Abstract
Botulinum neurotoxins (BoNTs) target presynaptic nerve terminals by recognizing specific neuronal surface receptors. Two homologous synaptic vesicle membrane proteins, synaptotagmins (Syts) I and II, bind toxins BoNT/B and G. However, a direct demonstration that Syts I/II mediate toxin binding and entry into neurons is lacking. We report that BoNT/B and G fail to bind and enter hippocampal neurons cultured from Syt I knockout mice. Wild-type Syts I and II (but not Syt I loss-of-function toxin-binding domain mutants) restored binding and entry of BoNT/B and G in Syt I–null neurons, thus demonstrating that Syts I/II are protein receptors for BoNT/B and G. Furthermore, mice lacking complex gangliosides exhibit reduced sensitivity to BoNT/G, and binding and entry of BoNT/A, B, and G into hippocampal neurons lacking gangliosides is diminished. These data suggest that gangliosides are the shared coreceptor for BoNT/A, B, and G, supporting a double-receptor model for these three BoNTs for which the protein receptors are known.
Introduction
Botulinum neurotoxins (BoNTs) are bacterial toxins produced by Clostridium botulinum (Schiavo et al., 2000). There are seven serotypes, BoNT/A–G, that share similar structures and functions. The active form of each toxin molecule is composed of a heavy chain (∼100 kD) and a light chain (∼50 kD) connected via a disulfide bond (Schiavo et al., 2000; Simpson, 2004). The heavy chain mediates the binding and entry of toxins into neurons through receptor-mediated endocytosis (Schiavo et al., 2000). Once inside neurons, the light chain translocates across the endosomal membrane into the cytosol (Schiavo et al., 2000; Koriazova and Montal, 2003; Fischer and Montal, 2007), where it functions as a protease that cleaves proteins required for exocytosis of synaptic vesicles. BoNT/A and E cleave the peripheral membrane protein SNAP-25 (synaptosomal-associated protein of 25 kD); BoNT/B, D, F, and G cleave the vesicle membrane protein synaptobrevin (Syb), and BoNT/C cleaves both SNAP-25 as well as the plasma membrane protein syntaxin (Schiavo et al., 1992, 1993a,b, 1994; Blasi et al., 1993a,b). SNAP-25, syntaxin, and Syb assemble together to form the core of a conserved membrane fusion machine that mediates the fusion of synaptic vesicles with the plasma membrane (Rothman and Warren, 1994; Sudhof, 2004; Jahn and Scheller, 2006). Cleavage of these proteins by BoNTs thus inhibits exocytosis of synaptic vesicles. Disruption of exocytosis at the neuromuscular junction causes flaccid paralysis (botulism), which can lead to death as a result of respiratory failure.
BoNTs are the most toxic substances known (Schiavo et al., 2000) and have been classified as a potential bioterrorism threat (Arnon et al., 2001). On the other hand, these toxins are also used to treat a variety of human diseases by attenuating overactive nerve terminals (Montecucco and Molgo, 2005; Verderio et al., 2006). The medical applications of BoNTs are not limited to motor neurons; these toxins can enter many types of neurons, and their use in the central nerve system is being explored (Montecucco and Molgo, 2005; Verderio et al., 2006).
A major focus has been to identify the receptors and pathways for each BoNT to understand how they recognize and enter neurons. The first reported binding proteins for a BoNT were two homologous synaptic vesicle membrane proteins, synaptotagmins (Syts) I and II, which bound BoNT/B (Nishiki et al., 1994, 1996). It was later found that BoNT/G, which shares high sequence similarity with BoNT/B, also bound Syts I/II (Rummel et al., 2004a). Several lines of evidence suggest that Syts I/II are the receptors for BoNT/B and G: (1) BoNT/B and G bind to the luminal region of Syts I/II with high affinity (Nishiki et al., 1994, 1996; Dong et al., 2003; Rummel et al., 2004a; Chai et al., 2006; Jin et al., 2006); (2) Syts I/II can mediate the entry of BoNT/B into PC12 cells, a neuroendocrine cell line (Dong et al., 2003); and (3) peptides containing the toxin-binding site protected motor nerve terminals from BoNT/B and G (Dong et al., 2003; Rummel et al., 2004a), and mutations within the Syt II–binding region of BoNT/B and G reduced the effect of these toxins on motor nerve terminals in phrenic nerve preparations (Rummel et al., 2007). These data support the idea that Syts I/II could serve as receptors for BoNT/B and G. However, it has also been reported that stimulation of synaptic vesicle exocytosis in cultured hippocampal neurons, which exposes Syt I to the cell surface, did not increase the functional entry of BoNT/B (Verderio et al., 1999). This report raised the interesting possibility that Syt is not the receptor for BoNT/B in neurons from the central nerve system (Verderio et al., 2006). A direct test of whether Syt I/II expression is required for the entry of BoNT/B and G into neurons has been lacking.
