Abstract
Background
Pulmonary hypoplasia (PH) is found in 15-20% of all neonatal autopsies, accounting for 2,850 deaths yearly. Development of engineered tissue substitutes that could functionally restore damaged tissue remains a unique opportunity for biotechnology. Recently, we isolated and characterized murine fetal pulmonary cells (FPC) and engineered 3-dimensional pulmonary tissue constructs in vitro. Our goal is to devise a reliable and reproducible method for delivering FPC into a live animal model of PH.
Materials and Methods
Three methods of delivery were explored: intraoral, intratracheal and intrapulmonary injection. Adult Swiss Webster mice were anesthetized and fluorescent labeled microspheres (20μm diameter) were delivered by intraoral and intratracheal injection. Subsequently, labeled FPC (Cell Tracker, CMPTX) were delivered by the same methods. In addition, direct transpleural intrapulmonary injection of FPC was performed.
Outcome analysis included survival, reproducibility, diffuse vs. confined location of the injected substance, and adequacy of delivery. Routine histological examination, fluorescent microscopy, and immunostaining were performed.
Results
Microspheres
We demonstrated reproducible, diffuse instillation via tracheotomy into the distal alveoli. Intraoral delivery appeared less reliable compared to direct intratracheal injection.
FPC
Intratracheal injection was a reliable method of delivery. Labeled FPC showed transepithelial migration after 7 days of in vivo culture. Intrapulmonary injection led to local accumulation of cells in sites of injection.
Conclusions
We demonstrate that delivery of FPC is feasible with intratracheal injection giving the most reliable, diffuse delivery throughout the lung. This represents the first step towards translational research with site-specific delivery for a cell-based therapeutic approach towards PH and similar pulmonary diseases.
Keywords: Cell-based therapy, pulmonary hypoplasia, murine fetal pulmonary cells, intratracheal injection, intraoral injection, intrapulmonary injection, transepithelial migration
Introduction
Preterm delivery with resultant developmental lung abnormalities (i.e., pulmonary hypoplasia) is a major problem in neonatology and accounts for more than 70% of perinatal mortality (1). The pathology of pulmonary hypoplasia includes reduced lung mass, insufficient surfactant production, poorly differentiated alveolar epithelium and a reduction of alveolar gas exchange (2).
The loss or failure of lung tissue is one of the most frequent, devastating, and costly problems in health care. The most frequently used and successful method of therapy is transplantation. However, the severe scarcity of donor organs, especially in the pediatric population, is a major limitation and has thus stimulated investigation into selective cell transplantation and other molecular based therapies (3-8).
Because of advances in tissue engineering, novel cell based therapies are emerging as an available treatment modality for damaged tissues (7, 8). Delivery of engineered cells into hypoplastic lung tissue has the potential to improve underdeveloped lung tissue and aid in restoring the process of natural tissue development. Drug delivery alone does not have the ability to achieve both of these affects.
We have recently been able to demonstrate histiotypic tissue construct formation from a mixed population of fetal pulmonary cell culture in vitro (9,10). After characterization of the cells, we further examined methods of introduction into the lung parenchyma. We began by investigating three different delivery methods for the in vivo administration of fetal pulmonary cells (FPC) into the pulmonary system.
Materials and Methods
The Institutional Animal Care and Usage Committee (IACUC #16150) at Drexel University approved all animal protocols in compliance with the Guide for the Care and Use of Laboratory Animals. Adult Swiss Webster female mice (Charles River Laboratories, Wilmington, VA) were injected with different substrates between March 2006 and December 2006.
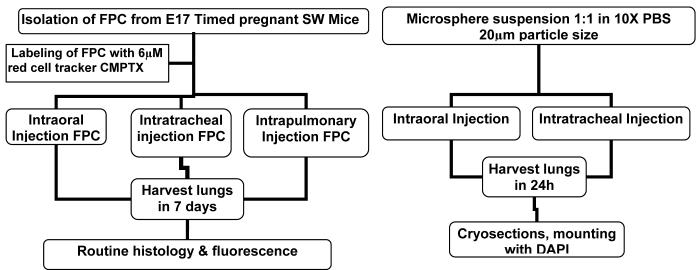
1. Methods of Injection (Figure 1)
Figure 1.
