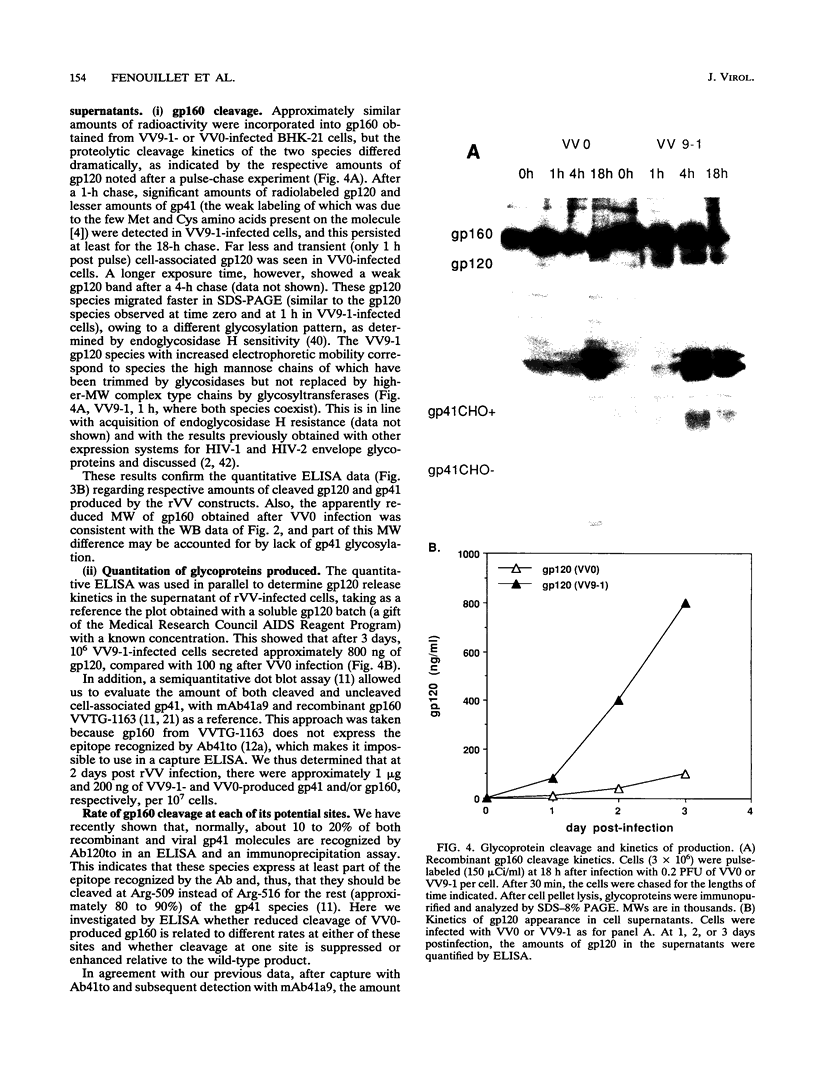
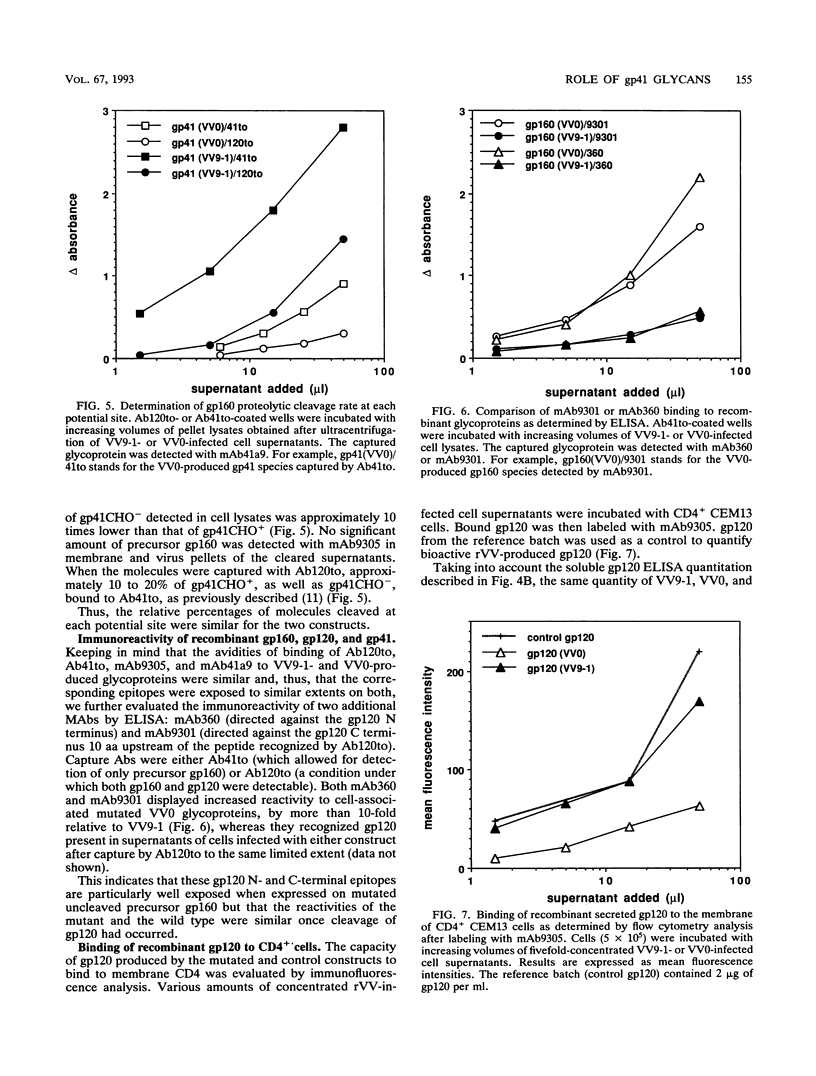
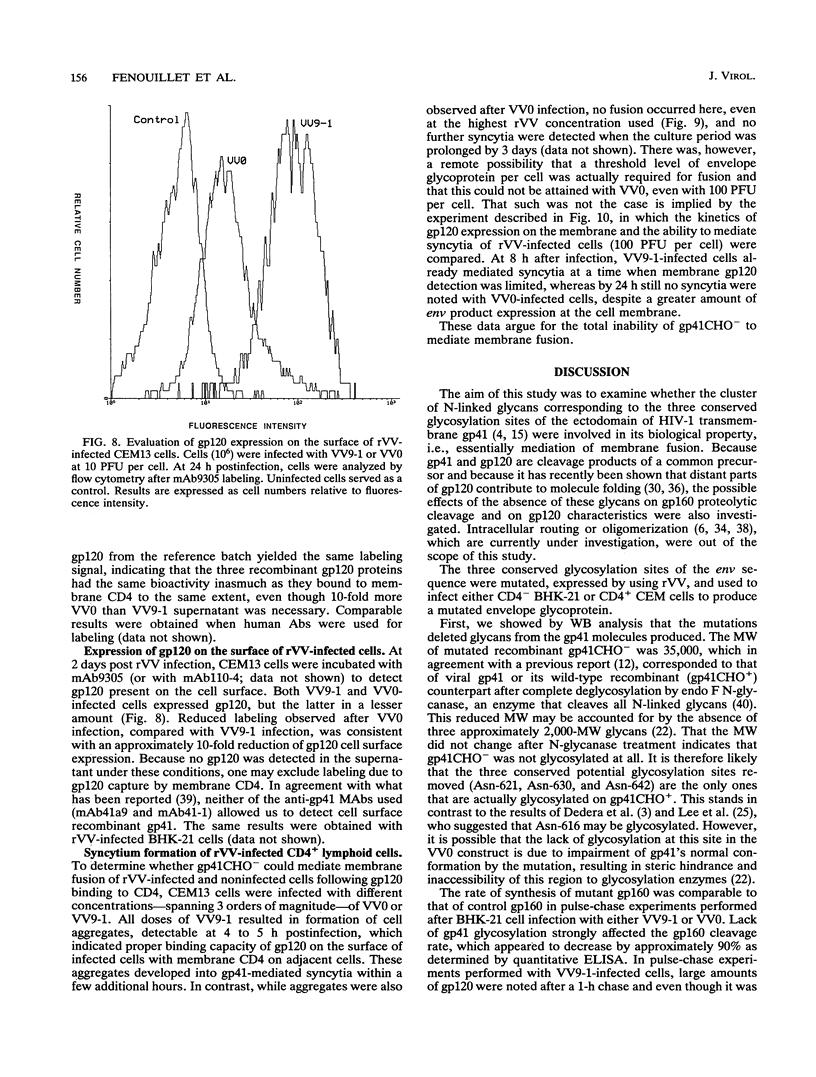
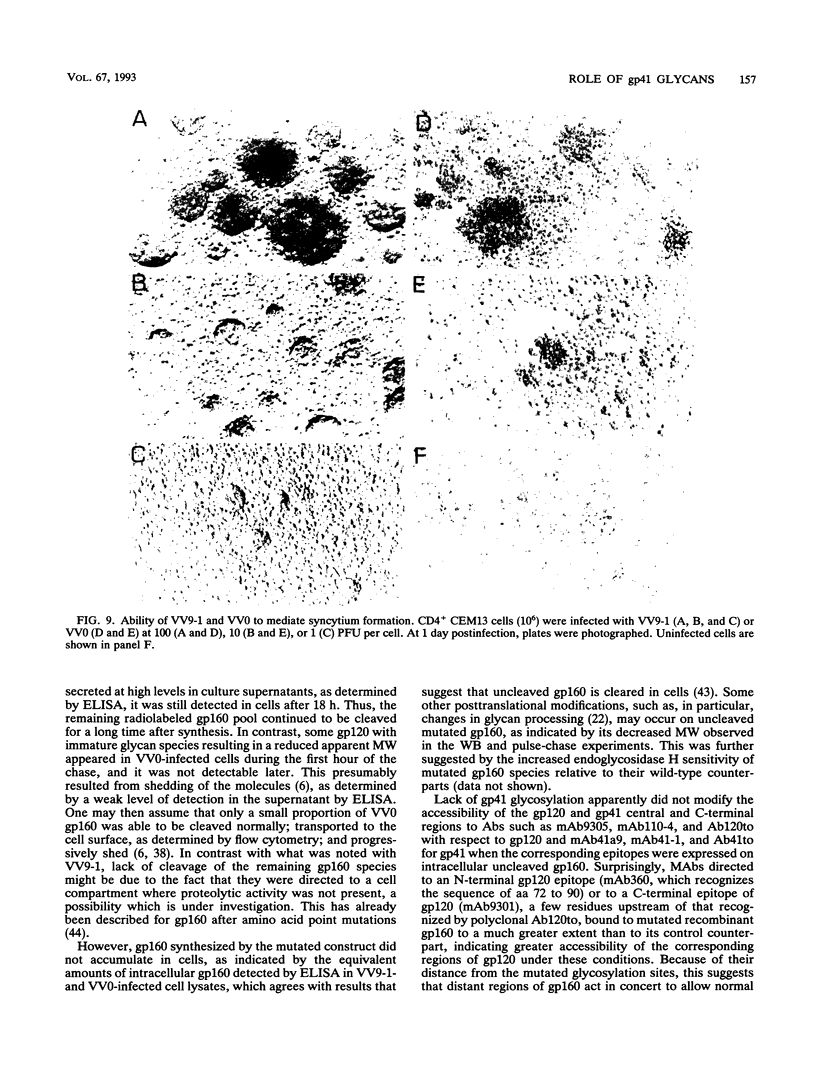
Abstract
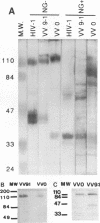
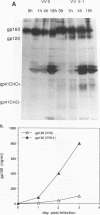
To examine the role of the glycans of human immunodeficiency virus type 1 transmembrane glycoprotein gp41, conserved glycosylation sites within the env sequence (Asn-621, Asn-630, and Asn-642) were mutated to Gln. The mutated and control wild-type env genes were introduced into recombinant vaccinia virus and used to infect BHK-21 or CD4+ CEM cells. Mutated gp41 appeared as a 35-kDa band in a Western blot (immunoblot), and it comigrated with the deglycosylated form of wild-type gp41. Proteolytic cleavage of the recombinant wild-type and mutant forms of the gp160 envelope glycoprotein precursor was analyzed by pulse-chase experiments and enzyme-linked immunosorbent assay: gp160 synthesis was similar whether cells were infected with control or mutated env-expressing recombinant vaccinia virus, but about 10-fold less cleaved gp120 and gp41 was produced by the mutated construct than the control construct. The rates of gp120-gp41 cleavage at each of the two potential sites appeared to be comparable in the two constructs. By using a panel of antibodies specific for gp41 and gp120 epitopes, it was shown that the overall immunoreactivities of control and mutated gp41 proteins were similar but that reactivity to epitopes at the C and N termini of gp120, as present on gp160 produced by the mutated construct, was enhanced. This was no longer observed for cleaved gp120 in supernatants. Both gp120 proteins, from control and mutated env, were expressed on the cell surface under a cleaved form and could bind to membrane CD4, as determined by quantitative immunofluorescence assay. In contrast, and despite sufficient expression of env products at the cell membrane, gp41 produced by the mutated construct was unable to induce membrane fusion. Therefore, while contradictory results reported in the literature suggest that gp41 individual glycosylation sites are dispensable for the bioactivity and conformation of env products, it appears that such is not the case when the whole gp41 glycan cluster is removed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosch V., Pawlita M. Mutational analysis of the human immunodeficiency virus type 1 env gene product proteolytic cleavage site. J Virol. 