In addition to protein receptors, a group of membrane glycosphingolipids called gangliosides has been proposed to serve as coreceptors for BoNTs (Montecucco, 1986). Complex forms of gangliosides called polysialiogangliosides (PSGs) have been shown to bind BoNT/A and B (Kitamura et al., 1980; Kozaki et al., 1998; Rummel et al., 2004b; Yowler and Schengrund, 2004), and mice that lack PSG expression displayed lower sensitivity to BoNT/A–C (Kitamura et al., 1999; Tsukamoto et al., 2005). It was also reported that phrenic nerve preparations from mice lacking PSG are less sensitive to BoNT/G, and mutations within the putative ganglioside-binding domain of BoNT/G reduced the activity of this toxin in the phrenic nerve preparation (Rummel et al., 2007). However, it has not been tested whether gangliosides are required for the toxicity of BoNT/G in vivo. Moreover, it has not been directly addressed whether disruption of ganglioside synthesis in neurons affects the binding of BoNTs or affects other steps in the action of the toxins.
In this paper, we study the binding and entry of BoNTs using hippocampal neurons cultured from Syt I knockout (KO) mice and ganglioside KO mice as loss-of-function models (Geppert et al., 1994; Liu et al., 1999). We found that expression of Syts I/II is essential for binding and entry of BoNT/B and G into hippocampal neurons, demonstrating that Syts I/II function as protein receptors for these two toxins. We further found that mice lacking gangliosides are less sensitive to BoNT/G than control mice and that gangliosides are important for the binding and entry of BoNT/A, B, and G into neurons. Together with previous findings that BoNT/A uses the synaptic vesicle protein SV2 as its protein receptor (Dong et al., 2006; Mahrhold et al., 2006), the data reported here further support a double-receptor model for BoNT/A, B, and G, the only three BoNTs for which protein receptors have been identified.
Results
Binding sites for BoNT/B are located in synaptic vesicles in hippocampal neurons
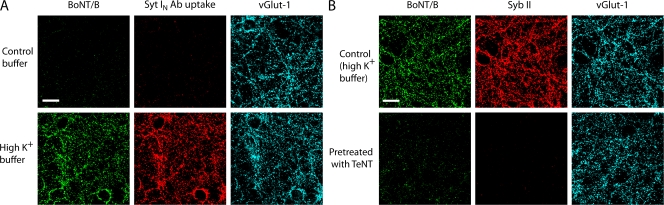
Because Syts I/II localize to synaptic vesicles in neurons, where they are thought to function as Ca2+ sensors that regulate exocytosis (Matthew et al., 1981; Brose et al., 1992; Chapman, 2002), we first addressed whether BoNT/B enters neurons via synaptic vesicle recycling. To test this, we used cultured hippocampal neurons as a model system and drove synaptic vesicle recycling by depolarization with high K+ buffer (56 mM K+). Cultured neurons were exposed to BoNT/B in either control or high K+ buffer. An antibody that recognizes the luminal domain of Syt I (Syt IN antibody), which does not interfere with BoNT/B–Syt I interactions (Dong et al., 2003), was included in the buffers to confirm vesicle recycling. As shown in Fig. 1 A, binding of both BoNT/B and Syt IN antibody was greatly increased after neurons were depolarized with high K+ buffer, indicating that vesicle exocytosis exposes binding sites for BoNT/B. To further confirm this finding, we pretreated neurons with tetanus neurotoxin (TeNT), which blocks synaptic vesicle exocytosis by cleaving Syb (Schiavo et al., 1992). As shown in Fig. 1 B, BoNT/B failed to bind neurons that had been pretreated with TeNT, suggesting that the binding sites for BoNT/B are located in the lumen of synaptic vesicles in hippocampal neurons.
