Abstract
In response to inflammatory stimulation, dendritic cells (DCs) have a remarkable pattern of differentiation (maturation) that exhibits specific mechanisms to control antigen processing and presentation. Here, we show that in response to lipopolysaccharides, protein synthesis is rapidly enhanced in DCs. This enhancement occurs via a PI3K-dependent signaling pathway and is key for DC activation. In addition, we show that later on, in a manner similar to viral or apoptotic stress, DC activation leads to the phosphorylation and proteolysis of important translation initiation factors, thus inhibiting cap-dependent translation. This inhibition correlates with major changes in the origin of the peptides presented by MHC class I and the ability of mature DCs to prevent cell death. Our observations have important implications in linking translation regulation with DC function and survival during the immune response.
Introduction
Dendritic cells (DCs) are regulators of the immune response whose antigen-processing activities are controlled in response to inflammatory stimuli (e.g., lipopolysaccharides [LPSs]) (Banchereau and Steinman, 1998). Among antigen-presenting cells, DCs are the most efficient at initiating antigen-specific responses, inducing differentiation of naive T cells. Upon stimulation, DCs begin a maturation process characterized by dramatic functional changes, such as cytokine production (e.g., IL-12) or up-regulation of antigen presentation capacity (Mellman and Steinman, 2001). TLR-4 signaling by LPS activates two major pathways resulting in DC maturation (Kaisho and Akira, 2001). The first results in IKK, JNK, and p38 stimulation through the MyD88-IRAK-TRAF6 pathway. The second involves the Toll-IL-1 receptor domain-containing adaptor-inducing IFN-β (TRIF) and IRF3, which leads to type I interferon expression and costimulatory molecule up-regulation. Both pathways are required for optimal NF-κB activation. DC activation results therefore in the enhanced ability to stimulate and polarize T cells in vitro and in vivo.
A large proportion of newly synthesized proteins termed DRiPs are abnormal and degraded rapidly, thus contributing importantly to the MHC class I restricted endogenous antigenic peptide pool (Princiotta et al., 2003). Upon activation, DCs store ubiquitinated DRiPs in cytosolic bodies called dendritic cell aggresome-like induced structures (DALIS) (Lelouard et al., 2002). Ubiquitinated DRiPs storage in DALIS delays their processing and could contribute to regulate MHC class I presentation (Herter et al., 2005; Pierre, 2005). DRiPs and DALIS formation are tightly linked to protein synthesis and quality control (Lelouard et al., 2004). Through this study we explore different aspects of mRNA translation regulation and its consequences for DC function.
Protein synthesis regulation can be achieved through phosphorylation, inhibition, and proteolysis of key translation factors (Gingras et al., 1999). Extracellular stimuli such as growth factors activate translation through phosphoinositide 3-kinase (PI3K) and Ras signaling pathways. In contrast, viruses and cellular stresses inhibit translation through the phosphorylation and/or proteolytic cleavage of initiation factors such as eIF2α and eIF4GI (Holcik and Sonenberg, 2005). In addition to a general decline in protein synthesis, these events allow the translation of specific viral- or stress-related mRNAs. These mRNAs bear a complex structural element called internal ribosome entry site (IRES) that can directly recruit ribosomes under stress conditions and bypass the need for a 7mGpppN cap, which is normally recognized by the translation initiation complex. Thus, cap-dependent and cap-independent translations are most often regulated in opposite ways, IRES-mediated translation being relatively inefficient under physiological conditions.
We demonstrate here that LPS stimulation has a profound effect on the intensity and quality of translation in DCs both in vitro and in vivo. Translation control is tightly coordinated with the state of DC activation and can act independently of transcription regulation. LPS-stimulated bone marrow–derived DCs first undergo a phase of rapid up-regulation of protein synthesis. We show that this translational activation mediated by the PI3K/AKT signal transduction pathway is necessary for cytokine production, costimulatory molecules, and MHC class II surface up-regulation, as well as for DALIS formation in the first hours of LPS stimulation. At later stages of maturation eIF2α phosphorylation together with an increased production and degradation of eIF4GI and the eIF4GI-like factor DAP5, are correlated with the inhibition of cap-dependent translation and an increased resistance to apoptosis of mature DCs. Inhibition of cap-dependent translation also has an impact on MHC class I restricted antigen presentation, which at late time of DC maturation loses its dependence on protein neo-synthesis. Thus, translation regulation in response to LPS is required for proper DC function and survival.
Results
Protein translation is regulated during DC maturation
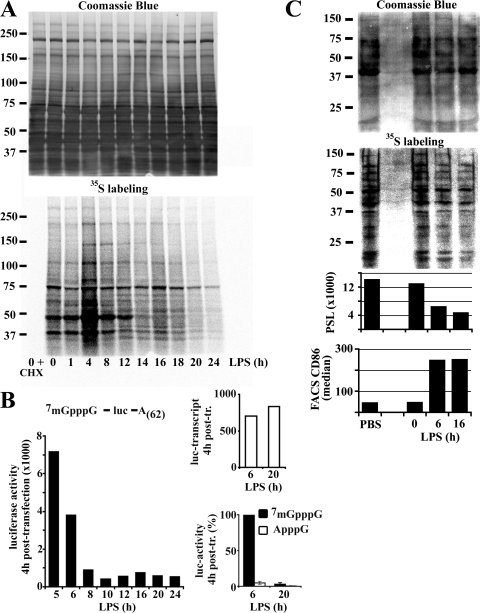
Protein translation was monitored by [35S]methionine/cysteine metabolic labeling in LPS-stimulated mouse bone marrow–derived DCs. Two events were observed (Fig. 1 A). First, an important increase in protein synthesis peaking at 4 h was seen rapidly upon stimulation. From this time point on, methionine/cysteine incorporation continuously decreased, reaching a lower level than in immature DCs (iDCs) after 16 h of maturation. To confirm this observation, a luciferase reporter mRNA was introduced in maturing DCs (mDCs) by transfection (Fig. 1 B). As in the pulse chase experiment, translation of luciferase, very active at 5 h of LPS activation, was progressively inhibited with maturation time. 7mGpppG-capped mRNA transfection efficiency was determined by quantitative PCR and found equivalent at the different times of activation. A replacement of 7mGpppG-cap by the cap analogue ApppG further demonstrated that mRNA capping is required to allow translation during the first hours of maturation. Thus, an enhancement of cap-mediated translation is observed at the initiation of maturation, immediately followed by a marked reduction, and this in a manner independent of the mRNA levels present. These results were confirmed by monitoring protein synthesis in freshly explanted splenic CD11c+ DCs from mice injected with LPS (Fig. 1 C). Autoradiography and corresponding phosphoimager quantification demonstrated the strong down-regulation of protein synthesis (threefold) in splenic dendritic cells isolated as soon as 3 h after LPS injection (6 h of total exposure including 3 h of manipulation). [35S]methionine/cysteine incorporation levels were inversely correlated with phenotypical maturation of DCs as monitored by CD86 surface staining (Fig. 1 C). This inhibition was maintained for at least 16 h of LPS treatment, thus confirming that maturing DCs down-regulate heavily their protein synthesis in vivo.
Figure 1.
Protein synthesis is regulated during DC maturation. (A) At the indicated time of LPS-induced maturation, DCs were pulsed for 10 min with 35S-labeled Pro-Mix. Soluble fractions were prepared and analyzed by Coomassie blue staining (top) and autoradiography (bottom) after SDS-PAGE. (B) A firefly luciferase reporter mRNA was transfected in DCs. Left: luciferase activity was measured 4 h after transfection at the indicated time of maturation (absolute values of one representative experiment). Bottom right: comparative levels of translation of luciferase mRNAs modified or not with an unmethylated cap-analogue in transfected 6 h and 20 h LPS-treated DCs. Error bars indicate the SD of three independent experiments. Due to variations of absolute values in the different experiments, the time point 6 h of 7mGpppG was defined as 100%. Transfection efficiency was determined by quantitative PCR (top right, absolute values). (C) Spleen DCs, isolated from mice treated with PBS as control or 10 μg LPS i.p. for different times as indicated, were metabolically labeled. After SDS-PAGE, soluble fractions were stained with Coomassie blue (top) and 35S incorporation was visualized by phosphoimaging (bottom) and quantified (PSL = photostimulated luminescence). The maturation status of the DCs is shown by FACS analysis of CD86 (bottom).
