Abstract
Heparin-binding EGF-like growth factor (HB-EGF) is synthesized as a type I transmembrane protein (proHB-EGF) and expressed on the cell surface. The ectodomain shedding of proHB-EGF at the extracellular region on the plasma membrane yields a soluble EGF receptor ligand and a transmembrane-cytoplasmic fragment (HB-EGF-CTF). The cytoplasmic domain of proHB-EGF (HB-EGF-cyto) interacts with transcriptional repressors to reverse their repressive activities. However, how HB-EGF-cyto accesses transcriptional repressors is yet unknown. The present study demonstrates that, after exposure to shedding stimuli, both HB-EGF-CTF and unshed proHB-EGF translocate to the nuclear envelope. Immunoelectron microscopy and digitonin-permeabilized cells showed that HB-EGF-cyto signals are at the inner nuclear membrane. A short sequence element within the HB-EGF-cyto allows a transmembrane protein to localize to the nuclear envelope. The dominant-active form of Rab5 and Rab11 suppressed nuclear envelope targeting. Collectively, these data demonstrate that membrane-anchored HB-EGF is targeted to the inner nuclear membrane via a retrograde membrane trafficking pathway.
Introduction
EGF family members are synthesized as type I transmembrane proteins. A member of the family, heparin-binding EGF-like growth factor (HB-EGF), which is a potent mitogen and chemotactic factor for various types of cells, expresses on the plasma membrane as a 20–30-kD precursor (proHB-EGF) containing an extracellular EGF-like domain, a transmembrane segment, and a short cytoplasmic tail (Higashiyama et al., 1991). The proHB-EGF is cleaved at the juxtamembrane domain via metalloprotease activation, yielding a soluble EGF receptor ligand and a C-terminal fragment containing transmembrane and cytoplasmic segments (HB-EGF-CTF). This process, called ectodomain shedding, can be stimulated by various physiological and pharmacological agonists, including 12-O-tetradecanoylphorbol-13-acetate (TPA) (Goishi et al., 1995), calcium ionophores (Dethlefsen et al., 1998), G protein–coupled receptor ligands (Prenzel et al., 1999), and cytokines and growth factors (Tanida et al., 2004; Nanba et al., 2008). Although the EGF-like domain alone is sufficient to elicit EGF receptor phosphorylation, recent studies have shown some biological activities for the conserved cytoplasmic tail of proHB-EGF (HB-EGF-cyto), which contains 24 amino acids. For instance, the multi-functional protein BAG-1 binds to HB-EGF-cyto and increases HB-EGF secretion (Lin et al., 2001). A serine residue in HB-EGF-cyto is phosphorylated in response to shedding stimuli (Wang et al., 2006). Our group has found that HB-EGF-cyto interacts with transcriptional repressors, promyelocytic leukemia zinc finger protein (PLZF) and B cell lymphoma 6 (Bcl6), resulting in the reversal of PLZF- and Bcl6-mediated gene repression (Nanba et al., 2003; Kinugasa et al., 2007). These data suggest that HB-EGF-cyto is able to function in the nucleus, thus raising an intriguing question: how is HB-EGF-cyto targeted to the nucleus?
The nuclear envelope (NE) consists of an inner nuclear membrane (INM) and an outer nuclear membrane (ONM), which are joined at the nuclear pore membrane. The ONM is structurally continuous with the peripheral ER. One model explaining the targeting of integral protein from the ER to the INM is that relocation is accomplished by passive diffusion in the interconnected ER, ONM, pore, and INM. The proteins are then tethered to the INM as a result of interactions with the INM resident proteins (for review see Holmer and Worman 2001). In other models, the trafficking of some integral membrane proteins to the INM requires energy (Ohba et al., 2004) and is mediated by multiple protein interactions (King et al., 2006; Saksena et al., 2006; Braunagel et al., 2007). This study shows that ectodomain-shedding stimuli trigger the translocation of membrane-anchored HB-EGF to the INM via a retrograde transmembrane trafficking pathway, where it can bind to transcriptional repressors.
