Abstract
The antipsychotic drug, haloperidol, elicits the expression of neurotensin and c-fos mRNA in the dorsal lateral region of the striatum and produces an acute cataleptic response in rodents that correlates with the motor side effects of haloperidol in humans. Mice harboring a targeted disruption of the RIIβ subunit of protein kinase A have a profound deficit in cAMP-stimulated kinase activity in the striatum. When treated with haloperidol, RIIβ mutant mice fail to induce either c-fos or neurotensin mRNA and the acute cataleptic response is blocked. However, both wild-type and mutant mice become cataleptic when neurotensin peptide is directly injected into the lateral ventricle, demonstrating that the kinase deficiency does not interfere with the action of neurotensin but rather its synthesis and release. These results establish a direct role for protein kinase A as a mediator of haloperidol induced gene induction and cataleptic behavior.
Antipsychotic drugs are a group of chemically diverse compounds that are used in the treatment of severe psychiatric disorders. The efficacy of haloperidol, the prototypic antipsychotic agent, in the treatment of the symptoms of schizophrenia is thought to result, at least in part, from its ability to bind to and antagonize dopamine D2-like receptors (1, 2) leading to an increase in cAMP (3). However, the pathways that connect the changes in cAMP levels to either therapeutic or toxic effects remain unclear.
Medium spiny neurons in the striatum express either D1 or D2 receptors (4). The D1 receptor is coupled via Gs to a stimulation of cAMP synthesis whereas the D2 receptor is Gi coupled and could lead to an inhibition of adenylate cyclase activity and a decrease in intracellular cAMP. D2 receptors are also coupled to the activation of K+ channels via the βγ subunits (5). Acute haloperidol administration leads to changes in neuropeptide gene expression in the striatum. One of the genes affected is that coding for neurotensin (NT), a tridecapeptide that is heterogeneously distributed in the central nervous system of many mammals including humans, where it functions as a neurotransmitter or neuromodulator (6, 7). Several lines of evidence suggest that NT may play an important role in the etiology and/or pharmacotherapy of schizophrenia and other neuropsychiatric disorders. NT regulates the dopaminergic neuronal systems, increasing the firing rate of nigrostriatal neurons as well as attenuating dopamine autoinhibition of nigrostriatal neurons. NT levels have been reported reduced in the cerebrospinal fluid of drug-free schizophrenic patients, but return to normal levels following haloperidol treatment (8).
Previous studies have shown that 7 h following a single dose of haloperidol, a dramatic increase in NT mRNA occurs in neurons of the dorsolateral striatum (DLST) of the rat brain, a component of the basal ganglia circuitry that has been implicated in the regulation of motor output (9, 10). Haloperidol administration also leads to the induction of c-fos mRNA within 30 min in DLST neurons (9). The promoter of the NT gene contains two putative AP-1 sites (11) where the binding of Fos-containing complexes might enhance the transcription of the NT gene.
In rodents, centrally administered NT also produces behavioral effects similar to those seen with clinically used neuroleptics such as haloperidol (8, 12, 13). One of those behavioral effects is catalepsy, which is manifest as an impaired ability to initiate movement. This cataleptic response in rodents is thought to represent a correlate of the acute extrapyramidal motor side effects (e.g., dystonia, parkinsonism) seen in humans following antipsychotic drug administration (14). Based on these and other neurochemical findings it has been postulated that NT plays a role as an endogenous neuroleptic (15).
The induction of c-fos and NT mRNA in the DLST as well as the cataleptic behavior are thought to be downstream consequences of haloperidol’s antagonism of dopamine D2 receptors and could result from increases in intracellular levels of cAMP and the activation of protein kinase A (PKA). The PKA holoenzyme is composed of a regulatory subunit dimer and two catalytic subunits. The regulatory subunits bind cAMP and release the active catalytic subunits that enter the nucleus and phosphorylate cAMP response element binding protein and other related transcription factors. Multiple isoforms of regulatory (RIα, RIβ, RIIα, RIIβ) and catalytic (Cα and Cβ) subunits are expressed in the mammalian brain and all are encoded by separate genes. Of these, the RIIβ subunit is the predominant regulatory subunit of PKA expressed in the striatum and has been shown to anchor the holoenzyme to the dendritic cytoskeleton (16, 17).