Protein translational increase at the onset of maturation is mediated by a PI3K signaling pathway
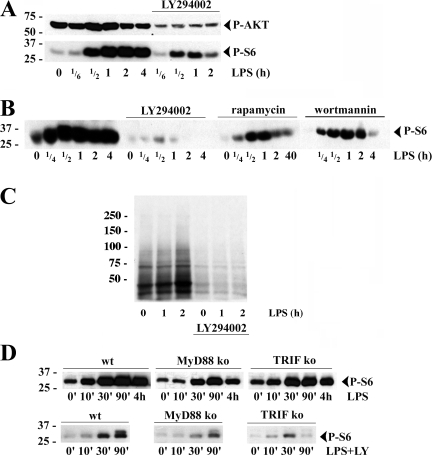
PI3K, AKT, and the mammalian target of rapamycin (mTOR) are major components of the transduction pathway controlling mRNA translation (von Manteuffel et al., 1997; Raught et al., 2000; Aoki et al., 2001; Ruggero and Sonenberg, 2005). Activation of this pathway leads to the phosphorylation of the S6 ribosomal protein by the cognate 70-kD S6 kinase (S6K1), which correlates well with the enhanced protein synthesis capacity of stimulated cells (Fingar et al., 2004). AKT phosphorylation was increased 15–30 min after LPS stimulation, followed by massive phosphorylation of S6, thus matching the rapid increase in protein synthesis (Fig. 2 A). Moreover, inhibition of the PI3K-dependent pathway with LY294002 (LY) inhibited efficiently AKT and S6 phosphorylation, thus confirming the importance of PI3K in initiating this signaling cascade in DCs. Wortmannin (another PI3K inhibitor) also inhibited S6 phosphorylation, although less efficiently than LY (Fig. 2 B). Inhibition of mTOR with rapamycin markedly reduced S6 phosphorylation, strongly suggesting that this kinase lying downstream of AKT and upstream of S6K1 is an important part of the signaling pathway leading to S6 phosphorylation upon LPS detection (Fig. 2 B). The implication of this pathway in controlling translation activation was further demonstrated by the limited [35S]methionine/cysteine incorporation observed in metabolically pulsed maturing DCs treated with LY, while control cells displayed the expected up-regulation of protein synthesis in response to LPS (Fig. 2 C).
Figure 2.
The PI3K/AKT signal transduction pathway is involved in S6 phosphorylation and translational increase during DC activation. (A) Immunoblot of S6 (top) and AKT (bottom) phosphorylation at the onset of DC maturation and its inhibition by LY 294002 (LY). (B) DCs were pretreated with wortmannin, LY, or rapamycin prior stimulation and immunoblotted for phospho-S6 (P-S6). (C) Translation was monitored in DCs by 35S-labeled Pro-Mix during the first 2 h of LPS activation with or without LY treatment. (D) Immunoblot of P-S6 in DCs derived from C57BL6 (WT), MyD88−/−, and TRIF−/− mice and stimulated with LPS for the indicated times, with (bottom) or without (top) pretreatment with LY.
Signal transduction upon TLR4 engagement by LPS is dependent on the two adaptors MyD88 and TRIF (Kaisho and Akira, 2001); although both adaptors are required for proper DC activation, their potential interaction and role in PI3K recruitment is unclear (Arbibe et al., 2000). Thus, we evaluated the individual contribution of these molecules to translation activation in response to LPS using DCs derived from MyD88 (−/−) or TRIF (−/−) deficient mice. As expected, TRIF−/− DCs and to a lesser level MyD88−/− DCs were impaired in their LPS-triggered maturation process (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200707166/DC1). In both transgenic strains, S6 phosphorylation levels after LPS activation were considerably decreased compared with wild-type control (Fig. 2 D). In MyD88−/− DCs, despite its reduced intensity, S6 phosphorylation peaked after 90 min of activation like in wild-type DCs (Fig. 2 D). In contrast, the peak of phosphorylation occurred earlier (30 min) in TRIF−/− DCs. Because S6 phosphorylation displayed an exacerbated sensitivity to LY treatment, we could perform a sharper analysis of the adaptor contribution by immunoblotting LY-treated DC extracts. The lower intensity and the peaks of activity in absence of MyD88 (90 min) and TRIF (30 min) were conserved under these conditions (Fig. 2 D, bottom), thus suggesting that the two adaptors synergize to allow the full phosphorylation of S6 upon LPS stimulation, but with some difference in their activation kinetics. MyD88 seems to initiate the response early after LPS detection (30 min peak in TRIF−/− DCs), whereas TRIF seems to be involved at later times (90 min peak in MYD88−/−) and thus complement MyD88 activity to increase and sustain the signaling response for longer period of activation. TLR4 signaling requires, therefore, both MyD88 and TRIF adaptors for the full activation of PI3K leading to S6 phosphorylation and a strong up-regulation of translation.
Up-regulation of maturation markers and DALIS formation are linked to PI3K signaling and translation activation
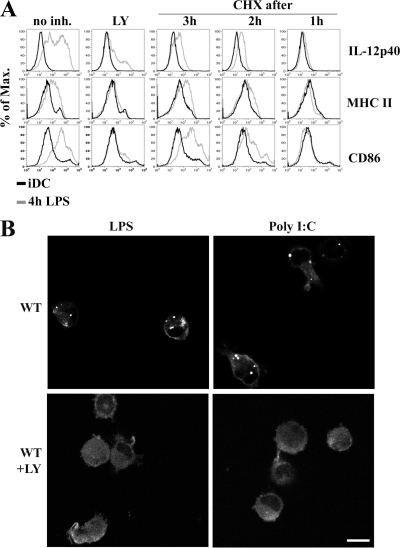
DC activation is phenotypically characterized by the surface up-regulation of MHC and costimulatory molecules, which are required for efficient antigen presentation and T cell activation. MHC class II molecule redistribution from the lysosomes of iDC to the cell surface of mDC is a key event in this process (Pierre et al., 1997). However, the respective contribution of preexisting and of newly synthesized molecules to this surface appearance is still debated. As translation is rapidly enhanced in response to LPS, we investigated the importance of PI3K signaling and protein synthesis in surface appearance of classical maturation markers such as CD86, MHC class II, and the production of IL-12, a key cytokine for DC function and T cell differentiation. LY treatment abrogated the surface appearance of the different markers and the production of IL-12, as illustrated by FACS staining of CD11c+ DCs activated with LPS (Fig. 3 A). Thus, PI3K activity is absolutely required for functional LPS activation of DCs. Based on our observation that LY has a potent inhibitory effect on protein synthesis in DCs, we next tested whether direct inhibition of protein synthesis with the translation inhibitor cycloheximide (CHX) could also negatively impact on DC maturation. Upon CHX addition, CD86 and MHC class II surface up-regulation as well as IL-12 production were fully inhibited after 3 h of treatment (Fig. 3 A). This further demonstrates that protein neo-synthesis is required to achieve proper DC activation.
Figure 3.
Importance of protein translational increase for DALIS formation and DC immunological functions. (A) Impaired MHC class II and CD86 surface expression up-regulation and cytokine production in maturing DCs upon treatment with LY or CHX. FACS staining of IL-12 synthesis and surface expression of MHC class II and CD86 in iDCs (black line) and mDCs (4 h light gray) are shown for different times of treatment with LY and CHX. (B) After 8 h of LPS (left) or poly I:C (right) stimulation in the absence or in the presence of the PI3K inhibitor LY294002, mice bone marrow–derived DCs were stained for ubiquitinated proteins (FK2) and visualized by confocal microscopy. Bar, 10 μm.