Results and discussion
TPA treatment induces relocalization of HB-EGF-cyto to the NE
Although HB-EGF-cyto interacts with transcriptional repressors to suppress their repressive activity (Nanba et al., 2003; Kinugasa et al., 2007), detailed analysis on the nature of HB-EGF-cyto has been hampered partly because of a lack of available specific antibodies. Thus, we raised mAbs against a synthetic peptide corresponding to HB-EGF-cyto and obtained one clone, designated mAbD, which specifically reacted with proHB-EGF and HB-EGF-CTF (Fig. 1 B). The mAbD reactivity was abrogated by deleting the C-terminal 10 amino acids (199–208) of HB-EGF. mAbD recognized the 15-mer peptide (194–208), but not the 10-mer peptide (199–208). Thus, the epitope was mapped in the 194–208 region (Fig. 1, B and C). We then performed immunofluorescence microscopy in cells expressing proHB-EGF. In the absence of ectodomain-shedding stimuli, or TPA, the mAbD gave signals predominantly on the plasma membrane in most of the cells examined and also in the perinuclear regions of some cells (Fig. 1 D). The perinuclear signal probably represents newly synthesized (pre)proHB-EGF because it colocalized with the Golgi apparatus marker, β1,4-galactosyltransferase, and disappeared after translation block by cycloheximide (unpublished data). After TPA treatment, mAbD staining almost completely disappeared from the plasma membrane and was found in the NE and reticular network. The NE signal obviously overlapped with lamin B, which resides at the nucleoplasmic face of the INM (Fig. 1 D), and the reticular network also was co-stained with the ER marker (unpublished data). These findings raised the possibility that after the ectodomain shedding on the plasma membrane, the membrane-anchored HB-EGF-cyto (maHB-EGF-cyto), which indicates both proHB-EGF and HB-EGF-CTF, translocated to the ER/NE. Notably, when anti-HB-EGF polyclonal antibodies (#H6), which recognize the extracellular EGF-like domain (Miyagawa et al., 1995), were used, essentially the same staining pattern as mAbD was observed (Fig. 1 E). Uncleavable HB-EGF mutant (L148G) was then used to test the ectodomain-shedding requirement. As a result, the mutant HB-EGF was internalized into the cytoplasm but not targeted to the NE (Fig. 1 F). In the cells stably expressing metalloproteinase domain lacking ADAM12, the ectodomain shedding of proHB-EGF was disturbed (Asakura et al., 2002) and the NE localization of HB-EGF-cyto was suppressed (unpublished data). Therefore, some ectodomain shedding is essential for the translocation of maHB-EGF-cyto into the NE and may trigger the activation of a retrograde sorting signal in HB-EGF-cyto.
Figure 1.
Membrane-anchored HB-EGF is targeted to the NE. (A) Schematic presentation of HB-EGF. The proHB-EGF is cleaved at the juxtamembrane domain, resulting in production of soluble HB-EGF and HB-EGF-CTF. mAbD specifically recognizes HB-EGF-cyto, and #H6 recognizes the extracellular domain of proHB-EGF (amino acids 54–73). The underline shows the epitope region. TM, transmembrane domain; Cyto, HB-EGF-cyto. (B) Total cell lysates expressing YFP-tagged wild-type or C-terminal 10 amino acids-deleted HB-EGF (Δ10C) were analyzed by immunoblotting with mAbD (left) or anti-GFP antibodies (right). *, proHB-EGF-YFP; **, HB-EGF-CTF-YFP; ***, YFP; arrows, degradation products. (C) Total cell lysate expressing GFP-fused C-terminal 10- or 15-amino acid regions of HB-EGF were probed with mAbD (left) or anti-GFP antibodies (right). (D and E) HT1080 cells transfected with HB-EGF were treated with TPA and double-stained with mAbD and anti-lamin B or mAbD and #H6 antibodies. (F) Uncleavable form of HB-EGF expressing cells were stained with mAbD.
maHB-EGF-cyto is targeted to the INM
To further analyze the NE translocation of maHB-EGF-cyto, another antibody was used. A V5-epitope tag was inserted just downstream from the transmembrane domains (termed HB-EGF-V5-C) because tagging at the C terminus interferes with the HB-EGF-cyto function (Nanba et al., 2003) and stabilizes phosphorylation in HB-EGF-cyto (Wang et al., 2006). The shedding of HB-EGF-V5-C was confirmed by Western blotting, and its efficiency was slightly lower than wild type (unpublished data). In the absence of shedding stimuli, the expression pattern of HB-EGF-V5-C in the plasma membrane and perinuclear area was essentially the same as that observed for wild type. Upon TPA stimulation, plasma membrane staining was decreased, and a strong signal at the NE and the reticular network was again observed and quantitated with statistical significance (Fig. 2 A; Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200710022/DC1). Because it is well known that various cytokines and growth factors induce the ectodomain shedding of proHB-EGF, we further used more physiological ligands, such as bFGF and EGF. As shown in Fig. 2 B, both bFGF and EGF induced the V5-signal translocation to the NE, whereas efficiency was lower than with TPA.
Figure 2.
HB-EGF is targeted to the INM. HB-EGF-V5-C was transiently transfected into the cells. (A and B) Cells were treated with either TPA, bFGF, or EGF, then stained with an anti-V5 mAb. (C) Cells were treated with TPA and extracted with Triton X-100, then fixed and stained with either anti-V5 mAb or #H6. (D) Cells were permeabilized with digitonin and fixed. The cells were incubated with (bottom) or without (top) Triton X-100 and labeled with mAbD and anti-lamin B antibodies. Bar, 10 μm. (E) Ultra-thin sections were stained with anti-V5 and 15-nm gold–conjugated second antibodies. PM; plasma membrane. Bar, 200 nm.