Figure 1.
Binding sites for BoNT/B are exposed upon exocytosis of synaptic vesicles in hippocampal neurons. (A) Rat hippocampal neurons were exposed to 10 nM BoNT/B and an antibody that recognizes the luminal domain of Syt I (Syt IN antibody; 1:200) in either control buffer (PBS without Ca2+) or high K+ buffer (PBS with 56 mM KCl and 1 mM Ca2+) for 5 min. Cells were then fixed for immunocytochemistry. Binding of BoNT/B was visualized using an anti-BoNT/B antibody. Excitatory synapses were labeled using an antibody that recognizes the vesicular glutamate transporter-1 (vGlut-1). The experiments described here were performed in at least three independent trials; representative examples of the data are shown. (B) Rat hippocampal neurons were treated with 15 nM TeNT for 24 h and were briefly exposed to 10 nM BoNT/B for 5 min in high K+ buffer. Control cells were not treated with TeNT. Immunostaining was performed for BoNT/B and Syb II, the substrate of TeNT. Binding of BoNT/B was detected only in neurons that were not treated with TeNT. Bars, 20 μm.
Hippocampal neurons from Syt I KO mice lack both Syts I and II and can actively recycle synaptic vesicles
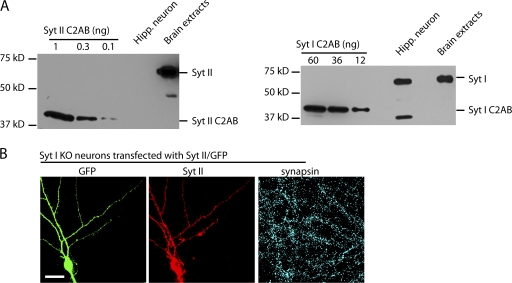
To determine whether Syts I/II are necessary for the binding and entry of BoNT/B to neurons, a loss of function model that lacks Syts I/II is needed. It is currently thought that most neurons express either Syt I or II, and Syt I is the predominant isoform expressed in the hippocampus (Ullrich et al., 1994; Pang et al., 2006; Fox and Sanes, 2007). Therefore, hippocampal neurons from Syt I KO mice would provide a potential loss of function model if these neurons do not express Syt II (Geppert et al., 1994). To determine this, we first examined the expression pattern of Syts I/II in wild-type (WT) hippocampal neurons. Lysates from cultured rat hippocampal neurons were analyzed by Western blotting using anti–Syt I and II antibodies. The cytoplasmic domains of Syt (Syt I C2AB and Syt II C2AB), which contain the peptide sequences used to raise the antibodies, were included as controls. Rat brain detergent extracts were also loaded as additional controls. Consistent with previous studies (Geppert et al., 1994; Ullrich et al., 1994), we found that Syt I is abundantly expressed in cultured hippocampal neurons (Fig. 2 A, right). The Syt II antibody detected 0.3 ng recombinant Syt II under these assay conditions, and this antibody detected a protein band at 65 kD, corresponding to the molecular weight of Syt II in brain detergent extracts. However, Syt II was not detected in lysates from cultured hippocampal neurons (Fig. 2 A, left).
Figure 2.
