Abstract
We show how nacre and pearl construction in bivalve and gastropod molluscs can be understood in terms of successive processes of controlled self-assembly from the molecular- to the macro-scale. This dynamics involves the physics of the formation of both solid and liquid crystals and of membranes and fluids to produce a nanostructured hierarchically constructed biological composite of polysaccharides, proteins and mineral, whose mechanical properties far surpass those of its component parts.
Keywords: biomineralization, nacre, molluscs, β-chitin
1. Introduction
Pearl and nacre, or mother of pearl, have been appreciated since antiquity for their beauty and have been studied scientifically for at least the past 150 years (Addadi & Weiner 1997). As a result of these researches, it is now understood that nacre is not just of interest for its aesthetic qualities, but also as a material of exceptional performance compared with the properties of its component parts (Jackson et al. 1988), and it has become a challenge to those engaged in fabricating biomimetic products to replicate the structure of this iconic biomineral (Mayer 2005; Sanchez et al. 2005).
As seen with the naked eye, nacre is an iridescent layer that composes the inner surface of the shell of numerous species of mollusc, while pearl is a similar coating formed around a foreign body trapped within the organism in many of these species; we shall henceforth use the term nacre to refer to both. It is a very stiff, strong and tough material (Jackson et al. 1988), fabricated by the organism as part of the armour of its shell, which generally comprises the nacreous inner layer, together with an outer layer with a different microstructure. One idea is that this structure could function like modern body or vehicle armour that combines a harder outer layer as a primary barrier to penetration with a tougher inner layer which dissipates energy and stops cracks, should the outer layer be breached, but it is not clear that the outer layer of the shell possesses the appropriate material properties, and it may be that the outer layer is laid down just to provide a firm base for the nacre assembly. Whatever selection pressures may have been brought to bear to produce nacre, its structure is certainly exquisitely detailed. The two classes of nacre-producing molluscs that are commonest, and have been most studied, are the bivalves and the gastropods and we choose here to study, compare and contrast nacre from species of these two taxa.
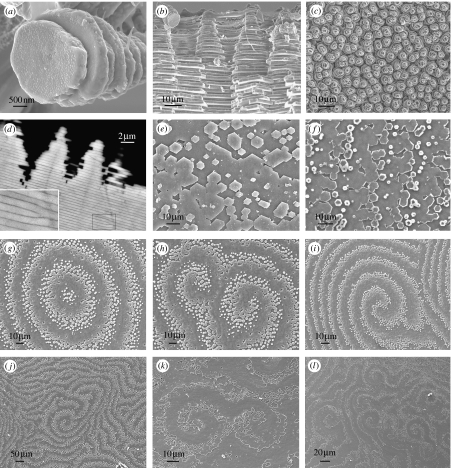
When the growth surface of nacre is viewed on the mesoscale, piles of tablets are seen to give rise to a landscape of columns in gastropods (figure 1a–d), while steps or terraces of tablets form arrangements of spirals, labyrinths and target patterns in bivalves (figure 1e–l). If we zoom in to observe nacre on the micro-scale, each tablet is found to be formed mainly of crystalline calcium carbonate in its aragonite polymorph and is bounded above and below by an organic mortar, the interlamellar membrane. In bivalves, the structure is that of a brick wall, with tablets in each layer offset with respect to those in the layers above and below them, while in gastropods the tablets are not offset but are piled one on top of another. On looking in yet more detail, now at the nanoscale, we find that each brick is a composite of aragonite mineral incorporating organic fibres, while the mortar is a composite of chitin crystals embedded in a proteinaceous matrix. Figure 2 summarizes the hierarchical structure of nacre from the molecular- to the macro-scale, whose development has been our concern in the present study.
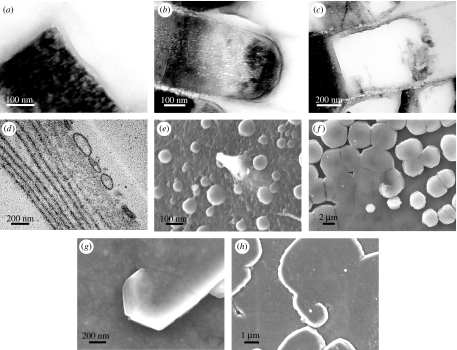
Figure 1.
Nacre morphology. Scanning electron micrographs of (a–d) gastropod nacre: Calliostoma zyziphynus showing (a) a tower of tablets and (b) a fractured transverse section. (c) Bolma rugosa displaying towers of tablets and (d) Gibbula pennanti back scattered electron scanning of a transverse section: (e–l) bivalve nacre; (e) Anodonta cygnea, (f) Atrina pectinata displaying growth fronts made up of tablets, (g–j) Pteria avicula and (k) and (l) Pteria hirundo, showing target, spiral and labyrinthine patterns at the mesoscale, respectively.
Figure 2.
Hierarchical construction of nacre. A sketch diagram showing the construction of nacre from a chitin molecule to a shell in both gastropods (top) and bivalves (bottom).
Although the assembly of nacre is programmed by the genetics of the organism in as much as the chemical species present, their concentrations and moment of appearance are all presumably under genetic control, the construction work goes on without a cellular template in the extrapallial space, a liquid filled cavity beyond the direct control of the cells, and thence must occur by the self-assembly of the component parts through physical and chemical mechanisms. The formation of nacre is thus genetically choreographed, but proceeds in a self-organized fashion from the dynamics of the physical and chemical processes that, beginning with molecular components, produce a series of structures each of which in turn serves as the building block for the next level in the hierarchical fabrication. We affirmed above that pearl and nacre are one and the same material. In fact, very little has been published recently in the open literature on pearl, as opposed to nacre structure. We assume that commercial pressures lead to much research on pearl being carried out in secret; moreover, the cost of samples to study is much greater with pearl than with nacre. However, earlier work demonstrates that the environments of pearl and nacre assembly are structurally identical, with the so-called pearl sac within which a pearl develops being functionally analogous to the mantle epithelium (Bevelander & Nakahara 1969), and our own investigations indicate that the pearl morphology follows that of the nacre of the same species of mollusc.
While others have investigated in detail one or another aspect of nacre construction and of the biological processes that beget it, the dynamics of the self-assembly is what interests us here. We have combined our own observations of nacre with scanning and transmission electron microscopy together with a careful examination of the results of others who have utilized many different techniques to study one or another aspect of nacre construction and have applied to this structural analysis a knowledge of the physics of solid and liquid crystals and of membranes and fluids to arrive at a fresh theoretical viewpoint for understanding the dynamics of nacre construction that we believe will provide fruitful insights for those engaged in unravelling the underlying molecular biology.