If maHB-EGF-cyto is targeted to the INM, cytoplasmic domains face the nucleoplasm and physically interact with proteins localized inside the nucleus. Therefore, it is essential to elucidate whether maHB-EGF-cyto localizes at the INM or ONM, so the following experiments were performed. First, since membrane proteins known to be targeted to INM resist Triton X-100 extraction (Gerace et al., 1982; Furukawa et al., 1995) because of tight interactions with nuclear structures (e.g., nuclear lamina or chromatin), maHB-EGF-cyto was tested to share this character. In non-TPA–treated cells, the HB-EGF-V5-C was readily extracted with Triton X-100. In contrast, in TPA-treated cells the HB-EGF-V5-C remained at the NE, while the signal at the reticular network was decreased by Triton X-100 extraction (Fig. 2 C). EGF or bFGF also induced the detergent-resistant NE localization (Fig. S1 C). Consequently, maHB-EGF-cyto becomes detergent-unextractable after translocation to the NE, suggesting that maHB-EGF-cyto could be at the INM. Second, an experiment was designed to take advantage of the fact that low concentrations of digitonin selectively permeabilize plasma membranes, while leaving nuclear membranes intact (Adam et al., 1990). Cells expressing HB-EGF-V5-C were permeabilized with digitonin, then fixed and labeled with anti-lamin B and mAbD antibodies. Lamin B and NE-targeted HB-EGF-V5-C signals were not detected (Fig. 2 D, top). After Triton X-100 permeabilization, the HB-EGF-V5-C signal in the NE was detected only in TPA-treated cells, whereas the lamin B signal was detected in both cells regardless of TPA treatment (Fig. 2 D, bottom). This result indicates that in TPA-treated cells HB-EGF-cyto is inside the nucleus, which required Triton X-100 permeabilization for antibody access, suggesting that maHB-EGF-cyto is localized at the INM. Next, immunoelectron microscopy showed compelling evidence that the HB-EGF-cyto signal was detected at the nucleoplasmic face of the NE in TPA-treated cells (Fig. 2 E). Collectively, we concluded that maHB-EGF-cyto can be localized at the INM upon shedding stimuli.
HB-EGF-cyto has the ability to localize to the NE
The transmembrane domain of lamina-associated polypeptide 2β (LAP2β), which is an integral INM protein, was used to identify the crucial amino acids in HB-EGF-cyto for NE localization. A C-terminal fragment of LAP2β (410–452, LAP2bTM) is responsible for membrane insertion, and a large hydrophilic domain (1–409) promotes localization at the INM. Consistent with previous data (Furukawa et al., 1995), GFP-tagged LAP2βTM (GFP-LAP2βTM) was distributed throughout the membrane structure in the cytoplasm. In contrast, insertion of HB-EGF-cyto into the GFP-Lap2βTM changed its localization in the reticular meshwork and the NE (Fig. 3, B and C), indicating that HB-EGF-cyto has the ability to allow the membrane protein to localize to the NE. Next, three fragments of HB-EGF-cyto were inserted into the GFP-LAP2βTM (Fig. 3 D). The GFP-LAP2βTM containing HB-EGF-cyto 185–198 and 185–203, but not 199–208, were localized at the NE and the reticular meshwork (Fig. 3, E and F), indicating that the 185–198 amino acid region is sufficient for NE localization. It should be noted that the NE-targeting activity of HB-EGF-cyto is constitutively active when it is fused to LAP2βTM; nevertheless, its activity is masked when proHB-EGF is newly synthesized on ER.
Figure 3.
HB-EGF-cyto has the ability to target to the NE. (A and D) Schematic presentation of the constructs. (B, E, and G) Indicated constructs were transfected and visualized with GFP. (C and F) Indicated constructs at the left-hand side were transfected and visualized with GFP and anti-lamin B antibodies. Though HB-EGF-cyto in LAP2β fusion protein has flipped orientation as compared with the native protein, it does not interfere with NE targeting. Bar, 10 μm.
It was recently reported that some integral INM proteins possess a basic sequence motif that resembles classical NLS and binds to karyopherin-α (King et al., 2006). However, interactions between HB-EGF-cyto and three importin α subtypes (human karyopherin-α) were not detected (unpublished data). Furthermore, GFP-fused cytoplasmic tail of HB-EGF was distributed throughout the cell, including the cytoplasm and nucleoplasm, as was GFP (Fig. 3 G). This indicates that HB-EGF-cyto has no detectable NLS activity for a soluble protein and that the transmembrane region is indispensable for NE targeting. Because HB-EGF-cyto delivers a membrane protein to the NE, it is feasible that both proHB-EGF and HB-EGF-CTF can be targeted to the NE.