One of the hallmarks of DC activation is the formation of DALIS. They are transient, forming as soon as 3 h after DC activation, peaking at 8–12 h and disappearing 24–36 h later (Lelouard et al., 2002). DALIS formation and maintenance require continuous protein synthesis and seem to be linked to MHC class I restricted antigen processing (Herter et al., 2005). The striking correlation of DALIS appearance and disappearance with the translation variations observed in mDCs led us to investigate the importance of protein synthesis regulation and PI3K signaling for their formation. DALIS were stained by the anti-polyubiquitinated proteins monoclonal antibody FK2 and detected by immunofluorescence confocal microscopy after LPS or poly I:C activation (Fig. 3 B, top). PI3K inhibition with LY completely blocked DALIS formation, independently of the mode of DC activation (Fig. 3 B, bottom). DALIS formation is therefore linked to PI3K signaling, probably through its translational up- regulation effect.
Translation inhibition correlates with eIF2α phosphorylation
Having demonstrated PI3K involvement in increasing translation in response to LPS, we next investigated by which molecular mechanism(s) translation down-regulation could be achieved and its consequences during late phase of DC maturation. Cap-mediated translation inhibition can be achieved through the regulation of translation initiation by phosphorylation or proteolysis of key translation factors (Gingras et al., 1999). Cell defense pathways take advantage of four different kinases (e.g., PKR) to regulate protein synthesis in response to different environmental stresses by phosphorylating the α subunit of the translation initiation factor 2 (eIF2-α) (Anderson and Kedersha, 2002; Gebauer and Hentze, 2004). Phosphorylated eIF2-α acts as a dominant-negative molecule and blocks the initiation of cap-dependent protein synthesis in stressed cells by inhibiting Met-tRNA recruitment.
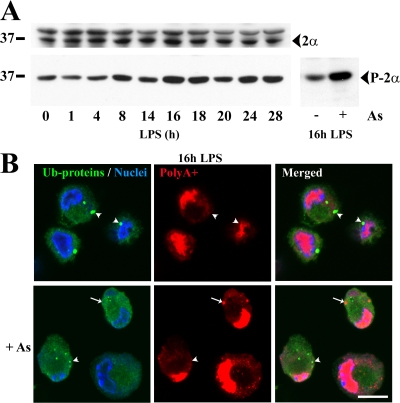
We monitored eIF2α phosphorylation levels by immunoblot in maturing DCs (Fig. 4 A). eIF2α phosphorylation increased between 4 and 8 h of DC maturation, suggesting that a stress-like response is induced by LPS. However, eIF2α phosphorylation in mDCs appeared limited when compared with control samples treated with arsenite (Fig. 4 A) in which translation was fully abrogated. Stress- or arsenite-induced eIF2α phosphorylation promotes the formation of stress granules (SGs), which serve as mRNA and preinitiation complex deposits until stress diminishes and protein synthesis can resume (Anderson and Kedersha, 2002). Thus, SG formation is a relatively good indicator of the eIF2α phosphorylation and associated translation inhibition levels. Immunofluorescence confocal microscopy was used to visualize SGs using a fluorescent oligo-dT probe in 16-h maturing DCs (Fig. 4 B). In absence of arsenite treatment, SGs were never observed during DC maturation, further supporting that the increase in eIF2α phosphorylation in maturing DCs is truly modest. Thus, in response to LPS, limited phosphorylation of eIF2α may modulate mRNA translation quantity and quality (Morley et al., 2005), although it is unlikely solely responsible for the dramatic translation inhibition observed during DC maturation.
Figure 4.
eIF2α phosphorylation increases during DC maturation without inducing SG formation. (A) eIF2α levels remained constant during DC maturation (top) while its phosphorylation increased (bottom left) to a lesser extent than in mDCs treated with 500 μM arsenite (bottom right), as monitored by immunoblot. (B) mDCs were treated (bottom) or not (top) with arsenite before being stained for ubiquitinated proteins (FK2; green), polyA+ mRNA (oligo-dT probe; red), and nuclei (TO-PRO-3; blue). SGs (red; arrows) induced upon arsenite treatment are not enriched in ubiquitinated proteins (green), while DALIS (green; arrowheads) formed upon LPS treatment are not enriched in mRNA (red). Because SGs induced by arsenite did not contain ubiquitinated proteins and conversely DALIS were not enriched in poly-adenylated mRNAs, these are distinct structures. Translation inhibition is responsible for the disappearance of DALIS in the arsenite-treated cells. Bar, 10 μm.
Alteration of eIF4GI during DC maturation
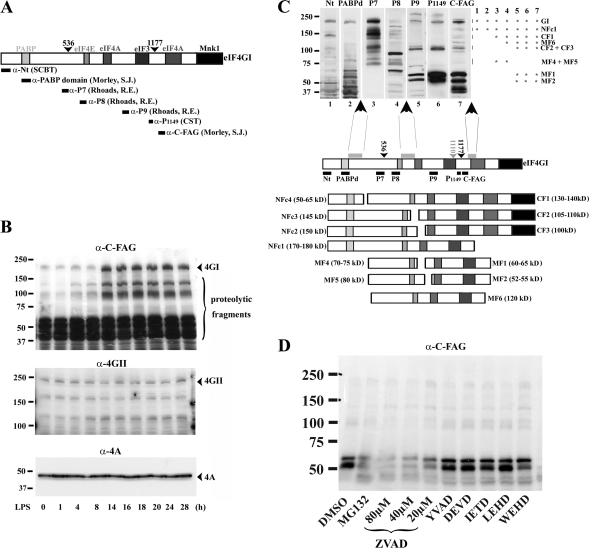
Alternatively, cap-dependent translation can also be inhibited by the cleavage of the scaffold translation initiation factor eIF4GI (Prevot et al., 2003; Holcik and Sonenberg, 2005; Spriggs et al., 2005) (Fig. 5 A). Proteolytic cleavage of eIF4GI by viral proteases or caspase-3 occurs during picornavirus and retrovirus infection as well as during cellular stress (Holcik and Sonnenberg, 2005). Proteolytic fragments of eIF4GI have been shown to compete with the recruitment of the complete cap-binding protein complex eIF4F by the mRNAs, thereby limiting cap-dependent translation and favoring the internal ribosome entry sites (IRES)–mediated translation of a small number of specific mRNAs (Bushell et al., 2000). Thus, we investigated by immunoblotting the fate of the scaffold translation initiation factor eIF4GI using an antibody recognizing a C-terminal epitope (α-C-FAG) (Cowan and Morley, 2004). Surprisingly, between 8 and 14 h of LPS activation, a time at which cap-dependent translation is down-regulated, eIF4GI levels increased sturdily (Fig. 5 B). In contrast, the levels of the closely related homologue eIF4GII and of eIF4A remained stable. The specific up-regulation of eIF4GI does probably not reflect a slower degradation rate because several smaller fragments weakly present in iDCs also increased after 8 h of LPS treatment. Interestingly, eIF4GI translation is enhanced in a context of general cap-dependent translation inhibition.
Figure 5.