1990 May;64(5):2337–2344. doi: 10.1128/jvi.64.5.2337-2344.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Mizukami T., Franchini G., Moss B. Synthesis, oligomerization, and biological activity of the human immunodeficiency virus type 2 envelope glycoprotein expressed by a recombinant vaccinia virus. Virology. 1990 Sep;178(1):134–142. doi: 10.1016/0042-6822(90)90386-6. [DOI] [PubMed] [Google Scholar]
- Dedera D. A., Gu R. L., Ratner L. Role of asparagine-linked glycosylation in human immunodeficiency virus type 1 transmembrane envelope function. Virology. 1992 Mar;187(1):377–382. doi: 10.1016/0042-6822(92)90331-i. [DOI] [PubMed] [Google Scholar]
- Dirckx L., Lindemann D., Ette R., Manzoni C., Moritz D., Mous J. Mutation of conserved N-glycosylation sites around the CD4-binding site of human immunodeficiency virus type 1 GP120 affects viral infectivity. Virus Res. 1990 Dec;18(1):9–20. doi: 10.1016/0168-1702(90)90085-p. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Doms R. W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991 Apr;65(4):2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Fenouillet E., Clerget-Raslain B., Gluckman J. C., Guétard D., Montagnier L., Bahraoui E. Role of N-linked glycans in the interaction between the envelope glycoprotein of human immunodeficiency virus and its CD4 cellular receptor. Structural enzymatic analysis. J Exp Med. 1989 Mar 1;169(3):807–822. doi: 10.1084/jem.169.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenouillet E., Gluckman J. C., Bahraoui E. Role of N-linked glycans of envelope glycoproteins in infectivity of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2841–2848. doi: 10.1128/jvi.64.6.2841-2848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenouillet E., Gluckman J. C. Effect of a glucosidase inhibitor on the bioactivity and immunoreactivity of human immunodeficiency virus type 1 envelope glycoprotein. J Gen Virol. 1991 Aug;72(Pt 8):1919–1926. doi: 10.1099/0022-1317-72-8-1919. [DOI] [PubMed] [Google Scholar]