K201 in HB-EGF-cyto is essential for the NE targeting
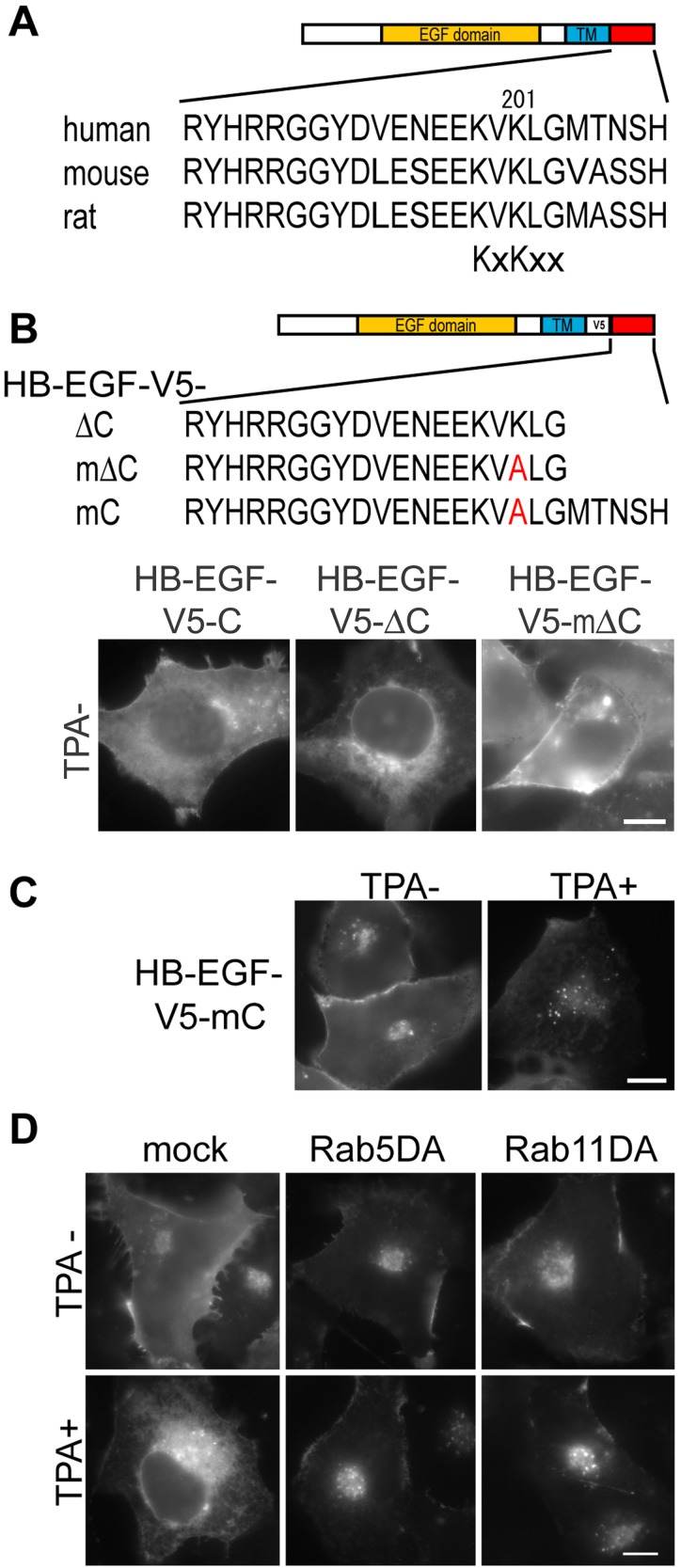
HB-EGF is synthesized at the ER-bound ribosome and subsequently transported to the cell surface. At this time, the ER/NE-targeting activity of HB-EGF-cyto must be inactivated. ER-resident integral membrane proteins are continuously exported by vesicular trafficking and recycled back from the Golgi apparatus. The retrieval of type I integral membrane proteins from the Golgi apparatus is directed by cytoplasmic K(X)KXX motifs located at the C terminus (for review see Duden, 2003). Interestingly, a conserved KXKXX ER retrieval signal-like sequence was identified in HB-EGF-cyto, although it was not exposed at the distal terminus (Fig. 4 A). The deletion of five amino acids from proHB-EGF (HB-EGF-V5-ΔC), exposing 199KVKLG203 at the C terminus, resulted in its localization to the ER even without TPA treatment. The deleted 5-amino acid region is not essential for plasma membrane localization because K201A point-mutated HB-EGF-V5-ΔC (HB-EGF-V5-mΔC) localized at the plasma membrane (Fig. 4 B). In Western blotting analysis, the molecular size of proHB-EGF-V5-ΔC was not altered by TPA treatment; that is, HB-EGF-V5-ΔC accumulated in the ER without ectodomain shedding (unpublished data). These data indicate that 199KVKLG203 is able to function as an ER retrieval signal when it is exposed at the distal C terminus. More importantly, point-mutated proHB-EGF at K201A (HB-EGF-V5-mC) was not targeted to the NE, even after TPA treatment (Fig. 4 C). Thus, K201 is essential for NE targeting. Some protein toxins (e.g., Pseudomonas exotoxin) are cleaved at the C terminus before activation of the ER retrieval signal (for review see Sandvig and van Deurs, 2002). A possible mechanism for activation of the ER retrieval signal of HB-EGF-cyto is that some level of ectodomain shedding may eventually induce proteolytic processing at the C terminus, thereby activating the ER retrieval signal, although proteolytically processed HB-EGF-cyto was not detected at this point by Western blotting analysis (unpublished data). Another conceivable mechanism is that protein modification(s) in HB-EGF-cyto after exposure to ectodomain-shedding stimuli may regulate the activation of retrograde transport; e.g., S207 reportedly is phosphorylated upon shedding stimulation (Wang et al., 2006).
