Abstract
Nature provides a wide range of materials with different functions and which may serve as a source of bio-inspiration for the materials scientist. The article takes the point of view that a successful translation of these ideas into the technical world requires more than the observation of nature. A thorough analysis of structure-function relations in natural tissues must precede the engineering of new bio-inspired materials. There are, indeed, many opportunities for lessons from the biological world: on growth and functional adaptation, about hierarchical structuring, on damage repair and self-healing. Biomimetic materials research is becoming a rapidly growing and enormously promising field. Serendipitous discovery from the observation of nature will be gradually replaced by a systematic approach involving the study of natural tissues in materials laboratories, the application of engineering principles to the further development of bio-inspired ideas and the generation of specific databases.
Keywords: bionic, bio-inspired, adaptive, self-healing, hierarchical materials
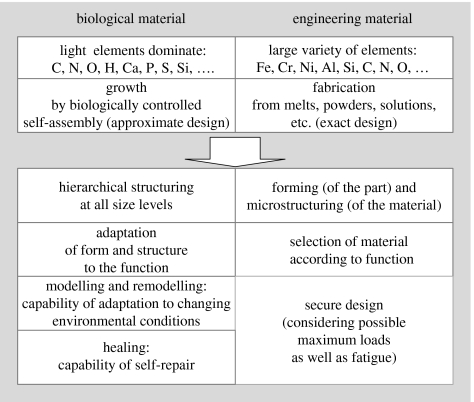
Biological materials constitute most of the body of plants and animals around us. They allow cells to function, eyes to capture and interpret light, plants to stand up to the light and animals to move or fly. This multitude of solutions has always inspired mankind to make materials and devices, which simplify many of our day-to-day functions. Biological structures are a constant source of inspiration for solving a variety of technical challenges in architecture (Kemp 2004), aerodynamics and mechanical engineering (Nachtigall 1998), as well as in materials science (Jeronimidis & Atkins 1995). Natural materials consist of relatively few constituent elements, which are used to synthesize a variety of polymers and minerals. On the contrary, human history is characterized by the use of many more elements. This led to the invention of materials with special properties, which are not used by nature. The ages of copper, bronze and iron were later followed by the industrial revolution based on steel and the information age based on silicon semiconductors. All these technical materials require high temperatures for fabrication and biological organisms have no access to them. Nevertheless, nature has developed—with comparatively poor base substances—a range of materials with remarkable functional properties. The key is a complex, often hierarchical, structuring of the natural materials (Lakes 1993; Tirrell 1994; Jeronimidis & Atkins 1995; Currey 2005), which results from the fact that natural materials grow according to a recipe stored in the genes, rather than being fabricated according to an exact design (figure 1).
Figure 1.
Biological and engineering materials are governed by a very different choice of base elements and by a different mode of fabrication. As a result, different strategies have to be pursued to achieve the desired functionality (below the arrow).
1. How can we learn from nature?
The design strategies of biological materials are not immediately applicable to the design of new engineering materials, since there are some remarkable differences between the strategies common in engineering and those used by nature (figure 1). The first major difference is in the range of choice of elements, which is far greater for the engineer. Elements such as iron, chromium and nickel are very rare in biological tissues and certainly not used in metallic form, as would be the case for steel. Iron is found in red blood cells, for instance, as an ion bound to the protein haemoglobin and its function is certainly not mechanical but rather to bind oxygen. Most of the structural materials used by nature are polymers or composites of polymers and ceramic particles. Such materials would generally not be the first choice of an engineer to build strong and long-lasting mechanical structures. Nevertheless, nature uses them to build trees and skeletons. The second major difference is the way in which materials are made. While the engineer selects a material to fabricate a part according to an exact design, nature goes the opposite way and grows both the material and the whole organism (a plant or an animal) using the principles of (biologically controlled) self-assembly. This provides control over the structure of the material at all levels of hierarchy and is certainly a key to the successful use of polymers and composites as structural materials.