Up-regulation and cleavage of eIF4GI during DC maturation. (A) Scheme of the murine eIF4GI largest isoform. Binding sites for PABP and the different translation initiation factors are boxed. The caspase-3 cleavage sites are indicated with arrowheads. Epitopes recognized by the different antibodies used throughout this study are organized according to their position in eIF4GI. (B) Conversely to eIF4GII (middle) and eIF4A (bottom), eIF4GI and associated fragments (top) are strongly increased after 8 h of maturation as monitored by immunoblot performed with α-C-FAG antibody. (C) Model of eIF4GI cleavage in mDCs. Extracts of mDCs were separated by SDS-PAGE and immunoblotted with different anti-eIF4GI antibodies. Fragment patterns were compared as follows: bands recognized by only one antibody were eliminated; other bands were analyzed according to antibody reactivity (illustrated by asterisks on the right of the immunoblot), molecular weight and comparison to the previously described cleavage products generated by caspase-3, proteasome, picornaviral, and HIV virus proteases. Three major proteolytic sensitive areas (gray boxes) and their putative products were deduced from antibody reactivity and molecular weights. NFc stands for N-terminal fragment cluster because several clustered bands are always displayed due to the existence of the multiple isoforms of eIF4GI. MF stands for middle fragment and CF for C-terminal fragment. (D) Alteration of eIF4GI during DC maturation is prevented by proteasome and pan-caspase inhibitors. Effects of protease inhibitors on eIF4GI fate in DCs were analyzed by immunoblot using the anti- C-FAG antibody. Conversely to MG132 and Z-VAD-FMK, specific caspase inhibitors (YVAD, DEVD, IETD, LEHD, WEHD) had no effect on accumulation of eIF4GI fragments.
eIF4GI mRNA contains an IRES sequence, which favors eIF4GI neosynthesis in response to the accumulation of several of its own specific proteolytic fragments (Byrd et al., 2005). Thus eIF4GI proteolysis was further investigated using antibodies detecting epitopes spread along the entire length of the 220-kD molecule (Fig. 5 A). They confirmed that levels of intact eIF4GI were increased, but also that the cleavage of the translation factor was strongly enhanced during DC maturation (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200707166/DC1). The higher molecular weight fragments migrating around 160 kD were recognized by all antibodies, and are therefore the result of a C-terminal cleavage that occurs downstream of the previously identified caspase-3 cleavage sites (Bushell et al., 2000). Based on the degradation patterns observed by immunoblot, it was possible to identify three major areas of cleavage along eIF4GI (Fig. 5 C).
The first cleavage site is positioned after the PABP-binding site and when cleaved, separates the N terminus of eIF4GI (e.g., NFc4) recognized by antibodies such as α-Nt and α-PABPd from the rest of the molecule. The second region is located after the eIF4E-binding site, thus allowing the production of fragments only recognized by α-P7 and α-P8 and containing the cap-binding protein eIF4E interaction domain (e.g., MF4 and MF5). The last cleavage domain was mapped in the vicinity, but distinct of the last known caspase-3 cleavage site, and produces fragments recognized by α-P9, α-phospho-S1149, and α-C-FAG. The smaller eIF4GI fragments (e.g., MF4 and MF5), although containing the eIF4E binding site, were found to have a low affinity for eIF4E because they were not retained efficiently on a 7mG-cap affinity column (Fig. S2 B). Conversely, higher molecular weight eIF4GI fragments (>100 kD), possessing several other binding domains, were efficiently retained on the column and could therefore compete in the cell with intact eIF4GI for the recruitment of the cap-binding protein eIF4E and associated mRNAs. Thus, these fragments are likely to participate to the down-regulation of cap-dependent translation occurring during DC activation.
Identification of the proteolytic activity responsible for eIF4GI cleavage
In addition to caspase-3 and viral proteases known to degrade eIF4GI, the caspase-like activity of the proteasome has been shown in vitro to cleave eIF4GI in multiple fragments (Baugh and Pilipenko, 2004). To identify the protease(s) responsible for eIF4GI fragmentation, we used a panel of specific proteasome and caspase inhibitors and performed an immunoblot on mDCs (Fig. 5 D). The lack of inhibitory effect observed in the presence of the caspase-3 inhibitor DEVD-FMK confirmed that this caspase is not involved in eIF4GI cleavage in mDCs. Although none of the other specific caspase inhibitors tested had any inhibitory activity, the pan-caspase inhibitor Z-VAD-FMK prevented the accumulation of eIF4GI fragments. The same effect was observed with the reversible proteasome inhibitor MG132. Thus, the caspase-like activity of proteasome is likely to mediate at least partially the degradation of eIF4GI and play an active role in inhibiting translation in mature DCs.
Consequences of eIF4GI cleavage: IRES-mediated translation activation
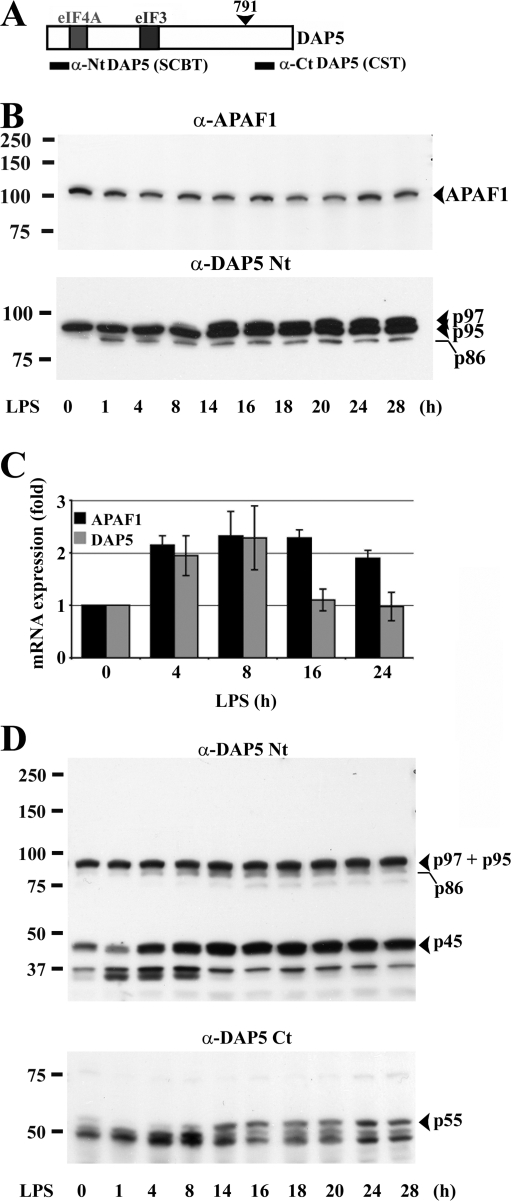
In addition of being partly responsible for cap-mediated translation inhibition in mature DCs, we wondered if eIF4GI cleavage could have other functional consequences in DCs. Indeed, the major 50- to 65-kD fragments of eIF4GI (MF1 and MF2) are predicted to contain the first RNA-helicase eIF4A and the ribosome adaptor eIF3 binding domains, and are therefore equivalent to the eIF4GI fragments generated by different retroviral proteases (Alvarez et al., 2003). These fragments, as well as those generated by apoptosis or cellular stress induction, are known to act as IRES-transacting factors (ITAFs) and are central in switching from m7G cap-dependent to IRES-mediated translation during viral infection or cellular stress (Holcik and Sonenberg, 2005; Spriggs et al., 2005). In addition to viral mRNAs, they have been shown to induce protein synthesis from the cellular mRNAs of the pro-apoptotic factor APAF1 and of the eIF4GI family member DAP5 (Fig. 6 A) (Nevins et al., 2003). We thus investigated whether APAF1 and DAP5 expression levels were modified during DC maturation by immunoblot (Fig. 6 B). APAF1 expression showed no significant variations upon DC maturation, whereas DAP5 expression was increased from 8 h of LPS activation. To examine whether the up-regulation of DAP5 could be due to transcriptional activation, quantitative PCR was performed (Fig. 6 C). DAP5 and APAF1 mRNAs were not induced more than twofold, DAP5 mRNA returning to basal levels after 8 h. Thus, mRNA transcription is not linearly correlated with the level of protein accumulation.
Figure 6.
DAP5 translational up-regulation and cleavage in mDCs. (A) Scheme of the murine DAP5. Binding sites for the different translation initiation factors are boxed. (B) Immunoblots of maturing DCs for APAF1 (top) and DAP5 (bottom). Conversely to APAF1, DAP5 p95/p97 is increased during LPS activation. (C) Transcriptional regulation of DAP5 and APAF1. mRNAs were isolated at different stages of DC maturation and quantitative PCR was performed. The mRNA expression levels are indicated as fold regulation normalized to iDCs (0 h). Error bars indicate the SD of three independent duplicated experiments. (D) Alteration of DAP5 during DC maturation. In addition to DAP5/p97, a 45-kD fragment (p45) detected by immunoblot with the α-DAP5 Nt antibody (top) is increased during DC maturation. A complementary fragment of 55 kD (p55) is also detected with the α-DAP5 Ct antibody (bottom).