Figure 4.
NE targeting of HB-EGF-cyto–containing fragments use the retrograde membrane traffic pathway. (A) Comparison of the cytoplasmic domain of proHB-EGF. (B and C) Schematic presentation of mutated proHB-EGF. Indicated constructs were transfected and visualized with anti-V5 mAb. (D) HB-EGF-V5-C was coexpressed with dominant-active Rab5 or Rab11. After TPA treatment, the cells were visualized with anti-V5 mAb.
The NE targeting of maHB-EGF-cyto utilizes membrane trafficking pathways
The involvement of Rab5 and Rab11, key regulators of the endocytic membrane trafficking pathway, were examined to further delineate the transport pathway of the maHB-EGF-cyto from the plasma membrane to the NE. Rab5 regulates membrane trafficking through early endosomes, and Rab11 functions in the endocytic recycling pathway via recycling endosomes (for review see Mellman, 1996; Maxfield and McGraw, 2004). When HB-EGF-V5-C was coexpressed with either a dominant-active form of Rab5 or Rab11, the TPA-induced ER/NE accumulation of HB-EGF-V5-C was obviously suppressed (Fig. 4 E). However, these Rab proteins, even when overexpressed, did not affect the nuclear transport of soluble proteins containing classical NLS (unpublished data). This suggests that the NE targeting of maHB-EGF-cyto utilizes Rab5- and Rab11-dependent retrograde membrane trafficking pathways—possibly from the plasma membrane, through early endosomes, followed by recycling endosomes to the Golgi apparatus/the ER.
The NE targeting of maHB-EGF-cyto from the plasma membrane is supposed to be controlled precisely at several levels with multiple sorting determinants. Based on our results, a model for this retrograde pathway is proposed in Fig. 5. An integral membrane protein, pro-HB-EGF, is primarily localized at the plasma membrane with a dynamic equilibration between endocytic and recycling membrane trafficking pathways. In response to shedding stimuli, endocytosed maHB-EGF-cyto cannot be recycled back to the plasma membrane, possibly due to the stimulation-dependent phosphorylation on Ser207, following the retrograde transport pathway to the Golgi apparatus through recycling endosomes. Subsequently, maHB-EGF-cyto may use a K(X)KXX ER retrieval signal-like sequence in HB-EGF-cyto to reach ER. A Lap2β experiment showed that the residue 185–198 is important in targeting the NE. ER-localized maHB-EGF-cyto can be laterally diffused between the peripheral ER, ONM, and INM via the nuclear pore complex because the cytoplasmic domain is small enough to pass through the nuclear pore complex. Alternatively, it can be transported from ONM to INM via active transport requiring protein interactions. At the INM, the HB-EGF-cyto faces the nucleoplasm and is expected to interact with transcriptional repressors such as Bcl6 and PLZF. It has been reported that a number of plasma membrane integral receptors accumulate in the nucleoplasm—the nuclear space unassociated with the NE—in some physiological situations. The cytoplasmic tails in some receptors are cleaved and can then migrate as soluble peptides into the nucleus (e.g., ErbB4, FGF receptor, and Notch; reviewed in Bryant and Stow, 2005). The other receptors (e.g., the EGF receptor) may have to escape from the membrane; however, the mechanisms are mostly unknown. Here, we first show that a membrane-anchored growth factor on the cell surface is targeted to the INM as an integral membrane protein where it could bind to transcriptional repressors. The transmembrane-free HB-EGF-cyto has never been detected and maHB-EGF-cyto is a major product after shedding stimuli in Western blotting (Nanba et al., 2003). Thus, it is feasible that the NE-accumulated maHB-EGF-cyto is responsible for gene activation. Recent studies show that proteins of the NE participate in the regulation of gene transcription on several levels (for review see Akhtar and Gasser, 2007; Brown and Silver, 2007; Shaklai et al., 2007). Therefore, the results of this study may provide a new insight into the functions of representative plasma membrane–anchored growth factors, such as the EGF family in transcriptional regulation.
