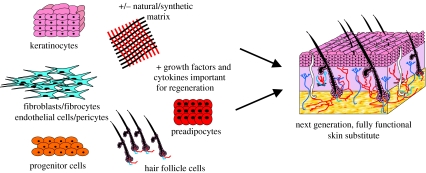
Abstract
Advanced therapies combating acute and chronic skin wounds are likely to be brought about using our knowledge of regenerative medicine coupled with appropriately tissue-engineered skin substitutes. At the present time, there are no models of an artificial skin that completely replicate normal uninjured skin. Natural biopolymers such as collagen and fibronectin have been investigated as potential sources of biomaterial to which cells can attach. The first generation of degradable polymers used in tissue engineering were adapted from other surgical uses and have drawbacks in terms of mechanical and degradation properties. This has led to the development of synthetic degradable gels primarily as a way to deliver cells and/or molecules in situ, the so-called smart matrix technology. Tissue or organ repair is usually accompanied by fibrotic reactions that result in the production of a scar. Certain mammalian tissues, however, have a capacity for complete regeneration without scarring; good examples include embryonic or foetal skin and the ear of the MRL/MpJ mouse. Investigations of these model systems reveal that in order to achieve such complete regeneration, the inflammatory response is altered such that the extent of fibrosis and scarring is diminished. From studies on the limited examples of mammalian regeneration, it may also be possible to exploit such models to further clarify the regenerative process. The challenge is to identify the factors and cytokines expressed during regeneration and incorporate them to create a smart matrix for use in a skin equivalent. Recent advances in the use of DNA microarray and proteomic technology are likely to aid the identification of such molecules. This, coupled with recent advances in non-viral gene delivery and stem cell technologies, may also contribute to novel approaches that would generate a skin replacement whose materials technology was based not only upon intelligent design, but also upon the molecules involved in the process of regeneration.
Keywords: skin, regeneration, stem cells, tissue engineering, scarring
1. Overview
Tissue engineering is emerging as an interdisciplinary field in biomedical engineering that aims to regenerate new biological material for replacing diseased or damaged tissues or organs. To achieve this, not only is a source of cells required, but also an artificial extracellular matrix (ECM) upon which the cells can be supported. In humans, skin represents approximately one-tenth of the body mass, and damage such as trauma, disease, burn or surgery to a part of this major organ has dramatic consequences. Engineering skin substitutes represent a prospective source of advanced therapy in combating acute and chronic skin wounds. At the present time, there are no models of bioengineered skin that completely replicate the anatomy, physiology, biological stability or aesthetic nature of uninjured skin. Skin substitutes should have some essential characteristics which include: being easy to handle and apply to the wound site; provide vital barrier function with appropriate water flux; be readily adherent; have appropriate physical and mechanical properties; undergo controlled degradation; be sterile, non-toxic, non-antigenic; and evoke minimal inflammatory reactivity. Additionally, they should incorporate into the host with minimal scarring and pain and facilitate angiogenesis, while still being cost effective. The ultimate goal of the tissue engineer is to satisfy most if not all of these criteria when producing novel, smart skin replacement therapies. Such matrices should attempt to model the properties of ECM in that, as far as it is feasible, they have the ability to release a multitude of growth factors, cytokines and bioactive peptide fragments in a temporally and spatially specific event-driven manner. This timed and focal release of cytokines, enzymes and pharmacological agents should promote optimal tissue repair and regeneration of full-thickness wounds. Tissue engineering generally requires an artificial ECM that can be assimilated into the body when the new tissue is regenerated. Materials for such a matrix could be naturally occurring substances such as collagen or could be prepared from biodegradable polymers. Resorption, along with adequate cell adhesion onto the matrix surface, gives the biological materials an attractive potential in tissue regeneration.
A major consideration when developing a tissue engineering strategy for promoting repair and regeneration is to identify suitable sources of cells and mechanisms by which they can function and interact properly. These cells would also have to be abundant enough to be able to carry out the regeneration process completely. Developmental biology conundrums such as regional specification of the embryo posed a great many questions 20 years ago but by now are almost solved. Unfortunately, progress towards a definitive understanding of morphogenetic movements, the absolute timing of developmental events and the regeneration of missing parts has been slower (Slack 2003). Recently, however, some of the major advances in molecular biology have been applied to the understanding of wound healing, development and regenerative processes (Harty et al. 2003). Tissue engineering as a discipline is becoming more aware of this knowledge base and there are now moves towards designing artificial tissues and organs using both cells and specifically designed materials. This review article outlines how our present understanding of materials science, regeneration biology and current advances in proteomics and genomics could be incorporated into a multidisciplinary translational research approach for developing novel smart matrices.
2. Skin and present concepts of tissue engineering
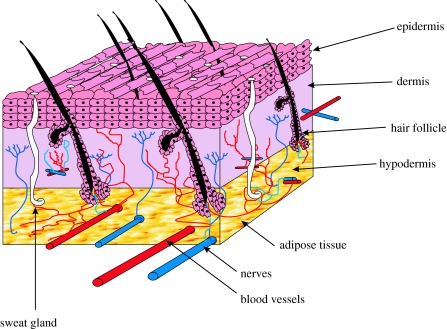
The skin, the largest organ of the body in vertebrates, is composed of the epidermis and dermis with a complex nerve and blood supply (figure 1). A third layer, the hypodermis, is composed mainly of fat and a layer of loose connective tissue. These three layers play an important role in protecting the body from any mechanical damage such as wounding. The epidermis is thin and totally cellular, but has sufficient thickness to provide vital barrier function. Mammalian epidermis and its appendages (hair, nail, sweat and sebaceous glands) maintain homeostasis by constant recycling of the basal cell layer. The epidermis is also exposed to ultraviolet radiation, and the resulting damage is one of the factors contributing to the constant sloughing of cells from the stratum corneum, which are replaced by migrating cells from the basal layers (Alonso & Fuchs 2003). The dermis situated directly below the epidermis constitutes the bulk of the skin and is composed of collagen with some elastin and glycosaminoglycans (GAGs). The major cell type present in the dermis is fibroblast, which is capable of producing remodelling enzymes such as proteases and collagenases, which play an important role in the wound healing process. The hypodermis is the layer located beneath the dermis and contains a considerable amount of adipose tissue that is well vascularized and contributes to both the mechanical and the thermoregulatory properties of the skin.
Figure 1.
A schematic of the structure of skin.
The challenge facing the tissue engineer is to combine novel materials with living cells to produce a skin equivalent which is both functional and durable, and allows the integration and manipulation of the cell biology of host cells and the multitude of signals that control their behaviour. Conventionally, tissue-engineered skin exists as cells grown in vitro and subsequently seeded onto a scaffold or some porous material which is then placed in vivo at the site of injury. At present, there are a number of different engineered skin substitutes available for clinical use, all of which fail to fulfil the criteria for fully functional skin (reviewed extensively in Supp & Boyce 2005).
3. Biomaterials: taking cues from nature to create artificial extracellular matrix
Engineering of skin substitutes implies deliberate design and fabrication according to specific functional objectives (Boyce & Warden 2002). So far, that design specification in skin has relied upon the creation of both artificial dermal and epidermal components which when combined produce a replacement skin, which can be grafted in place (reviewed in Supp & Boyce 2005). Materials used as artificial ECM to date include those derived from naturally occurring materials and those manufactured synthetically. Examples of natural materials include polypeptides, hydroxyapatites, hyaluronan, glycosaminoglycans (GAGs), fibronectin, collagen, chitosan and alginates. Such materials have the advantage that they have low toxicity and a low chronic inflammatory response. Examples of synthetic materials include polyglycolide, polylactide and polylactide coglycolide, which are used for sutures and meshes (Vats 2003); other examples include polytetrafluoroethylene and polyethylene terephthalate.
Matrices used routinely in therapeutic applications are made from polymers that are often resorbed or degraded in the body. Two key challenges exist here. First, the primary generation of degradable polymers widely used in tissue engineering were adapted from other surgical uses and have deficiencies in terms of mechanical and degradation properties (Griffith 2002). To overcome this, new classes of degradable materials are being developed, considered later in this review. The second major challenge is how to fabricate these polymers into scaffolds that have defined shapes and a complex porous internal architecture that can direct tissue growth (Griffith & Schwartz 2006). New technologies are emerging for accurate manufacture, creating materials of defined pore size using novel technologies using three-dimensional printing (Mironov et al. 2003; Seitz et al. 2005) and electrospinning (Luu et al. 2003; Li et al. 2005). Equally importantly, these new polymers and their degradation products must also be non-toxic and non-immunogenic upon implantation and degradation. A major disadvantage of synthetic materials is the lack of cell-recognition signals. Manufacturing processes are now being developed which will incorporate into biomaterials, cell-adhesion peptides, which are known to be involved in cellular interactions.
4. Natural biomaterial sources for tissue engineering
Cells adhere and interact with their environment using integrins, focal adhesions and their ability to stimulate downstream signalling pathways. Such mechanotransduction signals conveyed to cells via their adhesion to the matrix also clearly regulate the development of various tissues, and this is becoming a focus point in designing intelligent matrices. There are obviously many different naturally derived biomaterials that could be considered for use in a tissue engineering context. For the purposes of this review, we have concentrated on just two examples of frequently used natural scaffolds: collagen and fibronectin.
4.1 Collagen
One of the most abundant naturally occurring proteins responsible for maintaining structural integrity is collagen. Collagens present in the skin are mainly synthesized by fibroblasts and myofibroblasts. In tissues, such as skin, tendons and bone, that undergo shear and tensile stresses, collagen is arranged in fibrils. Type I collagen is present in the dermis, fasciae and tendons and is a major component of scar tissue. Of the 20 different types of collagen, collagens I, II, III, V and XI assemble into fibrils. Other collagens form networks, e.g. collagen IV, the major component of basement membrane. Collagens can also form transmembrane proteins, beaded structures or associate with fibril surfaces. Collagen has been used for some time in the design of skin substitutes (Supp & Boyce 2005) and recently has been used to create a model of endothelialized, reconstructed dermis that promotes the spontaneous formation of a human capillary-like network (Hudon et al. 2003; Tremblay et al. 2005). Butler & Orgill (2005) describe a tissue engineering technique that combines disaggregated autologous keratinocytes and a highly porous, acellular collagen–glycosaminoglycan matrix that has been shown in a porcine model to regenerate dermis and epidermis in vivo. Such skin substitute technology may have useful applications, if it can be proven to work in a clinical setting.
4.2 Fibronectin
A major multifunctional component of the ECM is fibronectin. In designing a fully functional skin substitute, there are a multitude of considerations, not least of which are the mechanical forces and interactions brought about by wound contraction. This involves a complex interplay between the fibroblast cytoskeleton and integrins with their ECM ligands. In dermal fibroblasts, most of these interactions are mediated by the β1-type integrins (Sethi et al. 2002). Integrins such as α5β1 and αvβ3 are the major receptors that direct fibronectin matrix assembly (Ruoslahti 1991; Wu et al. 1996). These and other integrins also interact with the actin cytoskeleton, another essential factor in matrix assembly (Hynes 1990). One of the major problems with currently available skin substitutes is their failure to vascularize. Mechanical stretching is known to affect fibronectin function (Rosso et al. 2005). Vogel & Baneyx (2003) have shown that excessive tension generated by cells in contact with biomaterials render fibronectin fibrils non-angiogenic, which may provide an explanation towards why biomaterials fail to vascularize. Fibrin, associated with fibronectin, has been shown to support keratinocyte and fibroblast growth both in vitro and in vivo, and may enhance cellular motility in the wound (Currie et al. 2001). When used as a delivery system for cultured keratinocytes and fibroblasts, fibrin glue may provide similar advantages to those proven with conventional skin grafts (Currie et al. 2001). Fibrin matrix has been shown to be a suitable delivery vehicle for exogenous growth factors that may in the future be used to accelerate wound healing (Gwak et al. 2005).
5. New age biopolymers
Protein components of the ECM, such as collagen, fibrin, laminin or elastin as well as mixtures of these components in Matrigel (Raeber et al. 2005), are widely used as cell-culture substrates due to their inherent resemblance to the natural ECM and complex signalling capabilities. Most biopolymers form complex three-dimensional fibrillar matrices in a self-assembly and/or enzyme-catalysed process. The matrix architecture can be altered by varying solid content or gelation conditions, or by adding molecules that induce fibre aggregation or interfere with the cross-linking mechanisms (Kuntz & Saltzman 1997; Hayen et al. 1999). The use of synthetic degradable gels is emerging primarily as a way to deliver cells and/or molecules in situ; these are the so-called smart matrices. A predominant approach, pioneered by Hubbell and co-workers, is the formation of photopolymerizable gels using polyethylene oxide-based macromer substrates (Desai & Hubbell 1991). Hubbell and colleagues have also explored a novel class of synthetic ECM analogues, which due to their homogeneous nanoscale microstructure were anticipated to restrict cell migration to proteolytic remodelling (Raeber et al. 2005). These molecularly engineered synthetic hydrogels are based on end-functionalized multiarm polyethylene glycol (PEG) macromers, reacted via Michael-type addition under mild conditions with cysteine-containing peptide sequences. This results in a hybrid network that can be formed from aqueous precursors in the presence of cells. Such approaches are particularly amenable to inclusion of biologically active ligands (Seliktar et al. 2004; Van de Wetering et al. 2005). PEG acts as an inert structural platform due to its hydrophilicity and resistance to protein adsorption. Hence, it exhibits the desired biological signals uniquely from incorporated peptides or proteins, with minimal structural or chemical background (Raeber et al. 2005). Similarly, these authors have linked linear RGD-containing peptides as dangling ends and protease-sensitive oligopeptides as elastically active network chains to the PEG macromers to mimic the two essential biological functionalities of an ECM analogue: cell adhesion and degradability by proteases (Raeber et al. 2005).
6. Culturing skin components in the laboratory
Severely damaged full-thickness skin is incapable of spontaneous regeneration in mammals. To create a fully functional skin substitute, complete recapitulation of ontogenesis must occur and this is not yet possible in vitro (Boyce & Warden 2002). However, the phenotype expressed by human skin cells in culture closely resembles wound healing physiology (Clark 1996), which includes cytogenesis, morphogenesis and histogenesis but not organogenesis. The challenge is not only that cells can be guided to follow the wound healing process, but also, more importantly, for them to drive the processes of developmental regeneration. Then it may be possible to provide full function to an artificial skin substitute produced in this way. There are some examples in the literature where individual components of the skin have been cultured and transplanted successfully but not as part of a fully functional skin replacement. Connective tissue cells are thought to repopulate grafts from the wound bed, but many skin substitutes contain dermal fibroblasts to facilitate such repair processes (Hansbrough et al. 1992; Parenteau et al. 1992; Boyce et al. 1993). Melanocytes have also been used to repopulate burn scars and for the treatment of vitiligo (Lerner et al. 1987) as well as being added to various polymer scaffolds (Boyce et al. 1993; Swope et al. 1997). However, full restoration of skin sensation has not been demonstrated with either split thickness skin grafts or engineered skin, and sweat and sebaceous glands have only been transplanted experimentally (Ward et al. 1989; Jahoda et al. 1996). It is these latter structures, along with nerves and blood vessels, that are likely to prove the most challenging since even with skin autografts these structures are neither restored nor regenerated.
To date, the approach has been to create a very simple skin substitute using cell populations within the skin. Disaggregated autologous keratinocytes and a highly porous, acellular, collagen–glycosaminoglycan matrix have been shown in a porcine model to regenerate a dermis and epidermis in vivo (Butler & Orgill 2005). During regeneration, a basement membrane of normal appearance forms at the dermoepidermal junction and vascularization of the construct occurs (Butler & Orgill 2005). A large number of parenchymal cells are required to repopulate wounds and restore skin structure and populations of keratinocytes and fibroblasts can be grown in relatively large numbers over a period of two to three weeks. A fundamental basis for generation of skin substitutes comes about from the utilization of this rapid growth of cultured cells. As soon as skin cells are prepared in sufficient quantity, they need organizing within the substitute to recreate the anatomical composition of normal skin. Currently, it is possible to create a polarized environment in which to do this with populations of skin cells. Human keratinocytes can be co-cultured with a dermal substitute in vitro and exposed to an air interface to create a stratified epithelium (Kivinen et al. 1999). In culture, keratinocytes behave just as they would in vivo with cornified cells migrating towards the air interface to form a squamous epithelial surface. The fibroblasts forming the majority of the dermal substitute slowly degrade the biopolymer and lay down their own ECM. As the cell numbers increase, there is a concomitant increase in the number of soluble factors released into the microenvironment. Keratinocytes and fibroblasts are known to produce a wide variety of cytokines including various growth factors and inflammatory mediators as well as different matrix polymers and catabolic enzymes (Boyce et al. 1996). Allowing such cell types to interact may stimulate paracrine mechanisms to initiate production of cell proliferation factors, such as insulin-like growth factors and platelet-derived growth factor (PDGF), transforming growth factor-α (TGF-α) and -β (TGF-β) and basic fibroblast growth factor (bFGF), known to be important not only for cell competency but also for the process of wound healing and tissue regeneration.
