebach D, Brenner M, Rueping M, Schwirzer B, Jaun B. Preparation and determination of X-ray crystal and NMR solution structures of γ2,3,4-peptides. Chem. Commun. 2001a;4:207–208. doi:10.1039/b008377l [Google Scholar]
- Seebach D, Rueping M, Arvidsson P.I, Kimmerlin T, Micuch P, Noti C, Langenegger D, Hoyer D. Linear, peptidase-resistant β2/β3-di- and α/β3-tetrapeptide derivatives with nanomolar affinities to a human somatostatin receptor. Helv. Chim. Acta. 2001b;84:3503–3510. doi:10.1002/1522-2675(20011114)84:11<3503::AID-HLCA3503>3.0.CO;2-A [Google Scholar]
- Seebach D, Beck A.K, Bierbaum D.J. The world of β- and γ-peptides comprised of homologated proteinogenic amino acids and other components. Chem. Biodivers. 2004;1:1111–1239. doi: 10.1002/cbdv.200490087. doi:10.1002/cbdv.200490087 [DOI] [PubMed] [Google Scholar]
- Seebach D, Hook D.F, Glattli A. Helices and other secondary structures of beta and gamma peptides. Biopolymers. 2006;84:23–37. doi: 10.1002/bip.20391. doi:10.1002/bip.20391 [DOI] [PubMed] [Google Scholar]
- Sharma G.V.M, Jadhav V.B, Ramakrishna K.V.S, Jayaprakash P, Narsimulu K, Subash V, Kunwar A.C. 12/10- and 11/13-mixed helices in x/γ- and β/γ-hybrid peptides containing C-linked carbo-γ-amino acids with alternating x- and γ-amino acids. J. Am. Chem. Soc. 2006;128:14 657–14 668. doi: 10.1021/ja064875a. doi:10.1021/ja064875a Web release date: 21-Oct-2006. [DOI] [PubMed] [Google Scholar]
- Sill G.J. The mechanism of action of gabapentin and pregabalin. Curr. Opin. Pharmacol. 2006;6:103–113. doi: 10.1016/j.coph.2005.11.003. doi:10.1016/j.coph.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Singh, S. K. 2001 Studies on triosephosphate isomerase and related (β/α) barrell proteins. Ph.D. thesis, Indian Institute of Science, Bangalore, India.
- Tanaka M, Oba M, Ichiki T, Seumune H. Solid-state conformation of a hybrid tripeptide between β-amino acid; 8-aminocyclooct-4-enecarboxylic acid and 2- aminoisobutyric acid. Chem. Pharm. Bull. 2001;49:1178–1181. doi: 10.1248/cpb.49.1178. doi:10.1248/cpb.49.1178 [DOI] [PubMed] [Google Scholar]
- Toniolo C, Benedetti E. The polypeptide 310-helix. Trends Biochem. Sci. 1991;16:350–353. doi: 10.1016/0968-0004(91)90142-i. doi:10.1016/0968-0004(91)90142-I [DOI] [PubMed] [Google Scholar]
- Vasudev P.G, Ananda K, Shamala N, Balaram P. C9 helices and ribbons in γ-peptides: crystal structures of gabapentin oligomers. Angew. Chem. Int. Ed. 2005;44:4972–4975. doi: 10.1002/anie.200501055. doi:10.1002/anie.200501055 [DOI] [PubMed] [Google Scholar]
- Venkatachalam C.M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968;6:1425–1436. doi: 10.1002/bip.1968.360061006. doi:10.1002/bip.1968.360061006 [DOI] [PubMed] [Google Scholar]
- Venkatraman J, Shankaramma S.C, Balaram P. Design of folded peptides. Chem. Rev. 2001;101:3131–3152. doi: 10.1021/cr000053z. doi:10.1021/cr000053z [DOI] [PubMed] [Google Scholar]
- Wilmot C.M, Thornton J.M. Analysis and predictions of different types of β-turns in proteins. J. Mol. Biol. 1988;203:221–232. doi: 10.1016/0022-2836(88)90103-9. doi:10.1016/0022-2836(88)90103-9 [DOI] [PubMed] [Google Scholar]
- Wolfe M.S, Citron M, Diehl T.S, Xia W.M, Donkar I.O, Selkoe D.J. A substrate based difluoro ketone selectively inhibits alzheimers γ-secretase activity. J. Med. Chem. 1998;41:6–9. doi: 10.1021/jm970621b. doi:10.1021/jm970621b [DOI] [PubMed] [Google Scholar]