Abstract
The settlement and adhesion of Navicula perminuta and Ulva linza to methyl-terminated alkanethiol self-assembled monolayers (SAMs) of increasing chain length has been investigated. Organisms were allowed to settle onto the monolayers and were subsequently exposed to hydrodynamic shear stress in order to determine their adhesion strength. Results show that as the SAM structure changes from amorphous to crystalline (C14), there is a marked change in the adhesion of N. perminuta and U. linza. Given that the SAMs in the series all exhibit similar contact angle behaviour and surface energy, it is hypothesized that the lubricity of the surface plays a role in determining the surface adhesion.
Keywords: adhesion, biofouling, alkanethiol, self-assembled monolayer, Ulva, Navicula
1. Introduction
Marine biofouling, the colonization of submerged structures by barnacles, macroalgae and microbial slimes, has major economic implications for military and commercial shipping (Callow & Callow 2002). Finding environmentally benign methods of controlling biofouling requires a greater understanding of the settlement, colonization and adhesion processes used by fouling organisms to select and adhere to submerged surfaces. For such purposes, the advantages of using well-defined model surfaces based on self-assembled monolayers (SAMs) have been well documented (e.g. Callow et al. 2000, 2005; Ista et al. 2004).
Ulva linza, a major macroalgal fouling species in temperate zones, colonizes new surfaces by the release of quadriflagellate zoospores. Spores swim freely in the water column, and on contacting a suitable surface they secrete a preformed, fast curing glycoprotein adhesive that surrounds the spore anchoring it to the surface (Callow et al. 1997). Once the adhesive is released, the spore is permanently secured to the substratum. Diatoms, a family of siliceous microalgae, form a major component of marine microbial biofilms. The method used by diatoms to colonize a new surface is quite distinct from that of the macroalgae. When in suspension, diatoms are non-motile, lacking flagella to swim with. They colonize new surfaces by passive processes, such as gravitational settlement and adhere through the production of a polysaccharide-based extracellular polymeric substance (EPS; reviewed by Chiovitti et al. 2006). Once adhered to a surface, diatoms are capable of a ‘gliding’ motility mediated by the production of EPS from a slit in the cell termed the raphe.
The physical properties of a surface have been shown to have a profound effect on the settlement and adhesion of fouling organisms. Microtopography and surface roughness influence both the settlement (Callow et al. 2002) and attachment strength (Granhag et al. 2004) of U. linza spores. Similarly, surface energy and wettability have been shown to influence the settlement behaviour of Ulva zoospores (Callow et al. 2000), the attachment of marine bacteria Cobetia marina (Ista et al. 2004) and the attachment strength of U. linza and the diatom Amphora spp. (Finlay et al. 2002). It is proposed that the influence of wettability is derived from differences in adhesive spreading on the surface altering the contact area (Callow et al. 2005). The influence of surface lubricity and modulus has also been shown to be of significance in determining the attachment strength of Ulva spores (Hoipkemeier-Wilson et al. 2004; Chaudhury et al. 2005).
In this paper, the settlement and adhesion of spores of U. linza, and a diatom Navicula perminuta, to methyl-terminated alkanethiol SAMs of different chain length has been investigated. Compounds 1–6 (figure 1) are alkanethiols varying in length from an octyl chain to an octadecyl chain, each compound being increased in length by one ethylene unit from the previous adsorbate. SAMs formed from compounds 1–6 all present a methyl-terminated surface to the aqueous phase and exhibit advancing water contact angles in the region of 111–115°. It is known that SAMs formed from compounds 1 (C8H17SH) and 2 (C10H21SH) exhibit a significant degree of alkyl chain mobility, whereas SAMs formed from compounds 3 (C12H25SH) to 6 (C18H37SH) are two-dimensional quasi-crystalline solids (Porter et al. 1987; Evans & Ulman 1990; Evans et al. 1991). As a result, SAMs formed from compounds 1 and 2 have alkyl chain structures that possess higher densities of gauche defects and are more readily deformable than those from compounds 3–6. Thus, the objective of investigating this SAM series was to assess how the settlement and adhesion of U. linza and N. perminuta is affected by changes in the SAM structure as the chain length is increased.