7. Commercially available skin substitutes
Serious injury to the skin, such as burns, trauma or chronic ulcers, requires immediate coverage to facilitate repair and restore skin function. The gold standard for skin replacement is the autologous skin graft in which an area of suitable skin is separated from the tissue bed and transplanted to the recipient area on the same individual from which it must receive a new blood supply. Grafts may be full thickness in which a complete section of epidermis and dermis is transplanted, or split thickness that includes only part of the dermis. Full thickness grafts are less likely to take than split skin, but provide improved coverage and decreased wound contraction (Rudolph 1976). Syngeneic grafts, performed between genetically identical individuals such as monozygotic twins or mice of the same inbred strain, take equally well as autologous grafts. Xenogeneic skin grafting involves the transfer of tissue between species, but like allogeneic transplantation (from a non-genetically identical individual of the same species) there are problems with graft rejection. Allografts of cadaver skin are used as a temporary cover for full thickness burns but are subject to rejection, because antigens present in the donor tissue may elicit an immune reaction in the recipient (Burd & Chiu 2005). Cadaver skin can be chemically treated using a three-step process that includes epidermis removal, cell solubilization and dry preservation, in order to decrease the antigenic components. This leaves an immunologically inert acellular dermal matrix (Alloderm, Lifecell Corporation, Branchburg, NJ), which aids the regeneration of the underlying dermis. Alloderm has been successfully used in the resurfacing of full-thickness burn wounds in combination with an ultra-thin autograft which replaces the epidermis (Tsai et al. 1999). Other clinically useful products include Integra, Dermagraft, Apligraf and Epicel and although they do not fully recreate the functions and aesthetics of skin, they have established milestones in treatments of skin problems and disorders (extensively reviewed by Supp & Boyce 2005; Ehrenreich & Ruszczak 2006).
Early studies carried out by Rheinwald & Green (1975), Bell et al. (1979) and Yannas & Burke (1980) formed the basis for the development of future dermal, epidermal and composite skin replacements. The current focus of research is towards developing a tissue-engineered skin equivalent that combines living cells with natural or synthetic cellular components (Philips 1998; Auger et al. 2004). Removal of suitable skin for autologous grafting is a painful process and is not always possible if, for example, the patient has extensive burns. An engineered skin substitute would need to fulfil specific criteria to replace the many functions of human skin (Boyce 2001). It should act as a barrier to micro-organisms, control fluid loss and have long-term elastic durability. It must also be histocompatible, support wound healing, lack antigenicity and toxicity, yet also be cost effective (Harlan et al. 2002; Ahsan & Nerem 2005; Donohue et al. 2005). Table 1 summarizes skin substitutes that are available or have been available in the past for the treatment of skin injuries in humans along with the advantages and disadvantages associated with their use. At present, no manufactured skin substitute has provided an outcome consistently comparable to an autograft.
Table 1.
Examples of commercially created skin substitutes.
| commercial product name and manufacturer | epidermal component | dermal component | advantages | disadvantages | |
|---|---|---|---|---|---|
| epidermal substitutes | Epicel Genzyme Tissue repair Corporation | cultured epidermal autograft grown from patient skin biopsy | none | large area of permanent wound coverage with little risk of rejection | three weeks required to produce fragile confluent sheets, susceptible to blistering post-grafting |
| Laserskin Fidia Advanced Biopolmers | cultured autologous keratinocytes from skin biopsy in a perforated hyaluronic acid membrane | none | a less fragile delivery system for keratinocytes. Hyaluronic acid/cell interaction properties improve mechanical stability | minimum of three weeks required to expand keratinocyte population | |
| EpiDex Modex Therapeutiques | cultured autologous outer root sheath hair follicle cells | none | cells have increased proliferative capacity and can be cryopreserved for repeat applications. Success in chronic ulcer treatment | substitute takes up to six weeks after harvesting to produce. Product fragile | |
| Myskin CellTran | cultured autologous keratinocytes on a PVC polymer coated with a plasma-polymerized surface | Myskin with dermal fibroblasts under development | PVC encourages keratinocyte attachment and proliferation, providing a more stable delivery platform. Keratinocytes can be thawed for repeated application | up to 14 days required for cell expansion. Repeated application needed for good clinical outcome | |
| dermal substitutes | Alloderm Life Cell Corporation | none | processed cadaver allograft skin | processing helps reduce antigenic components. Successful in resurfacing full-thickness burns | problems of graft rejection and disease transfer |
| Dermagraft Advanced Biohealing, Inc. | none | allogeneic neonatal fibroblasts on a three-dimensional bioabsorbable scaffold | neonatal fibroblast rapidly proliferate to produce collagen, GAGs and growth factors to aid wound healing | potential risk of rejection and disease risk from fibroblasts, although none reported | |
| Integra Johnson & Johnson | synthetic polysiloxane polymer | bovine type I collagen and GAGs | encourages ingrowth of fibroblasts and epithelial cells. Epidermal equivalent replaced after 14 days with an autograft | bovine collagen presents and antigenicity and disease risk. Three weeks required to expand the dermal autograft | |
| Transcyte Advanced Biohealing, Inc. | thin silicone layer (Biobrane) | collagen-coated nylon mesh seeded with neonatal allogeneic fibroblasts | successfully used to treat second- and third-degree burns. Dermal fibroblasts secrete collagen, GAGs and growth factors to aid wound healing | nylon mesh not biodegradable. Rejection and disease risk from fibroblasts | |
| Permacol Tissue Sciences Laboratories | none | porcine-derived acellular dermal matrix | non-immunogenic due to processing to remove non-collagenous and cellular material. Supports host fibroblast infiltration and revascularization | revascularization sometimes inefficient to support overlying epidermal graft | |
| composite substitutes | Apligraf Organogenesis | human allogeneic neonatal keratinocytes | human allogeneic neonatal foreskin fibroblasts in bovine type I collagen, ECM proteins and cytokines | graft take comparable to autografts with good cosmetic results. Improves granulation tissue deposition and no signs of rejection observed | risk of chronic graft rejection and disease from allogeneic keratinocytes and fibroblasts. Requires repeated applications |
| OrCel Ortec International | human allogeneic neonatal keratinocytes | human allogeneic neonatal foreskin fibroblasts in a bovine collagen sponge | provides a favourable environment for host cell migration and provides a source of cytokines and growth factors | not intended for use as a permanent skin replacement. Designed as a biological dressing. Risk of rejection and disease from bovine collagen and other cells. |
8. Problems with existing commercially available skin substitutes
8.1 Reduced vascularization
When a skin graft is placed on a recipient wound bed, it must acquire a blood supply to maintain long-term survival and integrate with the host tissue. Some existing skin substitutes do allow angiogenesis to occur, but there it is still an area for improvement, particularly in cases where vascularization is not rapid enough. The repeated failure of fabricated skin replacements to adequately vascularize has led to renewed efforts to understand autologous skin graft revascularization (O'Ceallaigh et al. 2006). This inability for substitutes to ‘take’ leads to cells in the replacement dying and ultimately the construct sloughs away from the host. Until recently, revascularization of skin autografts was thought to occur either by direct anastomosis between graft vessels and bed vessels or by ingrowth of bed vessels (angiogenesis) into the graft (O'Ceallaigh et al. 2006). These authors have recently shown that the initial onset of revascularization is attributable to early anastomoses between graft and bed vessels, mainly within the central area of the graft (O'Ceallaigh et al. 2006). An obvious implication of this work is that bioengineered skin substitutes incorporating prefabricated vessels may vascularize more rapidly in a fashion similar to autologous skin grafts.
8.2 Scarring
The process of scarring is discussed extensively later in this review. In the context of skin grafting and substitution, scarring at the graft margins is problematic, functionally, mechanically and aesthetically. Scar tissue is not identical to the tissue which it replaces and is usually of inferior functional quality. For example, scars in the skin are less resistant to ultraviolet radiation, and sweat glands and hair follicles do not grow back within scar tissue. Presently available skin substitutes that integrate well often suffer from scarring problems at the graft margins. The next generation of skin substitute should incorporate anti-scarring technologies to address this problem.
8.3 Absence of differentiated structures
Bioengineered skin substitutes are often relatively simple single layer or bilayered structures. If ‘take’ is successful, then the substitute offers a barrier function similar to normal skin. The absence of complexity with regard to differentiated structures means that presently available treatments offer none of the many other characteristics of functioning skin. There is a lack of temperature control provided in normal skin by sweat and sebaceous glands, as well as hair follicles. Additionally, in presently available substitutes, insulation and an adequate vascular supply from adipose tissue do not exist. Skin substitutes often lack melanocytes and thus skin pigmentation, they also do not have nerve supplies and so suffer from a lack of sensation, both temperature and pressure. Critically, skin substitutes have no resident Langerhans cells which play an important function in immune regulation in the skin. A key development to enhance present skin replacement therapy would be to develop strategies to incorporate or induce differentiated structures into skin constructs. There are, however, extensive studies that have incorporated melanocytes and Langerhans cells in skin substitutes for testing purposes and melanocytes have also been incorporated in therapeutic products for the treatment of vitiligo. A recent study by Hachiya et al. (2005) used mixed cell slurries containing keratinocytes and fibroblasts with melanocytes on the backs of severe immunodeficient mice to produce a skin substitute with spontaneously sorted melanocytes. This may offer a means of treating both structural and cosmetic aspects of skin conditions. One of the most remarkable examples of true organogenesis from adult tissue in culture is described by Zheng et al. (2005). Here, the authors inject a mixture of isolated neonatal dermal cells with epidermal aggregates into the dermis of nude mice. These are then able to interact and undergo relatively normal hair morphogenesis to give rise to cycling hair follicles within 8–12 days. Such approaches hold great hope for the future of incorporating differentiated structures in a new generation of skin substitutes.
8.4 Delay involved with cell culture
Cells for the epidermal and dermal components can take between two and three weeks to expand to sufficient numbers for grafting purposes. This problem is highlighted in many of the skin substitutes considered in table 1. New developments of protocols for rapid cell expansion would help alleviate such problems, e.g. in severely injured patients.
8.5 Persistence of cells in heterologous grafts
Ideally, skin cells are obtained from the patient (autologous) but in some cases, such as large surface area burns and with some skin disorders, this is not always possible. Epidermal and dermal cells isolated from donor skin (allogeneic) have been used to develop commercial skin substitutes such as Apligraf and have demonstrated that the presence of skin cells can aid wound healing (Falanga & Sabolinski 1999). The use of allogeneic skin cells offers a means to develop an ‘off-the-shelf’ skin replacement therapy for immediate application to injured skin. It is thought that cell age may affect the duration that allogeneic cells survive in vivo. Male allogeneic skin cells shown to persist for up to 2.5 years were from a neonatal source (Otto et al. 1995). Foetal allogeneic fibroblasts have also been shown to exhibit a similar spatial and temporal pattern of persistence to that seen with syngeneic cells and were shown to migrate and proliferate within a wound environment (Sandulache et al. 2003). Cell persistence is therefore a key characteristic to understand when developing the next generation skin replacement. Cell persistence may be desirable when covering a large area of missing skin but brings with it long-term safety challenges, e.g. demonstrating lack of subsequent transformation.
8.6 Biocompatibility, mechanical and handling properties
One of the prerequisites of bioengineered skin is that it should be biocompatible, i.e. it will support appropriate cellular activity, including the facilitation of molecular and mechanical signalling cascades, in order to optimize tissue regeneration. Additionally, the bioengineered product will not induce any undesirable local or systemic responses in the host, e.g. chronic inflammation. Skin replacements should also have appropriate mechanical and handling properties that make it as mechanically durable as skin but with handling properties that allow clinicians to manipulate it in a surgical setting. As discussed earlier, currently available skin substitutes do not mimic normal skin composition. The three-dimensional architecture and mechanical properties of skin replacements are therefore completely different to normal skin. This is in part explained by the fact that the manufacturing processes employed are not sophisticated enough to recapitulate the developmental morphogenesis used to create skin naturally. In designing the next generation substitute, greater attention must be paid to overcoming some of these fundamental problems.
8.7 Cell source
In sourcing cells for a skin substitute, there are essentially three locations from which cell types can be derived: local; systemic; and progenitor cell populations. Cells sourced locally include fibroblasts, keratinocytes, melanocytes, adipocytes and hair follicle cells. Systemic cells are populations of cells that are resident in the blood or bone marrow system, an example of which would be fibrocytes, known to play a key role in skin wound healing (Bucala et al. 1994; Abe et al. 2001). Progenitor cells are also resident locally in stem cell niches such as the hair follicle; they are resident in the bone marrow or could arise from embryonic stem (ES) cell lines cultured in vitro.
8.8 Development, safety and product costs
The early stages of bringing a new tissue-engineered product to the market place can be a costly exercise and this has resulted in many of the smaller companies that have developed skin replacements having to file for bankruptcy. A final consideration for an off-the-shelf skin replacement must be the cost of the research and development process, product safety, clinical trials, storage, shelf-life and ultimately the cost of producing, marketing and selling the product.
The components of bioengineered skin replacements are continually being developed and improved. Hopefully, many of the above problems will be overcome with advances in research and development technologies. The next generation of skin replacements will probably evolve from lessons learned from our understanding of wound repair and regeneration.
9. Wound repair, scar-free healing and foetal regeneration in mammals
In order to fully understand the differences between regeneration and the normal outcome of tissue repair, namely fibrosis and scarring, it is useful at this point to briefly review the processes of normal wound repair, the scarring process and to consider some examples of mammalian tissues, which undergo scar-free healing. In designing a smart skin replacement for grafting, it is obvious that the mechanisms of both wound healing and regeneration must be at the forefront of the tissue engineers' mind.
Tissue repair is normally a rapid process that has been devised through evolution to allow animals to escape danger and rapidly recover tissue integrity using scarring to join the wound edges or to fill tissue voids (Caplan 2003). Wound healing has often been described as a sequential mechanism and it is more specifically an event-driven process, whereby signals from one cell type set off cascades in other cell types, which propel the wound through the phases of healing (Sweitzer et al. 2006). Adult wound healing is essentially a repair process, which normally exhibits scarring. Tissue repair begins immediately with fibrin clot deposition at the site of injury, preventing haemorrhage from damaged blood vessels. Circulating platelets then aggregate at the site of injury and various inflammatory mediators, such as PDGF, TGF-α and TGF-β, epidermal growth factor (EGF) and FGFs, are released. These molecules are also believed to play major roles downstream in the wound repair process (Chettibi & Ferguson 1999). The inflammatory response of adult tissues to wounding is characterized by an early influx of neutrophils whose numbers steadily increase and reach a maximum 24–48 h post-wounding (reviewed in Chettibi & Ferguson 1999). As the neutrophil numbers begin to decline, macrophages take over and repopulate the wound site. Re-epithelialization also occurs at the same time, with keratinocytes migrating across the granulation tissue from deep within the dermis and the basal cells of the wound edge. As soon as the keratinocytes have re-established the barrier property of the skin, they resume a basal cell phenotype upon contact inhibition and differentiate into a stratified squamous keratinizing epidermis (Schaffer & Nanney 1996).
The final phases of the inflammatory response and epithelialization coincide with the migration of fibroblasts and endothelial cells and the formation of granulation tissue. Angiogenesis and fibroplasia then take place, with fibroblasts becoming the dominant cell type, laying down collagen and ECM. Remodelling of collagen occurs using matrix metalloproteinases produced by the fibroblasts and macrophages, and this is a phase that can last several months and, in the adult, is characterized by scar formation (Chettibi & Ferguson 1999). The balance of newly formed collagen with the destruction of old collagen establishes the final physical characteristics of the scar.
