Abstract
At present there are three protein families that share a common structural domain, the αβ/βα fold of class B β-lactamases: zinc β-lactamases, glyoxalases II, and A-type flavoproteins. A detailed inspection of their superimposed structures was undertaken and showed that although these proteins contain binuclear metal sites in spatially equivalent positions, there are some subtle differences within the first ligand sphere that determine a distinct composition of metals. Although zinc β-lactamases contain either a mono or a di-zinc center, the catalytically active form of glyoxalase II contains a mixed iron–zinc binuclear center, whereas A-type flavoproteins contain a di-iron site. These variations on the type of metal site found within a common fold are correlated with the subtle variations in the nature of the ligating amino acid residues and are discussed in terms of the different reactions catalyzed by each of the protein families. Correlation of these observations with sequence data results in the definition of a sequence motif that comprises the possible binuclear metal site ligands in this broad family. The evolution of the proteins sharing this common fold and factors modulating reactivity are also discussed.
Keywords: Bimetallic sites, β-lactamase, glyoxalase, iron, zinc, evolution, A-type flavoprotein
The class B β-lactamase metallohydrolase fold has an αβ/βα structure, consisting of a core unit of two β-sheets sandwich surrounded by solvent-exposed helices toward the external faces. At present this structural organization has been identified in three distinct types of proteins, for which adequate structural templates are available: metallo-β-lactamases (Carfi et al. 1995; Concha et al. 1996,2000; Ullah et al. 1998), glyoxalase, and rubredoxin:oxygen oxidoreductase (Frazão et al. 2000). Interestingly, despite having identical folds, all these proteins catalyze distinct reactions, with no apparent common features. The metallo-β-lactamases are zinc-dependent enzymes with antibiotic degrading activity, having a high efficiency for carbapenems, although they hydrolyze nearly all β-lactams. The three-dimensional structure of the Bacillus cereus 569H β-lactamase provided the first description of this metallohydrolase fold (Carfi et al. 1995). Since then, the resolution of three other crystal structures from the enzymes from Bacteroides fragilis (Concha et al. 1996), Stenotrophomonas maltophilia (Ullah et al. 1998), and Pseudomonas aeruginosa (Concha et al. 2000) has provided further details concerning the structural aspects of this protein family. In these enzymes, the active site is located at one edge of the internal ββ sandwich and comprises a binuclear zinc center. However, the occupancy of this site is distinct in the different proteins: whereas the Bt. fragilis, S. maltophilia enzymes have two high affinity zinc-binding sites, the B. cereus and P. aeruginosa lactamases have one high and one low affinity zinc-binding site (Wang et al. 1999a).
Glyoxalase II is a thiolesterase involved in a two-enzyme system that catalyzes the conversion of toxic 2-oxoaldehydes to the corresponding 2-hydroxycarboxylic acids using glutathione as a coenzyme (Thornalley 1990). The crystal structure of this protein showed that it is composed by two distinct structural domains, one of which is similar to the whole structure of metallo-β-lactamases (Cameron et al. 1999). This similarity had been anticipated from amino acid sequence comparisons, which have led to the suggestion that glyoxalase II enzymes would be members of the superfamily of hydrolases (Melino et al. 1998). The structure also revealed the presence of a binuclear metal site in a spatial location topologically equivalent to that of metallo-β-lactamases. The binuclear metal site in glyoxalase II proteins is promiscuous in terms of metals, being able to accommodate either zinc or iron (Cameron et al. 1999; Zang et al. 2001), and see below).