2. Material and methods
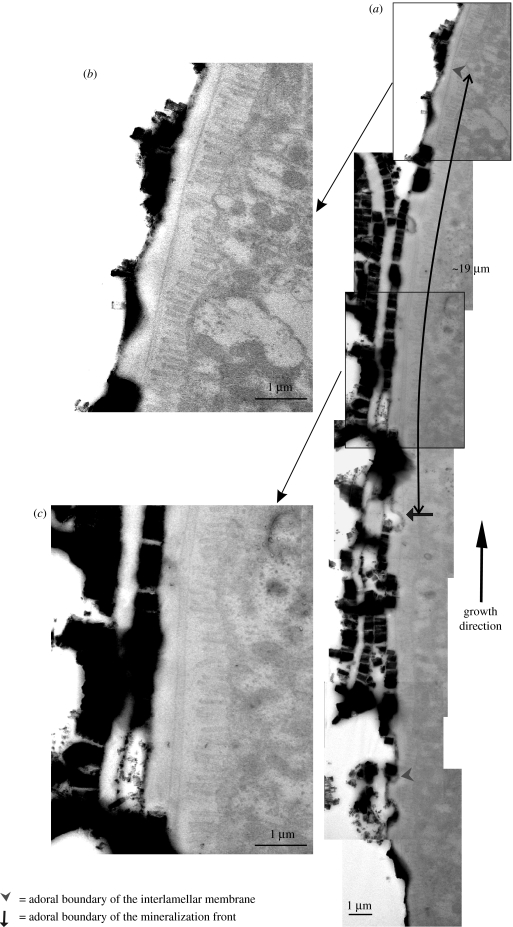
Living specimens were fixed in 2.5% glutaraldehyde in a 0.1 M cacodylate buffer. Field emission scanning electron microscope (Leo Gemini 1530) observations were carried out always on untreated specimens, both on the growth surface and on fractured and polished sections. High-resolution transmission electron microscope (Philips CM20) observations were carried out on samples of ours completely decalcified with 2% EDTA, and on specimens of the late Hiroshi Nakahara of Meikai University (figure 5), which were prepared according to the protocol described by Nakahara (1991). Samples were coated (Hitachi UHS evaporator) with carbon for both techniques.
Figure 5.
Interlamellar membrane formation in bivalves. (a) Collage of transmission electron micrographs of the growth front of nacre in Pinctada radiata, with blow-ups (b) and (c) of two areas of interest.
3. Results and discussion
3.1 Chitin crystallization
Our thesis is that nacre assembly begins at the molecular level with the fabrication of the polysaccharide chitin (N-acetyl-2-glucosamine), which is secreted by the animal from its mantle into the liquid filled extrapallial space between the mantle and the shell. Chitin is a biopolymer found abundantly in nature in three different crystalline polymorphic forms (α, β, and γ) and is second only to its close relative cellulose in its presence throughout the biosphere. α-Chitin, with an orthorhombic crystal structure of antiparallel polymer chains forming a three-dimensional hydrogen-bond network, is the polymorph generally obtained in abiotic synthesis and is the most common in nature, being present in arthropods (in insect cuticle, crustacean carapace, etc.), fungi and elsewhere. But the chitin found in molluscs is the β polymorph (Weiner & Traub 1980). While it is not yet established that chitin occurs in all molluscs, it has been found in each species in which it has been sought. β-Chitin, which has a monoclinic crystal structure of parallel polymer chains that form sheets of hydrogen-bonded molecules with weaker bonding from sheet to sheet (figure 3e), is less widespread than α-chitin, but it is also present in other phyla besides molluscs, and β-chitin crystallites up to 50 nm in diameter and hundreds of nanometres in length containing from tens to thousands of polymer molecules are produced in diatoms (Imai et al. 2003) and vestimentiferans (Gaill et al. 1992). Chitin biosynthesis is not yet completely understood (Cohen 2001; Merzendofer 2006), but it is thought that similar mechanisms are responsible for chitin as for cellulose crystallite formation, where structures in the cellular membrane termed rosettes extrude polymer chains from closely packed pores, whence they immediately crystallize by interchain hydrogen bonding into long thin needle crystallites (Imai et al. 2003; figure 3d). Note that it is simpler to fabricate β-chitin rather than α-chitin with this nanoscale-directed crystallization, as only one type of extruder is required, versus two for α-chitin with its antiparallel polymer chains.
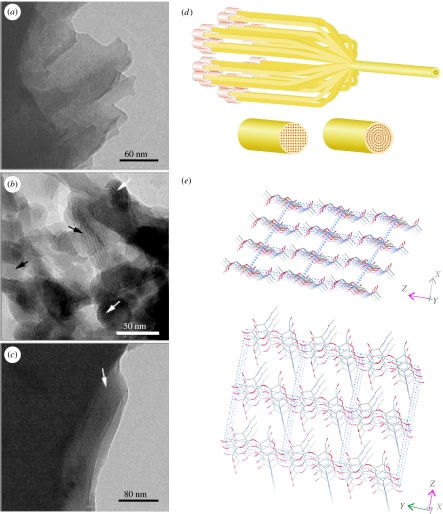
Figure 3.
Chitin in nacre. Cryo-transmission electron micrographs of a vitrified suspension of fixed and demineralized nacreous shell organic matrix fragments from Atrina. (a) A matrix fragment showing the homogenous texture and layered structure. (b) An aggregation of small fragments aligned such that, in some areas, lattice images are visible (black arrows). White arrows show net-like structures. (c) A fragment of the organic matrix after the suspension was refluxed in 1 M NaOH to remove the protein. The homogenous texture and layered structure are still preserved, as is the lattice image in part of this fragment (arrow). (a–c) are taken from figure 1 in Levi-Kalisman et al. (2001). (d) Sketch of the putative chitin crystallization process showing alternative possibilities for the alignment of the crystal planes dictated by the arrangement of the rosettes extruding the polymer chains. (e) Crystal structure of β-chitin (Dweltz et al. 1968).
Cryo-transmission electron micrographs of material from the bivalve Atrina serrata show the presence of these chitin crystallites in mollusc nacre. In figure 3a–c, taken from Levi-Kalisman et al. (2001), one can observe crystallites approximately 20–30 nm in diameter and up to hundreds of nanometres in length, consisting of hundreds of chitin polymers. The black arrows in that figure point to crystallites seen side-on, while white arrows indicate others viewed end-on. Levi-Kalisman et al. (2001) note that the distance separating lattice lines in this micrograph is approximately 2 nm. This is discrepant from the distance from sheet to sheet in the β-chitin crystal lattice of 0.9 nm. However, this intersheet spacing is 0.9 nm only for the anhydrous crystal. It is now well established that the weak intersheet interactions in β-chitin easily allow molecules to intercalate the lattice to form cocrystals or crystallosolvates (Saito et al. 2000). There are two β-chitin hydrates, a monohydrate and a dihydrate, with intersheet spacings of 1.0 and 1.1 nm (Gaill et al. 1992; Saito et al. 2002). And there are many other molecules that can expand the lattice much more; spacings of 2 nm have been shown for β-chitin intercalated with aliphatic amines (e.g. Noishiki et al. 2003). There is little doubt that the chemical processing of the chitin sample of figure 3, undertaken to remove any protein, will have produced such a chitin crystallosolvate. Therefore, we should neither be surprised at the measured 2 nm spacing nor conclude that it represents the in vivo situation, which may well be that of a chitin hydrate.
Vestimentiferan β-chitin crystallites have square cross-section (Gaill et al. 1992), hence it is interesting to see that the crystallites seen end-on in figure 3b appear to be round rather than faceted. Moreover, while X-ray diffraction studies of diatom β-chitin crystallites indicate that they are conventional crystals formed of a collection of parallel planes (Imai et al. 2003), the dense sheets of mollusc chitin, bright in the images in figure 3b and the dark intersheet regions show some signs of curvature. If this is not an artefact of the sample processing, it may be that the crystal structure here is not of the planar type, but instead displays a curved geometry (figure 3d), as occurs in some other materials with similarly weakly bonded sheets, such as clays and graphene carbon. We are carrying out investigations of mollusc β-chitin crystallites with X-ray diffraction to clarify this point.