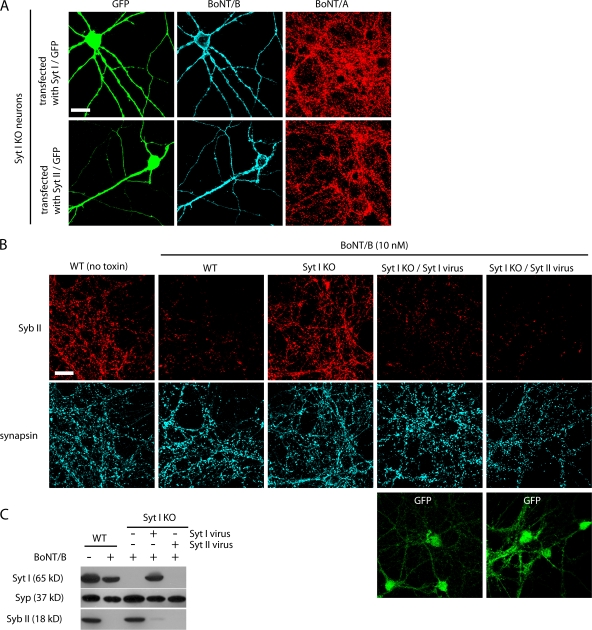
Hippocampal neurons from Syt I KO mice do not express Syt II. (A, left) 30 μg of cell lysates from cultured rat hippocampal neurons (14 DIV) and 3.5 μg of rat brain detergent extracts were analyzed by Western blotting using a monoclonal antibody against Syt II. A recombinant Syt II fragment containing the antibody epitope was loaded as a positive control (Syt II C2AB). Syt II was detected in brain detergent extracts but not in cultured hippocampal neurons. (right) The experiment was performed as described for the left panel except using an anti–Syt I antibody, recombinant Syt I fragment as a positive control, and 10 μg of hippocampal neuron lysates. (B) Cultured hippocampal neurons (14 DIV) from Syt I KO mice were transfected with a construct that expresses Syt II and GFP under the control of two separated promoters. The neurons were subsequently immunostained using anti–Syt II and antisynapsin antibodies. The Syt II antibody recognized Syt II in the transfected neuron, which also expresses GFP. Syt II was not detected in untransfected neurons. The experiments described here were performed in at least three independent trials; representative examples of the data are shown. Bar, 20 μm.
We further examined whether there is compensatory expression of Syt II in hippocampal neurons cultured from Syt I KO mice by immunostaining with an anti–Syt II antibody. To provide a positive control, we transfected Syt I KO neurons with a construct that expresses both Syt II and GFP under two separate promoters. We also stained neurons with a synapsin antibody to label all synapses. The anti–Syt II antibody specifically stained transfected neurons, but no signal was detected in untransfected cells (Fig. 2 B). Thus, hippocampal neurons from Syt I KO mice lack both Syt I and Syt II.
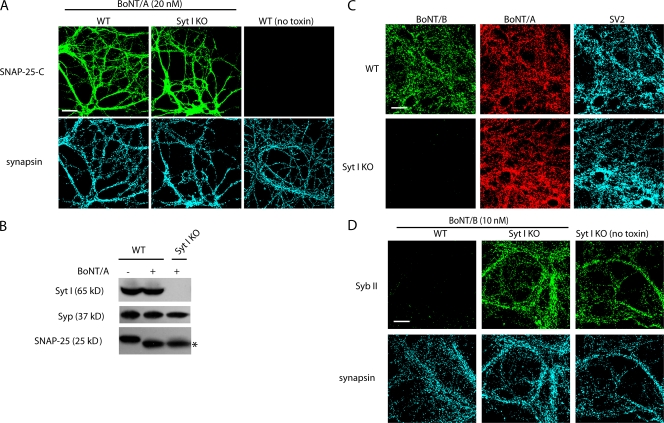
Because Syts I and II are essential for triggering the release of synaptic vesicles and also play important roles in endocytosis of synaptic vesicle components (Chapman, 2002; Pang et al., 2006), there is a concern that the synaptic vesicle recycling pathway might be disrupted in neurons lacking Syts I and II. Such defects would block binding and entry of any toxin that used the synaptic vesicle cycle to enter neurons. To address this concern, we tested whether BoNT/A, which uses the synaptic vesicle protein SV2 as its receptor (Dong et al., 2006), was able to enter Syt I KO neurons. The functional entry of BoNT/A into neurons was monitored by either staining cells with an antibody that recognizes only the SNAP-25 cleavage product generated by BoNT/A (anti–SNAP-25-C; Fig. 3 A; Dong et al., 2006) or by Western blot analysis using an anti–SNAP-25 antibody (Fig. 3 B). A similar degree of SNAP-25 cleavage was observed in Syt I KO and WT neurons. These results suggest that synaptic vesicle recycling is preserved in Syt I KO neurons under the conditions we used to load toxins (5 min in high K+ buffer). This conclusion is consistent with previous studies demonstrating that the synaptic vesicle cycle remains intact in Syt I KO hippocampal neurons but occurs with markedly slower kinetics (Nicholson-Tomishima and Ryan, 2004; Nishiki and Augustine, 2004). Thus, we conclude that hippocampal neurons from Syt I KO mice are a valid loss of function model to study the binding and entry of BoNT/B.