Rubredoxin:oxygen oxidoreductase (ROO) belongs to the superfamily of A-type flavoproteins (Chen et al. 1993a; Santos et al. 1993;Gomes et al. 1997,1999; Wasserfallen et al. 1998) and is the prototype enzyme for dioxygen detoxification in anaerobes. It operates as the terminal oxygen reductase of a scavenging pathway operating in the sulfate-reducing bacterium Desulfovibrio gigas (Chen et al. 1993a,b; Gomes et al. 1997). The recently determined crystallographic structure of ROO showed that it contains two distinct structural domains: one having a metallo-β-lactamase-like fold and the other a flavodoxin-like one (Frazão et al. 2000). Unlike the case of glyoxalase II enzymes, this structural similarity between ROO and metallo-β-lactamases could not be directly inferred from amino acid sequence comparisons. Like in the previous cases, a binuclear metal site is also associated with this fold, but this time a di-iron center is present. The latter has a completely novel coordination, as compared to other di-iron sites, and is responsible for the protein ability to directly reduce di-oxygen to water (Frazão et al. 2000).
Results and Discussion
Coordinates of structures from the three distinct protein families sharing a domain with the class B β-lactamase fold were retrieved from the Protein Data Bank and compared (Fig. 1 ▶): metallo-β-lactamases from B. cereus, 2bc2 (Fabiane et al. 1998), from Bt. fragilis, 1a7t (Fitzgerald et al. 1998), from S. maltophilia, 1sml (Ullah et al. 1998), and from P. aeruginosa, 1dd6 (Concha et al. 2000); human glyoxalase II, 1qh5 (Cameron et al. 1999); and D. gigas rubredoxin:oxygen oxidoreductase, 1e5d (Frazão et al. 2000). A global structure comparison involving available structures was performed using MODELLER (Sali and Blundell 1993). The positional root mean square deviations (rmsd) of the superimposed C-α atoms, the number of three-dimension superimposed residues and their percentage of sequence identity are shown in Table 1. Noteworthy, the first domains of ROO and glyoxalase are more closely related to each other than to the set of zinc β-lactamases. A detailed comparison of the protein topology was performed, and topology diagrams illustrating the common domain from representatives of the three families were generated (Fig. 2 ▶). All proteins exhibit a αβ/βα topology, with the N-β-sheet composed by two sections, antiparallel (3 to 5 chains) and parallel (3 or 4 chains) sections, and the C-β-sheet composed by antiparallel (4 chains) and parallel (2 chains) sections, except for ROO, which has an additional antiparallel chain at the end of the C-β-sheet. The external helices run parallel to each other on the amino side of the sandwich (3 helices, except for glyoxalase with only 2 helices) and a pair of antiparallel helices on the carboxyl side for lactamases, 1 helix in glyoxalase and 3 helices with mixed directions in ROO.
Fig. 1.
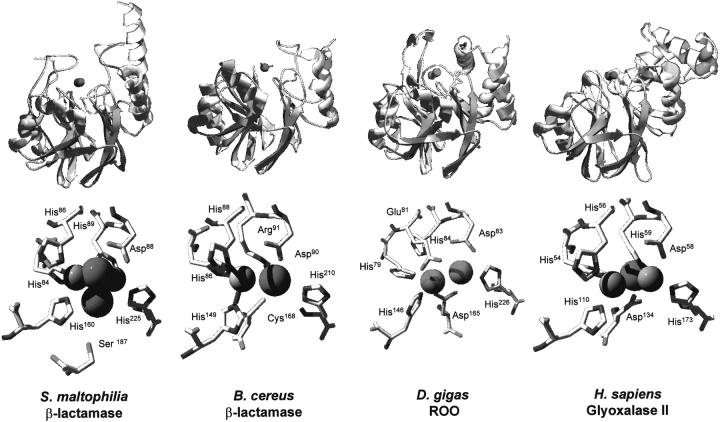
Comparison of the three-dimensional structures of proteins with the αβ/βα fold of class B β-lactamases. (Top) Ribbon representation of the regions of the studied proteins comprising the class B β-lactamase fold; (bottom) structures of bimetallic centers from the same proteins. The representative structures here depicted are the zinc β-lactamases from Bacillus cereus (2bc2) and S. maltophilia (1sml), and the structural domains of D. gigas rubredoxin:oxygen oxidoreductase (1e5d) and H. sapiens glyoxalase II (1qh5) that also have a αβ/βα lactamase-like fold. The protein structures were superimposed using Swiss-PdbViewer (Guex and Peitsch 1997) and are represented in identical configurations. For definition, metal site position 1 is on the left and position 2 on the right. Metal-ligating water molecules are represented as spheres. Rendering was done with POV-ray.