Schematic depicting the delivery of fluorescent microspheres and labeled FPC, harvesting and sample processing times.
1.1 Intraoral injection
Adult Swiss Webster mice were anesthetized with isofluorane. Direct laryngoscopy with a small spatula was utilized and a 24 gauge angiocathether was inserted into the oropharynx to deliver either microspheres (100μl suspension, microsphere solution diluted 1:1 in 10X PBS) or fluorescently labeled fetal pulmonary cells (10 million CMTPX CellTracker™ labeled FPC suspended in 100 μL of medium as described below).
1.2 Intratracheal injection
Adult Swiss Webster mice were anesthetized with isofluorane. A small incision was made over the anterior neck in a transverse fashion. Blunt dissection was utilized to identify the trachea and a 27 gauge needle was inserted between the tracheal rings. Microspheres or labeled FPC (10 million labeled FPC suspended in 100 μL of medium) were delivered as described below. Adequate injection was evidenced by visualization of the suspension through the tracheal tissue with minimal reflux of suspension back through the nose. Pain relief was obtained with buprenorphine (0.2 ml Buprenex diluted 1:100 in 10X PBS) injected subcutaneously before termination of the procedure. The incision site was closed with 4-0 silk sutures.
1.3 Intrapulmonary injection
Adult Swiss Webster mice were anesthetized with isofluorane and a skin incision was made over the right chest. The muscle layers were dissected sharply until the lung was visualized through the intercostal spaces. With a 27 gauge needle 10 million labeled FPC in 100 μl of 1 mg/ml collagen type I solution (BD Bioscience) were injected through the intercostal space directly into the lung parenchyma. The collagen solution was used as a delivery vehicle to localize the distribution of the engrafted cells, as the collagen solution gels rapidly at 37 degrees Celsius (°C). The skin was closed in an interrupted fashion with 4-0 silk sutures. Buprenorphine was administered subcutaneously for pain relief as described above.
2. Spheres and Labeled Fetal Pulmonary Cells (FPC) (Figure 1)
2. 1. Sphere delivery
One hundred μL of a solution containing fluorescent microspheres (microspheres diluted 1:1 in 10X PBS - yellow; 20μm diameter, Polysciences, Warrington PA) were administered with a 24 gauge angiocatheter intraorally or with at 27 gauge needle intratracheally. After the procedure, the animals were housed overnight. Lungs were harvested on the next day, washed in 10X PBS and fixed in paraformaldehyde overnight. Tissue was embedded in OCT compound (Triangle Biomedical Sciences, Durham, NC), snap-frozen at -80°C and stored at -80°C. Thirty micron sections were prepared with a cryostat. Slides were mounted with Vectashield™ mounting medium containing DAPI (Vector Laboratories, Burlington, CA) for nuclear counterstaining.
2. 2. Cellular delivery
Labeled FPC were administered intraorally, intratracheally or intrapulmonary, as described above.
2. 2. 1. Fetal Pulmonary Cell (FPC) Isolation
Murine FPC were obtained from the lungs of timed pregnant Swiss Webster mouse fetuses at gestational day 18 (Charles River Laboratories, Wilmington, VA) as previously described (9-11). Briefly, fetal lungs were surgically removed, rinsed in Hanks Balanced Salt Solution (HBSS; Cellgro, Herndon, VA) and minced. Following mincing, the tissue was first triturated in and then digested with 0.5% trypsin in PBS for 5 and 20 minutes, respectively. Following quenching of the trypsin with Dulbecco’s modified Eagle medium containing FBS (Cambrex; East Rutherford, NJ) and filtration through a 70 micron filter (BD Falcon; San Jose, CA), the cell suspension was pelleted for 5 min at 800 rpm. The pellet was resuspended for 30 s in 900 μL distilled water to remove the red blood cells by hypotonic lysis, followed by the addition of 100 μL of 10 × PBS (Cellgro). The cells were washed once more in 1 × Ca2+/Mg2+ containing PBS, and resuspended in complete medium (DMEM + 10% fetal bovine serum + antibiotics). A suspension of 10 million CMTPX CellTracker™ labeled FPC in 100 μL of medium or 100 μl of 1 mg/ml collagen type I solution was injected.