Scars are the end point of the normal continuum of mammalian tissue repair and arise after almost every dermal injury (Bayat et al. 2003). One of the key aims of regenerative medicine is to produce novel replacement skin which not only has the structural and functional properties of the original but also incorporates into the host tissue without any scarring. Scarring can be assessed clinically using the Vancouver scar scale (Powers et al. 1999) or the Manchester scar proforma (Beausang et al. 1998). There are three different types of scars: atrophic; hypertrophic; and keloidal. Atrophic scars are depressed and cause a valley or depression in the skin. Hypertrophic scars are elevated and may subside with time. Keloids are scar tissue which behaves like a benign tumour. Keloidal scars are elevated, expansive and continue to grow beyond the margin of the original wound. In addition to appearance, location and orientation are also important considerations in determining scar type. All types of scarring can occur on all areas of the body, but some areas such as the chest, knees and elbows are more susceptible to scarring.
Foetal wound repair, however, is essentially a regenerative process, characterized by an absence of scarring and fibrosis (see reviews by McCallion & Ferguson 1996; Ferguson et al. 1996; Garg & Longaker 2000; Ferguson & O'Kane 2004). These differences in the healing processes have sparked great interest and have lead to the development of several animal models in which foetal scarless healing has been described. These include the sheep, pig, rabbit, mouse, rat, guinea-pig, chicken, opossum and monkey (Adzick & Longaker 1991; reviewed in Chettibi & Ferguson 1999). Wounds made in foetal tissues heal via different mechanisms and result in much less scarring than an equivalent wound in adult tissues (Whitby & Ferguson 1991a,b).
The characteristics of scar-free healing after incisional wounding have been shown in various studies (Ferguson & O'Kane 2004). They include minimal inflammation and complete restoration of normal skin structure, with normal collagen deposition and regularly distributed hair follicles, capillaries and glands. Such healing is believed only to occur through a gestational age equivalent to the first third of human development; after that time, normal scarring as evident in the adult occurs (extensively reviewed in Ferguson & O'Kane 2004).
Originally, it was thought that the sterile aqueous environment provided by the amniotic fluid was important in scar-free healing; however, studies on marsupials such as the opossum which are raised in the mothers pouch proved otherwise (Armstrong & Ferguson 1995). In this model, incisional wounds were made at an equivalent time to the other incisional wounding studies and newborn opossums were found to heal without scarring despite not being kept within a sterile amniotic environment. In another study by Longaker et al. (1994), sheep skin from an adult or late foetus was grafted onto a young foetus in utero and then incisionally wounded; the newborn lamb had scar tissue formation. Repair and regeneration of the skin therefore appear to be correlated with the degree of skin differentiation and the inflammatory response to wounding (McCallion & Ferguson 1995).
Key to the process of scar-free healing is the correct deposition of ECM molecules such a hyaluronan, various collagen types and tenascin-C (Whitby & Ferguson 1991a,b; Lindblad 1998; Chin et al. 2000). Embryonic wounds which heal without scarring exhibit a number of major differences from adult wounds which scar including:
lack of fibrin clots and platelet degranulation,
markedly reduced inflammatory response, consisting of small numbers of poorly differentiated inflammatory cells, and
markedly elevated levels of molecules involved in skin morphogenesis and growth.
A consequence of this is that the growth factor profile of embryonic healing wounds is very different from that of an adult. Scar-free embryonic wounds show reduced levels of TGF-β1, TGF-β2 and PDGF (released in the adult from degranulating platelets and inflammatory cells) and elevated levels of TGF-β3 (a skin morphogenetic molecule). Experimental manipulation of the growth factor profile of adult wounds by exogenous addition of TGF-β3 or neutralization of TGF-β1, TGF-β2, PDGF, etc., results in markedly reduced or absent scarring (reviewed in Ferguson & O'Kane 2004). Such implications may be very important in artificial skin substitutes or grafts to enhance host take and minimize scarring at the perimeter and base of the graft.
10. Harnessing regenerative mechanisms to technologically advance current skin substitutes
Medical interest around regeneration has often focused on the repair of damaged adult tissues. The challenge faced here would be to incorporate molecules associated with the regeneration process into a smart matrix. There are only a few examples in vertebrate species of tissues where the initial phase of repair is followed by perfect functional and structural restoration of the organ. Regeneration has captured scientists' imaginations for well over 200 years since the early experiments carried out by Trembley in 1744 on hydra (Fallon 2003). Classically, when we consider regeneration in mammals, we think of liver regeneration (Michalopoulos & DeFrances 1997; Fausto 2000), rabbit ear regeneration (Goss & Grimes 1975), axolotl and Xenopus limb regeneration (Goss & Holt 1992; Gardiner & Bryant 1996), antler regeneration (Goss 1995; Allen et al. 2002), regeneration of the digit tip in a child (Douglas 1972; Illingworth 1974; reviewed in Han et al. 2005) or interdental papilla regeneration (reviewed in Chai & Slavkin 2003).
Regeneration is usually described as comprising one or both of two processes:
Epimorphic regeneration where replacement cells arise from undifferentiated cells (either stem cells or dedifferentiated cells) that form a blastema from which the new structures derive.
Morphallactic regeneration where new cells are derived from existing tissues by cell differentiation and/or migration.
Regeneration may have important links to development where undifferentiated stem cells are the source from which differentiated cell types arise in order to create (or recreate) functionally organized adult tissues. Recently, a murine model for mammalian wound repair and regeneration was discovered by Clark et al. (1998). Two millimetre through-and-through punch wounds made into the ears of MRL/MpJ mice closed with regeneration after 30 days, whereas they did not close in the control strain, C57BL/6 mice. MRL/MpJ-Faslpr mice, homozygous for the lymphoproliferation spontaneous mutation, show systemic autoimmunity, massive lymphadenopathy associated with proliferation of aberrant T cells, arthritis and immune complex glomerulonephrosis. The lpr defect is due to a mutation in the fas gene, which leads to an inability to mediate apoptosis of lymphocytes (Watanabe-Fukunaga et al. 1992). The MRL/MpJ strain, bred as a control for the MRL/MpJ-Faslpr strain, also exhibits autoimmune disorders but symptoms are manifested much later in life compared with those of the MRL/MpJ-Faslpr mice. The MRL/MpJ mouse has the same regenerative capacity as the −Faslpr strain, and since the autoimmune symptoms occur later, it is often the strain chosen to observe the regenerative mechanisms.
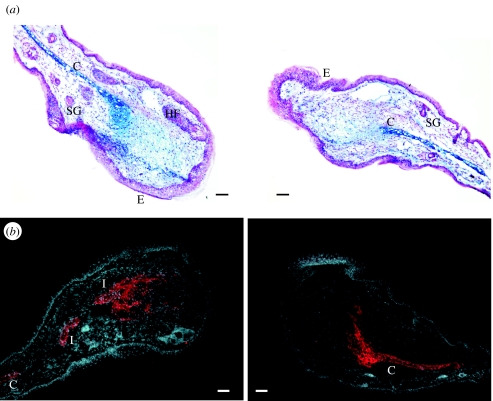
A further study by Rajnoch et al. (2003) details more specifically how the MRL/MpJ mouse ear wound closes in response to different traumas. These authors compared the effects of two different types of punches (a crude thumb punch and a clinical biopsy punch) on three strains of mice. MRL/MpJ ear wounds healed faster than either C57BL/6 or Balb/c mice. The MRL/MpJ mouse ears healed with enhanced blastema formation and markedly thickened tip epithelium. Rajnoch et al. (2003) postulated that the speed by which a punched ear hole closes might be an important factor before the onset of differentiation. The histological organization of the regenerating MRL/MpJ ear edges closely resembles the blastema of a regenerating limb (figure 2).
Figure 2.
Transverse sections through an MRL/MpJ ear 21 days post-biopsy punch wounding. The histological organization is blastema-like in structure. (a) shows an ear section stained with Alcian Blue and fast red. The cut end of the cartilage (C) can be seen clearly. Glycosaminoglycan deposits in the mesenchyme are stained blue. The apical epithelium (E) extends away from the cut cartilage. (b) shows a similar ear section stained with anti-Aggrecan-TRITC, a cartilage precursor molecule. The section is counterstained with the nuclear dye, DAPI. Note the formation of cartilage islands (I) in the mesenchyme and the infiltration of Aggrecan into the mesenchyme. HF, hair follicle; SG, sebaceous gland. Scale bar, 100 μm.
Furthermore, the regenerative ability of this strain may be restricted to the ear owing to its structure: a thin tissue covered on both sides by epithelium (Rajnoch et al. 2003). The small dimensions of the ear were thought to allow for the diffusion of growth factors and the establishment of appropriate gradients similar to embryonic development, whereas in large thick tissues, e.g. skin, this may not be possible (Rajnoch et al. 2003). This was confirmed in studies involving wounding both the ear and the back skin of the MRL/MpJ mouse, where the ear wounds completely regenerate, whereas the back skin wounds heal with a scar (Metcalfe et al. 2006). In keeping with studies of scar-free embryonic skin wound healing (Whitby & Ferguson 1991a,b; Ferguson et al. 1996; Cowin et al. 1998, 2001a) and the therapeutic manipulation of adult dermal healing to reduce scarring (Shah et al. 1995), the reduced inflammatory infiltrate seen with the biopsy as opposed to the crude thumb punch correlated with a faster, more regenerative repair process with reduced scarring. Other studies on athymic nude-nu mice have also shown that wound healing of the ear resembles regeneration (Gawronska-Kozak 2004). In this instance, it is thought that the absence of T-lymphocytes in wounded ears provides a microenvironment conducive to regeneration of mesenchymal tissues (Gawronska-Kozak 2004; Gawronska-Kozak et al. 2006).
The process of regeneration involves the complex tissue remodelling brought about by the formation of a blastema during healing, after which time, cartilage, sebaceous glands, hair follicles and blood networks reform. Thus, scarless healing accompanies blastema formation and regrowth of various tissues such as cartilage and other differentiated structures (Clark et al. 1998; Rajnoch et al. 2003; Metcalfe & Ferguson 2005). The tissue restoration process in the MRL/MpJ and nude-nu mice resembles foetal-like healing and is a rare example of regeneration among adult mammals. Understanding and characterizing the molecules involved in this regeneration from a tissue engineering perspective could lead to fundamental changes in skin substitute design.
11. Repair and regeneration share many common factors
A general problem in regeneration research is to understand how the events of tissue injury, which would normally result in simple repair, are subtly coupled and able to diverge towards the activation of plasticity in residual progenitor cells, resulting in tissue regeneration. The likelihood is that tissue repair and regeneration are not that dissimilar and, in fact, they share many common mechanisms that differ very subtly, a few examples of which will now be discussed.
11.1 Thrombin activation
The regulation of the fibrinolytic system is of critical importance during haemostasis, wound repair, neoplasia, inflammation and a variety of other biological processes. Successful haemostasis and wound repair is dependent on platelet adhesion and aggregation. Prothrombin activator converts plasma prothrombin into thrombin, which is integral to the blood-clotting process. Platelets release fibrinogen which once converted to fibrin by thrombin adds to the fibrin clot. In addition to activation of fibrin, thrombin facilitates migration of inflammatory cells to the site of injury by increasing vascular permeability. By this mechanism, factors and cells necessary for wound healing flow from the intravascular space and into the extravascular space.
In salamanders, it is thought that the local activation of thrombin is a key to the regenerative processes observed. Apart from the role that it plays in wound repair, thrombin is known to be an activator of S-phase re-entry in differentiated cells in newt myotubes (Imokawa & Brockes 2003; Imokawa et al. 2004; Brockes & Kumar 2005). A necessary aspect of this response is the inactivation of Rb by phosphorylation allowing transit through the G1/S restriction point (Imokawa et al. 2004). Cultured mouse myotubes missing both copies of the Rb gene can also be induced to re-enter S-phase. From these and other studies, an important parallel has been noted between newt and mouse myotubes—both are refractory to the familiar serum growth factors that act on mammalian myoblasts indicating that the post-mitotic arrest operates in both contexts (Tanaka et al. 1999). Responsiveness to thrombin-derived activity is a property of newt myotubes but not of mouse myotubes and the role of thrombin in dedifferentiation of newt myotubes is well documented (Tanaka et al. 1999). Lens regeneration is also a thrombin-dependent response (Imokawa et al. 2004). Lens regeneration in urodele amphibians such as the newt proceeds from the dorsal margin of the iris, where pigment epithelial cells (PEC) re-enter the cell cycle and transdifferentiate into lens (Imokawa et al. 2004). The axolotl, a related species that can regenerate its limb but not its lens, activates thrombin after amputation but not after lens removal. Apparently, all vertebrates possess PECs that are capable of transdifferentiating into lens under appropriate conditions, but only certain species of adult newts and fishes can regenerate the lens (reviewed extensively by Tsonis 2006). One theory for the newt's ability to regenerate the lens and the inability of the axolotl to do the same is that because the latter has lost the ability to activate thrombin on the dorsal iris, it no longer expresses tissue factor in this location (Imokawa & Brockes 2003).
11.2 Complement family members
The complement system plays an essential role in skin repair acting as host defence against infectious agents and in the inflammatory process. The complement pathway is activated during the inflammatory phase of wound healing. C5a, C6 and C7 are chemotactic agents for neutrophil migration. C3a, C4a and C5a cause degranulation of mast cells, leading to release of histamine and increased vascular permeability. The membrane attack complex composed of C5b, C6, C7, C8 and C9 is responsible for cytolysis.
Certain members of the complement family also appear to play a role in regeneration. There is increasing evidence that C5 plays an important role in mammalian liver regeneration, probably by mediating the release of IL-6 from Kupffer cells (Mastellos et al. 2001). Additionally, C3 and C5 are expressed in the newt limb blastema and C5 in the regenerating lens (Kimura et al. 2003; Tsonis et al. 2006). Positional memory is a critical aspect for the autonomy of limb regeneration, because it specifies the initial population of blastemal cells in relation to the extent of the axis to be regenerated (Brockes & Kumar 2005). An understanding of its molecular basis is important for our appreciation of how stem cells are specified to give rise to different structures, rather than to different cell types. Studies on limb regeneration have led to the identification of Prod 1, a gene that is regulated by proximodistal axis and retinoic acid (Brockes & Kumar 2005). Prod 1 is thought to act as a cue for local cell identity that is expressed in the normal limb and persists in blastemal cells. Prod 1 is apparently the newt orthologue of mammalian CD59, as evidenced by the prediction of secondary structure (Brockes & Kumar 2005). Interestingly, CD59 protein in mammals is associated with the inhibition of the terminal phase of complement activation (Murray & Robbins 1998).
11.3 TGF-β family isoforms
As a consequence of an altered inflammatory response and skin morphogenesis, the growth factor profile of a healing embryonic wound is very different qualitatively, quantitatively and temporally compared with an adult wound (Whitby & Ferguson 1991b; O'Kane & Ferguson 1997; Cowin et al. 2001a,b; Ferguson & O'Kane 2004). Embryonic wounds express very high levels of TGF-β3 and very low levels of TGF-β1 and TGF-β2. By contrast, adult wounds contain predominantly TGF-β1 (and TGF-β2), which is derived initially from degranulating platelets and subsequently from inflammatory cells such as monocytes and macrophages.
Application of neutralizing antibodies to TGF-β1 and/or TGF-β2 (preferably both) to healing adult rodent wounds results in markedly improved scarring (Shah et al. 1992, 1994a,b, 1995). Interestingly, pan-neutralization of all the three TGF-β isoforms (TGF-β1, TGF-β2 and TGF-β3) does not improve scarring, suggesting that neutralization of TGF-β3 may be detrimental (Shah et al. 1994a,b, 1995). By contrast, exogenous addition of TGF-β3 to healing adult wounds (to elevate levels similar to those seen in scar-free embryonic wounds) results in markedly improved or absent scarring during adult wound healing (Shah et al. 1995). From these studies, it can be seen that subtle alterations of the TGF-β isoform profile result in either scarring repair or scar-free healing.