3.2 The liquid-crystal core of the interlamellar membrane
At this stage in the construction of nacre, we have a colloid of chitin crystallites suspended in the extrapallial liquid. Above a certain critical concentration in solution, such rod-shaped particles are compelled by their mutual interactions to adopt a certain configuration in space. They form what is termed a liquid crystal, a mesophase in which the particles possess a certain amount of order, as in a crystal, while still being free to move, as in a liquid. This self-organization process has been studied in vitro with α-chitin crystallites of dimensions similar to the β-chitin crystallites of molluscs. When dispersed in a colloidal suspension, these form a so-called cholesteric liquid crystalline phase (Belamie et al. 2004); one in which the crystallites are parallel to the plane of the layers, and the crystallites in each layer are twisted with respect to those in the neighbouring layers. In these experiments, measurements of the liquid crystalline structure were taken after the system had come to equilibrium, by waiting from a few days up to a year. In the mollusc in vivo, on the other hand, a fresh layer of nacre is laid down every 1–24 h (Lin & Meyers 2005), so the system does not have time to arrive at equilibrium and, consequently, the liquid-crystalline ordering is only partial. It comprises a lamellar structure, but within the layers there is disorder, with the crystallites forming a mesh, like a logjam on a lake, as seen in figure 2. Scanning electron micrographs of the interlamellar membranes of molluscs display this felt-like structure (figure 4b,d). Simulations of the dynamics of the formation of a cholesteric phase of a liquid crystal are qualitatively consistent with this scenario; they show that the process consists first of the orientation of the material into layers followed by its alignment within and between layers (de Luca & Rey 2004). Liquid-crystal self-organization has been postulated to explain the structures of many fibrous bicomposites ranging from plant cell walls to bone to arthropod cuticle (Bouligand 1972; Neville 1993), and in particular, the so-called twisted plywood morphology seen in many of these instances is thought to originate as a cholesteric liquid crystal. Here, in nacre, the final morphology is somewhat different from twisted plywood, but both arise from cholesteric liquid crystals produced by the same self-organization mechanism.
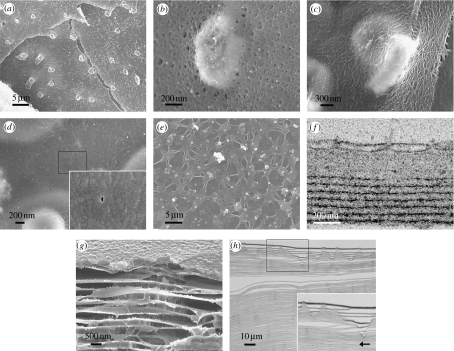
Figure 4.
Interlamellar membranes. Scanning electron micrographs of interlamellar membrane structures in (a,b) gastropods; (a) Gibbula umbilicalis with a membrane around towers of tablets and (b) Gibbula pennanti showing a closer view of a membrane around the top of a tower. (c,d) Bivalves; Anodonta cygnea, showing (c) a membrane draped across two tablets and (d) a close-up of the membrane displaying a pore (amplified in the inset). (e–h) Interlamellar membrane formation and surface membrane in gastropods; (e) scanning electron micrograph of the surface membrane in the gastropod, Calliostoma zyziphynus, viewed from the mantle side, (f) transmission electron micrograph of a section through the surface membrane (top) and the first few interlamellar membranes of Gibbula umbilicalis, (g) surface and interlamellar membranes in Clanculus jussieui and (h) sectional view of the overall structure in Gibbula umbilicalis (amplified in the inset), with the arrow indicating the growth direction.
The extrapallial space in molluscs is narrow, which only allows one or two additional liquid-crystalline layers to form at a time; in physical terms, this is liquid-crystal formation in a growing domain. The sequence of events in nacre formation diverges at this point between bivalves and gastropods; we shall deal first with bivalves. The chitin crystallites in the extrapallial liquid of bivalves self-organize as a liquid crystal to form a fresh layer above the last formed layer of interlamellar membrane. Transmission electron micrographs of transverse sections through the growing edge of bivalve nacre show how a fresh interlamellar membrane is laid down above an existing membrane in this way (Bevelander & Nakahara 1969; figure 5a). The new liquid-crystal layer aligns itself with those laid down earlier, spaced away from its nearest neighbour by a characteristic distance given by the strength of the interactions between the crystallites. In laboratory experiments with α-chitin crystallites, this interlayer spacing was 60–120 nm for different concentrations (Belamie et al. 2004), which may be compared with the approximately 90 nm spacing that we see in figure 5a for new β-chitin layers in the bivalve Pinctada radiata. In gastropods, there is a pre-existing, thick (approx. 100 nm) membrane lying between the mantle of the animal and the surface of the nacre. This, the surface membrane, was noted by Nakahara (1991) in his work on gastropod nacre, but has not, it seems, been characterized further. Possibly the surface membrane evolved to protect the growing nacre from the exterior environment when the body of the animal is retracted (Nakahara 1991), while this is not necessary in bivalves in which the growing surface does not come into contact with the exterior. It has a complex and beautiful structure (figure 4e). As the surface membrane lies between the mantle and the shell, all components of the nacre must pass through it. On the other hand, it must be sufficiently impermeable to prevent the ingress of seawater to the growing nacre beneath when the mantle is retracted. The resolution to this conundrum appears to be that the surface membrane is a dynamic structure, with material continually being added at its mantle side and leaving at the shell side. The surface membrane appears to serve as a guide or template for laying down a fresh liquid crystal layer in gastropods: a new interlamellar membrane periodically detaches itself at the shell side. The chitin crystallites aggregate into a new interlamellar membrane adjacent to the shell side of the surface membrane, as figure 4f displays. There is a slight tilt to the interlamellar membranes in gastropods, such that they detach first from the surface membrane in the adoral direction (figure 4h).
Layers of crystallites in liquid crystals are mathematically analogous to atomic or molecular terraces in crystals, and, just as defects are common in crystals, they are also widespread in liquid crystals. Cholesteric liquid crystals, as we have here, typically show screw and edge dislocations (Kléman 1989); indeed, these are what we find in the interlamellar membranes of bivalve nacre. Screw dislocations manifest themselves as the typical spiral patterns seen in scanning electron micrographs of bivalve nacre (figure 1h–l). Edge dislocations are hard to spot in micrographs of the growth surface and show up more clearly in sections through the material. The template function of the surface membrane in gastropods means that the defects are not so often introduced into the layer structure; it guides the production of new membranes so they form with fewer defects compared with the bivalves. However, edge dislocations do occur in gastropods (figure 1d). Bivalve nacre is seen to form both spiral and target patterns on its growth surface. While the former are the manifestations of screw dislocations propagating upwards, as fresh interlamellar membrane is laid down at the leading edge of the screw, the latter are how new layers of membrane nucleate and grow. The nucleation and growth of layers of liquid crystal can be analysed as the nucleation of crystals; in the same way as there is a correspondence between the defects in solid and liquid crystals, so there is with nucleation and growth too. Crystals typically nucleate and grow by a tangential growth mechanism, termed two-dimensional nucleation (Chernov 2003), in which a fresh layer of material is deposited atop an existing one, leading to growth hillocks formed of piles of layers, which are exactly what we observe here in the case of the liquid crystals forming the interlamellar membranes in nacre. We are presently working, following the analogy between tangential growth in crystals and liquid crystals, to model the formation of these interlamellar membranes.