2.2.2. Labeling
In order to identify the injected FPC in vivo, the cells were labeled in vitro with Cell Tracker (CMTPX, Molecular probes), a fluorescent probe that is retained in living cells through several generations. It is not transferred to adjacent cells. The labeling procedure, performed according to the manufacturer’s instructions, consisted of incubating the cells with 25 μM CMTPX for 30 minutes in serum-free medium, followed by three 5 minute washing steps in 1X PBS. The CMTPX CellTracker™ passes freely through cell membranes and, unlike some of the other CellTracker™ dyes, it does not require enzymatic activity once in the cell to activate fluorescence.
3. Housing, Harvesting & Sample Processing (Figure 1)
After the surgical procedure, the animals were housed for up to a week, depending on the protocol when the lungs were harvested (Figure 1). Following harvesting the lungs were washed in 10X PBS and fixed in paraformaldehyde overnight. Routine histology and immunostaining for surfactant protein C (ProSpC, type II alveolar epithelial cells) were performed. Identification of alveolar epithelial cells was carried out by immunofluorescence, as previously described (9,10,12).
4. Statistical Analysis
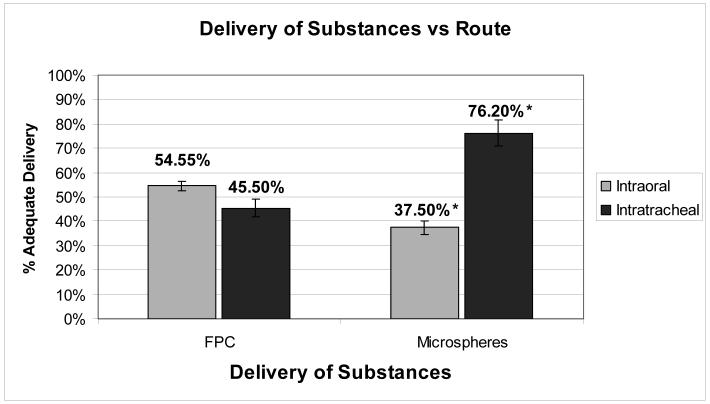
Comparison of intraoral (IO) vs. intratracheal (IT) injection was performed in both the microsphere group and the labeled FPC group (Figure 4). Examination of serial micrographs was performed and the percentage of animals with adequate delivery was calculated as the adequate delivery/inadequate delivery × 100. The adequate delivery percentage was then compared between groups (IO vs. IT) and analyzed statistically using Fisher’s exact test, with a p<0.05 considered statistically significant. Error bars represent variance.
Figure 4.
Labeled FPC and Microspheres delivery outcomes according to different delivery route (IO vs IT). Results for adequate delivery expressed in percentage value (*values are statistically significant with p<0.05). Error bars represent variance.
Results
Intraoral and Intratracheal Admininstration of Microspheres
Intraoral route
The first route explored for delivery of substances into the pulmonary parenchyma was the intraoral, which used cell-sized fluorescent microspheres as cell-surrogates. The quality of the injection was defined as “adequate delivery” if more than 10 microspheres were identified per 400x high power field (HPF). These results were assessed in randomly acquired images from harvested lungs of 8 mice. Three of the injections were identified as adequate (37.5%) and 5 as poor (62.5%). Figure 2 depicts adequate injections of microspheres in the intratracheal and intraoral groups, showing more than 10 microspheres per HPF in the proximal and distal airways.
Figure 2.