Bio-inspiration is not just a consequence of an observation of naturally occurring structures. The reason is that nature has a multitude of boundary conditions which we do not know a priori and which might all be important for the development of the structure observed. Therefore, we need to keep our eyes open and must be able to solve a particular problem set. Both the biological structure and the set of problems the structure is designed to solve can bio-inspire us. For example, if we consider the structure of our own femoral head to be a solution for a mechanical optimization problem (as hypothesized in the so-called Wolff law; Wolff 1892; Frost 2005), questions still remain like which mechanical property has been optimized (stiffness, toughness and defect tolerance) and what the possible influence of other boundary conditions is. It is well known that bone is also the body's ion reservoir and serves the calcium homeostasis. Rik Huiskes (2000) phrased the question, ‘If bone is the answer, what is the question?’. It is quite true that the structures we observe are probably good solutions found by a long adaptation process during evolution. Unfortunately, we do not exactly know which problem has been solved. It may be just to provide a strong material and also to meet some quite different biological constraints. This implies that we may not succeed if we follow without modifications the solutions found by nature as optimal for a certain unknown requirement. So, we have to carefully study the biological system and understand the structure–function relationship of the biological material in the context of its physical and biological constraints. Careful investigation of a biological system serving as the model is necessary for biomimetic materials research.
2. Growth and functional adaptation
Growth is a process that can be influenced by the external conditions including temperature, mechanical loading, and supply of light, water or nutrition. A living organism must necessarily possess the ability of adaptation to external needs, while possible external influences on a technical system must be typically anticipated in its design, often leading to considerable ‘over-design’ (figure 1). This aspect of functional adaptation is particularly fascinating for the materials scientist, since several undiscovered solutions of nature can serve as sources of inspiration. The subject was pioneered by D'Arcy Wentworth Thompson whose classical book in 1919 (with a second volume in 1942) ‘On Growth and Form’ was republished several times later (Thompson D'Arcy W 1992). This early text mostly relates the ‘form’ (or shape) of biological objects to their function. Even earlier, the relationships between anatomy (i.e. structure) and function of living systems had been explored by Leonardo da Vinci (1952) and Galileo Galilei (2005).
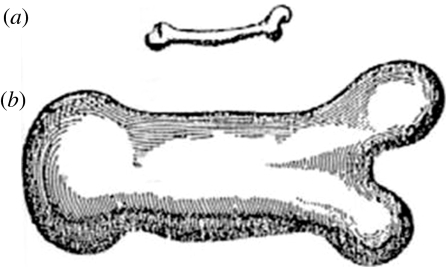
The latter is often considered the father of biomechanics. Among his many other discoveries, he recognized that the shape of an animal's bones are to some extent adapted to its weight. Long bones of larger animals typically have a smaller aspect ratio (figure 2). Galileo's explanation is a simple scaling argument, based on the fact that the weight of an animal scales with the third power of its linear dimension, while the structural strength of its bones scales with its cross-section, i.e. the square of the linear dimension. Hence, the aspect ratio of long bones has to decrease with the body weight of the animal (figure 2). This is also a good example of functional adaptation.
Figure 2.
Galileo's description of bones from (a) small and (b) large animals (Galilei 2005).
Different strategies in designing a material result from the two paradigms of ‘growth’ and ‘fabrication’ (figure 1). In the case of engineering materials, a machine part is designed and then the material is selected according to knowledge and experience regarding the functional requirements, taking into account possible changes in those requirements during service (e.g. typical or maximum loads) and fatigue (and other lifetime issues) of the material. In any case, the strategy is static, as the design is made in the beginning and must satisfy all needs during the lifetime. The fact that natural materials are growing rather than being fabricated leads to the possibility of a dynamic strategy: it is not the exact design of the organ that is stored in the genes, but rather a recipe to build it. This means that the final result is rather obtained by an algorithm than by the replication of a design. The advantage of this approach is that it allows flexibility at all levels. First, it permits adaptation to the function, while the body is growing. For example, a branch growing in the direction of the wind may grow differently than that in the opposite direction, without any change in the genetic code. Second, it allows the growth of hierarchical materials, where the microstructure at each position of the part is adapted to the local needs (Jeronimidis 2000). This is linked to the idea of robustness: nature has evolved structures that are capable of surviving/withstanding/adapting to a range of different environments, while man-made materials are generally less flexible in their use.