Figure 1.
Schematic of the molecular structures of compounds 1–6.
2. Material and methods
2.1 SAMs
Au substrates were prepared by thermally evaporating (Edwards Auto 306 Evaporator) a Cr adhesion promoter (approx. 5 nm) onto clean glass slides (VWR, Lutterworth, Leicestershire, UK), followed by the monitoring of approximately 100 nm of Au deposition using a quartz crystal microbalance thickness monitor employing deposition rates in the range of 0.05–0.10 nm s−1 for both Au and Cr. All glassware used in the SAM formation was cleaned prior to use by immersion in ‘piranha’ solution, a 3 : 7 mixture of 30% hydrogen peroxide (Fisher Scientific, laboratory reagent grade) and concentrated sulphuric acid (Fisher Scientific, analytical reagent grade), at room temperature for approximately 1 h. Cleaning of glassware with piranha solution was followed by rinsing with copious amounts of 18 MΩ deionized H2O (Elga UHQ-PS) and drying in an oven at 140oC. All Au substrates were cleaned prior to SAM formation by immersion in piranha solution at room temperature for 10 min. Cleaning of substrates with piranha solution was followed by rinsing with copious amounts of 18 MΩ deionized H2O (Elga UHQ-PS) and with C2H5OH. SAMs were prepared by immersing Cr-primed, Au-coated glass microscope slides in 1 mM C2H5OH (Fisher Scientific, HPLC grade) solutions of the SAM compounds for 24 h. The Au substrates were removed from the SAM solution and rinsed with copious amounts of C2H5OH, before being blown dry using Ar gas.
Dynamic H2O contact angles were measured using a custom-made stage apparatus, employing a charge-coupled device KP-M1E/K camera (Hitachi) and FTA Video Analysis software v. 1.96 (First Ten Angstroms) for analysis of the contact angle of a droplet of UHQ H2O at the three-phase intersection point. All data were collected under conditions of ambient temperature, pressure and humidity. A minimum of seven measurements were performed for each sample.
Ellipsometry measurements were performed using a multi-spectroscopic ellipsometer (Jobin-Yvon/Horiba), operating with DeltaPsi2 v. 2.0.8 software. The angle of incidence was set to 70°. The light wavelength range used for all measurements was 280–800 nm. All measurements were made under conditions of ambient temperature, pressure and humidity. SAM thicknesses are the averages of a minimum of six measurements, each made at a different location on the substrate.
XPS analysis of SAMs was performed using an Escalab 250 system (Thermo VG Scientific), operating with Avantage v. 1.85 software. An Al Kα X-ray source was used, providing a monochromatic X-ray beam with incident energy of 1486.68 eV. All measurements were made at a pressure of less than 5×10−9 mbar. Detailed scans of Au 4f7/4f5 (86 eV), S 2p (163 eV) and C 1 s (286 eV) were performed using a pass energy of 20 eV and a step size of 0.1 eV.
2.2 Settlement and removal of Ulva linza and Navicula perminuta
Six replicate SAMs of each chain length were used for the Ulva and the Navicula assay. All SAMs were rinsed in artificial seawater at pH 8.2 immediately prior to use. Reproductive plants of the green macroalga U. linza were collected from Wembury beach, Devon, England. Zoospores were released and the assay conducted according to the protocols detailed in Callow et al. (1997). Zoospore suspensions containing 1.5×106 spores ml−1 were allowed to settle onto SAMs for 45 min in the dark. Slides were rinsed to remove unsettled zoospores and three replicates were then fixed and washed as described by Callow et al. (1997). After rinsing, the remaining three replicates were exposed to 54 Pa wall shear stress in a fully turbulent water channel (Schultz et al. 2000) before fixation and washing. Spore density on the SAMs before and after exposure to flow was determined as described by Callow et al. (2002), using the autofluorescence of chlorophyll to locate settled spores with a Zeiss Axioskop 2 fluorescence microscope.