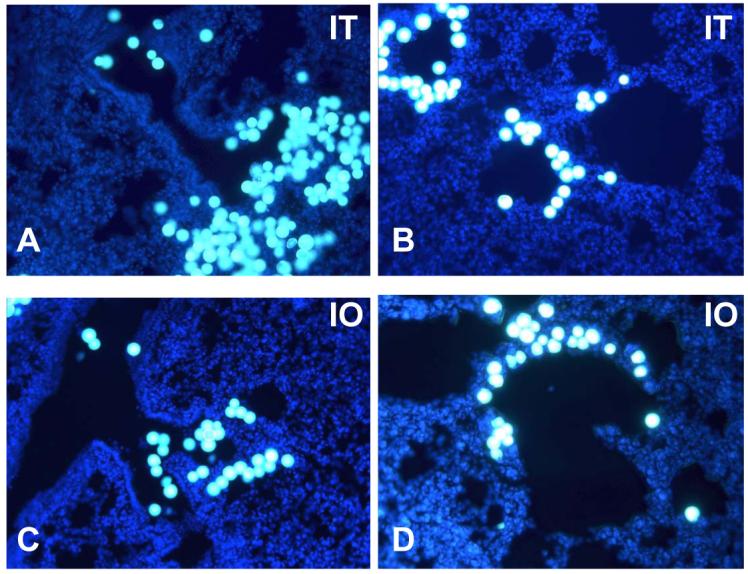
A-C: Microspheres: Fluorescent microscopy of intratracheally (IT) injected microspheres (yellow, particle size 20μm) evident in proximal (1A) (200x) and distal (1B) (400x) airway of adult mice. C and D: fluorescent microscopy of intraorally (IO) injected microspheres evident in proximal (1C) and distal (1D) airway of adult mice; with DAPI (blue) nuclei counterstaining.
Intratracheal route
In search of a more reliable and reproducible delivery route, the intratracheal route was explored, which ensured direct placement of the needle into the trachea. Microspheres were administered intratracheally to 23 mice. Two animals were excluded from the analysis due to intraoperative death. One animal died due to excessive dissection and pneumothorax and the second due to buprenorphine overdose. Of the 21 mice included, 16 were identified as adequate injections (76.2%) (Figure 2A,B) and 5 as poor (23.8%) (p<0.05).
Intraoral and Intratracheal Admininstration of Fetal Pulmonary Cells
Figure 3 depicts routine H&E and fluorescent micrographs of serial lung sections after intratracheal, intraoral and intrapulmonary delivery of labeled FPC. The percentages of animals with adequate delivery in the intratracheal and intraoral groups are shown in Figure 4.
Figure 3.
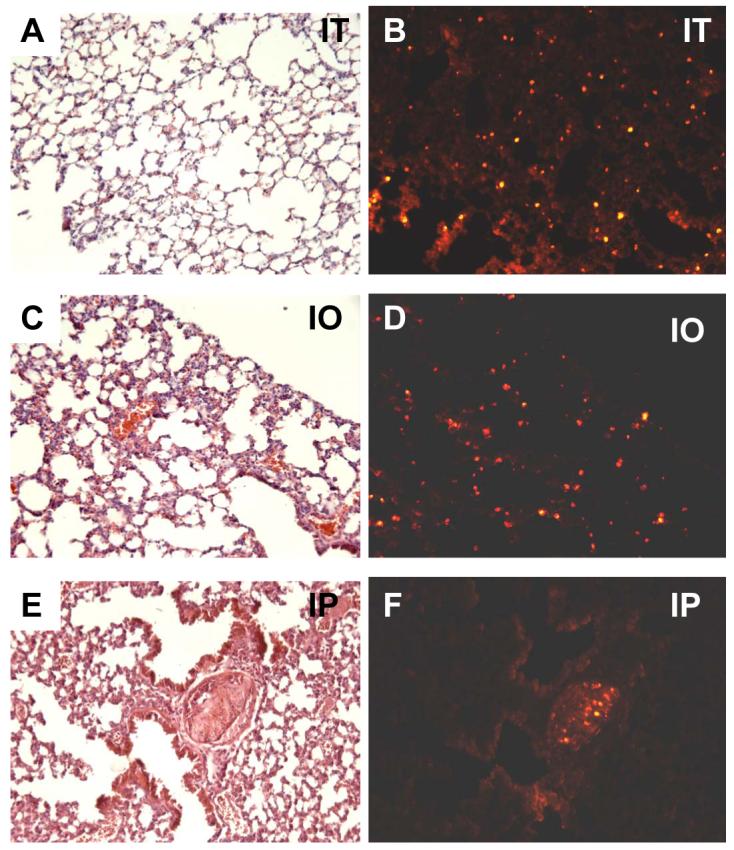
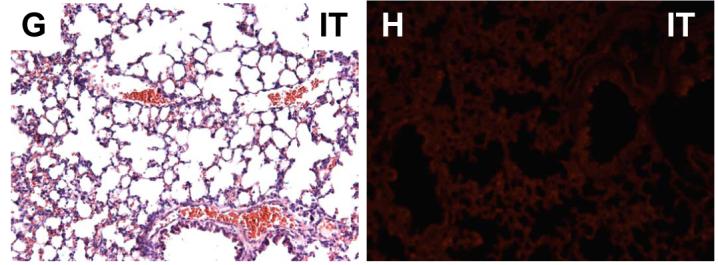
Representative distribution of engrafted fetal pulmonary cells in successful experiments using the three injection methods, as described in Materials and Methods. Shown are serial sections stained with H & E and the subsequent section photographed for the distribution of the CellTracker™ CMTPX-labeled FPC in lungs instilled via intratracheal injection (A,B); intraoral injection (C,D), and intrapulmonary injection (E,F). Panels G & H illustrate a control animal injected intratracheally with unlabeled FPC, (no CellTracker™ CMTPX-labeling used), depicting minimal tissue autofluorescence; all micrographs are 200x magnification.