11.4 The role of inflammation in wound repair and regeneration
Skin is the primary structure of the body's innate immunity, acting as a barrier to micro-organisms. The inflammatory response, initiated on injury to the skin as discussed earlier, is characterized by a series of events involving neutrophils, macrophages and monocytes which are all important for proper wound closure and adequate scar-forming repair mechanisms. Other innate receptors of the skin (dendritic cells) are also activated on wounding. They are located throughout the epithelium of the skin, where in their immature form they are attached by long cytoplasmic processes. The primary function of dendritic cells is to capture and present protein antigens to naive T-lymphocytes. Dendritic cells engulf micro-organisms and other materials and degrade them with their lysosomes. Peptides from microbial proteins are then bound to a groove of MHC-II molecules produced by macrophages, dendritic cells and B-lymphocytes. The peptide epitopes bound to the MHC-II molecules are then put on the surface of the dendritic cell, where they can be recognized by complementary shaped T-cell receptors and CD4 molecules on naive T4-lymphocytes.
The involvement of the immune system in the response to tissue injury has also raised the possibility that it might influence the outcome not only of tissue repair but also of tissue regeneration. A common hypothesis is that the process of inflammation may preclude the ability of a structure to regenerate. There is, however, emerging evidence of the immune system playing a more positive role in the regeneration of immune privileged sites such as the lens (Godwin & Brockes 2006). In various newt species, the ocular tissues such as the lens are regenerative and it has been recently shown that the response to local injury of the lens involves activation of antigen-presenting cells which traffic to the spleen and return to displace and engulf the lens, inducing regeneration from the dorsal iris (Godwin & Brockes 2006). Mammals have a very highly developed adaptive immunity and a relatively poor capacity to regenerate, whereas urodeles regenerate structures more easily but have a less robust immune system (reviewed in Godwin & Brockes 2006). According to these authors, immunomodulation of the injury response may play a positive or neutral role with respect to regeneration. The outcomes of wound repair and regeneration are profoundly different, but may be mechanistically linked by subtle differences in signalling pathways.
Understanding these subtle alterations between the various molecules and their pathways using model systems may begin to unravel the mechanisms underlying repair and regeneration. The likelihood is that the permissive environment necessary for regeneration to occur is similar to that observed during embryonic development. A more comprehensive appreciation of these permissive conditions has major implications for further advances in tissue engineering.
12. Isolating and characterizing genes and proteins involved in repair and regeneration
Alterations in gene expression patterns or in a DNA sequence can have profound effects on biological functions such as regeneration. These variations in gene expression are at the core of altered physiological and pathological processes. To that end, tissue-engineered scaffolds could provide a platform for enhanced regeneration using the scaffold for gene transfer or as a source of genetically altered cells (Vats 2003). Viral gene delivery has, until recently, been one of the only ways to effectively transfer genes therapeutically. Concerns over the safety of viruses as a gene carrier have led to the development of alternative gene delivery strategies. Hubbell and colleagues used fibrin as a surgical matrix to explore the potential of hypoxia-inducible factor-1α (HIF-1α) gene therapy in stimulating wound healing. Using a peptide-based gene delivery system, Trentin et al. (2006) expressed the HIF-1α δODD (oxygen-sensitive degradation domain) gene, translocated it to the nucleus under normoxic conditions and consequently upregulated vascular endothelial growth factor (VEGF)-A165 mRNA and protein levels in vitro. Peptide-DNA nanoparticles containing HIF-1α δODD entrapped in fibrin matrices were applied to full-thickness dermal wounds in the mouse. Angiogenesis was increased comparably strongly to that induced by VEGF-A165 protein alone (Trentin et al. 2006). These authors demonstrated that vessel maturity induced by HIF-1α δODD was significantly higher than that induced by VEGF-A165 protein, as shown by the stabilization of the neovessels with smooth muscle. This is one of many new approaches being developed to deliver genes non-virally and in a local way, representing a new therapeutic tool for the tissue engineer.
Recently, Larocca et al. (2002) have adapted filamentous phage vectors for targeted gene delivery to mammalian cells, enabling the delivery of growth factors such as FGF-2 and EGF. Targeted phage particles are specific for the appropriate target cell surface receptor and have distinct advantages over animal viral vectors, because they are simple, economical to produce at high titre, have no intrinsic trophism for mammalian cells and are relatively simple to genetically modify and evolve (Larocca et al. 2002). Other methodologies for targeted growth factor transfer include plasmid and adenoviral vectors immobilized in collagen–gelatin matrices. Such mixtures containing FGF-2 and FGF-6 were delivered to excisional muscle wounds, producing early angiogenic responses, increasing endothelial cell numbers that subsequently remodelled into arteriogenesis (Doukas et al. 2002). Muscle repair was also enhanced as FGF-treated wounds filled with regenerating myotubes. These biomatrix-gene delivery approaches continue to be developed and offer new approaches for tissue repair and regeneration.
DNA array technologies provide rapid and cost-effective methods of identifying gene expression and genetic variation. In the preparation of a skin substitute, it would be advantageous to know the genes that are involved in the process of both integration and regeneration. Cole et al. (2001) compared gene expression profiles in normal and injured skin from females undergoing breast reduction surgery. Specimens were collected at 30 min and 1 h after the initial injury. Representative cDNA from these samples was hybridized to microarray membranes containing cDNAs from 4000 genes. At 30 min, injury resulted in a consistent increase of 124 out of the 4000 genes and these genes were primarily involved in transcription and signalling (Cole et al. 2001). At 1 h, only 46 genes showed an increase in expression but a further 264 genes were significantly decreased, indicating silencing of many structural genes. In another study by Li et al. (2001), the expression of 8734 sequence-verified genes in response to ear punch was observed in MRL/MpJ-Faslpr mice compared with the slow-wound-repair strain, C57BL/6J mice. Many genes of unknown function (expressed sequence tags; ESTs) exhibited a more than twofold increase in MRL/MpJ-Fas(lpr) or C57BL/6J mice, suggesting that current understanding of the molecular events at the inflammatory stage of repair is still limited (Li et al. 2001). These authors concluded that fast-wound-repair in MRL/MpJ-Faslpr mice is mediated by a metabolic shift towards a low inflammatory response and an enhanced tissue repair. More recently, studies by Masinde et al. (2005) used restriction fragment differential display PCR to isolate genes differentially expressed in the MRL (good healer) mouse and the C57BL/6 (poor healer) mouse at different stages of wound healing. They identified 36 genes that were differentially expressed in the regenerating tissue of good and poor healer strains of which several genes are also genetically linked to wound healing. Microarray expression data have also recently been reported from studies on the PU.1 mouse. This mouse is genetically incapable of raising a normal inflammatory response, because it lacks macrophages and functioning neutrophils, but repairs skin wounds rapidly and with reduced fibrosis (Cooper et al. 2005). Cluster analysis of genes expressed after wounding wild-type mice versus PU.1 null mice distinguished between tissue repair genes and genes associated with inflammation (Cooper et al. 2005). These authors described several pools of genes, giving an insight into their likely functions during repair and hinting at potential therapeutic targets.
The use of stem cells as a component of a bioengineered substitute will be discussed elsewhere in this review; however, it is useful at this point to consider the power of DNA array technology when defining the ‘stem cell molecular signature’. Ivanova et al. (2002) and Ramalho-Santos et al. (2002) provided the first genome-wide transcript analysis of ES cells, haematopoietic stem cells and neural stem cells. Both groups generated about 200 genes that were upregulated in the tested stem cell populations. The analysis involved the use of Affymetrix mouse genome chips with up to 36 000 genes being able to be screened at once. Gosiewska et al. (2001) assessed the differential expression and regulation of ECM-associated genes in human foetal and neonatal fibroblasts. Foetal fibroblasts were found to secrete four- to tenfold more latent TGF-β1 and higher levels of collagen protein and mRNA for most types of collagen (particularly type III). mRNA for type V collagen was, however, significantly reduced in foetal cells. Approximately 20 other mRNAs for various cytokines, matrix molecules and receptors were also found to be similar between the two cell types (Gosiewska et al. 2001).
However, it must be noted that DNA/RNA analysis cannot predict the amount of a gene product that is made, if and when a gene will be translated, or the type and amount of post-translational modifications necessary for processes such as regeneration and wound healing. The Human Genome Project identified about 40 000 genes; these translate into approximately 300 000 to 1 million proteins when alternative splicing and post-translational modifications are considered. With the availability of DNA microarray analysis, permitting the expression of thousands of genes to be monitored simultaneously, it may be asked why proteomics is so important. Basically, proteins provide the structural and functional framework for cellular life; the genome in comparison is relatively static. However, there are several difficulties in the study of proteins that are not inherent in the study of nucleic acids. Proteins cannot be amplified like DNA; therefore, less abundant sequences are more difficult to detect. Additionally, proteins have secondary and tertiary structure that must often be maintained during their analysis. However, some studies have attempted an approach to determine protein profiles in skin regeneration. Li et al. (2000) identify the temporal protein profile of soft-tissue healing processes in the ear-punched tissue of regeneration strain, MRL/MpJ-Fas(lpr) mice, and compared it with the non-regeneration strain, C57BL/6J mice, using surface-enhanced laser desorption and ionization protein chip technology. Five candidate proteins were identified in which responses of MRL/MpJ-Fas(lpr) to the ear punch were two- to fourfold different compared with that of C57BL/6J mice (Li et al. 2000). Of the five candidate proteins, the amount of a 23.5 kDa protein in the ear-punched tissue was significantly correlated with the rate of ear healing in six representative strains of mice, making it a potential candidate for fast-wound-repair/regeneration. This protein was believed to be Ras-related protein RAB-8 (Li et al. 2000). Genomics and proteomics are therefore complementary fields, with proteomics defining the functional analysis. Although they constitute relatively new and under exploited disciplines, the future of bioengineering and regenerative medicine will be impacted greatly by both.
13. Incorporation of growth factors and cytokines in the creation of an intelligent skin substitute
Regeneration is characterized by a constantly changing environment in which cells are exposed to a complex pattern of molecular cues and signals, which impart positional information necessary for correct development. These cell signals trigger a series of events that in combination control cell proliferation, differentiation and cell death, such that a specific tissue can be delineated and its edges specifically defined. Since these molecules are often major components of early developmental pathways for cell specification, incorporating them into a tissue-engineered product could produce major advancements in regenerating adult structures such as skin. The number of morphogens that exist and are used in developmental and regenerative processes are obviously too numerous to catalogue in full. However, in this review, a few key growth factors and cell-signalling molecules will be considered, which if integrated into a bioengineered material could be critical to creating a fully functional skin replacement.
Platelet-derived growth factor (PDGF) is a potent activator for cells of mesenchymal origin, and a stimulator of chemotaxis, proliferation and new gene expression in monocytes, macrophages and fibroblasts, accelerating ECM deposition (Pierce et al. 1991; Shure et al. 1992). It has also been suggested that reduced levels of PDGF may play a role in the mechanism of scarless cutaneous repair (Whitby & Ferguson 1991a; Peled et al. 2001; Ferguson & O'Kane 2004). This family of growth factors exists in both homo- and heterodimeric forms and Pierce et al. (1989) reported that a single application of PDGF-BB to an incisional wound increased the neoangiogenesis through enhancement of endogenous PDGF-BB signalling (Kano et al. 2005). Gu et al. (2004) adenovirally delivered dose-related PDGF-B in a collagen-based biocompatible, gene-activated matrix to a rabbit dermal wound model. Dose-related increases in granulation tissue and cell proliferation were observed along with concordant expression of PDGF-B RNA and protein. No treatment-related changes in haematology, serum chemistry or histopathology were observed and no immunological responses against collagen were observed. These approaches for PDGF delivery have great potential in a tissue engineering context, where such insights into the mechanisms underlying the effects of growth factor co-stimulation could lead to a better design of therapeutic angiogenic strategies.
Cytokines of the transforming growth factor-β family (TGF-β) are multifunctional regulators of cell growth, differentiation and ECM formation (Roberts et al. 1990a,b). In mammals, there are three isoforms, TGF-β1, TGF-β2 and TGF-β3, and although they have up to 85% amino acid sequence homology, there are known differences in their potencies and biological activities in vivo. TGF-β is known to be the most potent growth factor involved in wound healing throughout the body (Whitby & Ferguson 1991a,b; Levine et al. 1993; Shah et al. 1995; Ashcroft et al. 1997; Cordeiro 2002; Cordeiro et al. 2003; Ferguson & O'Kane 2004). In particular, in relation to wound healing in the skin, TGF-β1 and TGF-β2 are implicated in cutaneous scarring, whereas TGF-β3 is known to have an anti-scarring effect (Shah et al. 1995; O'Kane & Ferguson 1997; Ferguson 2002; reviewed extensively in Ferguson & O'Kane 2004). One study has been performed looking at the effect of TGF-β1 delivered through a collagen scaffold (Pandit et al. 1999). Here, three 3×3 cm, full-thickness defects were created on the dorsi of 15 New Zealand White rabbits. Each rabbit had a control (no treatment), collagen scaffold and collagen scaffold with TGF-β1 (2 μg cm−2) and was observed for up to three weeks. In summary, a greater inflammatory response was found in the collagen scaffold-treated group, but the fastest epithelialization and contraction rates were associated with TGF-β and collagen (Pandit et al. 1999).
Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily. There are 15 members and although they are known for their role in bone and cartilage formation, they have diverse roles in many other developmental processes such as neuronal and dorsal/ventral specification. BMP-4, for instance, specifies the development of ventral structures, e.g. skin from ectoderm. In the developing limb bud, BMP-2 interacts with sonic hedgehog and fibroblast growth factor-4 (FGF-4) to allow chondrocyte and osteoclast precursors to form (Niswander & Martin 1993).
Fibroblast growth factors (FGFs) are a family of 21 isoforms with a broad spectrum of activities, including regulation of cell proliferation, differentiation and migration. FGFs 1, 2, 5, 7 and 10 are upregulated during adult cutaneous wound healing (Werner et al. 1992; Tagashira et al. 1997). Different FGFs are expressed throughout embryogenesis where they act as morphogens (Niswander & Martin 1992; Heikinheimo et al. 1994; Ohuchi et al. 1994; Rappolee et al. 1994). The FGFs are also necessary for proper limb development. Inactivating both FGF-4 and FGF-8 in the apical epidermal ridge (AER) produces severely deformed limb phenotypes. One hypothesis is that a principal function of AER–FGF signalling during normal limb development is to ensure that enough progenitor cells are available to form each element of the limb skeleton and perhaps in other tissues as well (Sun et al. 2002). FGF-1 and FGF-2 have also been implicated in limb regeneration in urodeles, since they both have been shown to control blastema cell proliferation (Zenjari et al. 1996; Ferretti et al. 2001). FGF-2 or basic FGF is released at the wound site by damaged endothelial cells and by macrophages, but if FGF-2 activity is blocked using monospecific antibodies raised against FGF-2, wound angiogenesis is almost completely blocked (Broadley et al. 1989). FGF-2 has also been implicated in scar-free healing (Spyrou & Naylor 2002). FGF-7 and FGF-10 are secreted by fibroblasts but act on keratinocytes to stimulate migration and proliferation (Tagashira et al. 1997). FGF-9 (glial-activating factor) on the other hand is neurotrophic (Kanda et al. 1999). An initial study by Kawai et al. (2000) has revealed that bFGF may have the ability to accelerate tissue regeneration in artificial dermis. These authors describe a dermal substitute which had gelatin–bFGF microspheres incorporated into its structure, the result of which accelerated fibroblast proliferation and capillary formation in a dose-dependent manner (Kawai et al. 2000).
However, the expression of FGFs during foetal skin development and scarless wound healing has not been properly characterized. Dang et al. (2003) hypothesized that differential expression of FGF isoforms and receptors occurs during foetal skin development and that this differential expression pattern may regulate the transition from scarless repair to healing with scar formation. After excisional wounding, expression of FGF-7 and FGF-10 was downregulated in scarless wounds, whereas FGF receptor 2 expression decreased in both scarless and scar-forming wounds. Expression of FGF isoforms 5 and 9 did not change in scarless wounds (Dang et al. 2003). These workers demonstrate an overall downregulation of FGF expression during scarless healing. Therefore, in an engineered substitute, it may be necessary to balance the levels of FGFs to ensure that there is no scarring but sufficient angiogenesis.