- Fenouillet E., Gluckman J. C. Immunological analysis of human immunodeficiency virus type 1 envelope glycoprotein proteolytic cleavage. Virology. 1992 Apr;187(2):825–828. doi: 10.1016/0042-6822(92)90487-a. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R., Ball J. M., Garry R. F., Griffin M. C., Montelaro R. C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989 Aug;5(4):431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- Geyer H., Holschbach C., Hunsmann G., Schneider J. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J Biol Chem. 1988 Aug 25;263(24):11760–11767. [PubMed] [Google Scholar]
- Gruters R. A., Neefjes J. J., Tersmette M., de Goede R. E., Tulp A., Huisman H. G., Miedema F., Ploegh H. L. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature. 1987 Nov 5;330(6143):74–77. doi: 10.1038/330074a0. [DOI] [PubMed] [Google Scholar]
- Guo H. G., Veronese F. M., Tschachler E., Pal R., Kalyanaraman V. S., Gallo R. C., Reitz M. S., Jr Characterization of an HIV-1 point mutant blocked in envelope glycoprotein cleavage. Virology. 1990 Jan;174(1):217–224. doi: 10.1016/0042-6822(90)90070-8. [DOI] [PubMed] [Google Scholar]
- Jones I. M., Jacob G. S. Anti-HIV drug mechanism. Nature. 1991 Jul 18;352(6332):198–198. doi: 10.1038/352198b0. [DOI] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Drillien R., Spehner D., Skory S., Schmitt D., Wiktor T., Koprowski H., Lecocq J. P. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984 Nov 8;312(5990):163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Rivière Y., Dott K., Schmitt D., Girard M., Montagnier L., Lecocq J. Improved antigenicity of the HIV env protein by cleavage site removal. Protein Eng. 1988 Sep;2(3):219–225. doi: 10.1093/protein/2.3.219. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Lee W. R., Syu W. J., Du B., Matsuda M., Tan S., Wolf A., Essex M., Lee T. H. Nonrandom distribution of gp120 N-linked glycosylation sites important for infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2213–2217. doi: 10.1073/pnas.89.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. R., Yu X. F., Syu W. J., Essex M., Lee T. H. Mutational analysis of conserved N-linked glycosylation sites of human immunodeficiency virus type 1 gp41. J Virol. 1992 Mar;66(3):1799–1803. doi: 10.1128/jvi.66.3.1799-1803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C. K., Spellman M. W., Riddle L., Harris R. J., Thomas J. N., Gregory T. J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990 Jun 25;265(18):10373–10382. [PubMed] [Google Scholar]