DAP5 is crucial for the regulation of cell survival after apoptotic stress. During apoptosis, DAP5 is activated by a cleavage generating an 86-kD fragment (p86), resembling those of eIF4GI containing both eIF3 and eIF4A domains (Fig. 5 A and Fig. 6 A) and also able to stimulate IRES-mediated translation (Holcik and Sonenberg, 2005). The intact form of DAP5 was resolved by immunoblot of the mDC extracts as a doublet (p97 and p95; Henis-Korenblit et al., 2000). Interestingly and in contrast to fibroblasts, which express constitutively both forms (Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200707166/DC1), iDCs displayed solely the p95 isoform, the DAP5/p97 isoform being induced only after 8 h of LPS activation (Fig. 6 B). Interestingly, although p86 was only weakly detected throughout the maturation process, a previously uncharacterized DAP5 proteolytic N-terminal fragment of 45 kD (p45) and a potential complementary C-terminal fragment of 55 kD (p55) were strongly increased during maturation (Fig. 6 D).
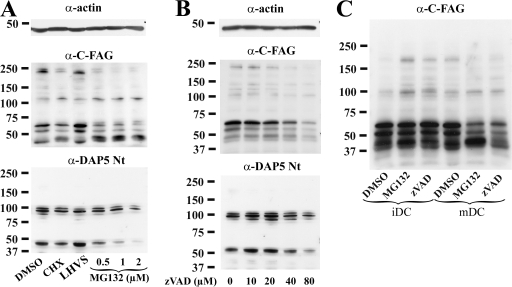
Loss of eIF4GI and DAP5 up-regulation upon inhibition of their degradation
In addition of inhibiting cap-mediated translation, the proteolytic cleavage of eIF4GI and/or DAP5 during maturation is likely to control their own expression at the translational level. Thus, conversely to a classical situation in which inhibiting the degradation of a given protein increases its total level, interfering with eIF4GI cleavage should prevent the maturation-induced up-regulation of eIF4GI and DAP5. In agreement with this hypothesis, when mDCs were treated with MG132, levels of eIF4GI and DAP5 were drastically reduced in a dose-dependent manner (Fig. 7 A). In contrast to MG132, LHVS, a general inhibitor of cysteine proteases, had no effect on the translation factors levels. Importantly, protein synthesis inhibition with CHX also reduced eIF4GI and DAP5 levels, thus confirming the importance of active translation to maintain steady-state levels of these molecules. To avoid potential indirect effect of MG132, the same experiment was conducted using Z-VAD-FMK to inhibit eIF4GI cleavage. A similar loss of eIF4GI and DAP5 was observed (Fig. 7 B). Thus, a strong link between proteolytic cleavage and increased production of the two translation factors exist in mDCs. Collectively, our results suggest that DC maturation promotes cleavage of eIF4GI and DAP5 into their potential ITAF fragments, thus boosting their own synthesis by favoring IRES-driven mRNA translation, in a global context of cap-mediated translation repression. Illustrating this conclusion and conversely to what occurred in mDCs, levels of eIF4GI and DAP5 increased upon MG132 or Z-VAD-FMK treatment in iDCs (Fig. 7 C). eIF4GI cleavage is therefore only functionally important in mature DCs.
Figure 7.
Up-regulation of eIF4GI and DAP5 in mDCs is dependent on their own proteolytic cleavage. Immunoblot detection of eIF4GI and DAP5 in 16 h LPS-activated DCs exposed to pharmacological agents. (A) Conversely to the cysteine protease inhibitor LHVS, the proteasome inhibitor MG132 induced a dose-dependent loss of eIF4GI and DAP5 in mDCs, in a similar fashion to translation inhibition (CHX). (B) Inhibition of caspase-like activities in mDCs with Z-VAD-FMK had a dose-dependent effect comparable to that of MG132 or CHX on eIF4GI and DAP5 levels. (C) In iDCs, MG132 and Z-VAD-FMK treatments favored the accumulation of intact eIF4GI, whereas in mDCs the two drugs promoted its loss, as detected with the anti-C-FAG antibody. Actin immunoblots are shown for equal loading control.
Translation regulation correlates with mDC survival
In addition to their influence on translation quality, eIF4GI and DAP5 fragments have been shown to be involved in cell death regulation by influencing the translation of pro- or anti-apoptotic factors in response to environmental cues (Holcik and Sonenberg, 2005). Lifespan of DCs can influence the duration of DC interaction with lymphocytes, thereby affecting the outcome of lymphocyte activation and immune responses. DCs have a relatively rapid turnover in vivo (Jung et al., 2002), and several lines of evidence indicate that apoptosis can regulate DC functions (Hou and Van Parijs, 2004). Induction of cell death in DCs has been shown to limit the priming of antigen-specific cytotoxic T cells, and apoptosis prevention potentially regulates the scope of immune responses and immune tolerance. We therefore tested if translation factor degradation could influence mDC survival.
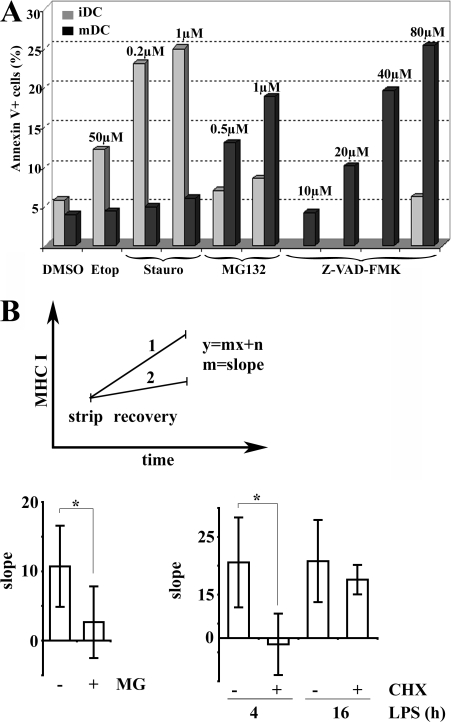
CD11c+ DC resistance to cell death was quantified by monitoring annexin V surface staining after treatment with MG132 or the pro-apoptotic drugs etoposide and staurosporine (Fig. 8 A). Although little LPS-induced death was observed, staurosporine treatment induced a strong annexin V labeling in 25% of the iDCs, whereas etoposide had a lesser effect. In contrast to iDCs, mDCs were not affected by the pro-apoptotic drugs, thus suggesting that maturation enhances their resistance to induced cell death. The contribution of eIF4GI and DAP5 proteolytic cleavage to this enhanced survival capacity of mDCs was evaluated using Z-VAD-FMK and MG132 at different doses (Fig. 8 A). Although MG132 treatment induced only a modest increase of cell death in iDCs, mDCs were extremely sensitive to the drug treatment. Most strikingly Z-VAD-FMK had no effect on iDCs even at higher doses (80 μM), while it efficiently promoted cell death in mDCs after a dose-dependent response in good correlation with the prevention of eIF4GI and DAP5 cleavage. Collectively, these results suggest that one of the functions of eIF4GI and DAP5 cleavage and translation inhibition in maturing DCs is to promote their survival, which otherwise might be affected by LPS stimulation.
Figure 8.