Figure 5.
A model of NE targeting. (1) proHB-EGF is predominantly expressed on the cell surface. (2) After shedding stimuli, the equilibration between endocytic and recycling could be changed. (3) maHB-EGF-cyto possibly follows a retrograde transport pathway to the Golgi apparatus through recycling endosomes, and then (4) is targeted to the ER. (5) Subsequently ER-localized HB-EGF-cyto is diffused or actively transported to the INM and finally tethered at the INM.
Materials and methods
Construction of expression plasmid
The plasmids coding full-length HB-EGF (pME18S-HB-EGF), YFP-fused HB-EGF (pEYFP-N1-HB-EGF), HB-EGF with the C-terminal 10 amino acids deleted (Δ10), and the uncleavable form of HB-EGF (pRCHB-EGF-L148G) are described in Nanba et al. (2003). GFP-tagged C-terminal 10- or 15-mer peptide regions in HB-EGF-cyto (199–208 or 194–208, respectively) were constructed by inserting oligonucleotides into the pEGFP-C1. For pME18S-HB-EGF-V5-C, the oligonucleotides corresponding to the V5-tag were inserted into the KpnI site of HB-EGF. For the construction of pEGFP-C1-hLAP2β and pEGFP-C1-hLAP2βTM, human LAP2β or LAP2βTM (402–454) was inserted into the pEGFP-C1. For the construction of pEGFP-C1-HB-EGF-cyto-hLAP2βTM, HB-EGF-cyto was inserted into the pEGFP-C1-hLAP2βTM. The dominant-active form of Rab5 and Rab11 plasmids were described previously (Misaki et al., 2007).
Antibodies
Anti-HB-EGF-cyto rat mAb was generated based on the rat lymph node method originally described by Sado and colleagues (Kishiro et al., 1995). A 10-wk-old female WKY/NCrj rat (Charles River Laboratories) was immunized with an emulsion containing a thyroglobulin-conjugated 24-mer synthetic peptide corresponding to HB-EGF-cyto (obtained from Immuno-Biological Laboratories) and Freund's complete adjuvant. After 3 wk, cells from the lymph nodes were fused with mouse myeloma Sp2/0-Ag14 cells. The hybridomas were screened by ELISA using GST-tagged HB-EGF-cyto, and one clone was selected (designated mAbD). Rabbit anti–human HB-EGF antibodies (#H6) were described by Miyagawa et al. (1995). Mouse anti-V5 mAb and goat anti-lamin B antibodies were obtained from Invitrogen and Santa Cruz Biotechnology, Inc., respectively. Rabbit anti-GFP polyclonal antibodies were obtained from Medical & Biological Laboratories.
Cell culture, transfection, and immunofluorescence
Human fibrosarcoma HT1080 cells were grown in MEM supplemented with 10% FCS (Hyclone) and 0.1 mM nonessential amino acids. 12-O-tetradecanoylphorbol-13-acetate, TPA (Sigma-Aldrich) was used at a final concentration of 100 nM for 30 min at 37°C. bFGF and EGF (R&D systems) were used at a final concentration of 50 ng/ml for 30 min at 37°C. Transfections were performed using LipofectAMINE 2000 (Invitrogen). After 16 h, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.4% Triton X-100 or 0.1% Tween 20 for 5 min. The primary antibodies were detected with Cy3- or FITC-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Image acquisition were performed using a microscope (IX70; Olympus) equipped with a camera (ORCA-ER; Hamamatsu) and a 60×/NA 1.4 lens.
To quantitate the nuclear envelope targeting, transiently HB-EGF-V5-C expressing cells were stained with anti-V5 mAb. The number of the cells showing nuclear envelope staining and the total number of cells expressing HB-EGF-V5-C were counted (>240 cells in total). Then the ratio of nuclear envelope accumulated cells was averaged (eight independent experiments) and shown as the mean ± SD (n = 8).