Intraoral route
Of a total of 11 mice administered labeled FPC intraorally, 6 were identified as adequate (54.5%) and 5 as poor (45.5%) (Figure 3C,D). Comparing the microsphere delivery intraorally to the labeled FPC delivery intraorally there was a trend for an improvement in the number of adequate injections from 37.5% to 54.5% (p=NS) (Figure 4).
Intratracheal route
A total of 23 mice were administered labeled FPC by intratracheal injection. One animal was excluded due to excessive dissection leading to pneumothorax and immediate intraoperative death. Of the 22 mice included, 10 were identified as adequate injections (45.5%) (Figure 3A,B; Figure 4), and 12 as poor injections (54.5%). Given the high rate of poor injections (54.5%), further intratracheal experiments with microspheres were carried out to improve the technique and limit amount of animal usage. To allow for comparison and standardization of tissue autofluorescence, an intratracheal experiment with unlabeled FPC was included (Figure 3G, H).
Transepithelial migration of FPC administered intratracheally
The intratracheally delivered labeled FPC showed evidence of transepithelial migration after 7 days of in vivo culture. Figure 5A and 5B shows fluorescent micrographs in which labeled FPC are seen not only in the alveolar lumen but also within the lung parenchyma. In addition, Figure 5C illustrates co-localization (yellow) of type II alveolar epithelial cells with prosurfactant protein C (green) and labeled FPC (red), which provides initial evidence that alveolar type II cells of donor origin may integrate into the lung epithelium/parenchyma.
Figure 5.