In the present study, mice bearing a targeted disruption of the RIIβ subunit gene of PKA were used to evaluate the importance of cAMP-dependent signaling in transducing the effects of haloperidol on striatal gene expression and behavioral responses. The construction of the RIIβ targeting vector and the generation of the RIIβ−/− mice will be described elsewhere (E.P.B. and R.L.I., unpublished data). We find that in mice lacking the RIIβ subunit, haloperidol fails to induce c-fos or NT gene expression in the DLST following haloperidol treatment. These mice are also unable to exhibit a cataleptic response to haloperidol, but they continue to respond to intracerebral injections of NT peptide. We conclude that the activation of the RIIβ isoform of PKA is required for the acute actions of haloperidol on both gene expression and behavior.
MATERIALS AND METHODS
Immunoblots.
All animals were 6–10 weeks old and were housed in a temperature controlled room on a 12-h dark/light cycle with free access to food and water. Immunoblot analysis of DLST homogenates from RIIβ mutant mice (−/−) and their wild-type controls (+/+) used antisera that specifically recognizes RIIβ, RIα, RIβ, or C. Protein for immunoblots was prepared from the DLST and probed with antisera to murine PKA subunits as described (18, 19).
Kinase Assay.
Kinase activity was assayed on cell homogenates as described (20) using Kemptide (21) as a substrate in the presence or absence of 5 μM cAMP. Residual activity in the presence of 4 μg/ml PKI (protein kinase inhibitor) peptide was subtracted.
In Situ Hybridization.
In situ hybridization was conducted as described (22). To generate a probe, a 450-bp fragment of the mouse NT cDNA was inserted into pGEM7Zf (+) vector kindly provided to us by Vaughn M. Gehle (University of Colorado). Antisense riboprobe for in situ hybridization was transcribed from BglII-linearized plasmid using SP6 RNA polymerase to generate a probe labeled with [35S]UTP. Sense controls were performed, and no specific signal was detected. A sense probe was generated from an EcoRV-linearized plasmid using T7 RNA polymerase. To generate a probe to detect c-fos mRNA an EcoRI/SalI fragment (nt 1–438) of c-fos cDNA was subcloned into pGEM3Z (Promega). Antisense riboprobe for in situ hybridization was transcribed from AvaI-linearized plasmid using SP6 RNA polymerase to generate a 294-bp fragment labeled with [35S]UTP. Sense controls were performed and no specific signal was detected. A sense probe was also synthesized from a HindIII-linearized plasmid using T7 RNA polymerase. In situ hybridization was carried out as described for NT mRNA with the hybridization temperature at 59°C and the wash temperature at 60°C.
Dopamine D2 Receptor Binding.
125I-labeled sulpride (2000 Ci/mmol; Amersham; 1 Ci = 37 GBq) was used to label dopamine D2 receptors according to the methods of Bouthenet et al. (23) with minor modifications. Brain sections adjacent to those used in in situ hybridization studies were used in this experiment. Sections were incubated for 45 min at room temperature with 250 μl of 50 mM Tris⋅HCl buffer (pH 7.4) containing 120 mM NaCl, 5 mM KCl, 1 mM CaCl2, 5.7 mM ascorbic acid, 0.01 mM 8-hydroxyquinoline, and 1 nM [125I]sulpride. Slides were subsequently washed in buffer at 4°C (two washes each of 2 min), dipped in deionized water, and dried promptly using a stream of cool dry air. Nonspecific binding, defined as binding in the presence of 10−6 M haloperidol, was less than 5% of the total binding. Labeled slides were stored overnight at 4°C in the presence of a desiccant and then apposed to hyperfilm βmax (Amersham) along with plastic standards containing known concentrations of 125I. After developing films, the density of [125I]sulpride binding to dopamine D2 receptors was determined using a microcomputer-based image analysis system (Image Research, St. Catherine’s, Ontario, Canada).
Behavioral Studies.