Vascular endothelial growth factor (VEGF) is induced during the initial phase of skin grafting, where endogenous fibrin clots are known to form a provisional matrix and to promote angiogenesis. Growth factors such as VEGF increase in such wounds to stimulate angiogenesis. There is some evidence in studies of diabetic mice that VEGF may promote healing; these mice fail to produce VEGF at the wound site and consequently healing is impaired (Frank et al. 1995). Fibrin rafts and fibrin glue have been used for skin grafting for some time; however, it remains unknown whether VEGF is induced when fibrin is used as a dermal substrate for cultured skin substitutes (Currie et al. 2001).
Recently, Hojo et al. (2003) investigated the effect of fibrin gel as a dermal substrate for a cultured skin substitute, using human keratinocytes and dermal fibroblasts. Experiments were performed using 12 cultured skin substitutes: four for histologic examination before transplantation; four for VEGF assay in vitro; and four for the transplantation to athymic mice. With in vivo transplantation, the fibrin-type cultured skin substitute showed an excellent take on the wound bed and a normally proliferating keratinocyte layer with emergence of vascular endothelial cells in the transplanted floor 3 days after transplantation. It would seem from this study that using fibrin as a dermal substrate for cultured skin substitute increases the secretion of VEGF, improves regeneration of mature epidermal structure after in vivo transplantation and promotes the migration of vascular endothelial cells.
Hypoxia is a potent stimulator of VEGF synthesis (Shweiki et al. 1992). Subsequent angiogenesis leads to the migration of new capillaries into the provisional matrix between the wound edges, initially a fibrin clot that is replaced by newly synthesized connective tissue. Angiopoietin-1 (Ang-1), another endothelial-specific growth factor, binds to and activates the receptor Tie-2, promotes endothelial cell survival and regulates the responsiveness of the endothelium to other cytokines. Therapeutic angiogenesis requires an understanding of how VEGF and Ang-1 interact to initiate neovascularization. Recently, Benest et al. (2006) have used adenovirus-mediated gene transfer into the adjacent fat pad of the rat mesentery to characterize induction of angiogenesis by VEGF and Ang-1. VEGF gene transfer produced short, narrow, highly branched and sprouting vessels, with normal pericyte coverage. Ang-1 gene transfer induced broader, longer neovessels with no increase in branching or sprouting, yet a significantly higher pericyte ensheathment. Combination of both Ang-1 and VEGF generated a significantly higher degree of functionally perfused, larger, less branched and more mature microvessels, resulting from increased efficiency of sprout to vessel formation (Benest et al. 2006). Thus, combinatorial additions of these factors to a bioengineered skin replacement could have profound effects upon successful neovascularization and take upon grafting.
Epidermal growth factor (EGF) has been implicated in wound healing and homeostasis in a number of tissues including colon, skin, mammary gland and liver (Wilcox & Derynck 1988). It is also known to be present at high levels in saliva and may accelerate healing of skin lesions in animals when they lick their wounds (Chettibi & Ferguson 1999). Topical application of EGF to human donor sites also appeared to accelerate wound healing (Falanga et al. 1992) and is also believed to stimulate keratinocytes to produce hyaluronan (Pienimaki et al. 2001). Within the last few years, a new class of ligand, the matrikine or matricryptin, has been characterized as subdomains of various ECM proteins capable of signalling to the cell through receptors, such as growth factor receptors (Tran et al. 2005). The EGF-like repeats of tenascin-C and laminin-5 signal to EGFR preferentially to upregulate migration during skin repair and tumour progression (Tran et al. 2005). It is thought that this new class of matrikine ligand will have an impact on dermatology and tissue engineering, as these proteins are altered in wound repair and skin diseases.
Hepatocyte growth factor/scatter factor (HGF/SF) is a pleiotrophic growth factor produced principally by cells of mesenchymal origin. HGF/SF is an important mitogen, morphogen and motogen known to play an important role in tumorigenesis and foetal development through the c-Met receptor tyrosine kinase (Boros & Miller 1995; Matsumoto et al. 1995; Zarnegar & Michalopoulos 1995). The association between HGF and c-Met receptor is known to be involved in morphogenesis and tissue organization during embryonic development and regeneration following tissue injury. HGF was first thought to be involved in cutaneous physiology and wound healing based on the observation that HGF stimulates keratinocyte migration and proliferation in vitro (Matsumoto et al. 1991; Sato et al. 1995). Transgenic expression of HGF in an epidermis-specific manner affects melanocyte development, leading to dermal melanocytosis (Kunisada et al. 2000). Cowin et al. (2001b) showed that expression of HGF and c-Met receptor increased in response to cutaneous wounding and transgenic overexpression of HGF resulted in increased vascularization and granulation tissue expansion (Toyoda et al. 2001). Recently, HGF has been implicated in enhancing the cutaneous wound healing processes of re-epithelialization, neovascularization and granulation tissue formation (Yoshida et al. 2003; Bevan et al. 2004).
Retinoic acid (RA) induces the ‘super-regeneration’ of organs that can already regenerate, such as the urodele amphibian limb, achieved by respecifying positional information in the limb (Maden & Hind 2003). In organs that cannot normally regenerate such as the adult mammalian lung, RA induces the complete regeneration of alveoli that have been destroyed by various noxious treatments. Another tissue that fails to regenerate is the mammalian central nervous system (CNS). RA does not induce neurite outgrowth as it does in the embryonic CNS, because one of the retinoic acid receptors, RARβ2, is not upregulated. However, when RARβ2 is transfected into the adult spinal cord in vitro, neurite outgrowth is stimulated (Maden & Hind 2003). Recent studies by Dmetrichuk et al. (2005) assessed the role of RA and one of its receptors, RARβ, in the reciprocal neurotrophic interactions between regenerating limb blastemas and spinal cord explants from the adult newt Notophthalmus viridescens. They found that endogenous RA is one of the trophic factors produced by the blastema and it is thought that it may be capable of guiding re-innervating axons to their targets. This may have important implications for the understanding of re-innervation of skin substitutes. Interestingly, from a skin engineering viewpoint in the diabetic mouse model, db/db, all trans-RAs have been shown to reverse the impaired wound healing displayed by this mouse (Kitano et al. 2001). RA therefore could be a candidate molecule for incorporation in modern tissue-engineered skin substitutes where cellular positional specification in addition to regeneration is necessary.
Homeobox (Hox) genes, although not strictly morphogens, are an evolutionarily conserved family of transcription factors and are attractive candidates for inclusion in a bioengineered product. Hox protein activity is essential during embryogenesis for the differentiation and specification of cell fate along the body axes (Krumlauf 1994; Manak & Scott 1994) and the Hox gene family have also been implicated as important factors in limb regeneration (Brockes 1997; Gardiner et al. 2002). Several Hox genes are expressed in mouse and human foetal and adult skin as well as nail and hair follicles (Stelnicki et al. 1997; Godwin & Capecchi 1998; Packer et al. 2000; Komuves et al. 2002). Particular attention has been drawn to Hoxb13, known to be downregulated during foetal scarless wound healing (Mack et al. 2003). This gene has been recently knocked out, the result of which is a mouse whose adult skin exhibits high levels of hyaluronan and enhanced wound healing (Mack et al. 2003). Hoxb13 overexpression in an adult organotypic epidermal model recapitulates actions of Hoxb13 reported in embryonic development (Mack et al. 2005). Epidermal cell proliferation is decreased, apoptosis increased and excessive terminal differentiation observed, as characterized by enhanced transglutaminase activity and excessive cornified envelope formation. Hoxb13 overexpression also produced abnormal phenotypes in the epidermal tissue that resembled certain pathological features of dysplastic skin diseases. Mack et al. (2005) suggest that Hoxb13 probably functions to promote epidermal differentiation, a critical process for skin regeneration and for the maintenance of normal barrier function.
The list of growth factors, cytokines and transcription factors that could be incorporated into a skin substitute as part of an intelligent design to facilitate skin regeneration are large; the above are just a few obvious examples. They do, however, represent an attractive group of factors, which if a suitable matrix could be found for defined release in both spatial and temporal dimensions could facilitate the rapid regeneration and integration of skin.
Alternatively a minimalistic engineering approach could be adopted to simulate embryonic development. Here, normal cell–cell interactions, e.g. epithelial–mesenchymal interactions, occur which initiate signalling cascades resulting in skin differentiation. If appropriate cell types could be identified and allowed to interact, then skin differentiation can result (Stenn 2003; Stenn & Cotsarelis 2005; Zheng et al. 2005). To this end, much recent interest has focused on stem cells.
14. Stem cells and their application to an engineered construct
Adult mesenchymal stem cells (MSC) are believed to be restricted in their ability to produce a wide range of replacement cells, but do nevertheless function as a source of cells for tissue repair and homeostasis. Embryonic stem cells on the other hand are totipotent and are able to differentiate into many different cell types. The ES cell represents an attractive and viable proposition for cell-replacement therapy. ES cells are derived from the inner cell mass of the embryonic blastocyst. These cells can be maintained indefinitely in vitro without loss of differentiation potential. To date, many of the studies on ES cells have been limited to mouse models, since human cell lines have only recently become available (Tuan et al. 2002). There are, however, many controversial ethical and technical problems that need to be overcome before the full potential of this type of cell can be realized (Ferrari et al. 1998; Gussoni et al. 1999; Peterson 2002). Defining the similarities and differences between the MSCs and ES cells will be especially relevant for applications of regenerative medicine and bioengineering.
Skin represents an ideal model system in which to investigate the use of stem cells as a source of cell-replacement therapy, because it contains one of the few well-characterized adult stem cell types, the keratinocytes. Adult somatic stem cells could resolve the problems that ES cells potentially have, namely if adult stem cells are transplanted back into the same individual, then there should not be any inherent problems with rejection. Treating patients with their own cells also avoids ethical and moral objections. Unless they are responding to trauma, adult stem cells typically divide infrequently to maintain homeostasis within their resident tissues (Alonso & Fuchs 2003). Since they are responsible for all cell replacement within a tissue, they are essential for tissue repair, wound healing and regeneration. Adult stem cells reside in specific niches and the niche exposes the stem cells to different differentiation cues—important in maintaining the stem cell state. Each stem cell division produces one replacement stem cell and one daughter cell. The latter is destined for differentiation and tissue regeneration.
One of the attractive features of skin from a bioengineering viewpoint is that skin keratinocytes can be maintained and propagated in the laboratory (Rheinwald & Green 1975, 1977). The cells that are contained in these primary cultures have a remarkable ability to proliferate, because they are capable of being passaged for many hundreds of generations without undergoing senescence (Alonso & Fuchs 2003). From the work of Morris & Potten (1994), it is known that slowly cycling cells in vivo are more clonogenic than actively dividing cells when placed into culture, suggesting that the less proliferative cells may be stem cells. The ability to maintain and grow such cells has lead to major advances in skin grafting technologies, such that burn patients routinely have cultured skin keratinocytes engrafted. (Ronfard et al. 2000; Brouard & Barrandon 2003; Gambardella & Barrandon 2003). Unfortunately, at present, the skin substitutes that are used are not fully functional in that they lack hair follicles, sweat and sebaceous glands as well as nerve and blood supplies. Whether long-term keratinocyte cultures contain multipotent stem cells which could produce hair follicles remains a subject of debate (Alonso & Fuchs 2003). The problem for the tissue engineer is that although the existence of skin stem cells is highly likely within such primary cell cultures, their isolation and characterization are proving to be a challenge. Fractionation by fluorescence-activated cell sorting has provided some insights. Jones & Watt (1993) found that some cells within the primary keratinocyte culture showed great proliferative capacity in vitro and are enriched for β1 integrins, and other studies by Li et al. (1998) and Kaur & Li (2000) show that similar cells have upregulated α6 integrin. Further characterization of these cells has shown that they adhere preferentially to ECM components, fibronectin (α5β1 receptor) and collagen IV (α2β1 receptor), suggesting that the reason why stem cells are kept in their niche is due to the very strong interaction with and adherence to basement membrane components (Watt 1998; Segre et al. 1999).
Another source of multipotent skin stem cells resides in the hair follicle bulge (Stenn & Cotsarelis 2005). This niche resides at the base of the permanent epithelial portion of the hair follicle which is the deepest, most protected place within the contiguous epithelial compartment (Alonso & Fuchs 2003). Kobayashi & Nishimura (1989) dissected dermal papillae cells and reconstituted them with fragments of hair follicle containing the hair bulge region. On transplantation under the kidney capsule of an athymic mouse, viable hair follicles formed. Other studies have shown that when the bulge cells of a normal mouse are surgically replaced with cells from a lac-Z mouse, the new chimeric hair follicles ubiquitously express lac-Z within all lineages of the hair follicle (Oshima et al. 2001). There are several studies which suggest that this compartment provides a source of multipotent stem cells which could be used for bioengineering hair follicles (Stenn & Cotsarelis 2005). When the skin suffers trauma such as burn injury or wounding, it is thought that the bulge cells migrate to the surface to aid re-epithelialization (Taylor et al. 2000). Thus, the regenerative role of bulge cells (or dissociated cells) from the skin is multiple; not only is it thought that they contribute to the production of sebaceous glands and epidermis, but also they are key to the formation of hair follicles (Zheng et al. 2005).
In defining cells for a bioengineered skin, apart from the keratinocyte and hair bud follicular cells, bone marrow is another logical candidate for sourcing cells to seed in a skin substitute (Badiavas & Falanga 2003). Bone marrow is known to contain inflammatory cell progenitors and multipotent stem cells. In some wounds, e.g. chronic ulcers or non-healing burns, it is thought that the mesenchymal cells that fill the dermis become phenotypically altered or senescent (Mendez et al. 1998; Vande Berg et al. 1998; Raffetto et al. 1999). The plasticity of bone marrow-derived stem cells means that they would have the inherent capacity to produce new skin cells if the conditions for growth were correct. Since bone marrow is a key to the process of haemopoiesis, it is also likely to contain hormones such as granulocyte–monocyte colony-stimulating factor which is thought to accelerate wound repair (Gillitzer & Goebeler 2001; Lingen 2001).
Another potentially important source of MSCs, known to be derived from bone marrow, is found in the circulating peripheral blood (for review see Tuan et al. 2002; Mori et al. 2005). Bucala et al. (1994) described a distinct population of blood-borne fibroblast-like cells that rapidly entered sites of tissue injury in a wound chamber model. The presence of fibroblasts in wound chambers had mainly been thought to be attributable to recruitment from surrounding subcutaneous tissue (Dunphy 1967). However, the presence of such large numbers of fibroblast-like cells coinciding with the entry of circulating inflammatory cells suggested that the cell population was arising from peripheral blood and not exclusively by slow migration from adjacent connective tissue (Bucala et al. 1994). This new cell type was termed fibrocyte with a distinct phenotype originally described as collagen+/vimentin+/CD34+ (Bucala et al. 1994). More recently, leucocyte-specific protein-1 (lsp-1) has been added to this profile as a fibrocyte marker in hypertrophic scars (Yang et al. 2005). Although these cells make up only 0.5% of peripheral blood leukocytes, they constitute 10% of the cells infiltrating subcutaneously implanted wound chambers in mice (Bucala et al. 1994). They are thought to have diverse roles in the wound healing process, inducing angiogenesis in vivo and in vitro (Hartlapp et al. 2001), produce chemoattractants to recruit CD4+ lymphocytes (Chesney et al. 1997) and express the chemokine receptor, CCR7, involved in the process of cell migration into the wound (Abe et al. 2001). Fibrocyte recruitment is also thought to be upregulated systemically in burn patients (Yang et al. 2002). The intercellular signals that modulate fibrocyte trafficking, proliferation and differentiation are only partially defined, but a better understanding of these signals is likely to enable new therapies to prevent pathologic fibrosis or to improve the tissue repair response (Quan et al. 2006).
The final consideration regarding cell sourcing is whether cells are autologous or heterologous. A rapid off-the-shelf tissue engineering product favours heterologous cell types and to date one of the most successful bioengineered products has been Dermagraft. This has been a classic example of a bioengineered product that has been both successful and, at the same time, distributed by three separate companies, Advanced Tissue Sciences, Smith and Nephew and Advanced Biohealing, Inc. Unlike many of the other skin substitutes, Dermagraft has a reasonable shelf-life; the cells cryopreserved within the construct survive for six months (reviewed extensively by Mansbridge 2006). This relatively long cell persistence, combined with a better understanding of cryopreservation techniques, bodes well for the persistence of stem cells in an engineered off-the-shelf product. The ultimate tissue engineering goal would be to combine the various cell types discussed earlier, with or without a natural or synthetic matrix in the presence of growth factors and cytokines known to promote regeneration (figure 3).