Triton X-100 extraction and digitonin permeabilization
Cells were extracted on ice in Transport buffer (TB: 20 mM Hepes, pH 7.3, 110 mM potassium acetate, 5 mM sodium acetate, 2 mM magnesium acetate, 1 mM EGTA, and 2 mM DTT) containing 1% Triton X-100 for 10 min. For digitonin permeabilization, the cells were incubated in TB containing 33 μg/ml digitonin on ice for 5 min. After extraction or permeabilization, the cells were washed and fixed with 4% paraformaldehyde in TB.
Immunogold electron microscopy
Cells were fixed with 2% paraformaldehyde/0.25% glutaraldehyde, dehydrated, and embedded in LR White resin. Ultra-thin sections were blocked with 1% BSA and incubated in anti-V5 mAb. The bound antibody was detected using gold-conjugated anti–mouse IgG (15 nm; BBInternational). After incubation, the grids were counterstained with 2% uranyl acetate and lead citrate and examined with a transmission electron microscope (JEM1230; JEOL).
Online supplemental material
Fig. S1 and Fig. S2 show the quantification of maHB-EGF-cyto NE targeting and low magnified immunoelectron microscopic localization of HB-EGF-cyto, respectively. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200710022/DC1.
Supplementary Material
Acknowledgments
We thank Dr. Fukuda (Tohoku University) for the gift of Rab constructs and are grateful to Dr. Ban and Dr. Misaki (Osaka University), Ms. Kanno (Ehime University) for technical assistance, and Higashiyama laboratory members.
This study was supported by Kato Memorial Bioscience Foundation to M. Hieda, Grants-in Aid for Scientific Research no.17570163 and no.19570182 to M. Hieda, no. 17014068 and no. 17390081 to S. Higashiyama from the Ministry of Education, Culture, Sports, Science and Technology and from JSPS, and PREST, JST, Japan. T. Taguchi was supported by the Core Research for Evolutional Science and Technology (CREST), Grants-in-aid for Scientific Research (18050019), and Senri Life Science Foundation Grants.
Abbreviations used in this paper: Bcl6, B-cell lymphoma 6; HB-EGF, heparin-binding EGF-like growth factor; INM, inner nuclear membrane; LAP2β, lamina-associated polypeptide 2β; NE, nuclear envelope; ONM, outer nuclear membrane; PLZF, promyelocytic leukemia zinc finger protein; TPA, phorbol ester 12-O-tetradecanoylphorbol-13-acetate.
References
- Adam, S.A., R.S. Marr, and L. Gerace. 1990. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J. Cell Biol. 111:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar, A., and S.M. Gasser. 2007. The nuclear envelope and transcriptional control. Nat. Rev. Genet. 8:507–517. [DOI] [PubMed] [Google Scholar]
- Asakura, M., M. Kitakaze, S. Takashima, Y. Liao, F.T. Yoshinaka, H. Ohmoto, K. Node, K. Yoshino, H. Ishiguro, H. Asanuma, et al. 2002. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 8:35–40. [DOI] [PubMed] [Google Scholar]
- Braunagel, S.C., S.T. Williamson, Q. Ding, X. Wu, and M.D. Summers. 2007. Early sorting of inner nuclear membrane proteins is conserved. Proc. Natl. Acad. Sci. USA. 104:9307–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, C.R., and P.A. Silver. 2007. Transcriptional regulation at the nuclear pore complex. Curr. Opin. Genet. Dev. 17:100–106. [DOI] [PubMed] [Google Scholar]
- Bryant, D.M., and J.L. Stow. 2005. Nuclear translocation of cell-surface receptors: lessons from fibroblast growth factor. Traffic. 6:947–954. [DOI] [PubMed] [Google Scholar]
- Dethlefsen, S.M., G. Raab, M.A. Moses, R.M. Adam, M. Klagsbrun, and M.R. Freeman. 1998. Extracellular calcium influx stimulates metalloproteinase cleavage and secretion of heparin-binding EGF-like growth factor independently of protein kinase C. J. Cell. Biochem. 69:143–153. [DOI] [PubMed] [Google Scholar]
- Duden, R. 2003. ER-to-Golgi transport: COP I and COP II function. Mol. Membr. Biol. 20:197–207. [DOI] [PubMed] [Google Scholar]
- Furukawa, K., N. Pante, U. Aebi, and L. Gerace. 1995. Cloning of a cDNA for lamina-associated polypeptide 2 (LAP2) and identification of regions that specify targeting to the nuclear envelope. EMBO J. 14:1626–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace, L., Y. Ottaviano, and C. Kondor-Koch. 1982. Identification of a major polypeptide of the nuclear pore complex. J. Cell Biol. 95:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goishi, K., S. Higashiyama, M. Klagsbrun, N. Nakano, T. Umata, M. Ishikawa, E. Mekada, and N. Taniguchi. 1995. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol. Biol. Cell. 6:967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama, S., J.A. Abraham, J. Miller, J.C. Fiddes, and M. Klagsbrun. 1991. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 251:936–939. [DOI] [PubMed] [Google Scholar]