- Machamer C. E., Florkiewicz R. Z., Rose J. K. A single N-linked oligosaccharide at either of the two normal sites is sufficient for transport of vesicular stomatitis virus G protein to the cell surface. Mol Cell Biol. 1985 Nov;5(11):3074–3083. doi: 10.1128/mcb.5.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Jones I. M., Stephens P. E., Clements G., Thomson S., Weiss R. A. Characterization of recombinant gp120 and gp160 from HIV-1: binding to monoclonal antibodies and soluble CD4. AIDS. 1990 Apr;4(4):307–315. doi: 10.1097/00002030-199004000-00004. [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Moore J. P., Fenouillet E., Jones I. M. Complementation of human immunodeficiency virus glycoprotein mutations in trans. J Gen Virol. 1992 Aug;73(Pt 8):1907–1913. doi: 10.1099/0022-1317-73-8-1907. [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Moore J. P., Wilkinson A. J., Jones I. M. Reduction in CD4 binding affinity associated with removal of a single glycosylation site in the external glycoprotein of HIV-2. Virology. 1991 Feb;180(2):853–856. doi: 10.1016/0042-6822(91)90106-l. [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Overton H. A., Moore J. P., Wilkinson A. J., Brady R. L., Lewis S. J., Jones I. M. Expression of HIV-1 gp120 and human soluble CD4 by recombinant baculoviruses and their interaction in vitro. AIDS Res Hum Retroviruses. 1990 Jun;6(6):765–773. doi: 10.1089/aid.1990.6.765. [DOI] [PubMed] [Google Scholar]
- Pal R., Hoke G. M., Sarngadharan M. G. Role of oligosaccharides in the processing and maturation of envelope glycoproteins of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 May;86(9):3384–3388. doi: 10.1073/pnas.86.9.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tilley S. A., Bona C., Zaghouani H., Gorny M. K., Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique C., Pham D., Tursz T., Dokhélar M. C. Human T-cell leukemia virus type I envelope protein maturation process: requirements for syncytium formation. J Virol. 