Figure 3.
A schematic of the requirements to create a fully functional skin substitute.
15. Future prospects
The ultimate goal of tissue engineering of the skin is to rapidly produce a construct that offers the complete regeneration of functional skin, including all the skin appendages (hair follicles, sweat glands and sensory organs) and layers (epidermis, dermis and fatty subcutus) with rapid take (vascularization) and the establishment of a functional vascular and nerve network and scar-free integration with the surrounding host tissue. Such a construct should allow the skin to fulfil its many normal functions: barrier formation; pigmentory defence against UV irradiation; thermoregulation; and mechanical and aesthetic functions. Some but not all of the functions can be restored with existing skin substitutes, e.g. cultured keratinocytes applied in fibrin, matrix or spray techniques, as cells or a cell layer, which can rapidly restore barrier function on a burn or major trauma; while cultures of keratinocytes on a dermal substitute, e.g. collagen/fibronectin mixture with or without fibroblasts, can additionally partially restore some mechanical functions. While autologous cells possess many potential advantages, they are neither available rapidly off-the-shelf for the treatment of burns, trauma, etc., nor do they lend themselves easily to commercially scalable treatment regimes. Heterologous cells, e.g. poorly differentiated or stem cells, which are not immunologically rejected and which are genetically stable to prevent subsequent tumour formation would appear to hold the promise of an off-the-shelf widely available therapy. Understanding how to engineer such cells using lessons learned from embryonic development and adult regeneration will be a key to developing the next generation enhanced tissue-engineered skin substitutes. On the one hand, these substitutes may be highly sophisticated, e.g. using biomaterial scaffolds carefully engineered to release in a time-dependent fashion, various signalling molecules, differentiation factors and protein domains engineered to facilitate cell migration and adhesion. Such substitutes may be further engineered to produce various three-dimensional patterns and densities. On the other hand, the substitutes may be minimally engineered—instead fulfilling the role of an easily handleable carrier (e.g. thermo-/hydrogel) for appropriate cells which when the carrier disappears in the body (by non-inflammatory dissociation) allows the cells to interact so as to regenerate all of the skin structures—such as that happens during embryonic development or adult regeneration. In this case, the substitute is minimally engineered so as to hold the cells in an undifferentiated state prior to delivery and, for this, then to disappear rapidly. Understanding and exploiting the mechanisms of embryonic development, stem cell biology and biomaterial engineering will be the key to achieving these next generation skin substitutes.
Acknowledgments
The authors are grateful to the BBSRC, MRC and EPSRC for their support of the UK Centre for Tissue Engineering.
References
- Abe R, Donnelly S.C, Peng T, Bucala R, Metz C.N. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J. Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Adzick N.S, Longaker M.T. Animal models for the study of fetal tissue repair. J. Surg. Res. 1991;51:216–222. doi: 10.1016/0022-4804(91)90097-6. [DOI] [PubMed] [Google Scholar]
- Ahsan T, Nerem R.M. Bioengineered tissues: the science, the technology, and the industry. Orthod. Craniofac. Res. 2005;8:134–140. doi: 10.1111/j.1601-6343.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- Allen S.P, Maden M, Price J.S. A role for retinoic acid in regulating the regeneration of deer antlers. Dev. Biol. 2002;251:409–423. doi: 10.1006/dbio.2002.0816. [DOI] [PubMed] [Google Scholar]
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- Armstrong J.R, Ferguson M.W. Ontogeny of the skin and the transition from scar-free to scarring phenotype during wound healing in the pouch young of a marsupial, Monodelphis domestica. Dev. Biol. 1995;169:242–260. doi: 10.1006/dbio.1995.1141. [DOI] [PubMed] [Google Scholar]
- Ashcroft G.S, Horan M.A, Ferguson M.W. The effects of ageing on wound healing: immunolocalisation of growth factors and their receptors in a murine incisional model. J. Anat. 1997;190:351–365. doi: 10.1046/j.1469-7580.1997.19030351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger F.A, Brthod F, Moulin V, Pouliot R, Germain L. Tissue-engineered skin substitutes: from in vitro constructs to in vivo applications. Biotechnol. Appl. Biochem. 2004;39:263–275. doi: 10.1042/BA20030229. [DOI] [PubMed] [Google Scholar]
- Badiavas E.V, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510–516. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- Bayat A, McGrouther D.A, Ferguson M.W. Skin scarring. Br. Med. J. 2003;326:88–92. doi: 10.1136/bmj.326.7380.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausang E, Floyd H, Dunn K.W, Orton C.I, Ferguson M.W.J. A new quantitative scale for clinical scar assessment. Plast. Reconstr. Surg. 1998;102:1954–1961. doi: 10.1097/00006534-199811000-00022. [DOI] [PubMed] [Google Scholar]
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl Acad. Soc. USA. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benest A.V, Salmo A.H, Wang W, Glover C.P, Uney J, Harper S.J, Bates D.O. VEGF and angiopoietin-1 stimulate different angiogenic phenotypes that combine to enhance functional neovascularization in adult tissue. Microcirculation. 2006;13:423–437. doi: 10.1080/10739680600775940. [DOI] [PubMed] [Google Scholar]
- Bevan D, Gherardi E, Fan T.P, Edwards D, Warn R. Diverse and potent activities of HGF/SF in skin wound repair. J. Pathol. 2004;203:831–838. doi: 10.1002/path.1578. [DOI] [PubMed] [Google Scholar]
- Boros P, Miller C.M. Hepatocyte growth factor: a multifunctional cytokine. Lancet. 1995;345:293–295. doi: 10.1016/S0140-6736(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Boyce S.T. Design principles for composition and performance of cultured skin substitutes. Burns. 2001;27:523–533. doi: 10.1016/S0305-4179(01)00019-5. [DOI] [PubMed] [Google Scholar]
- Boyce S.T, Warden G.D. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am. J. Surg. 2002;183:445–456. doi: 10.1016/S0002-9610(02)00813-9. [DOI] [PubMed] [Google Scholar]
- Boyce S.T, Medrano E.E, Abdel-Malek Z.A. Pigmentation and inhibition of wound contraction by cultured skin substitutes with adult melanocytes after transplantation to athymic mice. J. Invest. Dermatol. 1993;100:360–365. doi: 10.1111/1523-1747.ep12471822. [DOI] [PubMed] [Google Scholar]
- Boyce S.T, Supp A.P, Harriger M.D, Pickens W.L, Wickett R.R, Hoath S.B. Surface electrical capacitance as a noninvasive index of epidermal barrier in cultured skin substitutes in athymic mice. J. Invest. Dermatol. 1996;107:82–87. doi: 10.1111/1523-1747.ep12298286. [DOI] [PubMed] [Google Scholar]
- Broadley K.N, Aquino A.M, Hicks B, Ditesheim J.A, McGee G.S, Demetriou A.A, Woodward S.C, Davidson J.M. The diabetic rat as an impaired wound healing model: stimulatory effects of transforming growth factor-beta and basic fibroblast growth factor. Biotechnol. Ther. 1989;1:55–68. [PubMed] [Google Scholar]
- Brockes J.P. Amphibian limb regeneration: rebuilding a complex structure. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- Brockes J.P, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Brouard M, Barrandon Y. Controlling skin morphogenesis: hope and despair. Curr. Opin. Biotechnol. 2003;14:520–525. doi: 10.1016/j.copbio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Bucala R, Spiegel L.A, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol. Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- Burd A, Chiu T. Allogenic skin in the treatment of burns. Clin. Dermatol. 2005;23:376–387. doi: 10.1016/j.clindermatol.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Butler C.E, Orgill D.P. Simultaneous in vivo regeneration of neodermis, epidermis, and basement membrane. Adv. Biochem. Eng. Biotechnol. 2005;94:23–41. doi: 10.1007/b99998. [DOI] [PubMed] [Google Scholar]
- Caplan A.I. Embryonic development and the principles of tissue engineering. Novartis Found Symp. 2003;249:17–25. [PubMed] [Google Scholar]
- Chai Y, Slavkin H.C. Prospects for tooth regeneration in the 21st century: a perspective. Microse. Res. Tech. 2003;60:469–479. doi: 10.1002/jemt.10287. [DOI] [PubMed] [Google Scholar]
- Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc. Natl Acad. Sci. USA. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chettibi S, Ferguson M.W.J. Wound repair: an overview. In: Gallin J.I, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 3rd edn. Lippincott Williams & Wilkins; Philadelphia, PA: 1999. pp. 864–881. [Google Scholar]
- Chin G.S, Stelnicki E.J, Gittes G.K, Longaker M.T. Characteristics of fetal wound repair. In: Garg H.G, Longaker M.T, editors. Scarless wound healing. Marcel Dekker, Inc; New York, NY: 2000. pp. 239–262. [Google Scholar]
- Clark R.A. 2nd edn. Plenum Press; New York, NY: 1996. The molecular and cellular biology of wound repair. pp. 3–35. [Google Scholar]
- Clark L.D, Clark R.K, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin. Immunol. Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- Cole J, Tsou R, Wallace K, Gibran N, Isik F. Early gene expression profile of human skin to injury using high-density cDNA microarrays. Wound Repair Regen. 2001;9:360–370. doi: 10.1046/j.1524-475x.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- Cooper L, Johnson C, Burslem F, Martin P. Wound healing and inflammation genes revealed by array analysis of “macrophageless” PU.1 null mice. Genome Biol. 2005;6:R5. doi: 10.1186/gb-2004-6-1-r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro M.F. Transforming growth factor-beta function blocking already effective as therapeutic strategy. Circulation. 2002;106:130–135. doi: 10.1161/01.CIR.0000020689.12472.E0. [DOI] [PubMed] [Google Scholar]
- Cordeiro M.F, et al. Novel antisense oligonucleotides targeting TGF-beta inhibit in vivo scarring and improve surgical outcome. Gene Ther. 2003;10:59–71. doi: 10.1038/sj.gt.3301865. [DOI] [PubMed] [Google Scholar]
- Cowin A.J, Brosnan M.P, Holmes T.M, Ferguson M.W. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev. Dyn. 1998;212:385–393. doi: 10.1002/(SICI)1097-0177(199807)212:3%3C385::AID-AJA6%3E3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Cowin A.J, Holmes T.M, Brosnan M.P, Ferguson M.W. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur. J. Dermatol. 2001a;11:424–431. [PubMed] [Google Scholar]
- Cowin A.J, Kallincos N, Hatzirodos N, Robertson J.G, Pickering K.J, Couper J, Belford D.A. Hepatocyte growth factor and macrophage-stimulating protein are upregulated during excisional wound repair in rats. Cell Tissue Res. 2001b;306:239–250. doi: 10.1007/s004410100443. [DOI] [PubMed] [Google Scholar]
- Currie L.J, Sharpe J.R, Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast. Reconstr. Surg. 2001;108:1713–1726. doi: 10.1097/00006534-200111000-00045. [DOI] [PubMed] [Google Scholar]
- Dang C.M, Beanes S.R, Soo C, Ting K, Benhaim P, Hedrick M.H, Lorenz H.P. Decreased expression of fibroblast and keratinocyte growth factor isoforms and receptors during scarless repair. Plast. Reconstr. Surg. 2003;111:1969–1979. doi: 10.1097/01.PRS.0000054837.47432.E7. [DOI] [PubMed] [Google Scholar]
- Desai N.P, Hubbell J.A. Biological responses to polyethylene oxide modified polyethylene terephthalate surfaces. J. Biomed. Mater. Res. 1991;25:829–843. doi: 10.1002/jbm.820250704. [DOI] [PubMed] [Google Scholar]
- Dmetrichuk J.M, Spencer G.E, Carlone R.L. Retinoic acid-dependent attraction of adult spinal cord axons towards regenerating newt limb blastemas in vitro. Dev. Biol. 2005;281:112–120. doi: 10.1016/j.ydbio.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Donohue K.G, Carson P, Iriondo M, Zhou L, Saap L, Gibson K, Falanga V. Safety and efficacy of a bilayered skin construct in full-thickness surgical wounds. J. Dermatol. 2005;32:626–631. doi: 10.1111/j.1346-8138.2005.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Douglas B.S. Conservative management of guillotine amputation of the finger of children. Aust. Paediatr. J. 1972;8:86–89. doi: 10.1111/j.1440-1754.1972.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Doukas J, Blease K, Craig D, Ma C, Chandler L.A, Sosnowski B.A, Pierce G.F. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Mol. Ther. 2002;5:517–527. doi: 10.1006/mthe.2002.0579. [DOI] [PubMed] [Google Scholar]
- Dunphy J.E. The healing of wounds. Can. J. Surg. 1967;10:281–287. [PubMed] [Google Scholar]
- Ehrenreich M, Ruszczak Z. Update on dermal substitutes. Acta Dermatovenerol. Croat. 2006;14:172–187. [PubMed] [Google Scholar]
- Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair. Regen. 1999;7:201–207. doi: 10.1046/j.1524-475X.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- Falanga V, Eaglstein W.H, Bucalo B, Katz M.H, Harris B, Carson P. Topical use of human recombinant epidermal growth factor (h-EGF) in venous ulcers. J. Dermatol. Surg. Oncol. 1992;18:604–606. doi: 10.1111/j.1524-4725.1992.tb03514.x. [DOI] [PubMed] [Google Scholar]
- Fallon J.F. Editorial. Dev. Dyn. 2003;226:161. doi: 10.1002/dvdy.10252. [DOI] [Google Scholar]
- Fausto N. Liver regeneration. Hepatology. 2000;32(Suppl. 1):19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Ferguson M.W.J. Scar wars. Br. Dent. J. 2002;192:475. doi: 10.1038/sj.bdj.4801404a. [DOI] [PubMed] [Google Scholar]
- Ferguson M.W.J, O'Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Phil. Trans. R. Soc. B. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M.W.J, Whitby D.J, Shah M, Armstrong J, Siebert J.W, Longaker M.T. Scar formation: the spectral nature of fetal and adult wound repair. Plast. Reconstr. Surg. 1996;97:854–860. doi: 10.1097/00006534-199604000-00029. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Colletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow derived myogenic progenitors. Science. 1998;279:514–519. doi: 10.1126/science.279.5356.1528. [DOI] [Google Scholar]
- Ferretti P, Zhang F, Santos-Ruiz L, Clarke J.D.W. FGF signaling and blastema growth during amphibian tail regeneration. Int. J. Dev. Biol. 2001;45(S1):127–128. [Google Scholar]
- Frank S, Hubner G, Breier G, Longaker M.T, Greenhalgh D.G, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J. Biol. Chem. 1995;270:12 607–12 613. doi: 10.1074/jbc.270.46.27429. [DOI] [PubMed] [Google Scholar]
- Gambardella L, Barrandon Y. The multifaceted adult epidermal stem cell. Curr. Opin. Cell Biol. 2003;15:771–777. doi: 10.1016/j.ceb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Gardiner D.M, Bryant S.V. Molecular mechanisms in the control of limb regeneration: the role of homeobox genes. Int. J. Dev. Biol. 1996;40:797–805. [PubMed] [Google Scholar]
- Gardiner D.M, Endo T, Bryant S.V. The molecular basis of amphibian limb regeneration: integrating the old with the new. Semin. Cell Dev. Biol. 2002;13:345–352. doi: 10.1016/S1084952102000903. [DOI] [PubMed] [Google Scholar]
- Garg H.G, Longaker M.T. Marcel Dekker; New York, NY: 2000. Scarless wound healing. [Google Scholar]
- Gawronska-Kozak B. Regeneration in the ears of immunodeficient mice: identification and lineage analysis of mesenchymal stem cells. Tissue Eng. 2004;10:1251–1265. doi: 10.1089/ten.2004.10.1251. [DOI] [PubMed] [Google Scholar]