Adaptive growth has also been analysed in the book by Mattheck and Kubler, more specifically focusing on trees (Thompson D'Arcy W 1992; Mattheck & Kubler 1995), with the specific aim to extract useful engineering principles from the observation of natural structures. Adapting the form (of a whole part or organ, such as a branch or a vertebra) is the first aspect of functional adaptation. A second possibility, which relates more directly to materials science, is the functional adaptation of the microstructure of the material itself (such as the wood in the branch or the bone in the vertebra). This dual need for optimization of the part's form and the material's microstructure is well known for any engineering problem. However, in natural materials, shape and microstructure become intimately related due to their common origin, which is the growth of the organ. This aspect has been discussed in detail by Jeronimidis in his introductory chapters to a book on ‘Structural Biological Materials’ (Jeronimidis 2000). Growth implies that ‘form’ and ‘microstructure’ are created in the same process, but in a stepwise manner. The shape of a branch is created by the assembly of molecules to cells, and of cells to wood with a specific shape. Hence, at every size level, the branch is both form and material: the structure becomes hierarchical.
3. Hierarchical structuring
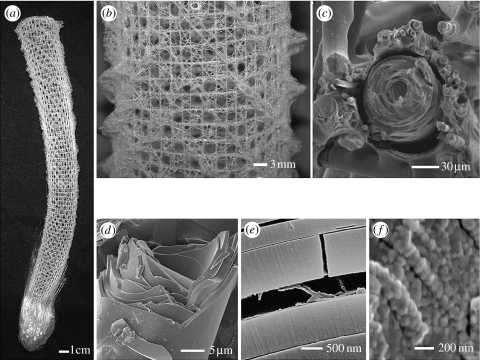
Hierarchical structuring is one of the consequences of the growth process of organs. Examples for hierarchical biological materials are bone (Rho et al. 1998; Weiner and Wagner 1998; Fratzl et al. 2004b; Peterlik et al. 2006), trees (Barnett & Jeronimidis 2003; Hoffmann et al. 2003; Keckes et al. 2003; Milwich et al. 2006), seashells (Kamat et al. 2000), spider silk (Vollrath & Knight 2001), the attachment systems of geckos (Arzt et al. 2003), superhydrophobic surfaces (Lotus effect; Barthlott & Neinhuis 1997; Neinhuis & Barthlott 1997; Furstner et al. 2005), optical microstructures (Aizenberg et al. 2001; Vukusic & Sambles 2003), the exoskeleton of arthropods (Raabe et al. 2005, 2006) or the skeleton of glass sponges (Aizenberg et al. 2005). Figure 3 shows an example of the hierarchical structure of the skeleton of the Euplectella glass sponge. Hierarchical structuring allows the construction of large and complex organs based on much smaller, often very similar, building blocks. Examples of such building blocks are collagen fibrils in bone which have units with a few hundred nanometre thickness and can be assembled to a variety of bones with very different functions (Weiner & Wagner 1998; Currey 2002; Fratzl et al. 2004b). Moreover, hierarchical structuring allows the adaptation and optimization of the material at each level of hierarchy to yield outstanding performance. For example, the extraordinary toughness of bone is due to the combined action of structural elements at the nanometre (Gao et al. 2003; Gupta et al. 2006b) and the micrometre levels (Peterlik et al. 2006).
Figure 3.
Several levels of hierarchy in the structure of the skeleton of the glass sponge Euplectella (Aizenberg et al. 2005; Weaver et al. in press). (a) whole basket, (b) woven glass fibres, (c) fibre bundle joined by glass matrix, (d) laminated structure of single glass fibre, (e) protein layer gluing successive glass layers, (f) colloidal structure of glass.
Clearly, hierarchical structuring provides a major opportunity for bio-inspired materials synthesis and adaptation of properties for specific functions (Tirrell 1994). Functionally graded materials are examples of materials with hierarchical structure. New functions may be obtained just by structuring a given material, instead of choosing a new material providing the desired function. One example for this strategy is composite materials that are omnipresent in nature. They feature lamellar structures, such as in seashells (Kamat et al. 2000; Tang et al. 2003; Fantner et al. 2006) or glass spicules (Aizenberg et al. 2005; Woesz et al. 2006), or fibrous structures, such as in bone (Weiner & Wagner 1998; Currey 2002; Peterlik et al. 2006) or wood (Barnett & Jeronimidis 2003; Hoffmann et al. 2003; Keckes et al. 2003). These structures carry many similarities with man-made fibre glass and ceramic laminates and it is highly remarkable that totally different strategies have converged at similar solutions in them. Moreover, interfaces play a crucial role in hierarchical composite materials. Joining elements by gluing (Smith et al. 1999; Tang et al. 2003; Fratzl et al. 2004a; Gupta et al. 2006a) is one aspect, while control of the synthesis of components, such as crystals, is another. For a while, this topic has been addressed in the research field of biomineralization (Mann 2001). Hierarchical hybrid materials can also provide movement and motility. Muscles and connective tissues are integrated to form a complex materials system which is motor and supporting structure at the same time. This may inspire materials scientists to invent new concepts for active biomimetic materials (Sidorenko et al. 2007).