Table 1.
Structural comparison of the proteins with a class B β-lactamase fold
| Bc | Bf | Pa | Sm | Hs | Dg | |
| BLM | BLM | BLM | BLM | GLY | ROO | |
| Bc BLM (2bcA) | 217 | 1.133 | 1.275 | 1.679 | 1.769 | 1.648 |
| Bf BLM (1a7t) | 210/33 | 227 | 1.190 | 1.639 | 1.480 | 1.655 |
| Pa BLM (dd6A) | 192/35 | 201/33 | 216 | 1.875 | 1.610 | 1.741 |
| Sm BLM (1sml) | 175/15 | 177/12 | 176/12 | 266 | 1.546 | 1.539 |
| Hs GLY (1qh5) | 147/16 | 146/20 | 142/17 | 143/15 | 173 | 1.438 |
| Dg ROO (1e5d) | 160/11 | 159/12 | 161/12 | 154/10 | 131/17 | 248 |
The values given above the diagonal are the positional root mean square distances for Cα atoms in Angstroms. The diagonal contains the number of structurally compared residues. The format of the values below the diagonal is the number of equivalent positions/sequence identity in the aligned region, in percentage.
(BLM) Metallo-β-lactamase; (GLY) glyoxalase; (ROO) rubredoxin:oxygen oxidoreductase; (Bc) Bacillus cereus; (Bf) Bt. fragilis; (Pa) P. aeruginosa; (Sm) S. maltophilia; (Hs) H. sapiens; (Dg) D. gigas.
Coordinates were retrieved from the Protein Data Bank, accession numbers and used chains are given between parentheses.
Fig. 2.
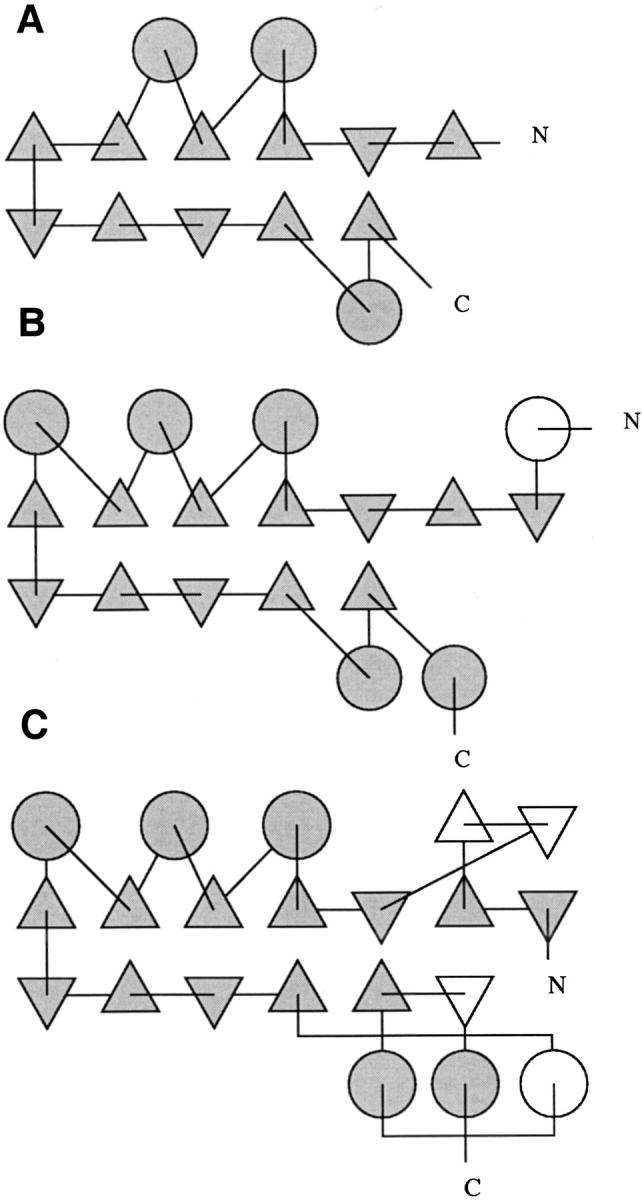
Topology diagrams of the common domain, from representative structures of (A) glyoxalases, with the first domain of human glyoxalase II, 1qh5 (Cameron et al. 1999); (B) metallo β-lactamases, with S. maltophilia β-lactamase, 1sml (Ullah et al. 1998); and (C) A-type flavoproteins, with the first domain of D. gigas rubredoxin:oxygen oxidoreductase (ROO), 1e5d (Frazão et al. 2000). Triangles represent β-chains, circles represent helices, N and C map peptide direction. Gray shading corresponds to common features among the families, white coloring corresponds to particular features of the represented structure or family. The domain is smallest in glyoxalase, with fewer β-chains and α-helices. The ROO domain shows an additional β-hairpin motif on its N-half and an additional helix and β-chain on the C-half. The S. maltophilia β-lactamase has an additional helical motif before the first β-chain. Figure prepared using output from Tops/EditTops (Westhead et al. 1999).