To measure catalepsy the animal was placed up against the side of the cage and care was taken not to touch the hindquarters. Catalepsy was scored when the animal maintained a fixed rearing posture against the side of the cage for a minimum of 20 s. For the intercerebroventricular (i.c.v.) injections, the peptide was injected into the right lateral ventricle through a cannula with a Hamilton microsyringe. The peptides were dissolved in saline, and 10 μg of peptide was injected in 3 μl. To verify ventricular delivery 3 μl of bromophenol blue was injected and its appearance in the right and left ventricles was confirmed.
RESULTS
Absence of RIIβ Protein and Reduction of PKA Activity in the DLST of RIIβ−/− Mice.
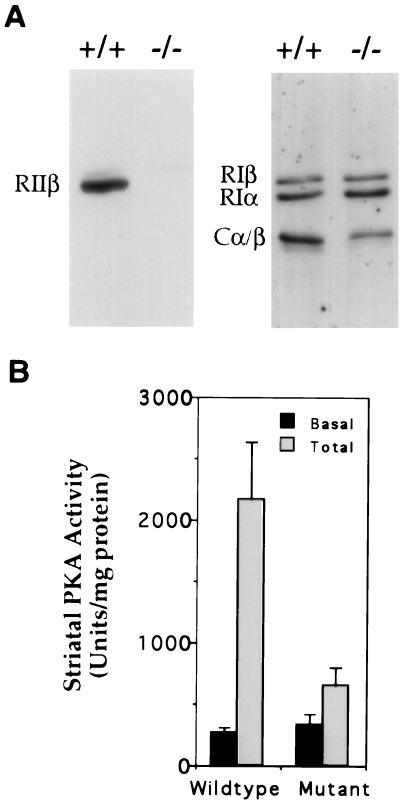
The complete absence of immunoreactive RIIβ protein in striatal extracts from mutant mice is shown in immunoblots using antisera directed against RIIβ in Fig. 1A. A modest increase was observed in both RIα and RIβ regulatory subunits, but the total level of C subunit declined dramatically. This led to a 75% decrease in cAMP-stimulated PKA activity with little change in basal activity (Fig. 1B). The loss of C subunit and PKA activity in the RIIβ−/− mice is likely due to instability of C subunit in the absence of R (24, 25).
Figure 1.
Absence of RIIβ protein and reduction of PKA activity in the DLST of RIIβ−/− mice. (A) Immunoblot analysis of DLST homogenates from RIIβ mutant (−/−) mice and their wild-type controls (+/+) using antisera that specifically recognizes RIIβ, RIα, RIβ, or C. (B) PKA activity was measured in the striatum of wild-type controls and mutant RIIβ mice. Each bar represents PKI inhibitable activity ± SD (n = 3).
Loss of Transcriptional Responsiveness to Haloperidol in RIIβ−/− Mice.
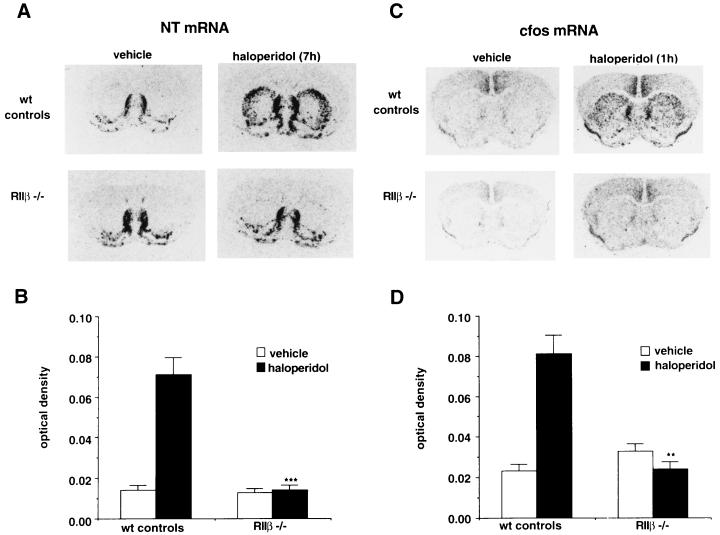
As expected, the basal expression of NT mRNA in the DLST was minimal in vehicle-treated animals in both the wild-type controls and the RIIβ−/− mice (Fig. 2 A and B). Consistent with previous experiments, haloperidol induces NT mRNA in the DLST of wild-type mice as shown by in situ hybridization. However, the induction of NT mRNA in response to haloperidol was completely absent in RIIβ−/− mice (Fig. 2 A and B). Expression of NT mRNA is unaffected in brain regions of RIIβ−/− mice where basal expression is higher than that of the striatum (e.g., septum, nucleus accumbens). To examine the possibility that the lack of NT mRNA induction was the result of a shift in the haloperidol dose–response curve in the RIIβ−/− mice, 2 mg/kg and 4 mg/kg of haloperidol were administered to the mice. The higher doses of haloperidol also failed to induce NT mRNA in the DLST of the RIIβ−/− mice (data not shown). When measured 7 h after injection, no significant difference was noted in circulating haloperidol levels in the plasma of RIIβ−/− mice and wild-type mice (data not shown). There results demonstrate a qualitative disruption of the haloperidol response in RIIβ−/− mice and suggest that the loss of PKA activity interrupts the drug response pathways.