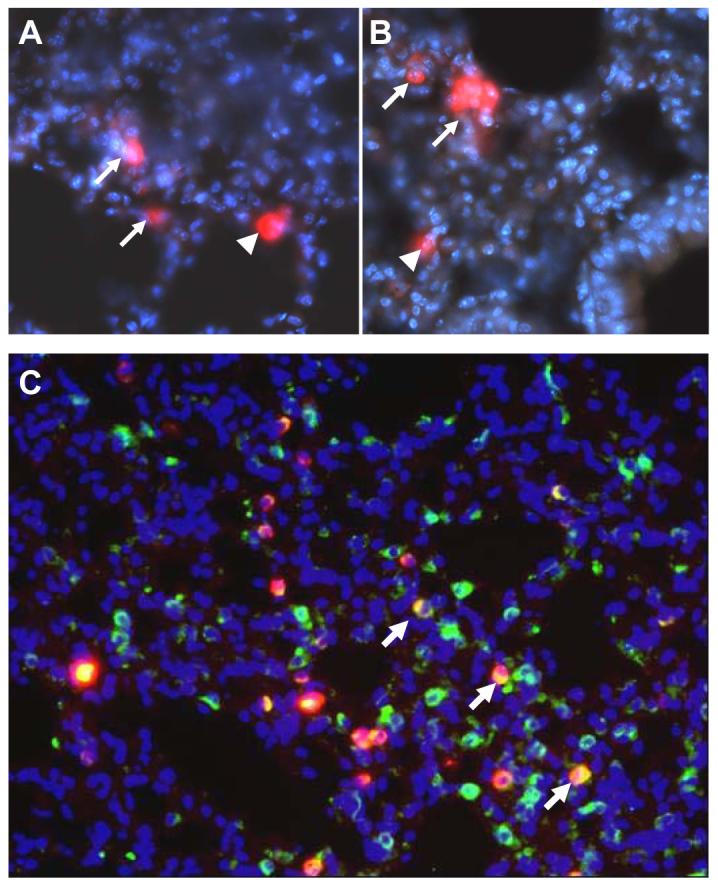
A-B: Fluorescent microscopy of intratracheally injected CellTracker™ CMTPX-labeled FPC (red) with evidence of transepithelial migration. Labeled FPC are seen not only in the alveolar lumen (arrowheads), but also within the lung parenchyma (arrows); all micrographs are 400x magnification. C: Fluorescent microscopy of intratracheally injected CellTracker™ CMTPX-labeled FPC (red). Immunoreactivity for Pro-Surfactant protein C is displayed in green (fluorescent stain for type II alveolar epithelial cells), while nuclei appear blue (DAPI blue nuclei staining). Yellow stains represent confluence of ProSpC and cell tracker stains (arrows); micrograph 200x magnification.
Intrapulmonary route
Experiments with microspheres were not carried out in this experimental subset since the intrapulmonary route delivers the substance directly into the lung parenchyma. Labeled FPC were administered intrapulmonary to 16 mice. The mortality rate associated with this procedure was 6.25%. As evidenced in Figures 3E and 3F, the intrapulmonary injection of labeled FPC resulted in the formation of pockets of cells that did not appear to disperse throughout the tissue. These cells did not form any discernable structures within the pockets. In addition, the pockets formed by intrapulmonary injection did not interface with the surrounding tissue and incited an apparent local fibrotic response (fibrous tissue in H&E section, Figure 3E). In these “successful” intrapulmonary injections, no hemorrhage on H&E staining occurred in the host lungs. However, due to the inability to visualize the path of the needle, 6.25% of intrapulmonary injections resulted in severe hemorrhage (Figure 6- red blood cells evident in H&E sections) and lung injury.
Figure 6.
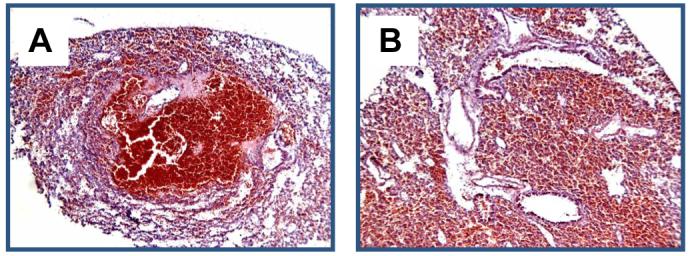
A and B: H&E micrographs showing intrapulmonary injection of CellTracker™ CMTPX-labeled FPC with noticeable hemorrhage. A, 100x magnification; B, 200x magnification.
Discussion
In this paper, we explored potential methods for delivering in vitro engineered fetal pulmonary cells as a first step towards translational research. Initially, we investigated the distribution of microspheres, chosen as particles that model cells; administered via intraoral and intratracheal injection into the airways as a means of testing the feasibility of the approach for distributing cells/particles into the respiratory tree. Secondly, we utilized fluorescently labeled isolated FPC and investigated intraoral and intratracheal injection attempting to achieve diffuse, intrapulmonary distribution. Furthermore we examined direct intrapulmonary injection in order to achieve localized delivery of labeled FPC. We evaluated our results based on animal survival, diffuse vs. local dissemination of the injected substance, and reproducibility as well as adequacy of delivery. The main results of this study indicate that a) delivery of cells into live animals via all three routes is feasible and b) the intratracheal route appears to be the most reliable and reproducible route for diffuse delivery of cells into the distal airways.