- Holmer, L., and H.J. Worman. 2001. Inner nuclear membrane proteins: functions and targeting. Cell. Mol. Life Sci. 58:1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, M.C., C.P. Lusk, and G. Blobel. 2006. Karyopherin-mediated import of integral inner nuclear membrane proteins. Nature. 442:1003–1007. [DOI] [PubMed] [Google Scholar]
- Kinugasa, Y., M. Hieda, M. Hori, and S. Higashiyama. 2007. The carboxyl-terminal fragment of proHB-EGF reversed Bcl6-mediated gene repression. J. Biol. Chem. 282:14797–14806. [DOI] [PubMed] [Google Scholar]
- Kishiro, Y., M. Kagawa, I. Naito, and Y. Sado. 1995. A novel method of preparing rat-monoclonal antibody-producing hybridomas by using rat medial iliac lymph node cells. Cell Struct. Funct. 20:151–156. [DOI] [PubMed] [Google Scholar]
- Lin, J., L. Hutchinson, S.M. Gaston, G. Raab, and M.R. Freeman. 2001. BAG-1 is a novel cytoplasmic binding partner of the membrane form of heparin-binding EGF-like growth factor: a unique role for proHB-EGF in cell survival regulation. J. Biol. Chem. 276:30127–30132. [DOI] [PubMed] [Google Scholar]
- Maxfield, F.R., and T.E. McGraw. 2004. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 5:121–132. [DOI] [PubMed] [Google Scholar]
- Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575–625. [DOI] [PubMed] [Google Scholar]
- Misaki, R., T. Nakagawa, M. Fukuda, N. Taniguchi, and T. Taguchi. 2007. Spatial segregation of degradation- and recycling-trafficking pathway in COS-1 cells. Biochem. Biophys. Res. Commun. 360:580–585. [DOI] [PubMed] [Google Scholar]
- Miyagawa, J., S. Higashiyama, S. Kawata, Y. Inui, S. Tamura, K. Yamamoto, M. Nishida, T. Nakamura, S. Yamashita, Y. Matsuzawa, and N. Taniguchi. 1995. Localization of heparin-binding EGF-like growth factor in the smooth muscle cells and macrophages of human atherosclerotic plaques. J. Clin. Invest. 95:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba, D., A. Mammoto, K. Hashimoto, and S. Higashiyama. 2003. Proteolytic release of the carboxy-terminal fragment of proHB-EGF causes nuclear export of PLZF. J. Cell Biol. 163:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba, D., H. Inoue, Y. Shigemi, Y. Shirakata, K. Hashimoto, and S. Higashiyama. 2008. An intermediary role of proHB-EGF shedding in growth factor-induced c-Myc gene expression. J. Cell. Physiol. 214:465–473. [DOI] [PubMed] [Google Scholar]
- Ohba, T., E.C. Schirmer, T. Nishimoto, and L. Gerace. 2004. Energy- and temperature-dependent transport of integral proteins to the inner nuclear membrane via the nuclear pore. J. Cell Biol. 167:1051–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenzel, N., E. Zwick, H. Daub, M. Leserer, R. Abraham, C. Wallasch, and A. Ullrich. 1999. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 402:884–888. [DOI] [PubMed] [Google Scholar]
- Saksena, S., M.D. Summers, J.K. Burks, A.E. Johnson, and S.C. Braunagel. 2006. Importin-alpha-16 is a translocon-associated protein involved in sorting membrane proteins to the nuclear envelope. Nat. Struct. Mol. Biol. 13:500–508. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., and B. van Deurs. 2002. Membrane traffic exploited by protein toxins. Annu. Rev. Cell Dev. Biol. 18:1–24. [DOI] [PubMed] [Google Scholar]
- Shaklai, S., N. Amariglio, G. Rechavi, and A.J. Simon. 2007. Gene silencing at the nuclear periphery. FEBS J. 274:1383–1392. [DOI] [PubMed] [Google Scholar]
- Tanida, S., T. Joh, K. Itoh, H. Kataoka, M. Sasaki, H. Ohara, T. Nakazawa, T. Nomura, Y. Kinugasa, H. Ohmoto, et al. 2004. The mechanism of cleavage of EGFR ligands induced by inflammatory cytokines in gastric cancer cells. Gastroenterology. 127:559–569. [DOI] [PubMed] [Google Scholar]
- Wang, X., H. Mizushima, S. Adachi, M. Ohishi, R. Iwamoto, and E. Mekada. 2006. Cytoplasmic domain phosphorylation of heparin-binding EGF-like growth factor. Cell Struct. Funct. 31:15–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.