Figure 2.
Induction of NT mRNA by haloperidol in the striatum. (A) In situ hybridization depicting the expression of NT mRNA 7 h following either vehicle or haloperidol (1 mg/kg, i.p.) treatment in wild-type (wt) controls (n = 12 and 23, vehicle and haloperidol, respectively) and RIIβ−/− (n = 7 and 11, vehicle and haloperidol, respectively). (B) Quantitation of the NT mRNA hybridization signal in the DLST was carried out by densitometric analysis. Each bar represents mean film optical density ± SEM. Statistical differences were determined using ANOVA; ∗∗∗, P < 0.001. (C) In situ hybridization for c-fos mRNA expression in the striatum 1 h following either vehicle or haloperidol treatment in wild-type controls (n = 8 and 9, vehicle and haloperidol, respectively) and RIIβ−/− (n = 4 and 4, vehicle and haloperidol, respectively) mice. (D) Graphical representation of the c-fos mRNA hybridization signal in the DLST using densitometric analysis. Each bar represents mean optical density ± SEM. Statistical differences were determined using ANOVA; ∗∗, P < 0.01.
Haloperidol leads to a dramatic induction of c-fos mRNA in the DLST, which peaks 1 h following haloperidol administration in rats (9). To examine the ability of haloperidol to induce c-fos mRNA in the mice, animals were treated with haloperidol and 1 h later c-fos mRNA was examined in the DLST. As shown in Fig. 2 C and D, haloperidol leads to induction of c-fos mRNA in the DLST of wild-type mice but failed to induce c-fos mRNA in the RIIβ−/− mice (Fig. 2 C and D). Thus, both an immediate early gene induction (c-fos) and a delayed gene induction (NT) are blocked in the RIIβ−/− mice. Previous studies have implicated c-fos as a regulator of the NT promoter (9), and our results are consistent with this idea.
Dopamine and D2 Receptors Are Unchanged in RIIβ−/− Mice.
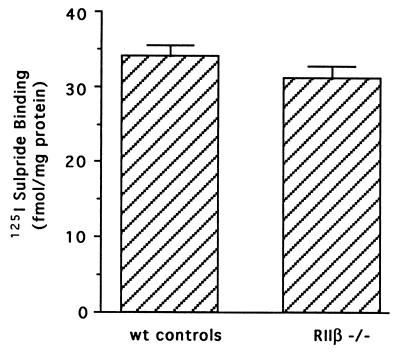
Because haloperidol acts by antagonizing dopamine D2 receptors, we wanted to be certain that striatal density of D2 receptors was comparable in the RIIβ−/− mice and wild-type mice. Therefore the density of D2-like receptors was examined in mice using [125I]sulpride, a well-characterized ligand for the D2 family of receptors (primarily D2 and D3). There was no difference in striatal D2-like receptor density noted in RIIβ−/− mice compared with the wild-type controls (Fig. 3), and striatal dopamine content was also unaffected in RIIβ−/− mice (E.P.B. and M.R.A., unpublished data). Thus, the lack of c-fos and NT mRNA induction by haloperidol in the RIIβ−/− mice does not result from differences in either dopamine or dopamine receptor levels but reflects a perturbation in overall signaling due to the loss of PKA.