Not surprisingly, a clear learning curve was found with each of the experimental procedures. The intratracheal method appeared to deliver the cells diffusely and reliably into the alveolar spaces. We believe that the direct access to the trachea close to the carina, lessens the distance the cells need to travel when compared with the intraoral delivery route. However, the intratracheal group resulted in a higher mortality rate than the intraoral group. Overall, the intraoral group mortality was 0%, whereas the mortality rate in the intratracheal group was 6.5%. Mortality cases occurred predominantly at the beginning of experimental procedures, suggesting they were related to the precise surgical technique. This indicates that intratracheal administration is more technically demanding, and follows a learning curve, with adequate delivery increasing from 45.5% (FPC group) to 76.2% (microsphere group) (p<0.05). (Figure 4).
As shown in Figure 3, administration of labeled FPC into the distal airways via direct intratracheal (tracheotomy) or intraoral injection resulted in a relatively uniform distribution of engrafted FPC in the peripheral airway tissue in adequate injections. This suggests that administration of cells via the airways is a viable method of distributing cells widely within the lung parenchyma. In addition, we demonstrate with the intratracheal delivery of labeled FPC, evidence of transepithelial migration of cells 7 days after injection (Figures 5A and 5B arrows). This finding is significant since it indicates that it is possible for cells delivered intratracheally to intercalate into the distal airways and enter the lung parenchyma. This phenomenon would be a requirement for cell based therapy of pulmonary hypoplasia to be successful. Initial phenotypic characterization of the labeled FPC indicated that a subpopulation of the injected FPC maintained type II alveolar cell phenotype. This suggests that the intratracheal method may be a useful means of instilling “engineered, optimized” cells in future cell-based pulmonary therapeutics, although the integration and functionality of specific pulmonary cell lineages in normal or diseased lung are unknown. We are currently studying the fate of engrafted cells in normal and diseased lungs in our laboratories.
In our study, we aimed to optimize the method of delivery and compare previously published methods of delivering cells into pulmonary tissue. Different types of cells have been delivered intratracheally or intrapulmonary (13-15) with no clear consensus regarding adequacy of delivery that would translate into potential successful therapeutic results. The intratracheal administration of mesenchymal stem cells as a means of treating endothelial dysfunction in pulmonary hypertension was described by Baber et al. (13). In their experiments, a similar concentration of cell suspension was injected intratracheally with successful results towards attenuation of pulmonary hypertension. In addition, the injected cells were followed for a period of 21 days. Similarly, Yang et al. (14) reported the intratracheal delivery of engineered dendritic cells as a means of treating lung epithelial cancers. The authors described the delivery of fewer amounts of unlabeled dendritic cells intratracheally resulting in increased local leukocyte infiltration, decreased tumor progression and increased survival due to dendritic cells’ effects within the cancerous lung tissue. Andrade et al. (15), in work most closely related to ours, delivered rat FPC into the pulmonary cavity as a potential method for the treatment of emphysema. They demonstrated adequate delivery of cells directly into the lung parenchyma, without any local inflammatory reaction. They followed in vivo implanted labeled rat FPC for a period of 35 days. However, the number of cells and the kinetics of cell migration were not of sufficient magnitude to be useful for tissue regeneration. Our results presented here suggest that differences among reported studies will be secondary to the fact that the technique requires a learning curve, and illustrates the fact that much work needs to be done before such procedures can be standardized for clinical practice.
In summary, the intratracheal route of delivery of substances was the most reliable and reproducible route for delivery of fetal pulmonary cells into the distal airway. In addition, the intratracheally delivered FPC showed transepithelial migration through the alveoli after seven days of in vivo culture. This finding suggests incorporation of intratracheally injected fetal pulmonary cells into the recipient lung parenchyma, with initial evidence of alveolar type II cell integration. Further research in this innovative area on the fate and functionality of engrafted cells will hopefully lead to the realization of cell-based pulmonary therapeutics for both pediatric and adult lung pathologies.