1992 Feb;66(2):906–913. doi: 10.1128/jvi.66.2.906-913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard S. R., Rosa M. D., Rosa J. J., Wiley D. C. Truncated variants of gp120 bind CD4 with high affinity and suggest a minimum CD4 binding region. EMBO J. 1992 Feb;11(2):585–591. doi: 10.1002/j.1460-2075.1992.tb05090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L. Glucosidase inhibitors for treatment of HIV-1 infection. AIDS Res Hum Retroviruses. 1992 Feb;8(2):165–173. doi: 10.1089/aid.1992.8.165. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Engleman E. G. Intracellular processing of the gp160 HIV-1 envelope precursor. Endoproteolytic cleavage occurs in a cis or medial compartment of the Golgi complex. J Biol Chem. 1990 Feb 15;265(5):2640–2649. [PubMed] [Google Scholar]
- Stevenson M., Meier C., Mann A. M., Chapman N., Wasiak A. Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS. Cell. 1988 May 6;53(3):483–496. doi: 10.1016/0092-8674(88)90168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Plummer T. H., Jr Enzymatic approaches for studying the structure, synthesis, and processing of glycoproteins. Methods Cell Biol. 1989;32:111–139. doi: 10.1016/s0091-679x(08)61169-3. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Vartanian J. P., Henry M., Chenciner N., Cheynier R., Delassus S., Martins L. P., Sala M., Nugeyre M. T., Guétard D. LAV revisited: origins of the early HIV-1 isolates from Institut Pasteur. Science. 1991 May 17;252(5008):961–965. doi: 10.1126/science.2035026. [DOI] [PubMed] [Google Scholar]
- Wells D. E., Compans R. W. Expression and characterization of a functional human immunodeficiency virus envelope glycoprotein in insect cells. Virology. 1990 Jun;176(2):575–586. doi: 10.1016/0042-6822(90)90028-p. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Bonifacino J. S., Potts B. J., Martin M. A., Klausner R. D. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Klimkait T., Frucht D. M., Bonifacino J. S., Martin M. A. Mutations within the human immunodeficiency virus type 1 gp160 envelope glycoprotein alter its intracellular transport and processing. Virology. 1991 Sep;184(1):319–329. doi: 10.1016/0042-6822(91)90848-6. [DOI] [PubMed] [Google Scholar]