Simple primary sequence database searches using the regions from these proteins that comprise the metallohydrolase fold fail to detect each other, a finding that is not surprising considering that there is a very low amino acid identity (<17%) between the representative members of the family. With the exception of some key residues that are involved in the bimetallic site binding (see below), a reasonable variability is allowed in other regions, which agrees well with the known evidence that there is a fourfold redundancy on the type of amino acids that determine a given secondary structure element (Taylor 1997). Thus, it is not surprising to find the same fold determined by different combinations of amino acids. However, remote sequence conservation and hindered common motifs may be successfully highlighted by inspecting databases of protein alignments derived from hidden Markov models (HMM). The InterPro integrated database (Apweiler et al. 2001), which gathers information from Pfam (Bateman et al. 1999), one such HMM-based repository, was queried using the sequences from the proteins whose structures were analyzed. They all gave hits toward the metallo-β-lactamase superfamily, a large group of sequences that harbors (1) β-lactamases; (2) thiolesterases members of the glyoxalase II family; (3) a competence protein essential for natural transformation in Neisseria gonorrhoeae, and; (4) the A-type flavoprotein members, although the latter are not yet included in the annotated description of the superfamily (InterPro Entry IPR001279).
Superimposition of the three-dimensional structures of the zinc β-lactamases, glyoxalase II, and ROO show that the respective metallic centers are all located in equivalent spatial positions. However, as mentioned above, these proteins have a distinct composition in terms of metals in the sites: metallo-β-lactamases contain either a mono or di-zinc center (Carfi et al. 1995; Concha et al. 1996,2000; Ullah et al. 1998), the catalytically active form of glyoxalase II contains a mixed iron–zinc binuclear center (Cameron et al. 1999; Zang et al. 2001), and ROO contains a di-iron site (Frazão et al. 2000) (Fig. 1 ▶, bottom). This variability on the type of metals present in the binuclear site is likely to be determined not only by the type of residues directly involved in metal coordination, but also by second shell ligands that contribute as orienting groups. Combining structure-oriented amino acid alignments and molecular superimposition allows a direct comparison of the residues involved in metal binding or present in the second coordination shell, and shows that there are subtle variations that account for the observed differences in the type of metals bound to the bimetallic site (Table 2).
Table 2.