Figure 3.
BoNT/B cannot bind or enter Syt I KO hippocampal neurons. (A) Cultured littermate wild-type (WT) and Syt I KO hippocampal neurons were briefly exposed to 20 nM BoNT/A for 5 min in high K+ buffer. Neurons were washed, further incubated for 6 h in culture medium, and fixed for immunostaining. Cleavage of SNAP-25 by BoNT/A was detected using a monoclonal antibody (anti–SNAP-25-C) that recognizes only the cleaved form of SNAP-25. This antibody revealed similar levels of immunostaining signals in WT and Syt I KO neurons, indicating that BoNT/A can enter Syt I KO neurons. (B) Experiments were performed as described in A except that neurons were harvested and subjected to Western blot analysis. Cleavage of SNAP-25 was detected using an antibody that recognizes both intact SNAP-25 as well as the cleavage product (indicated by an asterisk). Syp was assayed as an internal control for equal loading of lysates. (C) Cultured littermate WT and Syt I KO hippocampal neurons were simultaneously exposed to 20 nM BoNT/A and 10 nM BoNT/B for 5 min in high K+ buffer. Neurons were washed and fixed. Immunostaining was performed using human anti-BoNT/A, rabbit anti-BoNT/B, and mouse anti-SV2 antibodies. (D) WT and Syt I KO neurons were exposed to 10 nM BoNT/B for 5 min in high K+ buffer. Neurons were washed and further incubated for 24 h before fixation. Syb II was labeled using a monoclonal antibody. Cleavage of Syb II by BoNT/B resulted in the loss of Syb II immunofluorescence. All synapses were labeled with an anti-synapsin antibody. The experiments described here were performed in at least three independent trials; representative examples of the data are shown. Bars, 20 μm.
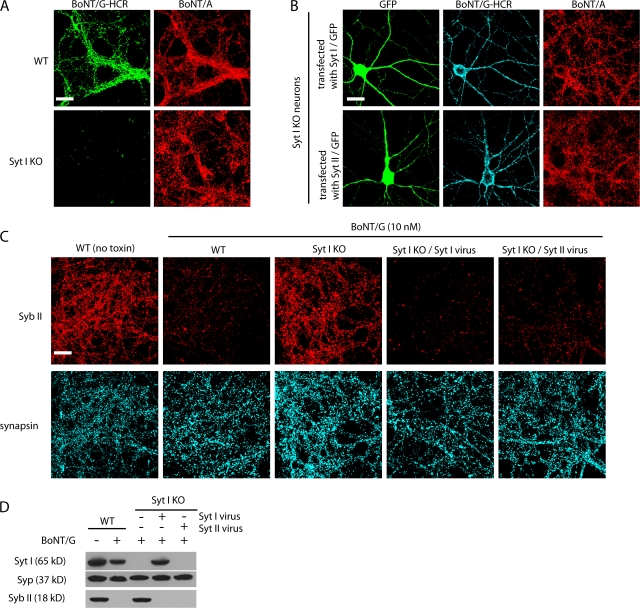
BoNT/B fails to bind or enter Syt I KO hippocampal neurons
Next, we tested whether the loss of Syt I/II expression in neurons affects the binding of BoNT/B. Hippocampal neurons from WT or Syt I KO littermates were exposed to BoNT/B and A in high K+ buffer for 5 min. Neurons were fixed, and toxin binding was detected by immunofluorescence using antibodies against BoNT/B and A. SV2 was also stained to mark all synapses. As shown in Fig. 3 C, BoNT/B failed to bind Syt I KO neurons. A similar degree of BoNT/A binding was observed in Syt I KO and WT neurons, thus providing an internal control for synaptic vesicle recycling in these neurons (Dong et al., 2006).