4. Damage repair and healing
Clearly, one of the most remarkable properties of biological materials is their capacity of self-repair. There are very different strategies associated with self-repair. At the smallest scale, there is the concept of sacrificial bonds between molecules that break and reform dynamically (Fantner et al. 2006). Bond breaking and reforming was found, for example, to occur upon deformation of wood (Keckes et al. 2003) and bone (Thompson et al. 2001; Fantner et al. 2005; Gupta et al. 2006a,b). This provides, in fact, the possibility for plastic deformation (without creating permanent damage) as in many metals and alloys. At higher levels, many organisms have the capability to remodel the material. In bone, for example, specialized cells (osteoclasts) are permanently removing material, while other cells (osteoblasts) are depositing new tissue. This cyclic replacement of the bone material has at least two consequences: first, it allows a continuous structural adaptation to changing external conditions and, second, damaged material may be removed and replaced by new tissue (Currey 2002; Fratzl et al. 2004b). In technical terms, this would mean that a sensor/actuator system is put in place to replace damaged material wherever needed. For example, a change in environmental conditions can be (partly) compensated by adapting the form and microstructure to the new conditions: the growth direction of a tree after a slight landslide (Mattheck & Kubler 1995, 1998) is an apt example. Finally, nature also can heal a fractured or critically damaged tissue. In most cases, wound healing is not a one-to-one replacement of a given tissue, but it rather starts with the formation of an intermediate tissue (based on a response to inflammation), followed by a scar tissue. An exception to this is bone tissue, which is able to regenerate completely and where the intermediate tissue (the callus) is eventually replaced by a material of the original type (Carter & Beaupre 2001). The science of self-healing materials is still in its complete infancy (White et al. 2001), but represents a major opportunity for biomimetic materials research.
5. Systematic biomimetic approach
As mentioned already, biomimetic materials research starts with the study of structure–function relationships in biological materials. Based on the strategies found in nature, bio-inspired materials may be developed. However, this approach has to some extent rely on serendipity, depending on what is actually found in the analysis of biological materials. Is it possible to make the biomimetic approach more systematic?
An example of this kind has been studied by Julian Vincent (2005). He analysed how the cuticle of arthropods were designed to cope with IR and UV irradiation, as well as with demands for sensory transmission, movement, etc., and proved that the similarity of the cuticle design with known technology is only approximately 20%, suggesting that engineering can actually learn from this structure. Most interestingly, the multifunctionality of the cuticle is achieved by controlling the local properties of the material rather than by changing its overall parameters (which would be the technical solution).
Another systematic approach is to store biomimetic solutions, once they are uncovered in the analysis of biological materials, into large databases, where they can then be retrieved by engineers in search of technical solutions. Such databases have previously been developed for materials selection (Ashby 2003) in technical design and have more recently been extended to the selection of both materials and processes (Ashby et al. 2004). Initial attempts have been made to establish a system into which all known biomimetic solutions can be placed, classified in terms of function (Vincent & Mann 2002; Vincent et al. 2006). Such tools will become extremely valuable for the development of bio-inspired materials and processes.
Finally, the verification of biological mechanisms by manufacture can lead to an iterative process between biology and engineering, in which the understanding gained from engineering may be fed back into biology. This mostly unexplored pathway offers the possibility that engineers can also contribute to biological sciences (Csete & Doyle 2002; Vincent 2003).