Comparison of the residues involved in binuclear metal site coordination and orientation
| Protein | Sequence motif | PDB code |
| Bf BLM | H99–x–H101–x–D103–C104–x57–H162–x18–C181–x41–H223 | 1a7t |
| Bc BLM | H86–x–H88–x–D90–R91–x57–H149–x18–C168–x41–H210 | 2bca |
| Ps BLM | H77–x–H79–x–D81–S82–x56–H139–x18–C158–x39–H197 | 1dd6 |
| Sm BLM | H84–x–H86–x–D88–H89–x70–H160–x26–S187–x37–H225 | 1sml |
| Hs GLY | H54–x–H56–x–D58–H59–x50–H110–x23–D134–x38–H173 | 1qh5 |
| Dg ROO | H79–x–E81–x–D83–H84–x61–H146–x18–D165–x60–H226 | 1e5d |
(BLM) Metallo-β-lactamase; (GLY) glyoxalase; (ROO) rubredoxin:oxygen oxidoreductase; (Bc) Bacillus cereus; (Bf) Bt. fragilis; (Pa) P. aeruginosa; (Sm) S. maltophilia; (Hs) H. sapiens; (Dg) D. gigas.
On most β-lactamases the zinc coordination around position 1 is tetrahedral, whereas it is trigonal pyramidal in position 2 (Fig. 1 ▶, bottom). Ligands of zinc-1 are histidines (e.g., His84, His86, and His160 for S. maltophilia, Table 2), whereas for zinc-2 there is some variability, although at least two ligation positions are always assured by an aspartate and a histidine (Asp88 and His225 in S. maltophilia, Table 2). In Bt. fragilis lactamase, a cysteine (Cys181) is also a ligand of zinc-2, which interestingly is also present on the B. cereus and P. aeruginosa proteins, although with a low affinity for the binding of the second zinc atom (Wang et al. 1999a,b; Concha et al. 2000). On the contrary, in S. maltophilia lactamase this cysteine has been replaced by a serine (Ser 187), which in this case does not act as a zinc-2 ligand, but rather as a second shell orienting residue ligand, that interacts with the metal with an intervening water molecule (Ullah et al. 1998). Thus, in metallolactamases the binuclear metal binding involves essentially soft imidazol, or thiol ligands, explaining the preference for zinc.
The metal-binding site of human glyoxalase II is similar to that of metallo β-lactamases, although not identical, especially concerning the position of two metal ligands. A major difference concerns the presence of an aspartate (Asp134) in a position equivalent to that in which a cysteine or a serine is found in lactamases (Fig. 1 ▶, bottom; Table 2). From the structure it becomes clear that this residue is able to interact with zinc-2 (Cameron et al. 1999). Another difference relates to the fact that position 2 has an additional histidine ligand (His59), which is also present on the S. maltophilia lactamase (His89), but is absent on the lactamases from Bt. fragilis and B. cereus. In these proteins, it is replaced, respectively, by an arginine and a cysteine, none of which are metal ligands. Although the available glyoxalase II structure indicates the presence of a di-zinc metal site (Cameron et al. 1999), it has recently been found that both zinc and iron can occupy the site and, even more interestingly, that a binuclear zinc–iron center is essential for substrate binding and catalysis (Zang et al. 2001). Considering the very high similarity of metal-binding ligands observed between glyoxalase II and the S. maltophilia lactamase, it may be concluded that the versatility in metal content is likely to be due to the presence of the additional aspartate, not present in β-lactamases. Accordingly, the ability to bind both zinc and iron is not observed in β-lactamases, which only bind zinc (Wang et al. 1999a). Consequently, it is the substitution of a soft ligand (histidine) by a harder one (the negatively charged and nonpolarizable aspartate) that increases the variability of the bimetallic site in glyoxalase II in respect to zinc and iron (the meaning of hardness relates to the susceptibility of ions to experience a charge shift in their electron shell through interaction with a coordination partner). This has led to a classification as hard for those being little affected and soft for the easily polarizable ones. Among the soft donors are, for example, thiolates and sulfides, whereas fluoride and negatively charged oxygen donors are classified as hard. Interactions between metal ions and ligand atoms can be interpreted in such a way that interactions between centers of the same type (i.e., hard/hard [highly ionic bond] and soft/soft [partly covalent bond]), are preferred (Basolo and Pearson, 1967)). From the analysis of the coordinating residues (Table 2) it may be anticipated that position 2 is the one likely to be more versatile in terms of the type of metal bound, as its harder character is preferred by iron over zinc.