Translation regulation influences DC survival and MHC class I presentation. (A) Effect of proteasome inhibition on DC survival. Surface annexin V staining was quantified by FACS on CD11c+ gated cells. iDCs (gray bars) were sensitive to the apoptosis inducers etoposide (Etop) and staurosporine (Stauro), whereas mDCs (black bars) remained unaffected. Conversely, MG132 and Z-VAD-FMK treatment induced apoptosis in mDCs and had little effect in iDCs. Data are from one representative experiment out of four performed. (B) Origin of MHC I ligands during DC maturation. Surface levels of peptide-loaded Kb molecules were monitored by FACS using the HB-176 antibody, which recognizes conformed H-2Kb molecules independently of their peptide ligands. Acid stripping was used to decrease surface peptide loaded H-2Kb and increase the sensitivity of detecting newly arriving surface MHC I molecules. Surface H-2Kb recovery over 2 h after strip was monitored with or without chemical treatment (MG132 or CHX) and data were plotted as a linear function where the x-value is time and the y-value the level of peptide-loaded MHC I. Slopes (recovery rate) of the linear function were compared to establish the importance of proteasome degradation and protein synthesis for MHC class I surface arrival during DC maturation (example shown in the top panel). Higher slopes indicate higher surface appearance of new peptide-loaded MHC I molecules. As expected, treatment of mDCs with the proteasome inhibitor MG132 decreased the MHC I recovery rate (MG, bottom left), whereas no difference was detected among 4 h and 16 h LPS-activated mDCs (bottom right). Inhibition of protein synthesis with CHX prevented the recovery of 4 h LPS-stimulated DC but not of 16 h stimulated DC (bottom right). Error bars indicate the SD of five independent experiments and the significance was determined by using t test (*, P < 0.05).
Origin of MHC class I ligands changes from newly synthesized to preexisting antigens during DC maturation
MHC class I restricted peptides are derived from rapidly degraded proteins such as DRiPs, and peptide generation is strongly dependent on protein synthesis (Princiotta et al., 2003; Qian et al., 2006a). The two phases of translation that we observed are thus likely to interfere directly with MHC class I presentation and consequently DC function. We therefore evaluated globally the generation of MHC class I/peptide complexes during DC maturation using acid stripping experiments followed by MHC class I/peptide recovery detected with the HB-176 mAb, which recognizes conformed H-2kb molecules independently of the nature of bound peptides (Qian et al., 2006b). We could confirm that proteasome activity is required for peptide-H-2kb recovery after acid stripping of the cells because the proteasome inhibitor MG132 abrogated the surface appearance of newly loaded MHC class I in maturing DCs (Fig. 8 B). Unexpectedly, we also observed that DCs during the reduced translation phase (16 h of LPS) had the same rate of MHC class I/peptide recovery than DCs during the enhanced translation phase (4 h of LPS). To further evaluate the direct contribution of protein synthesis to MHC class I loading, we compared recovery of MHC class I/peptide of acid-stripped DCs in the presence or absence of CHX (Fig. 8 B). Although translation inhibition had a strong impact on 4 h LPS-treated DCs, fully inhibiting peptide-H-2kb recovery, CHX had little effect on 16 h activated cells. The origin of the MHC class I/presented peptides is therefore changing dramatically during the course of LPS stimulation. Newly activated DCs are fully dependent on protein synthesis to generate MHC class I restricted peptides in agreement with the DRiPs hypothesis, while at later times of maturation DCs lose this dependency. It is tempting to speculate that these antigens are either exogenous antigens, which are cross-presented or stored DRiPs, which are released by DALIS during their breakdown induced by the protein synthesis inhibition. Thus translation regulation during maturation also has an important impact on antigen presentation by DCs.
Discussion
Our work provides an additional layer of analysis to the DC activation process. Although transcriptional regulation is necessary to acquire new functions, the regulation of gene expression at the level of translation provides the cell with the plasticity that is needed to respond to rapid changes in its environment. This has been shown recently in naive T helper-2 cells, which enter a stress response by blocking translation upon T cell receptor stimulation and retaining this state until restimulation (Scheu et al., 2006). Our results demonstrate that DC activation is divided in two distinct phases. A phase of protein synthesis increase during the first 4 h of DC maturation is followed by a phase of down-regulation involving cap-mediated translation inhibition. We have also provided examples of the importance of these two phases in DC function, illustrating how basic cellular processes such as regulation of mRNA translation can control cell differentiation and function.
Detection of LPS by TLR4 activates PI3K through the recruitment of the MyD88 and TRIF adaptors, leading to AKT and mTOR activation and finally ending by S6 protein phosphorylation by S6K1. This signal transduction pathway is also activated by growth factors in replicating cells. TLR signaling is therefore likely to control the cellular metabolism, in addition to its stimulating role on immunity. It is a puzzling observation because mDCs are not dividing and therefore do not need a high rate of protein synthesis to sustain proliferation. Putatively, a high rate of translation is required for achieving the massive cellular changes observed upon DC maturation such as MHC and costimulatory molecules up-regulation, as well as cytokine production. Interestingly, our observation that IL-12 secretion is prevented during PI3K inhibition by LY contrasts with previous results obtained by Fukao et al. (Fukao et al., 2002), in which DC treatment with wortmannin was found to enhance IL-12 production. We attribute this discrepancy to the use of wortmannin, which, at the concentration used, is less efficient than LY to inhibit PI3K in DCs (as shown in Fig. 2 B). Partial inhibition of the PI3K pathway could lead to potential compensation mechanisms able to promote IL-12 production.
The abrupt increase in protein synthesis is also likely to enhance markedly the proportion of DRiPs available for processing. In this context, DALIS probably represent an adapted stress response to these radical changes in protein synthesis and to the massive accumulation of DRiPs in such a short time lapse. DALIS are dependent on the signaling pathway inducing the translational boost, comprising the PI3K/AKT/mTOR signaling axis, which is key to achieve functional DC maturation. DALIS formation and disappearance follow the enhancement and inhibition of cap-mediated translation during DC maturation. By accumulating DRiPs, DALIS provide probably a mean to control the cytotoxic effects of these misfolded proteins, as well as their availability for degradation. Surprisingly, although the rate of surface arrival of peptide-loaded MHC class I does not fluctuate with maturation progression, the type of antigen presented changes dramatically. From fully dependent on newly synthesized antigens at early time of maturation, in agreement with the DRiPs hypothesis, MHC class I presentation switches to a pool of preexisting antigens at late stages of maturation. Thus, these antigens could be provided by decaying DALIS or alternatively they could be of exogenous origin and mostly cross-presented. In absence of a mean to control DALIS formation efficiently without affecting DC maturation, the elucidation of their exact function will remain unanswered. However, we could demonstrate that translation regulation affects the pool of antigen available for MHC class I presentation and that MHC class I restricted presentation is radically different in cells stimulated with LPS for different times.
A reduction in cap-dependent translation and a shift toward translation of certain IRES-containing mRNAs was observed after 8 h of maturation. Remarkably, this process is similar to the stress-induced attenuation of protein synthesis required for cell survival under harsh conditions. In addition to a mild phosphorylation of eIF2α, we observed a good correlation between eIF4GI and DAP5 proteolysis and the intensity of their translation during maturation. Moreover, protease inhibitors preventing eIF4GI and DAP5 cleavage had a strong inhibitory effect on the production of these molecules in a manner similar to protein synthesis inhibitors, thus definitely linking proteolysis and translation regulation. We could show that the protease(s) responsible for the cleavage of eIF4GI are different from the major known apoptotic caspases, such as caspase-3, which is responsible for translation inhibition during apoptosis. Based on MG132 effects and on a recent report (Baugh and Pilipenko, 2004), we further propose that proteasome activity could be responsible, at least in part, for the degradation of the translation initiation factors and, thus, exert a control on translation in mDCs. Interestingly, MG132 treatment is known to affect viral IRES activity (Baugh and Pilipenko, 2004) as well as to induce translation reprogramming of myoblasts (Cowan and Morley, 2004). The effect observed with the broad range inhibitor of caspases Z-VAD-FMK could be due either by inhibition of an additional unidentified protease or by inhibition of the caspase-like activity of the proteasome itself because a survey of eIF4GI primary sequence for sites potentially targeted by the proteasome caspase-like activity (Kisselev et al., 2003) indicates the presence of several consensus sites in areas matching eIF4GI cleavage map.