Cultures of the pennate diatom N. perminuta (Bacillariophyceae), originally isolated by Dr R. Wetherbee, were grown in an F2 medium at 18°C on a 16 : 8 h light : dark cycle. Exponentially growing cultures of Navicula were prepared and assayed as detailed in Pettitt et al. (2004). A cell suspension of 0.32 μg chla ml−1 was allowed to settle onto SAMs for 120 min, before slides were rinsed and processed as mentioned previously for the Ulva assay. Cell density before and after flow was determined by manual counts using an Olympus BH2 transmitted light microscope.
3. Results
SAMs formed from compounds 1–6, which are simple n-alkanethiols, will exhibit similar contact angles and similar surface energies (Bain et al. 1989). Leggett (2003) reported differences in the frictional properties of alkanethiol SAMs of increasing chain length, established using atomic force microscopy. The frictional properties of alkanethiol SAMs may be quantified by determining their coefficients of friction (μ), defined as the gradient of a graph of the friction force plotted as a function of the load applied perpendicular to the sample (Leggett et al. 2005). Friction coefficients of the SAMs studied here are shown in table 1. The largest coefficient of friction was measured for the shortest adsorbate, and the value of μ decreases as the alkyl chain length increases, which is in agreement with previous studies (McDermott et al. 1997).
Table 1.
Measured advancing water contact angles and friction coefficients for SAMs formed from compounds 1, 2, 3, 5 and 6 (Leggett 2003).
| alkyl chain length | advancing contact angle (deg.) | friction coefficient |
|---|---|---|
| 8 | 111±1 | 0.52±0.03 |
| 10 | 113±1 | 0.35±0.04 |
| 12 | 113±1 | 0.33±0.02 |
| 16 | 115±1 | 0.23±0.02 |
| 18 | 115±1 | 0.18±0.05 |
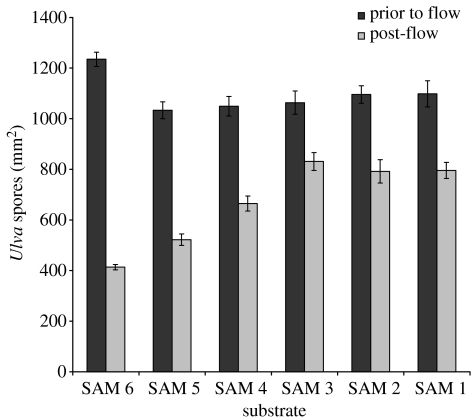
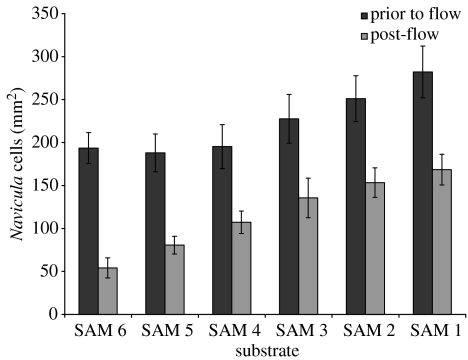
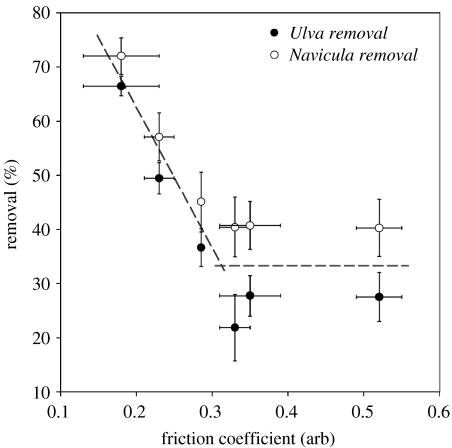
The density of Ulva spores and Navicula cells prior to and post-exposure to 54 Pa wall shear stress is shown in figures 2 and 3, respectively. The density of Ulva spores initially settled on different SAMs was very similar, but there appears to be a minor trend of increasing initial attachment of Navicula cells with reducing alkanethiol chain length. Irrespective of the level of initial settlement, when the adhesion strength of the attached cells of both organisms was examined by measuring the proportion of attached cells removed under flow, as a function of SAM friction coefficient, a clear trend was observed for both organisms (figure 4). Removal was approximately constant for friction coefficients greater than 0.3 (SAMs formed from compounds 1–3), but increased with decreasing friction coefficient, i.e. for friction coefficients less than 0.3.