3.3 The interlamellar membrane: a fibrous composite
A liquid-crystal layer of chitin crystallites then forms the core of each interlamellar membrane in nacre. However, this structure on its own is not strongly bonded together and thus would not have the material strength necessary for its purpose in nacre. This is remedied by the addition of proteins having an affinity for chitin. These are present in the extrapallial liquid, and cover the chitin layer, forming a coating that binds together and stabilizes the crystallites to form a strong composite material—a membrane. Chitin crystallites incorporated into a protein matrix have been noted, inter alia, in arthropod cuticle, nematode eggshell and vestimentiferan tube (Neville 1993; Gaill et al. 1992). Similar bonding is observed in other biological liquid crystals that would not be stable and useful in their biological environment in their original state (Vincent 2005), and glycoproteins that would fulfil the role of this protein matrix are known to be present in nacre (Pereira-Mouriès et al. 2002). Protein–chitin interactions may be facilitated by the coincidence of hydrogen-bonding sites along chitin and protein chains; the repeat distance is synchronized 2 : 3 between the aminosugars in chitin and the aminoacids in a protein with β-sheet conformation (Fraenkel & Rudall 1947; Neville 1993).
In bivalves, it is notable (figure 5a) that the distance between membranes rises from approximately 90 nm produced by the liquid-crystal interaction between chitin crystallites as new membrane is formed to approximately 500 nm of mature membrane prior to mineralization (Bevelander & Nakahara 1969). And in gastropods, a fresh membrane detaches from the surface membrane and positions itself with approximately the same spacing from its neighbour (Nakahara 1991; approx. 500 nm is typical in both bivalves and gastropods, but for some species this membrane spacing can be rather more, or less). The physical reason for this increase in the membrane spacing can be found in the above chemical process. The combination of hydrophobic chitin and relatively hydrophilic glycoproteins produces an amphiphilic structure in which glycoproteins shield the chitin from the aqueous extrapallial liquid by surrounding it. This adds a new component to the balance of attractive and repulsive forces between the membranes that determines their spacing. This balance is usually described in terms of van der Waals and electrostatic (Coulomb) forces (these are often grouped together as Derjaguin–Landau–Verwey–Overbeek (DLVO) interactions), hydration (solvation) repulsion and undulation (Helfrich) repulsion from steric interactions (Boal 2002). All depend on the molecular characteristics and solvent conditions, making a quantitative analysis of the spacing all but impossible to perform without a detailed knowledge of the system that as yet we do not possess. We can, however, affirm that similar stable lamellae form in amphiphile-water systems (Ikkala & ten Brinke 2004), and that the observed approximately 500 nm spacing in nacre is consistent with the layer spacing in lamellar amphiphilic systems. The increase, from approximately 90 to 500 nm, we can understand qualitatively in terms of an additional repulsion term in the force balance between membranes introduced by the electrostatic charges on the amphiphilic structure of the mature membrane. This change in the force balance from the original cholesteric liquid crystal to the mature membrane covered with protein would serve to repel the membrane from its neighbouring interlamellar membrane.
The interlamellar membrane then consists of chitin core of a felt-like mesh of fibres that are coated with protein to form a fibrous composite. What is seen in micrographs of the interlamellar membrane depends on the chemical treatment to which the sample has been subjected. While in some cases the bare chitin core may be visible, in others it may be coated with proteins; both glycoproteins and others. The complete chitin–protein composite is what is normally seen in our scanning electron micrographs of the interlamellar membrane, untreated except for carbon sputtering in figure 4. Depending upon the taxon in question, there may be pores visible in the membrane (Nakahara 1991). In gastropods these pores are invariably seen. They are of irregular shape but of a characteristic size and spacing, consistent with a distribution of crossed fibres (figure 4b). In bivalves, our scanning electron micrographs of the interlamellar membrane show either a smooth surface or a surface with patterning, but in either case, pores are far less frequent than in gastropods (figure 4c). The occasional pore that we find in bivalve membrane is minuscule (figure 4d). On the other hand, there are larger pores in scanning electron micrographs published by others of the interlamellar membrane of various species of bivalve (Nakahara 1991); these samples have possibly undergone treatment that lays bare the chitin core of the membrane. Another pertinent observation is that of a section through a pore in the bivalve, Pinctada maxima, which shows it to be filled with organic material (Rousseau et al. 2005b). It may be that the proteinaceous coating of the interlamellar membrane generally blocks the pores in bivalves, but not in gastropods. We shall discuss this further below when we come to mineralization.
3.4 The extrapallial liquid
The extrapallial liquid surrounding the membranes is an aqueous medium that must necessarily contain all the constituents of nacre: polysaccharides; proteins; and mineral. If we leave aside for a moment consideration of the mineral component, the other main element, together with chitin and glycoproteins, which has been distinguished in nacre is protein that has been identified with silk fibroin (Weiner & Traub 1980; Levi-Kalisman et al. 2001). It is probable that this is what appears in transmission electron micrographs as filamentary matter within the spaces between the membranes prior to mineralization (Bevelander & Nakahara 1969), and in atomic force micrographs of mineralized nacre as a spongiform material within the mineral tablets (Rousseau et al. 2005b). It may also adhere to the chitin–protein composite membranes and accumulate both around the mineral tablets, forming a so-called intertabular matrix (Addadi et al. 2006; figure 6a–c), and in gastropods, an organic core to the mineral tablets (Mutvei 1978; Nakahara 1983; figure 1d).
Figure 6.
Mineralization and its relationship to the organic components. (a–c) Interlamellar membranes and intertabular matrices of (a) Pinctada radiata, (b) Haliotis gigantea and (c) Monodonta labio. (d,e) Vesicles possibly containing mineral in (d) transmission and (e) scanning electron micrographs of Gibbula umbilicalis. (f–h) Mineral tablets at centres of membrane screw dislocations in (f) Pteria avicula, (g) Pteria hirundo and (h) Isognomon radiatus.
As we commented above, nacre growth takes place in a growing domain, which implies that fresh liquid is constantly being secreted into the extrapallial space as it is used up by being locked into the earlier grown nacre. We should not then think of the constituents of nacre discussed up to now having to cross the extrapallial space, rather that the components are secreted together with the extrapallial liquid, and as the quantity of extrapallial liquid becomes sufficient to form a new interlamellar membrane, they self-organize into the membrane structure. After a new interlamellar membrane has been formed, it moves within the extrapallial liquid to space itself at a given distance from the earlier membranes and, in gastropods, from the surface membrane. For this to occur, there must be bulk movement of liquid which implies both a sufficiently fluid nature of the extrapallial medium—it must not be vastly more viscous than water—and that there is a geometry to the system that permits this liquid flow. In gastropods, we have seen that the membranes remain porous, and so the extrapallial liquid can easily pass through them. In bivalves, on the other hand, most of the pores in the membranes may well be obturated by the stage of construction in which an interlamellar membrane respaces itself at approximately 500 nm from the initial approximately 90 nm from its neighbour (figure 5). The extrapallial liquid must then flow through the openings at the ends of a membrane, for which it is important that the membranes of bivalves contain as many defects as they do. A similar flow should occur in the reverse direction when some of the water is displaced from between the membranes during mineralization.