We further determined whether the loss of surface binding of BoNT/B corresponded to a decrease in functional entry of the toxin. Entry of BoNT/B results in the cleavage of Syb II, the predominant isoform of Syb in hippocampal neurons; cleavage was monitored by the loss of Syb II immunofluorescence (Baumert et al., 1989; Schiavo et al., 1992). WT and Syt I KO neurons were first exposed to BoNT/B for 5 min in high K+ buffer and were further incubated in the absence of toxin for 24 h. As shown in Fig. 3 D, we found that the Syb II immunofluorescence disappeared from most synapses in WT neurons after exposure to BoNT/B. In contrast, the Syb II signal remained intact in Syt I KO neurons, indicating that BoNT/B entry into neurons requires the expression of Syt I.
Expression of Syt I or II in Syt I KO neurons restores binding and entry of BoNT/B
To confirm that loss of binding and entry of BoNT/B in Syt I KO neurons was caused by the absence of Syts I/II, we performed rescue experiments. First, Syt I KO neurons were transiently transfected with plasmids to express either Syt I or II. Transfected cells were marked by GFP expression. We found that BoNT/B bound to cells that expressed either Syt I (Fig. 4 A, top) or Syt II (Fig. 4 A, bottom) but not to untransfected neurons. BoNT/A bound to both transfected and untransfected neurons, thus serving as a control.
Figure 4.
Expression of Syt I or II restores the binding and entry of BoNT/B into Syt I KO hippocampal neurons. (A) Syt I KO neurons were transfected with either Syt I or II and exposed to BoNT/A and BoNT/B as described in Fig. 3 A. Immunostaining was performed using anti-BoNT/A and B antibodies. The constructs that express Syt I or II also express GFP under the control of a separate promoter. BoNT/B bound only to neurons transfected with Syt I or II. BoNT/A bound to all synapses, thus serving as a control. (B) Syt I KO neurons were infected with lentiviruses that express either Syt I or II. Neurons were exposed to 10 nM BoNT/B for 5 min in high K+ buffer and were further incubated for 24 h before fixation. Immunostaining was performed as described in Fig. 3 A. GFP signals indicate infected neurons. BoNT/B failed to enter Syt I KO neurons but readily entered Syt I KO neurons that expressed either Syt I or II, as indicated by the loss of Syb II in these neurons. (C) Experiments were performed as described in B except that neurons were harvested, and cell lysates were subjected to Western blot analysis using antibodies against Syt I, Syb II, and Syp. The experiments described here were performed in at least three independent trials; representative examples of the data are shown. Bars, 20 μm.
Next, we determined whether the expression of Syt I or II in Syt I KO neurons restored the functional entry of BoNT/B. Entry of BoNT/B was determined by monitoring the levels of Syb II by immunostaining of neurons (Fig. 4 B) or by Western blot analysis of lysates from cultured neurons (Fig. 4 C). Syt I KO neurons were infected with lentiviruses that expressed either Syt I or II. The efficiency of infection was usually >90%. Infected neurons were exposed to BoNT/B for 5 min and were further incubated for 24 h. We found that the level of Syb II was greatly reduced by BoNT/B in neurons that expressed either Syt I or II virus (Fig. 4, B and C), demonstrating that BoNT/B entered infected neurons and cleaved Syb II.