6. Conclusions
Biomimetic materials research (sometimes also coined as material bionics or bio-inspired materials research), an old field, has now begun to develop very dynamically. One of the reasons is the growing interaction between biological and materials sciences. Indeed, bio-inspiration does not result from the observation of natural structures alone, but requires a thorough investigation of structure–function relationships in biological materials. Nature has evolved a number of strategies to create outstanding functional properties with comparatively cheap base materials. This is achieved by hierarchical structuring, adaptive growth instead of fabrication, and constant remodelling and healing. Biomimetic materials research creates numerous opportunities for devising new strategies to create multifunctional materials by hierarchical assembly, for the clever use of interfaces and the development of active or self-healing materials. Interdisciplinary teams will develop a portfolio of bio-inspired processes for obtaining new function by structuring and assembling of known elements. This will also require new approaches to the dissemination of knowledge, such as databases sorting materials and processes by function rather than by composition.
Acknowledgments
The author is grateful to many colleagues with whom he had the privilege to interact and collaborate over the years and whose work is partially referenced in this article. In particular, he would like to thank Yves Bréchet (Grenoble, France) for many intensive discussions on the subject of this paper.
References
- Aizenberg J, Tkachenko A, Weiner S, Addadi L, Hendler G. Calcitic microlenses as part of the photoreceptor system in brittlestars. Nature. 2001;412:819–822. doi: 10.1038/35090573. [DOI] [PubMed] [Google Scholar]
- Aizenberg J, Weaver J.C, Thanawala M.S, Sundar V.C, Morse D.E, Fratzl P. Skeleton of Euplectella sp.: structural hierarchy from the nanoscale to the macroscale. Science. 2005;309:275–278. doi: 10.1126/science.1112255. [DOI] [PubMed] [Google Scholar]
- Arzt E, Gorb S, Spolenak R. From micro to nano contacts in biological attachment devices. Proc. Natl Acad. Sci. USA. 2003;100:10 603–10 606. doi: 10.1073/pnas.1534701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby M.F. 3rd edn. Butterworth-Heinemann; Oxford, UK: 2003. Materials selection in mechanical design. [Google Scholar]
- Ashby M.F, Bréchet Y.J.M, Cebona D, Salvo L. Selection strategies for materials and processes. Mater. Design. 2004;25:51–67. doi: 10.1016/S0261-3069(03)00159-6. [DOI] [Google Scholar]
- Barnett J, Jeronimidis G. Blackwell; London, UK: 2003. Wood quality and its biological basis. [Google Scholar]
- Barthlott W, Neinhuis C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta. 1997;202:1–8. doi: 10.1007/s004250050096. [DOI] [Google Scholar]
- Carter D.R, Beaupre G.R. Cambridge University Press; Cambridge, UK: 2001. Skeletal function and form: mechanobiology of skeletal development, aging, and regeneration. [Google Scholar]
- Csete M.E, Doyle J.C. Reverse engineering of biological complexity. Science. 2002;295:1664–1669. doi: 10.1126/science.1069981. [DOI] [PubMed] [Google Scholar]
- Currey J.D. Princeton University Press; Princeton, NJ: 2002. Bones—structure and mechanics. [Google Scholar]
- Currey J.D. Materials science—hierarchies in biomineral structures. Science. 2005;309:253–254. doi: 10.1126/science.1113954. [DOI] [PubMed] [Google Scholar]
- Da Vinci, L. 1952 The notebooks of Leonardo da Vinci (transl. Bell, R. C.). Oxford.