The necessity for the extrapallial liquid to be of sufficiently low viscosity for the membrane to be able to increase its spacing implies that the silk fibroin should aggregate only after this event takes place. Possibly, the aggregation might be induced by a change in pH or ionic strength (Foo et al. 2006) produced by the alteration in the extrapallial liquid composition as the glycoproteins coat the chitin. In other words, the silk fibroin would form a gel only after the interlamellar membrane structure has been completely established. Such an interpretation is supported by the observation that the extrapallial liquid is homogeneous outside the membranes, and only within them are filaments seen (Bevelander & Nakahara 1969).
3.5 Mineralization
We finally arrive at the stage of nacre construction at which mineralization takes place. The aragonite mineral component of nacre is by far the greatest portion of its three ingredients. The other two, polysaccharide and protein, constitute together less than 5% by either mass or volume (Jackson et al. 1988), but they form the linchpin of the whole assembly; the entire skeleton of nacre has been constructed up to this point without the mineral part, and it is only now, at the culmination of the process, that mineralization occurs. Of course, this should not be taken to imply that the role of the mineral component is unimportant. Far from it, its existence is vital to providing the desired material properties of the nacre, but its presence has not been required to form the structure of the material.
The mineralization of nacre proceeds with calcium carbonate occupying the space between the interlamellar membranes. It seems that the mineral is initially present in growing nacre in an amorphous form (Nassif et al. 2005) that would be unstable in an abiotic environment. It is speculated that calcium carbonate may be transported in this amorphous state to its crystallization site within the partially completed assembly, having been previously formed elsewhere in the mantle (Addadi et al. 2006). We have noted in scanning electron micrographs the presence of spherical structures associated with the surface membrane of gastropods (figure 6e). When demineralized and viewed in a slice with transmission electron microscopy (figure 6d), we see they are hollow with a membrane around them. In other words, they are vesicles. Might they contain mineral and be involved in this transport? This has yet to be established. It is clear that mineral-carrying vesicles would provide a local source of raw material, and hence prevent the depletion of calcium carbonate within the membranes and the concomitant slowdown in mineralization that would inevitably occur if it were being supplied only remotely by the mantle and had to diffuse across the extrapallial space from there. The advantage of employing amorphous calcium carbonate as a precursor phase would be that it is easy to alter the physical conditions to achieve the desired crystalline form. We note that we have only seen vesicles thus far in gastropods and only on the mantle side of the surface membrane. They appear to deform into lamellar structures thereon (figure 6d), and we suppose the mineral phase—if that is in fact what they contain—to be liberated into the extrapallial liquid on the shell side as a fresh interlamellar membrane detaches. Our transmission electron micrographs are of demineralized samples, so we are seeing in figure 6d the organic membrane of the vesicle; this might be composed of the glycoproteins that are associated with the chitin.
Calcium carbonate crystallized in an abiotic environment under the conditions in which it is deposited in nacre forms the calcite polymorph, but it is the aragonite polymorph that is found in nacre. Thus, it is clear that the crystal polymorphism is being controlled by the system; and indeed, proteins present in nacre cause calcium carbonate to crystallize in vitro as aragonite (Belcher et al. 1996; Falini et al. 1996). Aragonite is rather harder than calcite, which may be the reason for this control over the polymorphism. Each brick or tablet is a composite of aragonite incorporating the organic matrix described above (Rousseau et al. 2005b). This must add considerably to its resistance against fracture, and it is apposite to note the similarity with the structure of adobe bricks of mud reinforced with straw used in some traditional architecture for the same reasons of improved material properties over mud alone. The intertabular matrix surrounding each tablet (figure 6a–c) may be produced by compositional zoning—partial segregation of the organic matter—during mineralization. Work on crystal growth in gels has shown behaviours ranging from the total incorporation of a fibrous network during crystal growth to complete segregation, depending on the nature of the crystal and of the foreign material (Henisch 1996; Grassman et al. 2003). In gastropods, tablets have an organic core (Mutvei 1978); as we may note in figure 1d. One possible explanation for this is that the tablet begins to grow when close to the surface membrane, when the concentration of organic material in the extrapallial liquid is high, so this is incorporated as a central organic-rich core. As growth continues, the concentration of organic material drops and the outer regions of the tablet are more mineral rich. Gastropod tablets are also sectored (Mutvei 1978), and compositional zoning as the sectors grow may be important. The tablets develop following the shape of the membranes that contain them, first growing upwards and outwards from points on the lower membrane until they contact the upper membrane, from when on they continue to grow outwards only until they contact their neighbouring crystals in the same layer, and in so doing they form a random close packing within each layer. All these observations imply that there is a variety of proteins present to both promote and inhibit mineralization (Marin & Luquet 2004); there would be an initial promotion of mineralization as a fresh tablet is formed, while when it contacts the membrane above it, further growth would be inhibited in that direction. Nacre then has in common with many other examples of biominerals that there is exquisite control over crystallization. A particularly interesting example of this is the morphology of the tablets present at the centres of screw dislocations in the interlamellar membranes of bivalves, which follows the spiral geometry of the membranes (figure 6f–h).
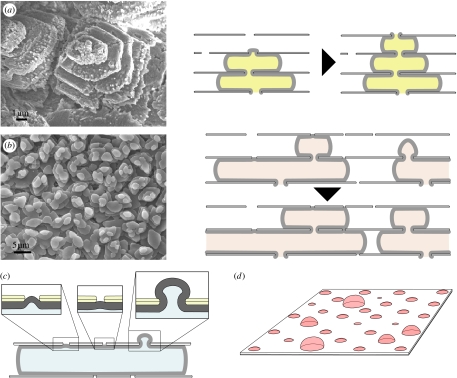
Both bivalve and gastropod nacres grow at different levels simultaneously, with deeper levels in the structure being completed as new upper levels are begun. In bivalves, this proceeds by new tablets in an upper layer initiating their growth from the edges of growing tablets in the layer below, forming an interlocking structure of brickwork (Rousseau et al. 2005a; Checa & Rodríguez-Navarro 2005). Mineralization is rather different in gastropods, in which the tablets in upper layers are centred on those in lower layers, forming columns (Nakahara 1983). In both cases, a tablet only begins to grow in an upper layer once there is one present in the layer beneath it, and tablets in a vertical line in both gastropod (DiMasi & Sarikayai 2004) and bivalve nacre (Hou & Feng 2003; Checa & Rodríguez-Navarro 2005; Checa et al. 2006) show coincident crystal axes (figure 7a,b). Thus, it is clear that either the mineral itself or some other substance that carries with it the information regarding the mineral crystal axes must pass from one layer to the next. In other words, there is either homo- or heteroepitaxy involved. The differences in the quantity and size of pores in the interlamellar membranes of bivalves and gastropods could be the key to clarifying these questions (Hou & Feng 2003). We have seen that the pores in the interlamellar membrane of gastropods remain open even after it has been coated with protein. Thus, it would not be a great surprise to find in gastropod nacre the aragonite itself passing as a mineral bridge from layer to layer. Indeed, there is strong, albeit not yet conclusive, evidence for this (Schäffer et al. 1997; Song et al. 2003). Following the mineral bridge hypothesis, since pores are large and plentiful, as soon as a tablet in the layer below reaches the membrane above it continues to grow through these pores (figure 7a), and there is then no shortage of mineral bridges to continue the growth of a tablet in the upper layer with the same crystalline orientation (DiMasi & Sarikaya 2004). As gastropod tablets are sectored (Mutvei 1978), the transmission of the structure of sectors would be expected to occur via various mineral bridges per tablet. On the other hand, in bivalves, most pores in the interlamellar matrix appear to be filled only with protein and not with mineral (Rousseau et al. 2005b), and there is as yet no evidence of mineral bridges. However, the tablets in different layers do retain the same crystalline orientation (Hou & Feng 2003; Checa & Rodríguez-Navarro 2005; Checa et al. 2006; figure 7b). It is difficult to envisage how the intertabular organic matrix could transmit information regarding the crystal orientation necessary for heteroepitaxy to take place, given that it itself does not have a fixed crystalline orientation, and furthermore that it is in contact with amorphous rather than crystalline calcium carbonate (Nassif et al. 2005). The simplest explanation is that there are mineral bridges in bivalves too. If these are more widely spaced than in gastropods, this could explain the observation that in bivalves new tablets on an upper layer grow at the borders of one on a lower layer (cf. figure 7b): as the lower tablet grows outwards, it arrives at a pore in the membrane above it, whereon it grows through the pore and initiates the tablet above, which is then naturally found at the border of the lower one, as we sketch in figure 7b. This is consistent with our observation that open bivalve pores are rather infrequent (figure 4d). It is clear too that pore size must influence the growth rate of a mineral bridge, as we sketch in figure 7c,d.