Cell death appears to be a normal response to counter-balance the activation of the proliferation machinery (including protein synthesis enhancement). The establishment of anti-apoptotic conditions during DC maturation seems dependent, at least in part, on the switch in the quality of translation induced by proteolytic cleavage of eIF4GI and DAP5. LPS detection by DCs is therefore integrated in a binary response containing a growth factor-like and a stress-like phase. This response is adapted to the need for rapid changes in the physiology of DCs after pathogen detection. Marked up-regulation of protein synthesis probably favors apoptosis through the accumulation of misfolded proteins. DCs therefore take advantage of the second phase to enhance their survival rate and augment their capacity to interact with other cells during their migration. Whether this phase is directly triggered by LPS or indirectly by an autocrine cytokine loop remains to be evaluated. IL-6 could cause some of these changes because it has been shown to favor some IRES-mediated translation (Yamagiwa et al., 2004).
Materials and methods
Cell culture
Male C57BL/6 mice 7–8 wk old were purchased from Charles River Laboratories. MyD88 and TRIF−/− mice were obtained from L. Alexopoulou (CIML, Marseille, France). Bone marrow–derived DCs were cultured as described previously (Lelouard et al., 2004). Maturation was induced using 100 ng/ml LPS or 20 μg/ml poly I:C.
Chemicals
All chemicals were purchased from Sigma-Aldrich, except Z-VAD-FMK, Z-DEVD-FMK, Ac-YVAD-CMK, and Ac-LEHD-FMK (from Bachem); Z-IETD-FMK, Z-WEHD-FMK, rapamycin, and LY294002 (from Calbiochem); and MG132 (from BIOMOL International, L.P.). iDCs were treated with 100 μM LY294002 or 1 mM rapamycin for 1 h before LPS treatment. Wortmannin was used at 1 μM for 30 min before activation. 0.5 mM sodium arsenite was added for 30 min to iDCs or to 16 h LPS-treated DCs. 10–80 μM Z-VAD-FMK; 0.5–2 μM MG132; 2 μM LHVS (gift of H. Ploegh, Massachusetts Institute of Technology, Cambridge, MA); 100 μM Z-DEVD-FMK, Ac-YVAD-CMK, Ac-LEHD-FMK; 40 μM Z-IETD-FMK, Z-WEHD-FMK; 25 μM CHX; 50 μM etoposide; or 0.2–1 μM staurosporin was added for 10 h to iDC or to 6 h LPS-treated DC.
Immunoblotting
50 μg of Triton X-100–soluble material was loaded on 2–12% gradient SDS-PAGE before immunoblotting and chemiluminescence detection (Pierce Chemical Co.). Polyclonal antibodies against eIF4A, Nt eIF4GI, and Nt DAP5 were from Santa Cruz Biotechnology, Inc.; polyclonal antibodies against phospho-S6, phospho-S6K1, S6, phospho-AKT, eIF2α, and Ct DAP5 were from Cell Signaling Technology; polyclonal antibody against phospho-eIF2α was from Invitrogen. Polyclonal antibodies specific for eIF4GI peptides P7, P8, and P9 were a gift of R. Rhoads (Louisiana State University, Baton Rouge, LA). Others polyclonal antibodies specific for eIF4GI and eIF4GII were gifts of S.J. Morley (University of Sussex, Brighton, UK). Secondary antibodies were from Jackson ImmunoResearch Laboratories and from Invitrogen. Immunofluorescence and confocal microscopy was performed with a microscope (LSM 510; Carl Zeiss, Inc.) using a 63× objective and accompanying imaging software.
MHC class I surface recovery by acid striping assay
5 × 106 cells were washed in PBS/0.1%BSA and resuspended in 0.5 ml of 0.2 M citric acid/0.2 M Na2HPO4 buffer (pH 3.0) and incubated on ice for 2 min. The cell suspension was neutralized by adding excess of ice-cold PBS/BSA and after centrifugation cells were immediately stained for FACS analysis or for recovering resuspended in LPS-free medium with or without drugs. Cell surface Kb molecules were measured by FACS after staining with HB176 (gift of Günter Hämmerling and Franck Momburg, Heidelberg).
m7G-cap binding assay
500 μg of soluble fraction were diluted in 300 μl lysis buffer and incubated with 20 μl packed 7-Methyl GTP-Sepharose 4B (GE Healthcare) for 2 h at 4°C. After washing with lysis buffer, the beads were applied to SDS-PAGE and immunoblot was performed as above.
Plasmids and in vitro transcription
Constructs are in a pBluescript-KS (Stratagene) vector. Plasmids encoding firefly luciferase, pT3LUC(pA), was the gift of M. Hentze (EMBL, Heidelberg). 20 μg plasmid DNA were linearized and purified with the QIAquick PCR purification KIT (QIAGEN). 10 μl linearized DNA were in vitro transcribed in the presence of either 7mGpppG or ApppG (Ambion) using the Riboprobe in vitro Transcription System-T3 (Promega). In vitro transcribed mRNA was purified using the RNeasy Mini kit (QIAGEN).
mRNA transfection
iDCs were transfected on d 5 of culture. Before transfection, iDCs were washed twice with ice-cold PBS. DCs were then adjusted to a final cell density of 20 × 106 cells/ml. 200 μl of the cell suspension (4 × 106 cells) was preincubated in a 4-mm gap electroporation cuvette (Bio-Rad Laboratories) for 5 min on ice. In vitro–transcribed mRNA (5 μg) was added to the cell suspension and cells were pulsed with a Gene Pulser II apparatus (Bio-Rad Laboratories), using a voltage of 500 V, a capacitance of 50 μF, and pulse times ranging from 2 to 4 ms. After electroporation, cells were immediately resuspended in fresh prewarmed culture medium. After 4 h, cells were harvested and luciferase activity was determined with a Luciferase Reporter Assay System (Promega) in a Wallace 1420 multilabel counter VICTOR2.
Online supplemental material
Figures presenting the effect of MyD88 and TRIF deficiency on DC maturation, the cap-binding activity of eIF4GI fragments and DAP5 isoforms expression in DCs are displayed as supplementary figures.Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200707166/DC1.
Supplemental Material
Acknowledgments
We are particularly indebted to Robert Rhoads, Simon Morley, Donald Degracia, Matthias Hentze, and Frank Momburg for antibody and reagents gift. We also thank for expert technical assistance the PICsL imaging core facility.
This work is supported by grants to PP from the Ministère de la Recherche et de la Technologie (ACI BCMS), La Ligue Nationale Contre le Cancer and the Human Frontier of Science Program. EKS is supported by the MRT. MC is supported by the Swiss National Research Fund. PP is part of the EMBO Young Investigator Program and of the DC-THERA FP6 NoE.
H. Lelouard and E.K. Schmidt contributed equally to this paper.
Abbreviations used in this paper: CHX, cycloheximide; DALIS, dendritic cell aggresome-like induced structures; DC, dendritic cell; iDC, immature DC; IRES, internal ribosome entry site; LPS, lipopolysaccharide; mDC, maturing DC; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide-3-kinase; TRIF, Toll-IL-1 receptor domain-containing adaptor-inducing IFN-β.