Acknowledgements
This present study was supported by grants from the National Institutes of Health (NIH-R21, CMF/PIL) and the St. Christopher’s Foundation (CMF).
The authors thank Dr. David Chimento for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Compernolle V, Brusselmans K, Acker T, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fetal respiratory distress in premature mice. Nat Med. 2002;8:702–10. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 2.Leinwand MJ, Tefft JD, Zhao J, et al. Nitrofen inhibition of pulmonary growth and development occurs in early embryonic mouse. Journal of Pediatric Surgery. 2002;37:1263–68. doi: 10.1053/jpsu.2002.34978. [DOI] [PubMed] [Google Scholar]
- 3.Pollok JM, Vacanti JP. Tissue engineering. Semin Pediatr Surg. 1996 Aug;5(3):191–6. [PubMed] [Google Scholar]
- 4.Kim SS, Vacanti JP. The current status of tissue engineering as potential therapy. Semin Pediatr Surg. 1999 Aug;8(3):119–23. doi: 10.1016/s1055-8586(99)70012-0. [DOI] [PubMed] [Google Scholar]
- 5.Nerem RM. Tissue engineering: confronting the transplantation crisis. Proc Inst Mech Eng [H] 2000;214(1):95–9. doi: 10.1243/0954411001535273. [DOI] [PubMed] [Google Scholar]
- 6.Stock UA, Vacanti JP. Tissue engineering: current state and prospects. Annu Rev Med. 2001;52:443–51. doi: 10.1146/annurev.med.52.1.443. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs JR, Nasseri BA, Vacanti JP. Tissue engineering: a 21st century solution to surgical reconstruction. Ann Thorac Surg. 2001 Aug;72(2):577–91. doi: 10.1016/s0003-4975(01)02820-x. [DOI] [PubMed] [Google Scholar]
- 8.Nerem RM. Cellular engineering. Ann Biomed Eng. 1991;19(5):529–45. doi: 10.1007/BF02367396. [DOI] [PubMed] [Google Scholar]
- 9.Mondrinos MJ, Koutzaki S, Jiwanmall E, Li M, Dechadarevian J, Lelkes PI, Finck CM. Engineering Three-Dimensional Pulmonary Tissue Constructs. Tissue Engineering. 2006 Apr;12(4):717–728. doi: 10.1089/ten.2006.12.717. [DOI] [PubMed] [Google Scholar]
- 10.Mondrinos MJ, Koutzaki S, Finck CM, Lelkes PI. A Tissue Engineered Model of Developing Distal Lung Tissue. AJP Lung. 2006 doi: 10.1152/ajplung.00403.2006. under revision. [DOI] [PubMed] [Google Scholar]
- 11.Bates SR, Gonzales LW, Tao JQ, Rueckert P, Ballard PL, Fisher AB. Recovery of rat type II cell surfactant components during primary cell culture. Am J Physiol Lung Cell Mol Physiol. 2002 Feb;282(2):L267–76. doi: 10.1152/ajplung.00227.2001. [DOI] [PubMed] [Google Scholar]
- 12.Yuli I, Lelkes PI. Neutral endopeptidase activity in the interaction of N-formyl-L-methionyl-L-leucyl-L-phenylalanine with human polymorphonuclear leukocytes. Eur J Biochem. 1991 Oct 15;201(2):421–30. doi: 10.1111/j.1432-1033.1991.tb16300.x. [DOI] [PubMed] [Google Scholar]
- 13.Baber SR, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007 Feb;292(2):H1120–8. doi: 10.1152/ajpheart.00173.2006. Epub 2006 Sept 15. [DOI] [PubMed] [Google Scholar]
- 14.Yang SC, et al. Intrapulmonary administration of CCL21 gene-modified dendritic cells reduces burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res. 2006 Mar 15;66:3205–13. doi: 10.1158/0008-5472.CAN-05-3619. [DOI] [PubMed] [Google Scholar]
- 15.Andrade CF, Wong AP, et al. Cell based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol. doi: 10.1152/ajplung.00175.2006. Epub 2006 Oct 6. [DOI] [PubMed] [Google Scholar]