Figure 3.
The density of dopamine D2 receptors are normal in the striatum of RIIβ−/− mice. [125I]Sulpride binding in the striatum of wild-type controls and RIIβ−/− mice was determined by quantitative autoradiography using 125I standards to calibrate the optical density and convert to tissue equivalent ligand concentrations. Each bar represents specific binding of [125I]sulpride ±SEM.
RIIβ−/− Mice Are Behaviorally Unresponsive to Haloperidol.
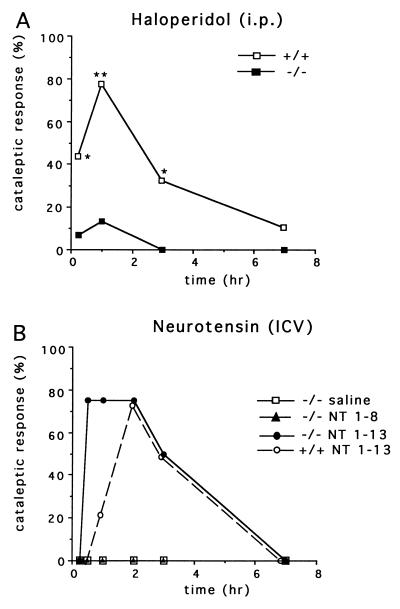
After determining that striatal neurons of RIIβ−/− mice are transcriptionally unresponsive to haloperidol, we next determined whether these animals could still exhibit a behavioral response. In previous experiments it has been shown that haloperidol produces a cataleptic response in rodents. The efficacy of haloperidol in eliciting cataleptic behavior in mutant and control mice was assessed by placing animals in a rearing position against the side of the cage. If the mouse maintained this posture for >20 s, it was scored as cataleptic. As expected, none of the animals displayed a cataleptic response after saline injection (data not shown). About 80% of wild-type mice became cataleptic at 1 h after injection of 4 mg/kg haloperidol. However, the cataleptic response of RIIβ−/− mice to haloperidol administration is severely attenuated (Fig. 4A). Because it has been shown in previous experiments that NT administered i.c.v. also produces catalepsy in rodents, we examined the cataleptic response to NT in the RIIβ−/− mice. Interestingly, the RIIβ−/− mice are fully able to respond to injected NT peptide and in fact respond at earlier time points than wild-type mice, suggesting an augmented sensitivity. Fig. 4B shows that 10 μg of active NT peptide (NT 1–13) given i.c.v. elicits catalepsy in 75% (3 of 4 mice) of RIIβ−/− mice within 30 min of administration, whereas saline and an inactive NT fragment (NT 1–8) produced no response. In wild-type mice, comparable rates of catalepsy (75%, 3 of 4) were not seen until 2 h after injection of NT peptide. The increased sensitivity to NT in RIIβ−/− mice could be due to a chronic loss of NT release at nerve terminals and a subsequent compensatory increase in receptor-coupled signaling. Our results demonstrate that the RIIβ mutation interrupts the drug-induced cataleptic pathway upstream of the NT receptor and further suggests that released NT may be directly responsible for the cataleptic response.
Figure 4.
The cataleptic response to haloperidol is significantly attenuated in RIIβ−/− mice, but they remain responsive to centrally administered NT. (A) Catalepsy was measured 15 min, 1 h, 3 h, and 7 h following administration of haloperidol (4 mg/kg, i.p.) in wild-type controls (n = 9) and RIIβ−/− (n = 15). Graph represents the percent of mice exhibiting catalepsy. Comparisons between groups were determined using Mann–Whitney U test; ∗, P < 0.05; ∗∗, P < 0.01. (B) Catalepsy was measured in RIIβ−/− (n = 4) mice at various times following administration of active NT 1–13, inactive NT 1–8, or saline. Wild-type mice (n = 4) were injected with the active NT 1–13 as a control.