Figure 7.
Pores in the interlamellar membranes: scanning electron micrographs and sketches showing the influence of pore spacing on morphology of mineralization in (a) gastropods (Gibbula umbilicalis) and (b) bivalves (Nucula nitidosa); (c,d) how pore size may influence the rate of growth of mineral bridges.
We have noted that gastropod nacre in formation contains piles of mineral tablets, while in bivalves there are steps of tablets forming spiral, labyrinthine and target patterns. The final question we must ask ourselves in terms of the dynamics of nacre assembly is how the membrane morphology described above leads to the mineral morphologies seen in figure 1. This is attributable directly to the differences in the formation processes of the interlamellar membranes. While in gastropods mineralization happens when an entire interlamellar membrane has formed and has separated as a whole within the extrapallial liquid, in bivalves it occurs after the membrane respacing has taken place, and membrane formation continues some tens of micrometres ahead of the front of growing mineral tablets within the interlamellar space formed by that membrane. If we imagine an early stage of nacre formation in both taxa, we see that in gastropods nucleation of mineral can take place over an entire two-dimensional surface more or less simultaneously, which leads to a random pattern of nucleation over the surface producing a distribution of tablets that form the bases of the piles we see growing up in figure 1a–d. In bivalves, on the other hand, the nucleation of mineral commences behind the growth fronts of the first interlamellar membranes, i.e. on one-dimensional lines following these growth fronts. As the fronts advance, encounter each other, join, initiate spiral dislocations and so on, the mineralization front follows behind, and these events are reflected in the morphology seen in figure 1e–l. It is intriguing that in the cephalopod, Nautilus, the nacre appears to have a morphology intermediate between that of gastropods and bivalves; in some regions, there appear the towers characteristic of gastropods, while in others there are the steps seen in bivalves (Mitchell & Phakey 1995). It would be most interesting to look more closely at this case to see how the two dynamics are blended in the cephalopod.
4. Conclusions
Nacre is a biocomposite involving three components: mineral (aragonite); polysaccharide (chitin); and protein (glycoproteins, silk fibroin and others). Many other natural composites involve two of these three components, but in having all three together, nacre is a member of the most advanced class of biological composite materials. Each of the components forms composite structures on different scales: the mineral grows through an intracrystalline protein matrix and the membranes between crystal layers have a polysaccharide core covered in a proteinaceous coating. Moreover, nacre assembly takes place in both bivalves and gastropods outside the cells that manufacture the component parts. Thus, any control exercised by the cells themselves can only be remote, and nacre is the result of the pooled secretions of neighbouring cells; the lack of faults coinciding with cell boundaries is a demonstration of the self-assembled nature of the material. We have seen that nacre is constructed in a manner that would seem, to a bricklayer, to be the most peculiar way of building a brick wall: a nacre wall is built by first depositing the mortar and only subsequently growing the bricks within it. This underscores a crucial point to be understood about nacre: it is frequently seen as a biomineral—with the emphasis on mineral—while in fact it is the few per cent of organic materials constituting the skeleton of the material within which the mineral is deposited which is the primum mobile for the self-assembly.
The spirals and target patterns of the mesoscale structure of bivalve nacre have long been noted, and for decades attempts have been made to assimilate the phenomenon to other instances of similar patterning (Wada 1966). What was lacking in those efforts, however, was firstly the understanding that the most visible aspects of the patterning, the aragonite tablets, are merely elements adorning the underlying membranes, and secondly and more fundamentally, a means of linking any physicomathematical theory of the growth of the patterns—spirals, target patterns and so on—to the underlying biology. In the intervening period, it is not just our knowledge of molluscan biology that has improved; the basic understanding of crystallization, of liquid crystals and of membrane and fluid physics has increased beyond all recognition and has allowed us here to make the necessary connections between the physics and the biology. To some extent, our analysis is a return to the ideas current in the field of nacre research over 30 years ago, in that it was understood then that the interlamellar membranes are present before mineralization (Bevelander & Nakahara 1969), while in the meantime this has sometimes been disputed. Certainly, we are following the long tradition of structural analysis in studies of nacre, which lately has tended to give way to molecular biology. We would argue that both biological approaches—molecular biology and structural analysis—are vital to understanding nacre formation. In the physics, a similar interdisciplinarity is also necessary. In the preceding sections, we have shown that several different areas of physics—crystallization, liquid crystals, membranes and fluids—must all be drawn upon to understand the dynamics of nacre assembly. Each has contributed some understanding of a particular component: the initial chitin crystallization; the subsequent formation of a liquid crystal; its transformation into a protein-coated membrane; and the part played by the extrapallial liquid. In the ultimate section, we have shown that mineralization pulls all these strands together. This has allowed us to put forward a coherent physical model for the dynamics of nacre self-assembly.
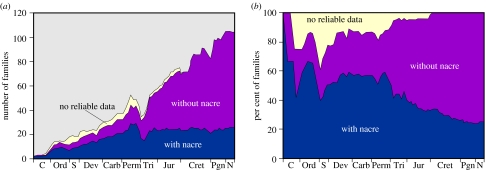
Nacre is an ancient material found in the earliest fossil molluscs. It is a high-performance biocomposite, but it is also a high-cost material. Possibly because it is energetically expensive for the organism to fabricate, it is seen in the fossil record to be gradually losing ‘market share’ in molluscs in favour of cheaper, but less high-performance materials (figure 8). An intriguing question, given what we have seen above of the similarities and differences among bivalve, gastropod and cephalopod nacres, is the nature of their evolutionary relationship. Sequencing the genomes of these taxa would certainly provide much useful information in this regard. There is still much to be understood of the genetic basis of nacre construction (Wilt et al. 2003), and we hope that this clarification of the physical aspects of the assembly will aid those studying the molecular biology.