- Fantner G.E, et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 2005;4:612–616. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- Fantner G.E, et al. Sacrificial bonds and hidden length: unraveling molecular mesostructures in tough materials. Biophys. J. 2006;90:1411–1418. doi: 10.1529/biophysj.105.069344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratzl P, Burgert I, Gupta H.S. On the role of interface polymers for the mechanics of natural polymeric composites. Phys. Chem. Chem. Phys. 2004a;6:5575–5579. doi: 10.1039/b411986j. [DOI] [Google Scholar]
- Fratzl P, Gupta H.S, Paschalis E.P, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J. Mater. Chem. 2004b;14:2115–2123. doi: 10.1039/b402005g. [DOI] [Google Scholar]
- Frost H.M. Skeletal structural adaptations to mechanical usage. 1. Redefining Wolff's law. Anat. Rec. 2005;226:403–413. doi: 10.1002/ar.1092260402. [DOI] [PubMed] [Google Scholar]
- Furstner R, Barthlott W, Neinhuis C, Walzel P. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir. 2005;21:956–961. doi: 10.1021/la0401011. [DOI] [PubMed] [Google Scholar]
- Galilei G. On the shoulders of giants. In: Hawking S, editor. Dialogues concerning two new sciences. Running Press Book Publishers; Philadelphia, PA: 2005. [Google Scholar]
- Gao H, Ji B, Jäger I.L, Arzt E, Fratzl P. Materials become insensitive to flaws at nanoscale: lessons from nature. Proc. Natl Acad. Sci. USA. 2003;100:5597–5600. doi: 10.1073/pnas.0631609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, H. S., Fratzl, P., Kerschnitzki, M., Benecke, G., Wagermaier, W. & Kirchner, H. O. K. 2006a Evidence for an elementary process in bone plasticity with an activation enthalpy of 1 eV. J. R. Soc. Interface. [DOI] [PMC free article] [PubMed]
- Gupta H.S, Seto J, Wagermaier W, Zaslansky P, Boesecke P, Fratzl P. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc. Natl Acad. Sci. USA. 2006b;103:17 741–17 746. doi: 10.1073/pnas.0604237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Chabbert B, Monties B, Speck T. Mechanical, biochemical and ultrastructural properties of wood and their changes during ontogeny in the two tropical lianas Bauhinia guianensis and Condylocarpon guianense. Planta. 2003;217:32–40. doi: 10.1007/s00425-002-0967-2. [DOI] [PubMed] [Google Scholar]
- Huiskes R. If bone is the answer, then what is the question? J. Anat. 2000;197:145–156. doi: 10.1046/j.1469-7580.2000.19720145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimidis G. In: Structural biological materials, design and structure–property relationships. Elices M, editor. ch. 1 and 2. Pergamon; Amsterdam, The Netherlands: 2000. [Google Scholar]
- Jeronimidis G, Atkins A.G. Mechanics of biological materials and structures—Nature's lessons for the engineer. J. Mech. Eng. Sci. 1995;209:221–235. [Google Scholar]
- Kamat S, Su X, Ballarini R, Heuer A.H. Structural basis for the fracture toughness of the shell of the conch Strombus gigas. Nature. 2000;405:1036–1040. doi: 10.1038/35016535. [DOI] [PubMed] [Google Scholar]
- Keckes J, et al. Cell-wall recovery after irreversible deformation of wood. Nat. Mater. 2003;2:810–814. doi: 10.1038/nmat1019. [DOI] [PubMed] [Google Scholar]
- Kemp, M. 2004 Structural intuitions and metamorphic thinking in art, architecture and science. In Metamorph—9th Int. Architecture Exhibition Focus, pp. 30–43. Fondazione La Biennale di Venezia.
- Lakes R. Materials with structural hierarchy. Nature. 1993;361:511–515. doi: 10.1038/361511a0. [DOI] [Google Scholar]
- Mann S. Oxford University Press; Oxford, UK: 2001. Biomineralization—principles and concepts in bioinorganic chemistry. [Google Scholar]
- Mattheck C, Bethge K. The structural optimization of trees. Naturwissenschaften. 1998;85:1–10. doi: 10.1007/s001140050443. [DOI] [Google Scholar]
- Mattheck C, Kubler H. Springer; Berlin, Germany: 1995. The internal optimization of trees. [Google Scholar]
- Milwich M, Speck T, Speck O, Stegmaier T, Planck H. Biomimetics and technical textiles: solving engineering problems with the help of nature's wisdom. Am. J. Bot. 2006;93:1295–1305. doi: 10.3732/ajb.93.10.1455. [DOI] [PubMed] [Google Scholar]