The variability in metal composition of the binuclear site is further expanded when analyzing ROO. This enzyme contains a binuclear iron site with an unprecedented coordination (Frazão et al. 2000) (Fig. 1 ▶, Table 2): (1) as in glyoxalase II, an aspartate (Asp 165) acting as a ligand of Fe-2 replaces the cysteine and serine found in β-lactamases; (2) the second histidine from the H-x-H-x-D motif common to β-lactamases and glyoxalase II is replaced by a glutamate (Glu81); and (3) although conserved in ROO, the histidine that is a ligand of the metal in position 2 in β-lactamases and glyoxalase II is no longer a metal ligand (Frazão et al. 2000). Thus, on the bimetallic site in ROO, two soft ligands (imidazole or thiolate) were replaced for two harder ones (carboxylates), favoring iron over zinc. This feature found in ROO typifies what is observed among the A-type family of flavoproteins (ATF), in which this set of ligands is conserved (Gomes et al. 1997,1999).
Conclusions
Metallo β-lactamases, glyoxalase II, and an A-type flavoprotein, three distinct types of proteins all sharing a common fold, and containing a bimetallic site were structurally compared in terms of the residues involved in the first and second coordination spheres. From this study it can be concluded that metallo β-lactamases bind exclusively zinc atoms in their bimetallic sites thanks to the excess of soft histidine ligands. The introduction of an aspartate as a ligand on glyoxalase II induces promiscuity at the bimetallic site, whose catalytically active form incorporates a mixed zinc–iron center. On the other extreme, in the A-type flavoprotein ROO two histidines are replaced by two carboxylate-containing residues, which modulate the affinity of the site toward iron. Altogether, and as expected on chemical grounds, the increasing hardness of ligands drives the specificity of the bimetallic sites in proteins having a metallohydrolase fold toward the incorporation of iron.
As a consequence, the sequence motif H-x-[EH]-x-D-[CRSH]-x50–70-H-x15–30–[CSD]-x30–70-H that gathers the distinct ligand combinations and orienting residues observed between these protein is proposed. Taking into account the extension of the gaps defined in the motif, it is also possible to analyze distant homologs. This motif extends a previous proposal (Melino et al. 1998) as it accounts for the multiple possibilities at a given ligand position that are observed in zinc- or in iron-containing bimetallic sites. For example, among the residues in the third cluster of the motif ([CSD]), C and S are typical of zinc β-lactamases, but not of the iron-ligating proteins, in which a D is always found. Also, it highlights the fact that minor modifications on the amino acids involved in metal coordination dictate which type of metal is bound and thus the catalytic function of the site. Furthermore, this comparison shows how successive substitutions of one amino acid involved in metal coordination lead from a di-zinc to a di-iron site, passing by a mixed metal center, keeping the same structural fold. This change in metal composition correlates with the activity of the enzymes, on going from a mainly Lewis acid catalysis by the zinc site (in β-lactamases) to a redox active di-iron site, capable of reducing dioxygen to water (in ROO).
These subtle modifications are accompanied by other alterations that altogether determine the different reactivities of these proteins: (1) glyoxalase II and ROO are both modular proteins that is, apart from the common class B lactamase fold they also have distinct structural domains (a predominantly α-helical domain in glyoxalase) (Cameron et al. 1999) and a flavodoxin-like region in ROO (Frazão et al. 2000); (2) in ROO the substrate-binding groove required for lactamase activity is not conserved, as in this space additional β-hairpin and helical motifs are present; (3) in glyoxalase II, a substrate analog binds at the domain interface (Cameron et al. 1999); and (4) the dimeric arrangement of ROO not only brings together the protein redox cofactors but also occludes the di-iron site from any possible substrate (Frazão et al. 2000). Altogether, this evidence points to a possible evolutionary link between these proteins, and illustrates how nature dramatically modulates the reactivity of a given metal in a common fold, accordingly to functional requirements.