Figure 8.
Palaeontological presence of nacre. The number (a) and percentage (b) of families of bivalves employing nacre in different geological periods. Approximately two-thirds of the nacre data come from Carter (1990). Other data are from personal communications (E. M. Harper 2006) and our own work. The temporal distribution of the bivalve families is from Skelton & Benton (1993).
Knowledge of the dynamics of nacre self-assembly provides clues as to how to control its construction, which would allow us to change its optical and mechanical properties. The optical properties of the material are attributed to interference in some cases and diffraction in others; the surface of nacre has been found to act as a diffraction grating (Liu et al. 1999), while pearl colour has been discovered to arise from interference within the nanocomposite structure of the aragonite tablets (Snow et al. 2004). An aim of altering the parameters of nacre construction might then be to control, say pearl colour, or to change the optical properties of nacre for other, technical rather than aesthetic, reasons. Its mechanical properties too might be amenable to alteration in this way. This would be a step on the way to a future biomimetic nanotechnology in which we would have learnt from biology how to construct self-assembled structures on the molecular scale (Kato 2002; Whitesides & Grzybowski 2002). As Feynman (1960) put it in his visionary discussion of nanotechnology, and as molluscs long ago discovered, there is plenty of room at the bottom.
Acknowledgments
We thank José Gavira, Fernando Gervilla, Chris Previti, Ignacio Sainz and Ana Villacampa for interesting discussions, Juan de Dios Bueno for sample preparation, Stephen Hyde for procuring some useful references, Mitsuo Kakei for providing us with material from the late Hiroshi Nakahara and Luis Sánchez for obtaining some gastropod specimens. J.H.E.C. is supported by the Spanish Ministerio de Educación y Ciencia grant CTQ2004-04648, and A.G.C. is supported by the Spanish Ministerio de Educación y Ciencia grant CGL2004-00802 and by Andalusian Research Group RMN190.
References
- Addadi L, Weiner S. A pavement of pearl. Nature. 1997;389:912–915. doi: 10.1038/40010. [DOI] [Google Scholar]
- Addadi L, Joester D, Nudelman F, Weiner S. Mollusk shell formation: a source of new concepts for understanding biomineralization processes. Chem. Eur. J. 2006;12:980–987. doi: 10.1002/chem.200500980. [DOI] [PubMed] [Google Scholar]
- Belamie E, Davidson P, Giraud-Guille M.M. Structure and chirality of the nematic phase in α-chitin suspensions. J. Phys. Chem. B. 2004;108:14 991–15 000. doi: 10.1021/jp048152u. [DOI] [Google Scholar]
- Belcher A.M, Wu X.H, Christensen R.J, Hansma P.K, Stucky G.D, Morse D.E. Control of crystal phase switching and orientation by soluble mollusk shell protein. Nature. 1996;381:56–58. doi: 10.1038/381056a0. [DOI] [Google Scholar]
- Bevelander G, Nakahara H. An electron miscroscope study of the formation of the nacreous layer in the shell of certain bivalve molluscs. Calc. Tissue Res. 1969;3:84–92. doi: 10.1007/BF02058648. [DOI] [PubMed] [Google Scholar]
- Boal D. Cambridge University Press; Cambridge, UK: 2002. Mechanics of the cell. [Google Scholar]
- Bouligand Y. Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell. 1972;4:189–217. doi: 10.1016/s0040-8166(72)80042-9. [DOI] [PubMed] [Google Scholar]
- Carter J.G. Evolutionary significance of shell microstructure in the Palaeotaxodonta, Pteriomorphia and Isofilibranchia (Bivalvia: Mollusca) In: Carter J.G, editor. Skeletal biomineralisation: patterns, processes and evolutionary trends. vol. 1. Van Nostrand Reinhold Co; New York, NY: 1990. pp. 135–296. [Google Scholar]
- Checa A.G, Rodríguez-Navarro A.B. Self-organisation of nacre in the shells of Pterioida (Bivalvia: Mollusca) Biomaterials. 2005;26:1071–1079. doi: 10.1016/j.biomaterials.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Checa A.G, Okamoto T, Ramírez J. Organization pattern of nacre in Pteriidae (Bivalvia: Mollusca) explained by crystal competition. Proc. R. Soc. B. 2006;273:1329–1337. doi: 10.1098/rspb.2005.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernov A.A. Protein crystals and their growth. J. Struct. Biol. 2003;142:3–21. doi: 10.1016/S1047-8477(03)00034-0. [DOI] [PubMed] [Google Scholar]
- Cohen E. Chitin synthesis and inhibition: a revisit. Pest Manag. Sci. 2001;57:946–950. doi: 10.1002/ps.363. [DOI] [PubMed] [Google Scholar]
- de Luca G, Rey A.D. Chiral front propagation in liquid-crystalline materials: formation of the planar monodomain twisted plywood architecture of biological fibrous composites. Phys. Rev. E. 2004;69:011706. doi: 10.1103/PhysRevE.69.011706. [DOI] [PubMed] [Google Scholar]
- DiMasi E, Sarikaya M. Synchrotron X-ray microbeam diffraction from abalone shell. J. Mater. Res. 2004;19:1471–1476. doi: 10.1557/JMR.2004.0196. [DOI] [Google Scholar]
- Dweltz N.E, Colvin J.R, McInnes A.G. Studies on chitan (β-(1→4)-linked 2-acetamido-2-deoxy-D-glucan) fibers of the diatom Thalassiosira fluviatilis, Hustedt. III. The structure of chitan from X-ray diffraction and electron microscope observations. Can. J. Chem. 1968;46:1513–1521. [Google Scholar]
- Falini G, Albeck S, Weiner S, Addadi L. Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science. 1996;271:67–69. doi: 10.1126/science.271.5245.67. [DOI] [Google Scholar]
- Feynman R.P. There's plenty of room at the bottom. Eng. Sci. 1960;23:22–36. [Google Scholar]
- Foo C.W.P, Bini E, Hensman J, Knight D.P, Lewis R.V, Kaplan D.L. Role of pH and charge on silk protein assembly in insects and spiders. Appl. Phys. A. 2006;82:223–233. doi: 10.1007/s00339-005-3426-7. [DOI] [Google Scholar]
- Fraenkel G, Rudall K.M. The structure of insect cuticles. Proc. R. Soc. B. 1947;134:111–143. doi: 10.1098/rspb.1947.0006. [DOI] [PubMed] [Google Scholar]
- Gaill F, Persson J, Sugiyama J, Vuong R, Chanzy H. The chitin system in the tubes of deep sea hydrothermal vent worms. J. Struct. Biol. 1992;109:116–128. doi: 10.1016/1047-8477(92)90043-A. [DOI] [Google Scholar]
- Grassman O, Neder R.B, Putnis A, Löbmann P. Biomimetic control of crystal assembly by growth in an organic hydrogel network. Am. Miner. 2003;88:647–652. [Google Scholar]