Figure 2.
Density of attached Ulva spores on SAMs of varying chain length, prior to (dark columns) and post (light columns) exposure to 54 Pa wall shear stress. N=30; error bars, ±2 s.e.
Figure 3.
Density of attached Navicula cells on SAMs of varying chain length, prior to (dark columns) and post (light columns) exposure to 54 Pa wall shear stress. N=30; error bars, ±2 s.e.
Figure 4.
Removal (%) of Navicula cells and Ulva spores by 54 Pa wall shear stress, as a function of SAM friction coefficient. Removal: N=30, error bars, 2 s.e. derived from arcsine transformed data. As the friction coefficient of compound 4 was not measured by Leggett (2003), a value has been interpolated from the equation y=−0.0301x+0.7067 (r2=0.9051).
4. Discussion and conclusions
It has previously been established that long alkyl chain moieties within a SAM promote well-ordered molecular packing, while shorter chain lengths promote a loss of molecular organization (Porter et al. 1987; Evans et al. 1991). Evans & Ulman (1990) stated that SAMs formed from alkanethiols with chain lengths less than 10 methylene units would be disordered, while those formed from greater than 12 methylene units would be ordered. Hence, SAMs formed from the shorter chain compounds, 1 and 2, exhibit higher densities of gauche defects and more mobile alkyl chains than those formed from compounds 3–6. At a chain length of 12 methylene units, the monolayer is thought to adopt a two-dimensional crystalline arrangement, in which the adsorbates are almost entirely trans-extended. This point corresponds to the onset of increasing organism removal in figure 4. It is proposed that this change in surface structure is the cause for the increase in organism removal.
The adhesion of N. perminuta and U. linza to a surface relies on the secretion of adhesive molecules that surround the cell and wet the surface. The chemical and physical properties of a surface will affect the interaction of this adhesive with the surface. Lee et al. (2000) reported that shorter, less ordered SAMs exhibit greater frictional forces, as measured using AFM, than crystalline, ordered SAMs, discussing the effect of intermolecular packing on the modulus of the SAM. A disordered SAM will have a lower modulus than a crystalline SAM; therefore, it can provide a greater surface area for interaction, in this case with the adhesive of a Navicula cell or Ulva spore. On the application of a shear stress, we may therefore anticipate lower removal from the amorphous SAM as a result of the increased area over which the diatom or spore is adhered.
Additionally, the reaction of the SAM surface to the application of energy (shear stress) may also affect the removal of the adhered spores/cells. The less well-ordered, short-chain SAMs will provide a greater number of channels for energy dissipation than crystalline SAMs, for example through molecular motions such as the formation of gauche defects and the rotation of carbon–carbon bonds. Owing to the increased number of van der Waals forces between chains in crystalline SAMs, which will increase with increasing chain length, a greater energy input is required to deform these SAMs. When an attached diatom or spore is subjected to a shear stress, if the surface onto which it has adhered is amorphous, more energy will be dissipated through the surface than if the surface were crystalline. Given that the adhesive secreted by a cell has a constant strength on either a crystalline SAM or an amorphous SAM, it appears that cells on the amorphous SAMs will adhere more strongly than cells on the crystalline SAMs. This may explain why reduced adhesion strength (i.e. higher removal under shear stress) of Navicula and Ulva is seen on the crystalline SAMs than on the amorphous SAMs.
In conclusion, the adhesion strength of N. perminuta and U. linza to alkanethiol SAMs was found to decrease with increasing alkyl chain length for SAMs with alkyl chain lengths greater than 12 methylene units, which are two-dimensional quasi-crystalline solids. It is proposed that as the alkyl chain length increases, the dissipation of energy through the SAM, upon application of a hydrodynamic force, becomes less favourable, leading to greater disruption of the adhesive bond between the adhesive secreted by the organism and the SAM. Hence, the adhesive bond fails more readily for longer alkyl chain lengths.