Acknowledgments
Maria Arménia Carrondo (ITQB) is gratefully acknowledged for critically reading of the manuscript. This work was supported by grant 36558 from Fundação para a Ciência e Tecnologia, Portugal.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.31202.
References
- Apweiler, R., Attwood, T.K., Bairoch, A., Bateman, A., Birney, E., Biswas, M., Bucher, P., Cerutti, L., Corpet, F., Croning, M.D., Durbin, R., Falquet, L., Fleischmann, W., Gouzy, J., Hermjakob, H., Hulo, N., Jonassen, I., Kahn, D., Kanapin, A., Karavidopoulou, Y., Lopez, R., Marx, B., Mulder, N.J., Oinn, T.M., Pagni, M., and Servant, F. 2001. The InterPro database, an integrated documentation resource for protein families, domains and functional sites. Nucleic Acids Res. 29 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basolo, F. and Pearson, R.G. 1967. Mechanisms of inorganic reactions—a study of metal complexes in solution, Wiley Eastern Limited, New Dehli, India.
- Bateman, A., Birney, E., Durbin, R., Eddy, S.R., Finn, R.D., and Sonnhammer, E.L. 1999. Pfam 3.1: 1313 multiple alignments and profile HMMs match the majority of proteins. Nucleic Acids Res. 27 260–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, A.D., Ridderstrom, M., Olin, B., and Mannervik, B. 1999. Crystal structure of human glyoxalase II and its complex with a glutathione thiolester substrate analogue. Structure Fold Des. 7 1067–1078. [DOI] [PubMed] [Google Scholar]
- Carfi, A., Pares, S., Duee, E., Galleni, M., Duez, C., Frere, J.M., and Dideberg, O. 1995. The 3-D structure of a zinc metallo-beta-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 14 4914–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L., Liu, M.Y., LeGall, J., Fareleira, P., Santos, H., and Xavier, A.V. 1993a. Rubredoxin oxidase, a new flavo-hemo-protein, is the site of oxygen reduction to water by the `strict anaerobe` Desulfovibrio gigas. Biochem. Biophys. Res. Commun. 193 100–105. [DOI] [PubMed] [Google Scholar]
- ———. 1993b. Purification and characterization of an NADH-rubredoxin oxidoreductase involved in the utilization of oxygen by Desulfovibrio gigas. Eur. J. Biochem. 216 443–448. [DOI] [PubMed] [Google Scholar]
- Concha, N.O., Rasmussen, B.A., Bush, K., and Herzberg, O. 1996. Crystal structure of the wide-spectrum binuclear zinc beta-lactamase from Bacteroides fragilis. Structure 4 823–836. [DOI] [PubMed] [Google Scholar]
- Concha, N.O., Janson, C.A., Rowling, P., Pearson, S., Cheever, C.A., Clarke, B.P., Lewis, C., Galleni, M., Frere, J.M., Payne, D.J., Bateson, J.H., and Abdel-Meguid, S.S. 2000. Crystal structure of the IMP-1 metallo beta-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry 39 4288–4298. [DOI] [PubMed] [Google Scholar]
- Fabiane, S.M., Sohi, M.K., Wan, T., Payne, D.J., Bateson, J.H., Mitchell, T., and Sutton, B.J. 1998. Crystal structure of the zinc-dependent beta-lactamase from Bacillus cereus at 1.9 A resolution: Binuclear active site with features of a mononuclear enzyme. Biochemistry 37 12404–12411. [DOI] [PubMed] [Google Scholar]