- Henisch H.K. Dover; New York, NY: 1996. Crystal growth in gels. [Google Scholar]
- Hou W.T, Feng Q.L. Crystal orientation preference and formation mechanism of nacreous layer in mussel. J. Cryst. Growth. 2003;258:402–408. doi: 10.1016/S0022-0248(03)01551-3. [DOI] [Google Scholar]
- Ikkala O, ten Brinke G. Hierarchical self-assembly in polymeric complexes: towards functional materials. Chem. Commun. 2004:2131–2137. doi: 10.1039/b403983a. [DOI] [PubMed] [Google Scholar]
- Imai T, Watanabe T, Yui T, Sugiyama J. The directionality of chitin biosynthesis: a revisit. Biochem. J. 2003;374:755–760. doi: 10.1042/BJ20030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A.P, Vincent J.F.V, Turner R.M. The mechanical design of nacre. Proc. R. Soc. B. 1988;234:415–440. [Google Scholar]
- Kato T. Self-assembly of phase-segregated liquid crystal structures. Science. 2002;295:2414–2418. doi: 10.1126/science.1070967. [DOI] [PubMed] [Google Scholar]
- Kléman M. Defects in liquid crystals. Rep. Prog. Phys. 1989;52:555–654. doi: 10.1088/0034-4885/52/5/002. [DOI] [Google Scholar]
- Levi-Kalisman Y, Falini G, Addadi L, Weiner S. Structure of the nacreous organic matrix of a bivalve mollusk shell examined in the hydrated state using cryo-TEM. J. Struct. Biol. 2001;135:8–17. doi: 10.1006/jsbi.2001.4372. [DOI] [PubMed] [Google Scholar]
- Lin A, Meyers M.A. Growth and structure in abalone shell. Mater. Sci. Eng. 2005;390:27–41. doi: 10.1016/j.msea.2004.06.072. [DOI] [Google Scholar]
- Liu Y, Shigley J.E, Hurwit K.N. Iridescence color of a shell of the mollusk Pinctada margaretifera caused by diffraction. Opt. Exp. 1999;4:177–182. doi: 10.1364/oe.4.000177. [DOI] [PubMed] [Google Scholar]
- Marin F, Luquet G. Molluscan shell proteins. CR Palevol. 2004;3:469–492. doi: 10.1016/j.crpv.2004.07.009. [DOI] [Google Scholar]
- Mayer G. Rigid biological systems as models for synthetic composites. Science. 2005;298:1144–1147. doi: 10.1126/science.1116994. [DOI] [PubMed] [Google Scholar]
- Merzendofer H. Insect chitin synthases: a review. J. Comp. Physiol. B. 2006;176:1–15. doi: 10.1007/s00360-005-0005-3. [DOI] [PubMed] [Google Scholar]
- Mitchell P.R, Phakey P.P. Notes on the microstructure of the Nautilus shell. Scan. Microscop. 1995;9:215–230. [Google Scholar]
- Mutvei H. Ultrastructural characteristics of the nacre in some gastropods. Zool. Scripta. 1978;7:287–296. [Google Scholar]
- Nakahara H. Calcification of gastropod nacre. In: Westbroek P, de Jong E.W, editors. Biomineralization and biological metal accumulation. Reidel; Dordrecht, The Netherlands: 1983. pp. 225–230. [Google Scholar]
- Nakahara H. Nacre formation in bivalve and gastropod molluscs. In: Suga S, Nakahara H, editors. Mechanisms and phylogeny of mineralization in biological systems. Springer; Berlin, Germany: 1991. pp. 343–350. ch. 4.2. [Google Scholar]
- Nassif N, Pinna N, Gehrke N, Antonietti M, Jäger C, Colfen H. Amorphous layer around aragonite platelets in nacre. Proc. Natl Acad. Sci. USA. 2005;102:12 653–12 655. doi: 10.1073/pnas.0502577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville A.C. Cambridge University Press; Cambridge, UK: 1993. Biology of fibrous composites. [Google Scholar]
- Noishiki Y, Nishiyama Y, Wada M, Okada S, Kuga S. Inclusion complex of β-chitin and aliphatic amines. Biomacromolecule. 2003;4:944–949. doi: 10.1021/bm034024k. [DOI] [PubMed] [Google Scholar]
- Pereira-Mouriès L, Almeida M.J, Ribeiro C, Peduzzi J, Barthélemy M, Milet C, Lopez E. Soluble silk-like organic matrix in the nacreous layer of the bivalve Pinctada maxima. Eur. J. Biochem. 2002;269:4994–5003. doi: 10.1046/j.1432-1033.2002.03203.x. [DOI] [PubMed] [Google Scholar]
- Rousseau M, Lopez E, Couté A, Mascarel G, Smith D.C, Naslain R, Bourrat X. Sheet nacre growth mechanism: a Voronoi model. J. Struct. Biol. 2005a;149:149–157. doi: 10.1016/j.jsb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Rousseau M, Lopez E, Stempflé P, Brendlé M, Franke L, Guette A, Naslain R, Bourrat X. Multiscale structure of sheet nacre. Biomaterials. 2005b;26:6254–6262. doi: 10.1016/j.biomaterials.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Saito Y, Okano T, Gaill F, Chanzy H, Putaux J.L. Structural data on the intra-crystalline swelling of β-chitin. Int. J. Biol. Macromol. 2000;28:81–88. doi: 10.1016/S0141-8130(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kumagai H, Wada M, Kuga S. Thermally reversible hydration of β-chitin. Biomacromolecules. 2002;3:407–410. doi: 10.1021/bm015646d. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Arribart H, Giraud-Guille M.M. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005;4:1–12. doi: 10.1038/nmat1305. [DOI] [PubMed] [Google Scholar]
- Schäffer T.E, et al. Does abalone nacre form by heteroepitaxial nucleation or by growth through mineral bridges? Chem. Mater. 1997;9:1731–1740. doi: 10.1021/cm960429i. [DOI] [Google Scholar]
- Skelton P.W, Benton M.J. Mollusca: Rostroconchia, Scaphopoda and Bivalvia. In: Benton M.J, editor. The fossil record 2. Chapman and Hall; London, UK: 1993. pp. 237–263. [Google Scholar]
- Snow M.R, Pring A, Self P, Losic D, Shapter J. The origin of the color of pearls in iridescence from nano-composite structures of the nacre. Am. Miner. 2004;89:1353–1358. [Google Scholar]
- Song F, Soh A.K, Bai Y.L. Structural and mechanical properties of the organic matrix layers of nacre. Biomaterials. 2003;24:3623–3631. doi: 10.1016/S0142-9612(03)00215-1. [DOI] [PubMed] [Google Scholar]
- Vincent J.F.V. Making biological materials. J. Bionics Eng. 2005;2:209–237. [Google Scholar]
- Wada K. Spiral growth of nacre. Nature. 1966;211:1427. doi: 10.1038/2111427a0. [DOI] [Google Scholar]
- Weiner S, Traub W. X-ray diffraction study of the insoluble organic matrix of mollusk shells. FEBS Lett. 1980;111:311–316. doi: 10.1016/0014-5793(80)80817-9. [DOI] [Google Scholar]
- Whitesides G.M, Grzybowski B. Self-assembly at all scales. Science. 2002;295:2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- Wilt F.H, Killian C.E, Livingston B.T. Development of calcareous skeletal elements in invertebrates. Differentiation. 2003;71:237–250. doi: 10.1046/j.1432-0436.2003.7104501.x. [DOI] [PubMed] [Google Scholar]