- Gawronska-Kozak B, Bogacki M, Rim J.S, Monroe W.T, Manuel J.A. Scarless skin repair in immunodeficient mice. Wound Repair Regen. 2006;14:265–276. doi: 10.1111/j.1743-6109.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- Godwin J.W, Brockes J.P. Regeneration, tissue injury and the immune response. J. Anat. 2006;209:423–432. doi: 10.1111/j.1469-7580.2006.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin A.R, Capecchi M.R. Hoxc13 mutant mice lack external hair. Genes Dev. 1998;12:11–20. doi: 10.1101/gad.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosiewska A, Yi C.F, Brown L.J, Cullen B, Silcock D, Geesin J.C. Differential expression and regulation of extracellular matrix-associated genes in fetal and neonatal fibroblasts. Wound Repair Regen. 2001;9:213–222. doi: 10.1046/j.1524-475x.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- Goss R.J. Future directions in antler research. Anat. Rec. 1995;241:291–302. doi: 10.1002/ar.1092410302. [DOI] [PubMed] [Google Scholar]
- Goss R.J, Grimes L.N. Epidermal downgrowths in regenerating rabbit ear holes. J. Morphol. 1975;146:533–542. doi: 10.1002/jmor.1051460408. [DOI] [PubMed] [Google Scholar]
- Goss R.J, Holt R. Epimorphic vs. tissue regeneration in Xenopus forelimbs. J. Exp. Zool. 1992;261:451–457. doi: 10.1002/jez.1402610412. [DOI] [PubMed] [Google Scholar]
- Griffith L.G. Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann. NY Acad. Sci. 2002;961:83–95. doi: 10.1111/j.1749-6632.2002.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Griffith L.G, Schwartz M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell. Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- Gu D.L, Nguyen T, Gonzalez A.M, Printz M.A, Pierce G.F, Sosnowski B.A, Philips M.L, Chandler L.A. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Mol. Ther. 2004;9:699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneka Y, Strickland C.D, Buzney E.A, Khan M.K, Flint A.F, Kumkel L.M, Mulligan R.C. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Gwak S.J, Kim S.S, Sung K, Han J, Choi C.Y, Kim B.S. Synergistic effect of keratinocyte transplantation and epidermal growth factor delivery on epidermal regeneration. Cell Transplant. 2005;14:809–817. doi: 10.3727/000000005783982521. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Sriwiriyanont P, Kaiho E, Kitahara T, Takema Y, Tsuboi R. An in vivo mouse model of human skin substitute containing spontaneously sorted melanocytes demonstrates physiological changes after UVB irradiation. J. Invest. Dermatol. 2005;125:364–372. doi: 10.1111/j.0022-202X.2005.23832.x. [DOI] [PubMed] [Google Scholar]
- Han M, Yang X, Taylor G, Burdsal C.A, Anderson R.A, Muneoka K. Limb regeneration in higher vertebrates: developing a roadmap. Anat. Rec. (Part B: New Anat) 2005;287B:14–24. doi: 10.1002/ar.b.20082. [DOI] [PubMed] [Google Scholar]
- Hansbrough J.F, Dore C, Hansbrough W.B. Clinical trials of a living dermal tissue replacement placed beneath meshed, split thickness skin grafts on excised wounds. J. Burn Care Rehab. 1992;13:519–529. doi: 10.1097/00004630-199209000-00004. [DOI] [PubMed] [Google Scholar]
- Harlan D.M, Karp C.L, Matzinger P, Munn D.H, Ransohoff R.M, Metzger D.W. Immunological concerns with bioengineering approaches. Ann. NY Acad. Sci. 2002;961:323–330. doi: 10.1111/j.1749-6632.2002.tb03114.x. [DOI] [PubMed] [Google Scholar]
- Hartlapp I, Abe R, Saeed R.W, Peng T, Voelter W, Bucala R, Metz C.N. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15:2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- Harty M, Neff A.W, King M.W, Mescher A.L. Regeneration or scarring: an immunologic perspective. Dev. Dyn. 2003;226:268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- Hayen W, Goebeler M, Kumar S, Riessen R, Nehls V. Hyaluronan stimulates tumor cell migration by modulating the fibrin fiber architecture. J. Cell Sci. 1999;112:2241–2251. doi: 10.1242/jcs.112.13.2241. [DOI] [PubMed] [Google Scholar]
- Heikinheimo M, Lawshe A, Shackleford G.M, Wilson D.B, MacArthur C.A. Fgf-8 expression in the post-gastrulation mouse suggests roles in the development of the face, limbs and central nervous system. Mech. Dev. 1994;48:129–138. doi: 10.1016/0925-4773(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Hojo M, Inokuchi S, Kidokoro M, Fukuyama N, Tanaka E, Tsuji C, Miyasaka M, Tanino R, Nakazawa H. Induction of vascular endothelial growth factor by fibrin as a dermal substrate for cultured skin substitute. Plast. Reconstr. Surg. 2003;111:1638–1645. doi: 10.1097/01.PRS.0000053842.90564.26. [DOI] [PubMed] [Google Scholar]
- Hudon V, Berthod F, Black A.F, Damour O, Germain L, Auger F.A. A tissue engineered endothelialized dermis to study the modulation of angiogenic and angiostatic molecules on capillary-like tube formation in vitro. Br. J. Dermatol. 2003;148:1094–1104. doi: 10.1046/j.1365-2133.2003.05298.x. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. Springer; New York, NY: 1990. Fibronectins; p. 546. [Google Scholar]
- Illingworth C.M. Trapped fingers and amputated finger tips in children. J. Pediatr. Surg. 1974;9:853–858. doi: 10.1016/S0022-3468(74)80220-4. [DOI] [PubMed] [Google Scholar]
- Imokawa Y, Brockes J.P. Selective activation of thrombin is a critical determinant for vertebrate lens regeneration. Curr. Biol. 2003;13:877–881. doi: 10.1016/S0960-9822(03)00294-X. [DOI] [PubMed] [Google Scholar]
- Imokawa Y, Simon A, Brockes J.P. A critical role for thrombin in vertebrate lens regeneration. Phil. Trans. R. Soc. B. 2004;359:765–776. doi: 10.1098/rstb.2004.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N.B, Dimos J.T, Schaniel C, Hackney J.A, Moore K.A, Lemischka I.R. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- Jahoda C.A, Oliver R.F, Reynolds A.J, Forrester J.C, Horne K.A. Human hair follicle regeneration following amputation and grafting into the nude mouse. J. Invest. Dermatol. 1996;107:804–807. doi: 10.1111/1523-1747.ep12330565. [DOI] [PubMed] [Google Scholar]
- Jones P.H, Watt F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-K. [DOI] [PubMed] [Google Scholar]
- Kanda T, Iwasaki T, Nakamura S, Ueki A, Kurokawa T, Ikeda K, Mizusawa H. FGF-9 is an autocrine/paracrine neurotrophic substance for spinal motoneurons. Int. J. Dev. Neurosci. 1999;17:191–200. doi: 10.1016/S0736-5748(99)00026-X. [DOI] [PubMed] [Google Scholar]
- Kano M.R, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, Miyazonon T, Miyazawa K. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFR(beta) signaling. J. Cell Sci. 2005;118:3759–3768. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- Kaur P, Li A. Adhesive properties of human basal epidermal cells: and analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J. Invest. Dermatol. 2000;114:413–420. doi: 10.1046/j.1523-1747.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- Kawai K, Suzuki S, Tabata Y, Ikada Y, Nishimura Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21:489–499. doi: 10.1016/S0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Madhavan M, Call M.K, Santiago W, Tsonis P.A, Lambris J.D, Del Rio-Tsonis K. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J. Immunol. 2003;170:2331–2339. doi: 10.4049/jimmunol.170.5.2331. [DOI] [PubMed] [Google Scholar]
- Kitano Y, Yoshimura K, Uchida G, Sato K, Harii K. Pretreatment with topical all-trans-retinoic acid is beneficial for wound healing in genetically diabetic mice. Arch. Dermatol. Res. 2001;293:515–521. doi: 10.1007/PL00007466. [DOI] [PubMed] [Google Scholar]
- Kivinen P.K, Hyttinen M, Harvima R.J, Naukkarinen A, Horsmanheimo M, Harvima I.T. Quantitative digital image analysis applied to demonstrate the stratified distribution of involucrin in organ cultured human skin. Arch. Dermatol. Res. 1999;291:217–223. doi: 10.1007/s004030050397. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Nishimura E. Ectopic growth of mouse whiskers from implanted lengths of plucked vibrissae follicles. J. Invest. Dermatol. 1989;92:278–282. doi: 10.1111/1523-1747.ep12276858. [DOI] [PubMed] [Google Scholar]
- Komuves L.G, et al. HOXB4 homeodomain protein is expressed in developing epidermis and skin disorders and modulates keratinocyte proliferation. Dev. Dyn. 2002;224:58–68. doi: 10.1002/dvdy.10085. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Kunisada T, et al. Keratinocyte expression of transgenic hepatocyte growth factor affects melanocyte development, leading to dermal melanocytosis. Mech. Dev. 2000;94:67–78. doi: 10.1016/S0925-4773(00)00308-7. [DOI] [PubMed] [Google Scholar]
- Kuntz R.M, Saltzman W.M. Neutrophil motility in extracellular matrix gels: mesh size and adhesion affect speed of migration. Biophys. J. 1997;72:1472–1480. doi: 10.1016/S0006-3495(97)78793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca D, Burg M.A, Jensen-Pergakes K, Ravey E.P, Gonzalez A.M, Baird A. Evolving phage vectors for cell targeted gene delivery. Curr. Pharm. Biotechnol. 2002;3:45–57. doi: 10.2174/1389201023378490. [DOI] [PubMed] [Google Scholar]
- Lerner A.B, Halaban R, Klaus S.N, Moellmann G.E. Transplantation of human melanocytes. J. Invest. Dermatol. 1987;89:219–224. doi: 10.1111/1523-1747.ep12470973. [DOI] [PubMed] [Google Scholar]
- Levine J.H, Moses H.L, Gold L.I, Nanney L.B. Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am. J. Pathol. 1993;143:368–380. [PMC free article] [PubMed] [Google Scholar]
- Li A, Simmons P.J, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl Acad. Sci. USA. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mohan S, Gu W, Miyakoshi N, Baylink D.J. Differential protein profile in the ear-punched tissue of regeneration and non-regeneration strains of mice: a novel approach to explore the candidate genes for soft-tissue regeneration. Biochim. Biophys. Acta. 2000;1524:102–109. doi: 10.1016/s0304-4165(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Li X, Mohan S, Gu W, Baylink D.J. Analysis of gene expression in the wound repair/regeneration process. Mamm. Genome. 2001;12:52–59. doi: 10.1007/s003350010230. [DOI] [PubMed] [Google Scholar]
- Li M, Mondrinos M.J, Gandhi M.R, Ko F.K, Weiss A.S, Lelkes P.I. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26:5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Lindblad W.J. Perspective article: collagen expression by novel cell populations in the dermal wound environment. Wound Repair Regen. 1998;6:186–193. doi: 10.1046/j.1524-475X.1998.60304.x. [DOI] [PubMed] [Google Scholar]
- Lingen M.W. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch. Pathol. Lab. Med. 2001;125:67–71. doi: 10.5858/2001-125-0067-ROLAEC. [DOI] [PubMed] [Google Scholar]
- Longaker M.T, Whitby D.J, Ferguson M.W, Lorenz H.P, Harrison M.R, Adzick N.S. Adult skin wounds in the fetal environment heal with scar formation. Ann. Surg. 1994;219:65–72. doi: 10.1097/00000658-199401000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu Y.K, Kim K, Hsiao B.S, Chu B, Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Control Release. 2003;89:341–353. doi: 10.1016/S0168-3659(03)00097-X. [DOI] [PubMed] [Google Scholar]
- Mack J.A, Abramson S.R, Ben Y, Coffin J.C, Rothrock J.K, Maytin E.V, Hascall V.C, Largman C, Stelnicki E.J. Hoxb13 knockout adult skin exhibits high levels of hyaluronan and enhanced wound healing. FASEB J. 2003;17:1352–1354. doi: 10.1096/fj.02-0959fje. [DOI] [PubMed] [Google Scholar]
- Mack J.A, Li L, Sato N, Hascall V.C, Maytin E.V. Hoxb13 up-regulates transglutaminase activity and drives terminal differentiation in an epidermal organotypic model. J. Biol. Chem. 2005;280:29 904–29 911. doi: 10.1074/jbc.M505262200. [DOI] [PubMed] [Google Scholar]
- Maden M, Hind M. Retinoic acid, a regeneration-inducing molecule. Dev. Dyn. 2003;226:237–244. doi: 10.1002/dvdy.10222. [DOI] [PubMed] [Google Scholar]
- Manak J.R, Scott M.P. A class act: conservation of homeodomain protein functions. Dev. Suppl. 1994:61–77. [PubMed] [Google Scholar]
- Mansbridge J. Commercial considerations in tissue engineering. J. Anat. 2006;209:527–532. doi: 10.1111/j.1469-7580.2006.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masinde G.L, Li X, Baylink D.J, Nguyen B, Mohan S. Isolation of wound healing/regeneration genes using restrictive fragment differential display-PCR in MRL/MPJ and C57BL/6 mice. Biochem. Biophys. Res. Commun. 2005;330:117–122. doi: 10.1016/j.bbrc.2005.02.143. [DOI] [PubMed] [Google Scholar]
- Mastellos D, Papadimitriou J.C, Franchini S, Tsonis P.A, Lambris J.D. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J. Immunol. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Hashimoto K, Yoshikawa K, Nakamura T. Marked stimulation of growth and motility of human keratinocytes by hepatocyte growth factor. Exp. Cell Res. 1991;196:114–120. doi: 10.1016/0014-4827(91)90462-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Kagoshima M, Nakamura T. Hepatocyte growth factor as a potent survival factor for rat pheochromocytoma PC12 cells. Exp. Cell Res. 1995;220:71–78. doi: 10.1006/excr.1995.1293. [DOI] [PubMed] [Google Scholar]
- McCallion R.L, Ferguson M.W.J. Fetal wound healing and the development of antiscarring therapies for adult wound healing. In: Clark R.A.F, editor. The molecular and cellular biology of wound repair. Plenum Press; New York, NY: 1996. pp. 561–600. [Google Scholar]
- Mendez M.V, Stanley A, Park H.Y, Shon K, Phillips T, Menzoian J.O. Fibroblasts cultured from venous ulcers display cellular characteristics of senescence. J. Vasc. Surg. 1998;28:876–883. doi: 10.1016/S0741-5214(98)70064-3. [DOI] [PubMed] [Google Scholar]
- Metcalfe A.D, Ferguson M.W. Harnessing wound healing and regeneration for tissue engineering. Biochem. Soc. Trans. 2005;33:413–417. doi: 10.1042/BST0330413. [DOI] [PubMed] [Google Scholar]
- Metcalfe A.D, Willis H, Beare A, Ferguson M.W. Characterizing regeneration in the vertebrate ear. J. Anat. 2006;209:439–446. doi: 10.1111/j.1469-7580.2006.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G.K, DeFrances M.C. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Mironov V, Boland T, Trusk T, Forgacs G, Markwald R.R. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21:157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Mori L, Bellini A, Stacey M.A, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp. Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Morris R.J, Potten C.S. Slowly cycling (label-retaining) epidermal cells behave like clonogenic stem cells in vitro. Cell Prolif. 1994;27:279–289. doi: 10.1111/j.1365-2184.1994.tb01425.x. [DOI] [PubMed] [Google Scholar]
- Murray E.W, Robbins S.M. Antibody cross-linking of the glycosylphosphatidylinositol-linked protein CD59 on hematopoietic cells induces signaling pathways resembling activation by complement. J. Biol. Chem. 1998;273:25 279–25 284. doi: 10.1074/jbc.273.39.25279. [DOI] [PubMed] [Google Scholar]
- Niswander L, Martin G.R. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992;114:755–768. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- Niswander L, Martin G.R. FGF-4 and BMP-2 have opposite effects on limb growth. Nature. 1993;361:68–71. doi: 10.1038/361068a0. [DOI] [PubMed] [Google Scholar]
- O'Ceallaigh S, Herrick S.E, Bluff J.E, McGrouther D.A, Ferguson M.W. Quantification of total and perfused blood vessels in murine skin autografts using a fluorescent double-labeling technique. Plast. Reconstr. Surg. 2006;117:140–151. doi: 10.1097/01.prs.0000185611.87601.b8. [DOI] [PubMed] [Google Scholar]