Acknowledgments
The authors acknowledge support from the AMBIO project (NMP-CT-2005-011827) funded by the European Commission's 6th Framework Programme. The views expressed in this publication reflect only those of the authors and the Commission is not liable for any use that may be made of the information contained therein. The University of Birmingham, ACORN (A Collaboration on Research into Nanoparticles) and EPSRC are acknowledged for financial support for J.B. We also acknowledge the assistance and the advice given by Prof. Stephen D. Evans and Dr Kevin Critchley at The University of Leeds, School of Physics and Astronomy, as well as Dr Shuqing Sun at the University of Sheffield, School of Chemistry.
References
- Bain C.D, Troughton E.B, Tao Y.-T, Evall J, Whitesides G.M, Nuzzo R.G. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 1989;111:321–335. doi: 10.1021/ja00183a049. [DOI] [Google Scholar]
- Callow M.E, Callow J.A. Marine biofouling: a sticky problem. Biologist. 2002;49:1–5. [PubMed] [Google Scholar]
- Callow M.E, Callow J.A, Pickett-Heaps J.D, Wetherbee R. Primary adhesion of Enteromorpha (chlorophyta, ulvales) propagules: quantitative settlement studies and video microscopy. J. Phycol. 1997;33:938–947. doi: 10.1111/j.0022-3646.1997.00938.x. [DOI] [Google Scholar]
- Callow M.E, Callow J.A, Ista L.K, Coleman S.E, Nolasco A.C, López G.P. Use of self-assembled monolayes of different wettabilities to study surface selection and primary adhesion processes of green algal (Enteromorpha) zoospores. Appl. Environ. Microbiol. 2000;66:3249–3254. doi: 10.1128/AEM.66.8.3249-3254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow M.E, Jennings A.R, Brennan A.B, Seegert C.E, Gibson A.L, Wilson L, Feinberg A.W, Baney R, Callow J.A. Microtopographic cues for settlement of zoospores of the green fouling alga Enteromorpha. Biofouling. 2002;18:237–245. doi: 10.1080/08927010290014908. [DOI] [Google Scholar]
- Callow J.A, Callow M.E, Ista L.K, Lopez G.P, Chaudhury M.K. The influence of surface energy on the wetting behaviour of the spore adhesive of the marine alga Ulva linza (synonym Enteromorpha linza) J. R. Soc. Interface. 2005;2:319–325. doi: 10.1098/rsif.2005.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury M.K, Finlay J, Chung J.Y, Callow M.E, Callow J.A. The influence of elastic modulus and thickness on the release of the soft-fouling green alga Ulva linza (syn Enteromorpha linza) from poly(dimethylsiloxane) (PDMS) model networks. Biofouling. 2005;21:41–48. doi: 10.1080/08927010500044377. [DOI] [PubMed] [Google Scholar]
- Chiovitti T, Dugdale T.M, Wetherbee R. Diatom adhesives: molecular and mechanical properties. In: Smith A.M, Callow J.A, editors. Biological adhesives. Springer; Berlin, Germany: 2006. pp. 79–103. [Google Scholar]
- Evans S.D, Ulman A. Surface potential studies of alkyl-thiol monolayers adsorbed on gold. Chem. Phys. Lett. 1990;170:462–466. doi: 10.1016/S0009-2614(90)87085-6. [DOI] [Google Scholar]
- Evans S.D, Urankar E, Ulman A, Ferris N. Self-assembled monolayers of alkanethiols containing a polar aromatic group: effects of the dipole position on molecular packing, orientation, and surface wetting properties. J. Am. Chem. Soc. 1991;113:4121–4131. doi: 10.1021/ja00011a010. [DOI] [Google Scholar]