DISCUSSION
The results of the present study point to a critical role of cAMP-dependent signaling in the transcriptional and behavioral responses to haloperidol that are believed to involve the motor striatum. Specifically, RIIβ−/− mice have an impaired ability to transduce cAMP-dependent signals in the striatum and consequently fail to induce c-fos and NT gene expression in the DLST following haloperidol treatment. This deficit is likely to be due to the loss of RIIβ PKA in the striatum but because RIIβ is also expressed in other brain regions including the cortex, hippocampus, and hypothalamus, we cannot rule out defects in these other brain regions that may affect the phenotype. Mice bearing mutations in either the Cβ or RIβ subunits of PKA maintain PKA activity in the striatum and exhibit normal transcriptional and behavioral responses to haloperidol (data not shown). The failure of haloperidol to induce NT gene transcription in the RIIβ−/− mice may be partially dependent on the loss of the earlier c-fos induction. Interference with c-fos expression using antisense oligonucleotides reduces haloperidol-induced NT gene expression in rat DLST (26) and an AP-1 binding site has been characterized in the NT gene promoter (27). The potential role of PKA as a cAMP response element binding protein (CREB) kinase and regulator of c-fos gene transcription has been well documented by others (28), although it is important to keep in mind that both calmodulin-regulated kinases and components of the mitogen-activated protein kinase pathway can also phosphorylate CREB and lead to c-fos induction (29, 30).
It appears that antipsychotic drugs mediate their extrapyramidal motor side effects by antagonizing D2 receptors in the dorsal lateral region of the striatum. These effects are likely to involve PKA activation because D2 receptor antagonists mediate at least some of their effects by allowing intracellular levels of cAMP to increase (3). Although haloperidol is not completely specific for D2 receptors, we believe that the effects we are observing are D2 receptor-mediated for the following reasons. First, we have shown that irreversible antagonism of D2 receptors with an agent such as n-ethoxycarbonyl-2-ethoxy-1–2-dihydroxyquinolone (EEDQ) nearly abolishes the effects of haloperidol on induction of NT gene expression (31). Second, we have tested a more specific D2 receptor antagonist, raclopride, and find essentially the same results as seen with haloperidol. The RIIβ mutant mice are resistant to raclopride’s effects on both NT expression and the induction of catalepsy (S. Sullivan and G.S.M., unpublished observations). Third, a D2 receptor mutant mouse has been created and found to be resistant to haloperidol-induced catalepsy (M. Low, Vollum Institute, Portland OR, personal communication) substantiating the role of the D2 receptor in the motor side effects of haloperidol. The loss of NT responsiveness to haloperidol in the RIIβ−/− mice cannot be attributed to a reduction in the density of D2 receptors since iodosulpride binding indicated that D2 receptor levels were unchanged.
The dopamine-responsive neurons in the striatum include those expressing either D2 or D1 receptors and pharmacological studies have indicated a strong interaction between the D2 and D1 pathways in the regulation of locomotor and behavioral responses (32). D1 antagonists will also produce catalepsy in rodents and our preliminary studies indicate that the D1 antagonist, SCH23390, produces catalepsy in both wild-type and RIIβ mutant mice (S. Sullivan and G.S.M., unpublished observations). These results indicate that the RIIβ mutation does not preclude a cataleptic response to pharmacological agents but that it specifically interrupts the actions of D2 receptor antagonists.
What is the relationship between the loss of haloperidol-induced NT gene transcription and the acute lack of cataleptic behavior? The cataleptic behavior begins within 15 min of haloperidol administration whereas NT gene transcription does not reach a maximum until 6–7 h later, suggesting that the behavioral response depends upon release of presynthesized NT followed by restoration of NT stores. Our hypothesis is that in RIIβ−/− mice, normal transcription of the NT gene is suppressed in D2 receptor containing neurons in the DLST. This leads to a reduction in NT peptide available for secretion at nerve terminals in regions such as the globus pallidus. Whether the disruption of PKA in the D2 receptor-containing neurons also affects the ability of dopamine to modulate nerve firing and release of γ-aminobutyric acid requires further study.