References
- Alvarez, E., L. Menendez-Arias, and L. Carrasco. 2003. The eukaryotic translation initiation factor 4GI is cleaved by different retroviral proteases. J. Virol. 77:12392–12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, P., and N. Kedersha. 2002. Stressful initiations. J. Cell Sci. 115:3227–3234. [DOI] [PubMed] [Google Scholar]
- Aoki, M., E. Blazek, and P.K. Vogt. 2001. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc. Natl. Acad. Sci. USA. 98:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbibe, L., J.P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P.J. Godowski, R.J. Ulevitch, and U.G. Knaus. 2000. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 1:533–540. [DOI] [PubMed] [Google Scholar]
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- Baugh, J.M., and E.V. Pilipenko. 2004. 20S proteasome differentially alters translation of different mRNAs via the cleavage of eIF4F and eIF3. Mol. Cell. 16:575–586. [DOI] [PubMed] [Google Scholar]
- Bushell, M., D. Poncet, W.E. Marissen, H. Flotow, R.E. Lloyd, M.J. Clemens, and S.J. Morley. 2000. Cleavage of polypeptide chain initiation factor eIF4GI during apoptosis in lymphoma cells: characterisation of an internal fragment generated by caspase-3-mediated cleavage. Cell Death Differ. 7:628–636. [DOI] [PubMed] [Google Scholar]
- Byrd, M.P., M. Zamora, and R.E. Lloyd. 2005. Translation of eukaryotic translation initiation factor 4GI (eIF4GI) proceeds from multiple mRNAs containing a novel cap-dependent internal ribosome entry site (IRES) that is active during poliovirus infection. J. Biol. Chem. 280:18610–18622. [DOI] [PubMed] [Google Scholar]
- Cowan, J.L., and S.J. Morley. 2004. The proteasome inhibitor, MG132, promotes the reprogramming of translation in C2C12 myoblasts and facilitates the association of hsp25 with the eIF4F complex. Eur. J. Biochem. 271:3596–3611. [DOI] [PubMed] [Google Scholar]
- Fingar, D.C., C.J. Richardson, A.R. Tee, L. Cheatham, C. Tsou, and J. Blenis. 2004. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol. Cell. Biol. 24:200–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao, T., M. Tanabe, Y. Terauchi, T. Ota, S. Matsuda, T. Asano, T. Kadowaki, T. Takeuchi, and S. Koyasu. 2002. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 3:875–881. [DOI] [PubMed] [Google Scholar]
- Gebauer, F., and M.W. Hentze. 2004. Molecular mechanisms of translational control. Nat. Rev. Mol. Cell Biol. 5:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras, A.C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913–963. [DOI] [PubMed] [Google Scholar]
- Henis-Korenblit, S., N.L. Strumpf, D. Goldstaub, and A. Kimchi. 2000. A novel form of DAP5 protein accumulates in apoptotic cells as a result of caspase cleavage and internal ribosome entry site-mediated translation. Mol. Cell. Biol. 20:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter, S., P. Osterloh, N. Hilf, G. Rechtsteiner, J. Hohfeld, H.G. Rammensee, and H. Schild. 2005. Dendritic cell aggresome-like-induced structure formation and delayed antigen presentation coincide in influenza virus-infected dendritic cells. J. Immunol. 175:891–898. [DOI] [PubMed] [Google Scholar]
- Holcik, M., and N. Sonenberg. 2005. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 6:318–327. [DOI] [PubMed] [Google Scholar]
- Hou, W.S., and L. Van Parijs. 2004. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat. Immunol. 5:583–589. [DOI] [PubMed] [Google Scholar]
- Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, et al. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 17:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78–83. [DOI] [PubMed] [Google Scholar]
- Kisselev, A.F., M. Garcia-Calvo, H.S. Overkleeft, E. Peterson, M.W. Pennington, H.L. Ploegh, N.A. Thornberry, and A.L. Goldberg. 2003. The caspase-like sites of proteasomes, their substrate specificity, new inhibitors and substrates, and allosteric interactions with the trypsin-like sites. J. Biol. Chem. 278:35869–35877. [DOI] [PubMed] [Google Scholar]
- Lelouard, H., E. Gatti, F. Cappello, O. Gresser, V. Camosseto, and P. Pierre. 2002. Transient aggregation of ubiquitinated proteins during dendritic cell maturation. Nature. 417:177–182. [DOI] [PubMed] [Google Scholar]
- Lelouard, H., V. Ferrand, D. Marguet, J. Bania, V. Camosseto, A. David, E. Gatti, and P. Pierre. 2004. Dendritic cell aggresome-like induced structures are dedicated areas for ubiquitination and storage of newly synthesized defective proteins. J. Cell Biol. 164:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. [DOI] [PubMed] [Google Scholar]
- Morley, S.J., M.J. Coldwell, and M.J. Clemens. 2005. Initiation factor modifications in the preapoptotic phase. Cell Death Differ. 12:571–584. [DOI] [PubMed] [Google Scholar]
- Nevins, T.A., Z.M. Harder, R.G. Korneluk, and M. Holcik. 2003. Distinct regulation of internal ribosome entry site-mediated translation following cellular stress is mediated by apoptotic fragments of eIF4G translation initiation factor family members eIF4GI and p97/DAP5/NAT1. J. Biol. Chem. 278:3572–3579. [DOI] [PubMed] [Google Scholar]
- Pierre, P. 2005. Dendritic cells, DRiPs, and DALIS in the control of antigen processing. Immunol. Rev. 207:184–190. [DOI] [PubMed] [Google Scholar]
- Pierre, P., S.J. Turley, E. Gatti, M. Hull, J. Meltzer, A. Mirza, K. Inaba, R.M. Steinman, and I. Mellman. 1997. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 388:787–792. [DOI] [PubMed] [Google Scholar]
- Prevot, D., J.L. Darlix, and T. Ohlmann. 2003. Conducting the initiation of protein synthesis: the role of eIF4G. Biol. Cell. 95:141–156. [DOI] [PubMed] [Google Scholar]
- Princiotta, M.F., D. Finzi, S.B. Qian, J. Gibbs, S. Schuchmann, F. Buttgereit, J.R. Bennink, and J.W. Yewdell. 2003. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 18:343–354. [DOI] [PubMed] [Google Scholar]
- Qian, S.B., M.F. Princiotta, J.R. Bennink, and J.W. Yewdell. 2006. a. Characterization of rapidly degraded polypeptides in mammalian cells reveals a novel layer of nascent protein quality control. J. Biol. Chem. 281:392–400. [DOI] [PubMed] [Google Scholar]
- Qian, S.B., E. Reits, J. Neefjes, J.M. Deslich, J.R. Bennink, and J.W. Yewdell. 2006. b. Tight linkage between translation and MHC class I peptide ligand generation implies specialized antigen processing for defective ribosomal products. J. Immunol. 177:227–233. [DOI] [PubMed] [Google Scholar]
- Raught, B., A.C. Gingras, S.P. Gygi, H. Imataka, S. Morino, A. Gradi, R. Aebersold, and N. Sonenberg. 2000. Serum-stimulated, rapamycin-sensitive phosphorylation sites in the eukaryotic translation initiation factor 4GI. EMBO J. 19:434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero, D., and N. Sonenberg. 2005. The Akt of translational control. Oncogene. 24:7426–7434. [DOI] [PubMed] [Google Scholar]
- Scheu, S., D.B. Stetson, R.L. Reinhardt, J.H. Leber, M. Mohrs, and R.M. Locksley. 2006. Activation of the integrated stress response during T helper cell differentiation. Nat. Immunol. 7:644–651. [DOI] [PubMed] [Google Scholar]
- Spriggs, K.A., M. Bushell, S.A. Mitchell, and A.E. Willis. 2005. Internal ribosome entry segment-mediated translation during apoptosis: the role of IRES-trans-acting factors. Cell Death Differ. 12:585–591. [DOI] [PubMed] [Google Scholar]
- von Manteuffel, S.R., P.B. Dennis, N. Pullen, A.C. Gingras, N. Sonenberg, and G. Thomas. 1997. The insulin-induced signalling pathway leading to S6 and initiation factor 4E binding protein 1 phosphorylation bifurcates at a rapamycin-sensitive point immediately upstream of p70s6k. Mol. Cell. Biol. 17:5426–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagiwa, Y., C. Marienfeld, F. Meng, M. Holcik, and T. Patel. 2004. Translational regulation of x-linked inhibitor of apoptosis protein by interleukin-6: a novel mechanism of tumor cell survival. Cancer Res. 64:1293–1298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.