- Nachtigall W. Springer; Berlin, Germany: 1998. Bionik—Grundlagen und Beispiele für Ingenieure und Naturwissenschaftler. [Google Scholar]
- Neinhuis C, Barthlott W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997;79:667–677. doi: 10.1006/anbo.1997.0400. [DOI] [Google Scholar]
- Peterlik H, Roschger P, Klaushofer K, Fratzl P. From brittle to ductile fracture of bone. Nat. Mater. 2006;5:52–55. doi: 10.1038/nmat1545. [DOI] [PubMed] [Google Scholar]
- Raabe D, Sachs C, Romano P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Materialia. 2005;53:4281–4292. doi: 10.1016/j.actamat.2005.05.027. [DOI] [Google Scholar]
- Raabe D, Romano P, Sachs C, Fabritius H, Al-Sawalmih A, Yi S.-B, Servos G, Hartwig H.G. Microstructure and crystallographic texture of the chitin–protein network in the biological composite material of the exoskeleton of the lobster Homarus americanus. Mater. Sci. Eng. A. 2006;421:143–153. doi: 10.1016/j.msea.2005.09.115. [DOI] [Google Scholar]
- Rho J.Y, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998;20:92–102. doi: 10.1016/S1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- Sidorenko A, Krupenkin T, Taylor A, Fratzl P, Aizenberg J. Reversible switching of hydrogel-actuated nanostructures into complex micropatterns. Science. 2007;315:487–490. doi: 10.1126/science.1135516. [DOI] [PubMed] [Google Scholar]
- Smith B.L, et al. Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature. 1999;399:761–763. doi: 10.1038/21607. [DOI] [Google Scholar]
- Tang Z, Kotov N.A, Magonov S, Ozturk B. Nanostructured artificial nacre. Nat. Mater. 2003;2:413–418. doi: 10.1038/nmat906. [DOI] [PubMed] [Google Scholar]
- Thompson D'Arcy W. Dover Publications; New York, NY: 1992. On growth and form—the complete revised edition. [Google Scholar]
- Thompson J.B, Kindt J.H, Drake B, Hansma H.G, Morse D.E, Hansma P.K. Bone indentation recovery time correlates with bond reforming time. Nature. 2001;414:773–776. doi: 10.1038/414773a. [DOI] [PubMed] [Google Scholar]
- Tirrell D.A. National Academy Press; Washington, DC: 1994. Hierarchical structures in biology as a guide for new materials technology. [Google Scholar]
- Vincent J.F.V. Biomimetic modelling. Phil. Trans. R. Soc. B. 2003;358:1597–1603. doi: 10.1098/rstb.2003.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.F.V. Deconstructing the design of a biological material. J. Theor. Biol. 2005;236:73–78. doi: 10.1016/j.jtbi.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Vincent J.F.V, Mann D.L. Systematic technology transfer from biology to engineering. Phil. Trans. R. Soc. A. 2002;360:159–173. doi: 10.1098/rsta.2001.0923. [DOI] [PubMed] [Google Scholar]
- Vincent J.F.V, Bogatyreva O.A, Bogatyrev N.R, Bowyer A, Pahl A.-K. Biomimetics: its practice and theory. J. R. Soc. Interface. 2006;3:471–482. doi: 10.1098/rsif.2006.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath F, Knight D.P. Liquid crystalline spinning of spider silk. Nature. 2001;410:541–548. doi: 10.1038/35069000. [DOI] [PubMed] [Google Scholar]
- Vukusic P, Sambles J.R. Photonic structures in biology. Nature. 2003;424:852–855. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]
- Weaver, J. C. et al. In press. Hierarchical assembly of the siliceous skeletal lattice of the hexactinellid sponge Euplectella aspergillum J. Struct. Biol. [DOI] [PubMed]
- Weiner S, Wagner H.D. The material bone: structure mechanical function relations. Annu. Rev. Mater. Sci. 1998;28:271–298. doi: 10.1146/annurev.matsci.28.1.271. [DOI] [Google Scholar]
- White S.R, Sottos N.R, Geubelle P.H, Moore J.S, Kessler M.R, Sriram S.R, Brown E.N, Viswanathan S. Autonomic healing of polymer composites. Nature. 2001;409:794–797. doi: 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- Woesz A, Weaver J.C, Kazanci M, Dauphin Y, Aizenberg J, Morse D.E, Fratzl P. Micromechanical properties of biological silica in skeletons of deep-sea sponges. J. Mater. Res. 2006;21:2068–2078. doi: 10.1557/jmr.2006.0251. [DOI] [Google Scholar]
- Wolff J. Hirschwald, A; Berlin, Germany: 1892. Das Gesetz der Transformation der Knochen. [Google Scholar]