The predominant output of the striatum is extensively modulated by dopaminergic inputs. Haloperidol binds to dopamine D2 receptors, relieves the negative inhibition on adenylate cyclase, and is likely to unmask the influence of receptors coupled to Gs-mediated activation of adenylate cyclase. When viewed in this light, it becomes important to determine which receptors colocalize with dopamine D2 receptor positive cells and may be coupled to an increase in cAMP. Candidate receptors for which evidence of colocalization exist include 5HT6 and 5HT4 receptors (33) and the adenosine A2 receptor (34, 35). Blockade of adenosine A2 receptors can prevent haloperidol-induced catalepsy and NT gene expression in the DLST in rats (R. Ward and D.M.D., unpublished data).
The RIIβ−/− mice provide a genetic model in which it is possible to trace a drug–receptor interaction through associated intracellular signaling mechanisms that lead to a downstream behavioral response. The data show that PKA plays an essential role in mediating antipsychotic drug effects involving the motor striatum, and this understanding may lead to novel approaches to attempt to minimize the extrapyramidal side effects observed with many of these antipsychotic drugs. These findings also raise the possibility that certain forms of antipsychotic drug resistance could result from alterations in the various components of the cAMP signal transduction pathway.
Acknowledgments
We thank Cong Xu and Thong Su for their excellent technical assistance. We also thank Jyoti Watters for her helpful scientific discussions. We gratefully acknowledge the laboratory of Maurice Dysken (Veterans Affairs Medical Center, Minneapolis) for performing the haloperidol RIA. This research was supported by a National Alliance for Research on Schizophrenia and Depression Distinguished Investigator Award and by National Institutes of Health Grant NS20311 (to D.M.D.), Public Health Service National Research Service Award T32 GM07270 from National Institute of General Medical Sciences and United Negro College Fund/Merck Fellowship (to M.R.A.), and National Institutes of Health Grant GM32875 (to G.S.M.).
ABBREVIATIONS
- NT
neurotensin
- DLST
dorsolateral striatum
- PKA
protein kinase A
References
- 1.Creese I, Burt D R, Snyder S H. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 2.Seeman P, Lee T, Chau Wong M, Wong K. Nature (London) 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko M, Sato K, Horikoshi R, Yaginuma M, Yaginuma N, Shiragata M, Kumashiro H. Prostaglandins Leukotrienes Essent Fatty Acids. 1992;46:53–57. doi: 10.1016/0952-3278(92)90059-r. [DOI] [PubMed] [Google Scholar]
- 4.Gerfen C R, Engber T M, Mahan L C, Susel Z, Chase T N, Monsoma F J J, Sibley D R. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 5.Einhorn L C, Oxford G S. J Physiol (London) 1993;462:563–578. doi: 10.1113/jphysiol.1993.sp019569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhl G R, Snyder S H. Life Sci. 1976;19:1827–1832. doi: 10.1016/0024-3205(76)90114-4. [DOI] [PubMed] [Google Scholar]
- 7.Iversen L L, Iversen S D, Bloom F, Douglas C, Brown M, Vale W. Nature (London) 1978;273:161–163. doi: 10.1038/273161a0. [DOI] [PubMed] [Google Scholar]
- 8.Widerlov E, Lindstrom L H, Besev G, Manberg P J, Nemeroff C B, Breese G R, Kizer J S, Prange A J., Jr Am J Psychiatry. 1982;139:1122–1126. doi: 10.1176/ajp.139.9.1122. [DOI] [PubMed] [Google Scholar]