- Frazão, C., Silva, G., Gomes, C.M., Matias, P., Coelho, R., Sieker, L., Macedo, S., Liu, M.Y., Oliveira, S., Teixeira, M., Xavier, A.V., Rodrigues-Pousada, C., Carrondo, M.A., and Le Gall, J. 2000. Structure of a dioxygen reduction enzyme from Desulfovibrio gigas. Nat. Struct. Biol. 7 1041–1045. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, P.M., Wu, J.K., and Toney, J.H. 1998. Unanticipated inhibition of the metallo-beta-lactamase from Bacteroides fragilis by 4-morpholineethanesulfonic acid (MES): A crystallographic study at 1.85-A resolution. Biochemistry 37 6791–6800. [DOI] [PubMed] [Google Scholar]
- Gomes, C.M., Silva, G., Oliveira, S., LeGall, J., Liu, M.Y., Xavier, A.V., Rodrigues-Pousada, C., and Teixeira, M. 1997. Studies on the redox centers of the terminal oxidase from Desulfovibrio gigas and evidence for its interaction with rubredoxin. J. Biol. Chem. 272 22502–22508. [DOI] [PubMed] [Google Scholar]
- Gomes, C.M., Teixeira, M., and Wasserfallen, A. 1999. The family of A-type flavoproteins: New members and definition of unique sequence fingerprints. Rudolf Weber-Agency for Scientific Publications, Berlin, Germany.
- Guex, N. and Peitsch, M.C. 1997. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 18 2714–2723. [DOI] [PubMed] [Google Scholar]
- Melino, S., Capo, C., Dragani, B., Aceto, A., and Petruzzelli, R. 1998. A zinc-binding motif conserved in glyoxalase II, beta-lactamase and arylsulfatases. Trends Biochem. Sci. 23 381–382. [DOI] [PubMed] [Google Scholar]
- Sali, A. and Blundell, T.L. 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234 779–815. [DOI] [PubMed] [Google Scholar]
- Santos, H., Fareleira, P., Xavier, A.V., Chen, L., Liu, M.Y., and LeGall, J. 1993. Aerobic metabolism of carbon reserves by the `obligate anaerobe` Desulfovibrio gigas. Biochem. Biophys. Res. Commun. 195 551–557. [DOI] [PubMed] [Google Scholar]
- Taylor, W.R. 1997. Evolution and relationships of protein families, in DNA and protein sequence analysis—a practical approach. IRL Press, Oxford.
- Thornalley, P.J. 1990. The glyoxalase system: New developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem. J. 269 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah, J.H., Walsh, T.R., Taylor, I.A., Emery, D.C., Verma, C.S., Gamblin, S.J., and Spencer, J. 1998. The crystal structure of the L1 metallo-beta-lactamase from Stenotrophomonas maltophilia at 1.7 A resolution. J. Mol. Biol. 284 125–136. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Fast, W., Valentine, A.M., and Benkovic, S.J. 1999a. Metallo-beta-lactamase: Structure and mechanism. Curr. Opin. Chem. Biol. 3 614–622. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Fast, W., and Benkovic, S.J. 1999b. On the mechanism of the metallo-beta-lactamase from Bacteroides fragilis. Biochemistry 38 10013–10023. [DOI] [PubMed] [Google Scholar]
- Wasserfallen, A., Ragettli, S., Jouanneau, Y., and Leisinger, T. 1998. A family of flavoproteins in the domains Archaea and Bacteria. Eur. J. Biochem. 254 325–332. [DOI] [PubMed] [Google Scholar]
- Westhead, D.R., Slidel, T.W., Flores, T.P., and Thornton, J.M. 1999. Protein structural topology: Automated analysis and diagrammatic representation. Protein Sci. 8 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, T.M., Hollman, D.A., Crawford, P.A., Crowder, M.W., and Makaroff, C.A. 2001. Arabidopsis glyoxalase II contains a zinc/iron binuclear metal center that is essential for substrate binding and catalysis. J. Biol. Chem. 276 4788–4795. [DOI] [PubMed] [Google Scholar]