- O'Kane S, Ferguson M.W. Transforming growth factor beta's and wound healing. Int. J. Biochem. Cell Biol. 1997;29:63–78. doi: 10.1016/S1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Yoshioka H, Tanaka A, Kawakami Y, Nohno T, Noji S. Involvement of androgen-induced growth factor (FGF-8) gene in mouse embryogenesis and morphogenesis. Biochem. Biophys. Res. Commun. 1994;204:882–888. doi: 10.1006/bbrc.1994.2542. [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/S0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Otto W.R, Nanchahal J, Lu Q.L, Boddy N, Dover R. Survival of allogeneic cells in cultured organotypic skin grafts. Plast. Reconstr. Surg. 1995;96:166–176. doi: 10.1097/00006534-199507000-00025. [DOI] [PubMed] [Google Scholar]
- Packer A.I, Jane-Wit D, McLean L, Panteleyev A.A, Christiano A.M, Wolgemuth D.J. Hoxa4 expression in developing mouse hair follicles and skin. Mech. Dev. 2000;99:153–157. doi: 10.1016/S0925-4773(00)00471-8. [DOI] [PubMed] [Google Scholar]
- Pandit A, Ashar R, Feldman D. The effect of TGF-beta delivered through a collagen scaffold on wound healing. J. Invest. Surg. 1999;12:89–100. doi: 10.1080/089419399272647. [DOI] [PubMed] [Google Scholar]
- Parenteau N.L, Bilbo P, Nolte C.J, Mason V.S, Rosenberg M. The organotypic culture of human skin keratinocytes and fibroblasts to achieve form and function. Cytotechnology. 1992;9:163–171. doi: 10.1007/BF02521744. [DOI] [PubMed] [Google Scholar]
- Peled Z.M, Rhee S.J, Hsu M, Chang J, Krummel T.M, Longaker M.T. The ontogeny of scarless healing II: EGF and PDGF-B gene expression in fetal rat skin and fibroblasts as a function of gestational age. Ann. Plast. Surg. 2001;47:417–424. doi: 10.1097/00000637-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Peterson D.A. Stem cells in brain plasticity and repair. Curr. Opin. Pharmacol. 2002;2:34–42. doi: 10.1016/S1471-4892(01)00118-7. [DOI] [PubMed] [Google Scholar]
- Philips T.J. New skin for old: developments in biological skin substitutes. Arch. Dermatol. 1998;134:344–349. doi: 10.1001/archderm.134.3.344. [DOI] [PubMed] [Google Scholar]
- Pienimaki J.P, et al. Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J. Biol. Chem. 2001;276:20 428–20 435. doi: 10.1074/jbc.M007601200. [DOI] [PubMed] [Google Scholar]
- Pierce G.F, Mustoe T.A, Lingelbach J, Masakowski V.R, Griffin G.L, Senior R.M, Deuel T.F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J. Cell Biol. 1989;109:429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G.F, Mustoe T.A, Altrock B.W, Deuel T.F, Thomason A. Role of platelet-derived growth factor in wound healing. J. Cell Biochem. 1991;45:319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- Powers P.S, Sarkar S, Goldgof D.B, Cruse C.W, Tsap L.V. Scar assessment: current problems and future solutions. J. Burn Care Rehab. 1999;20:54–60. doi: 10.1097/00004630-199901001-00011. [DOI] [PubMed] [Google Scholar]
- Quan T.E, Cowper S.E, Bucala R. The role of circulating fibrocytes in fibrosis. Curr. Rheumatol. Rep. 2006;8:145–150. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- Raeber G.P, Lutolf M.P, Hubbell J.A. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys. J. 2005;89:1374–1388. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffetto J.D, Mendez M.V, Phillips T.J, Park H.Y, Menzoian J.O. The effect of passage number on fibroblast cellular senescence in patients with chronic venous insufficiency with and without ulcer. Am. J. Surg. 1999;178:107–112. doi: 10.1016/S0002-9610(99)00134-8. [DOI] [PubMed] [Google Scholar]
- Rajnoch C, Ferguson S, Metcalfe A.D, Herrick S.E, Willis H.S, Ferguson M.W. Regeneration of the ear following wounding in different mouse strains is dependent on the severity of wound trauma. Dev. Dyn. 2003;226:388–397. doi: 10.1002/dvdy.10242. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan R.C, Melton D.A. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Rappolee D.A, Basilico C, Patel Y, Werb Z. Expression and function of FGF-4 in peri-implantation development in mouse embryos. Development. 1994;120:2259–2269. doi: 10.1242/dev.120.8.2259. [DOI] [PubMed] [Google Scholar]
- Rheinwald J.G, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinising colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/S0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rheinwald J.G, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Roberts A.B, Heine U.I, Flanders K.C, Sporn M.B. Transforming growth factor-beta. Major role in regulation of extracellular matrix. Ann. NY Acad. Sci. 1990a;580:225–232. doi: 10.1111/j.1749-6632.1990.tb17931.x. [DOI] [PubMed] [Google Scholar]
- Roberts A.B, Flanders K.C, Heine U.I, Jakowlew S, Kondaiah P, Kim S.J, Sporn M.B. Transforming growth factor-beta: multifunctional regulator of differentiation and development. Phil. Trans. R. Soc. B. 1990b;327:145–154. doi: 10.1098/rstb.1990.0050. [DOI] [PubMed] [Google Scholar]
- Ronfard V, Rives J.M, Neveux Y, Carsin H, Barrandon Y. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation. 2000;70:1588–1598. doi: 10.1097/00007890-200012150-00009. [DOI] [PubMed] [Google Scholar]
- Rosso F, Marino G, Giordano A, Barbarisi M, Parmegiani D, Barbarisi A. Smart materials as scaffolds for tissue engineering. J. Cell. Physiol. 2005;203:465–470. doi: 10.1002/jcp.20270. [DOI] [PubMed] [Google Scholar]
- Rudolph R. The effect of skin graft preparation on wound contraction. Surg. Gynecol. Obstet. 1976;142:49–56. [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J. Clin. Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandulache V.C, Zhou Z, Sherman A, Dohar J.E, Hebda P.A. Impact of transplanted fibroblasts on rabbit skin wounds. Arch Otolaryngol. Head Neck Surg. 2003;129:345–350. doi: 10.1001/archotol.129.3.345. [DOI] [PubMed] [Google Scholar]
- Sato C, Tsuboi R, Shi C.M, Rubin J.S, Ogawa H. Comparative study of hepatocyte growth factor/scatter factor and keratinocyte growth factor effects on human keratinocytes. J. Invest. Dermatol. 1995;104:958–963. doi: 10.1111/1523-1747.ep12606221. [DOI] [PubMed] [Google Scholar]
- Schaffer C.J, Nanney L.B. Cell biology of wound healing. Int. Rev. Cytol. 1996;169:151–181. doi: 10.1016/s0074-7696(08)61986-5. [DOI] [PubMed] [Google Scholar]
- Segre J.A, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005;74B:782–788. doi: 10.1002/jbm.b.30291. [DOI] [PubMed] [Google Scholar]
- Seliktar D, Zisch A.H, Lutof M.P, Wrana J.L, Hubbell J.A. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J. Biomed. Mater. Res. A. 2004;68:704–716. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- Sethi K.K, Yannas I.V, Mudera V, Eastwood M, McFarland C, Brown R.A. Evidence for sequential utilization of fibronectin, vitronectin, and collagen during fibroblast-mediated collagen contraction. Wound Repair Regen. 2002;10:397–408. doi: 10.1046/j.1524-475X.2002.10609.x. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman D.M, Ferguson M.W. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992;339:213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman D.M, Ferguson M.W. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J. Cell Sci. 1994a;107:1137–1157. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman D.M, Ferguson M.W. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1994b;339:213–214. doi: 10.1016/0140-6736(92)90009-R. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman D.M, Ferguson M.W. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Shure D, Senior R.M, Griffin G.L, Deue'l T.F. PDGF AA homodimers are potent chemoattractants for fibroblasts and neutrophils, and for monocytes activated by lymphocytes or cytokines. Biochem. Biophys. Res. Commun. 1992;186:1510–1514. doi: 10.1016/S0006-291X(05)81577-3. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Slack J.M. Regeneration research today. Dev. Dyn. 2003;226:162–166. doi: 10.1002/dvdy.10232. [DOI] [PubMed] [Google Scholar]
- Spyrou G.E, Naylor I.L. The effect of basic fibroblast growth factor on scarring. Br. J. Plast. Surg. 2002;55:275–282. doi: 10.1054/bjps.2002.3831. [DOI] [PubMed] [Google Scholar]
- Stelnicki E.J, Komuves L.G, Holmes D, Clavin W, Harrison M.R, Adzick N.S, Largman C. The human homeobox genes MSX-1, MSX-2, and MOX-1 are differentially expressed in the dermis and epidermis in fetal and adult skin. Differentiation. 1997;62:33–41. doi: 10.1046/j.1432-0436.1997.6210033.x. [DOI] [PubMed] [Google Scholar]
- Stenn K.S. Molecular insights into the hair follicle and its pathology: a review of recent developments. Int. J. Dermatol. 2003;42:40–43. doi: 10.1046/j.1365-4362.2003.01726.x. [DOI] [PubMed] [Google Scholar]
- Stenn K.S, Cotsarelis G. Bioengineering the hair follicle: fringe benefits of stem cell technology. Curr. Opin. Biotechnol. 2005;16:493–497. doi: 10.1016/j.copbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Sun X, Mariani F.V, Martin G.R. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- Supp D.M, Boyce S.T. Engineered skin substitutes: practices and potentials. Clin. Dermatol. 2005;23:403–412. doi: 10.1016/j.clindermatol.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Sweitzer S.M, Fann S.A, Borg T.K, Baynes J.W, Yost M.J. What is the future of diabetic wound care? Diabetes Educ. 2006;32:197–210. doi: 10.1177/0145721706286897. [DOI] [PubMed] [Google Scholar]
- Swope V.B, Supp A.P, Cornelius J.R, Babcock G.F, Boyce S.T. Regulation of pigmentation in cultured skin substitutes by cytometric sorting of melanocytes and keratinocytes. J. Invest. Dermatol. 1997;109:289–295. doi: 10.1111/1523-1747.ep12335766. [DOI] [PubMed] [Google Scholar]
- Tagashira S, Harada H, Katsumata T, Itoh N, Nakatsuka M. Cloning of mouse FGF10 and up-regulation of its gene expression during wound healing. Gene. 1997;197:399–404. doi: 10.1016/S0378-1119(97)00187-X. [DOI] [PubMed] [Google Scholar]
- Tanaka E.M, Drechsel D.N, Brockes J.P. Thrombin regulates S-phase re-entry by cultured newt myotubes. Curr. Biol. 1999;9:792–799. doi: 10.1016/S0960-9822(99)80362-5. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer M.S, Jensen P.J, Sun T.T, Lavker R.M. Involvement of follicular stem cells in forming not only the follicle but the epidermis. Cell. 2000;102:451–461. doi: 10.1016/S0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Takayama H, Horiguchi N, Otsuka T, Fukusato T, Merlino G, Takagi H, Mori M. Overexpression of hepatocyte growth factor/scatter factor promotes vascularization and granulation tissue formation in vivo. FEBS Lett. 2001;509:95–100. doi: 10.1016/S0014-5793(01)03126-X. [DOI] [PubMed] [Google Scholar]
- Tran K.T, Lamb P, Deng J.S. Matrikines and matricryptins: implications for cutaneous cancers and skin repair. J. Dermatol. Sci. 2005;40:11–20. doi: 10.1016/j.jdermsci.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Tremblay P.L, Hudon V, Berthod F, Germain L, Auger F.A. Inosculation of tissue engineered capillaries with the host's vasculature in a reconstructed skin transplanted on mice. Am. J. Transplant. 2005;5:1002–1010. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- Trentin D, Hall H, Wechsler S, Hubbel J.A. Peptide-matrix-mediated gene transfer of an oxygen-insensitive hypoxia-inducible factor-1alpha variant for local induction of angiogenesis. Proc. Natl Acad. Sci. USA. 2006;103:2506–2511. doi: 10.1073/pnas.0505964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.C, Lin S.D, Lai C.S, Lin T.M. The use of composite acellular allodemis-ultrathin autograft on joint area in major burns patients—one year follow up. Kaohsiung J. Med. Sci. 1999;15:651–658. [PubMed] [Google Scholar]
- Tsonis P.A. How to build and rebuild a lens. J. Anat. 2006;209:433–438. doi: 10.1111/j.1469-7580.2006.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis P.A, Lambris J.D, Del Rio-Tsonis K. To regeneration…with complement. Adv. Exp. Med. Biol. 2006;586:63–70. doi: 10.1007/0-387-34134-X_5. [DOI] [PubMed] [Google Scholar]
- Tuan R.S, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2002;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Wetering P, Metters A.T, Schoenmakers R.G, Hubbell J.A. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J. Control. Release. 2005;102:619–627. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Vande Berg J.S, Rudolph R, Hollan C, Haywood-Reid P.L. Fibroblast senescence in pressure ulcers. Wound Repair Regen. 1998;6:38–49. doi: 10.1046/j.1524-475X.1998.60107.x. [DOI] [PubMed] [Google Scholar]
- Vats A. Scaffolds and biomaterials for tissue engineering: a review of clinical applications. Clin. Otolaryngol. Allied Sci. 2003;28:165–172. doi: 10.1046/j.1365-2273.2003.00686.x. [DOI] [PubMed] [Google Scholar]
- Vogel V, Baneyx G. The tissue engineering puzzle: a molecular perspective. Ann. Rev. Biomed. Eng. 2003;5:441–463. doi: 10.1146/annurev.bioeng.5.040202.121615. [DOI] [PubMed] [Google Scholar]
- Ward R.S, Saffle J.R, Schnebly W.A, Hayes-Lundy C, Reddy R. Sensory loss over grafted areas in patients with burns. J. Burn Care Rehab. 1989;10:536–538. doi: 10.1097/00004630-198911000-00015. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan C.I, Copeland N.G, Jenkins N.A, Nagata S. Lymphoproliferation disorder in mice is explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watt F.M. Epidermal stem cells: markers, patterning and the control of stem cell fate. Phil. Trans. R. Soc. B. 1998;353:831–837. doi: 10.1098/rstb.1998.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Peters K.G, Longaker M.T, Fuller-Pace F, Banda M.J, Williams L.T. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc. Natl Acad. Sci. USA. 1992;89:6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby D.J, Ferguson M.W. Immunohistochemical localization of growth factors in fetal wound healing. Dev. Biol. 1991a;147:207–215. doi: 10.1016/S0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Whitby D.J, Ferguson M.W. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development. 1991b;112:651–668. doi: 10.1242/dev.112.2.651. [DOI] [PubMed] [Google Scholar]
- Wilcox J.N, Derynck R. Developmental expression of transforming growth factors alpha and beta in mouse fetus. Mol. Cell Biol. 1988;8:3415–3422. doi: 10.1128/mcb.8.8.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Bauer J.S, Juliano R.L, McDonald J.A. Identification of anew biological function for the integrin alpha v beta 3: initaition of fibronectin matrix assembly. J. Cell Sci. 1996;108:821–829. [Google Scholar]
- Yang L, Scott P.G, Giuffre J, Shankowsky H.A, Ghahary A, Tredget E.E. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab. Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- Yang L, Scott P.G, Dodd C, Medina A, Jiao H, Shankowsky H.A, Ghahary A, Tredget E.E. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- Yannas I.V, Burke J.F. Design of an artificial skin. I Basic design principles. J. Biomed. Mater. Res. 1980;14:65–81. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Yamaguchi Y, Itami S, Yoshikawa K, Tabata Y, Matsumoto K, Nakamura T. Neutralization of hepatocyte growth factor leads to retarded cutaneous wound healing associated with decreased neovascularization and granulation tissue formation. J. Invest. Dermatol. 2003;120:335–343. doi: 10.1046/j.1523-1747.2003.12039.x. [DOI] [PubMed] [Google Scholar]
- Zarnegar R, Michalopoulos G.K. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J. Cell Biol. 1995;129:1177–1180. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenjari C, Boilly-Marer Y, Desbiens X, Oudghir M, Hondermarck H, Boilly B. Experimental evidence for FGF-1 control of blastema cell proliferation during limb regeneration of the amphibian Pleurodeles waltl. Int. J. Dev. Biol. 1996;40:965–971. [PubMed] [Google Scholar]
- Zheng Y, Du X, Wang W, Boucher M, Parimoo S, Stenn K. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J. Invest. Dermatol. 2005;124:867–876. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]