- 9.Merchant K M, Dorsa D M. Proc Natl Acad Sci USA. 1993;90:3447–3451. doi: 10.1073/pnas.90.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merchant K M, Dobner P R, Dorsa D M. J Neurosci. 1992;12:652–663. doi: 10.1523/JNEUROSCI.12-02-00652.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kislauskis E, Bullock B, McNeil S, Dobner P R. J Biol Chem. 1988;263:4963–4968. [PubMed] [Google Scholar]
- 12.Ervin G N, Birkemo L S, Nemeroff C B, Prange A J., Jr Nature (London) 1981;291:73–76. doi: 10.1038/291073a0. [DOI] [PubMed] [Google Scholar]
- 13.Bissette G, Nemeroff C B, Loosen P T, Prange A J, Jr, Lipton M A. Nature (London) 1976;262:607–609. doi: 10.1038/262607a0. [DOI] [PubMed] [Google Scholar]
- 14.Snijders R, Kramarcy N R, Hurd R W, Nemeroff C B, Dunn A J. Neuropharmacology. 1982;21:465–468. doi: 10.1016/0028-3908(82)90032-6. [DOI] [PubMed] [Google Scholar]
- 15.Nemeroff C B. Biol Psychiatry. 1980;15:283–302. [PubMed] [Google Scholar]
- 16.Glantz S B, Amat J A, Rubin C S. Mol Biol Cell. 1992;3:1215–1228. doi: 10.1091/mbc.3.11.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadd G, McKnight G S. Neuron. 1989;3:71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- 18.Brandon E P, Zhuo M, Huang Y Y, Qi M, Gerhold K A, Burton K A, Kandel E R, McKnight G S, Idzerda R L. Proc Natl Acad Sci USA. 1995;92:8851–8855. doi: 10.1073/pnas.92.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi M, Zhou M, Skalhegg B S, Brandon E P, Kandel E R, McKnight G S, Idzerda R L. Proc Natl Acad Sci USA. 1996;93:1571–1576. doi: 10.1073/pnas.93.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clegg C H, Correll L A, Cadd G G, McKnight G S. J Biol Chem. 1987;262:13111–13119. [PubMed] [Google Scholar]
- 21.Kemp B E, Graves D J, Benjamini E, Krebs E G. J Biol Chem. 1977;252:4888–4894. [PubMed] [Google Scholar]
- 22.Merchant K M, Dobie D J, Dorsa D M. Ann NY Acad Sci. 1992;668:54–69. doi: 10.1111/j.1749-6632.1992.tb27339.x. [DOI] [PubMed] [Google Scholar]
- 23.Bouthenet M L, Souil E, Martres M P, Sokoloff P, Giros B, Schwartz J C. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- 24.Cummings D E, Brandon E P, Planas J V, Motamed K, Idzerda R L, McKnight G S. Nature (London) 1996;382:622–626. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 25.Amieux P S, Cummings D E, Motamed K L, Brandon E P, Wailes L A, Le K, Idzerda R L, McKnight G S. J Biol Chem. 1997;272:3993–3998. doi: 10.1074/jbc.272.7.3993. [DOI] [PubMed] [Google Scholar]
- 26.Merchant K M. Mol Cell Neurosci. 1994;5:336–344. doi: 10.1006/mcne.1994.1040. [DOI] [PubMed] [Google Scholar]
- 27.Kislauskis E, Dobner P R. Neuron. 1990;4:783–795. doi: 10.1016/0896-6273(90)90205-t. [DOI] [PubMed] [Google Scholar]
- 28.Sheng M, McFadden G, Greenburg M E. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- 29.Xing J, Ginty D D, Greenburg M E. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 30.Matthews R P, Guthrie C R, Wailes L M, Zhao X, Means A R, McKnight G S. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams M R, Dobie D J, Merchant K M, Unis A, Dorsa D M. Peptides. 1997;18:527–535. doi: 10.1016/s0196-9781(97)87957-0. [DOI] [PubMed] [Google Scholar]
- 32.Kalivas P W, Stewart J. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 33.Hoyer D, Clarke D E, Fozard J R, Hartig P R, Martin G R, Mylecharane E J, Saxena P R, Humphrey P P. Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 34.Latini S, Pazzagli M, Pepeu G, Pedata F. Gen Pharmacol. 1996;27:925–933. doi: 10.1016/0306-3623(96)00044-4. [DOI] [PubMed] [Google Scholar]
- 35.van Calker D, Muller M, Hamprecht B. J Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]