- Finlay J.A, Callow M.E, Ista L.K, Lopez G.P, Callow J.A. The influence of surface wettability on the adhesion strength of settled spores of the green alga Enteromorpha and the diatom Amphora. Integr. Comp. Biol. 2002;42:1116–1122. doi: 10.1093/icb/42.6.1116. [DOI] [PubMed] [Google Scholar]
- Granhag L.M, Finlay J.A, Jonsson P.R, Callow J.A, Callow M.E. Roughness-dependent removal of settled spores of the green alga Ulva (syn Enteromorpha) exposed to hydrodynamic forces from a water jet. Biofouling. 2004;20:117–122. doi: 10.1080/08927010410001715482. [DOI] [PubMed] [Google Scholar]
- Higgins M.J, Molino P, Mulvaney P, Wetherbee R. The structure and nanomechanical properties of the adhesive mucilage that mediates diatom–substratum adhesion and motility. J. Phycol. 2003;39:1181–1193. doi: 10.1111/j.0022-3646.2003.03-027.x. [DOI] [Google Scholar]
- Hoipkemeier-Wilson L, Schumacher J.F, Carman M.L, Gibson A.L, Feinberg A.W, Callow M.E, Finlay J.A, Callow J.A, Brennan A.B. Antifouling potential of lubricious, micro-engineered, PDMS elastomers against zoospores of the green fouling alga Ulva (Enteromorpha) Biofouling. 2004;20:53–63. doi: 10.1080/08927010410001662689. [DOI] [PubMed] [Google Scholar]
- Ista L.K, Callow M.E, Finlay J.A, Coleman S.E, Nolasco A.C, Simons R.H, Callow J.A, Lopez G.P. Effect of substratum surface chemistry and surface energy on attachment of marine bacteria and algal spores. Appl. Environ. Microbiol. 2004;70:4151–4157. doi: 10.1128/AEM.70.7.4151-4157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Shon Y.-S, Colorado R, Jr, Guenard R.L, Lee T.R, Perry S.S. The influence of packing densities and surface order on the frictional properties of alkanethiol self-assembled monolayers (SAMs) on gold: a comparison of SAMs derived from normal and spirodialkanethiols. Langmuir. 2000;16:2220–2224. doi: 10.1021/la9909345. [DOI] [Google Scholar]
- Leggett G.J. Friction force microscopy of self-assembled monolayers: probing molecular organisation at the nanometre scale. Anal. Chim. Acta. 2003;479:17–38. doi: 10.1016/S0003-2670(02)01575-1. [DOI] [Google Scholar]
- Leggett G.J, Brewer N.J, Chong K.S.L. Friction force microscopy: towards quantitative analysis of molecular organisation with nanometre spatial resolution. Phys. Chem. Chem. Phys. 2005;7:1107–1120. doi: 10.1039/b417136p. [DOI] [PubMed] [Google Scholar]
- McDermott M.T, Green J.-B.D, Porter M.D. Scanning force microscopic exploration of the lubrication capabilities of n-alkanethiolate monolayers chemisorbed at gold: structural basis of microscopic friction and wear. Langmuir. 1997;13:2504–2510. doi: 10.1021/la962099m. [DOI] [Google Scholar]
- Pettitt M.E, Henry S.L, Callow M.E, Callow J.A, Clare A.S. Activity of commercial enzymes on settlement and adhesion of cypris larvae of the barnacle Balanus amphitrite, spores of the green alga Ulva linza, and the diatom Navicula perminuta. Biofouling. 2004;20:299–311. doi: 10.1080/08927010400027068. [DOI] [PubMed] [Google Scholar]
- Porter M.D, Bright T.B, Allara D.L, Chidsey C.E.D. Spontaneously organised molecular assemblies. 4. Characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry. J. Am. Chem. Soc. 1987;109:3559–3568. doi: 10.1021/ja00246a011. [DOI] [Google Scholar]
- Schultz M.P, Finlay J.A, Callow M.E, Callow J.A. A turbulent channel flow apparatus for the determination of the adhesion strength of microfouling organisms. Biofouling. 2000;15:243–251. [Google Scholar]