BoNT/G enters neurons via recycling synaptic vesicles
Syts I/II have also been suggested to serve as the receptors for BoNT/G, which is closely related to BoNT/B (Rummel et al., 2004a). Data supporting this idea include the following: (1) BoNT/G binds directly to the luminal domain of Syts I and II (Rummel et al., 2004a); (2) recombinant Syt luminal fragments can reduce the toxicity of BoNT/G at motor nerve terminals (Rummel et al., 2004a); and (3) mutations within the putative Syt-binding domain of BoNT/G reduced the activity of BoNT/G at motor nerve terminals (Rummel et al., 2007). Although these experiments are consistent with a model in which Syts I/II are the receptors for BoNT/G, direct experiments that address whether Syts I/II are required for functional entry of BoNT/G into neurons have not been reported.
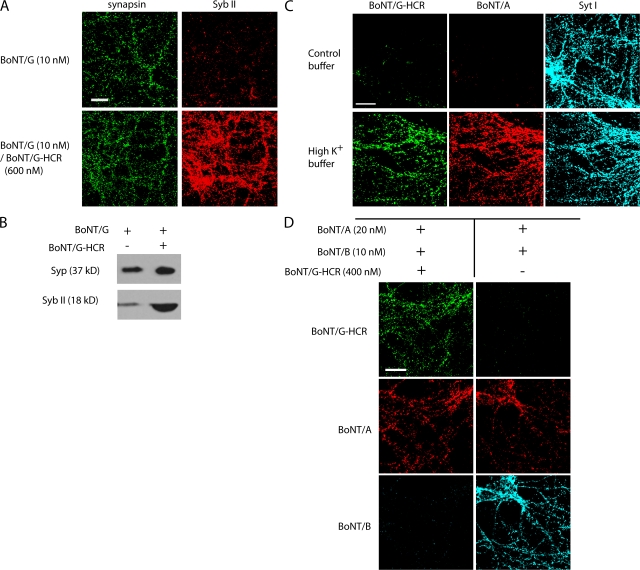
To study whether Syts I/II mediate the entry of BoNT/G, we first sought to determine whether BoNT/G enters neurons via recycling synaptic vesicles. Because an anti-BoNT/G antibody is not currently available, we monitored the binding of an epitope-tagged receptor-binding domain of this toxin to neurons. This receptor-binding domain of BoNT/G was purified as a FLAG-tagged recombinant protein (BoNT/G-HCR; ∼50 kD; see Materials and methods for details). This BoNT/G-HCR fragment was capable of antagonizing the binding of BoNT/G to its receptor on neurons because Syb II, the substrate protein of BoNT/G (Schiavo et al., 1994), was protected from cleavage by BoNT/G in the presence of high concentrations of BoNT/G-HCR (Fig. 5, A and B). Using this fragment, we found that stimulation of neurons with high K+ significantly increased the binding of BoNT/G-HCR, suggesting that the binding site for BoNT/G is localized to synaptic vesicles (Fig. 5 C). Furthermore, we found that excess levels of BoNT/G-HCR blocked the binding of BoNT/B to neurons (Fig. 5 D). This competition is specific to BoNT/B because the binding of BoNT/A was not affected (Fig. 5 D). These results suggest that BoNT/G recognizes the same binding site on neurons as BoNT/B.
Figure 5.
The receptor-binding domain of BoNT/G (BoNT/G-HCR) binds to hippocampal neurons in an activity-dependent manner and blocks binding and entry of BoNT/B and G. (A) Cultured rat hippocampal neurons were exposed to 10 nM BoNT/G with or without high concentrations of BoNT/G-HCR (600 nM) for 5 min in high K+ buffer. Cells were further incubated for 24 h before fixation. Immunostaining was performed using anti–Syb II and anti-synapsin antibodies. (B) Experiments were performed as described in A except that cells were harvested and subjected to Western blot analysis to detect Syp and Syb II. The presence of BoNT/G-HCR protected Syb II from cleavage by BoNT/G, presumably by competing with the binding of BoNT/G to neurons. (C) Rat hippocampal neurons were exposed to both 20 nM BoNT/A and 100 nM BoNT/G-HCR for 5 min in either control buffer or high K+ buffer. Cells were washed, and immunostaining was performed using anti-BoNT/A antibody and anti-FLAG antibody, which recognizes the FLAG tag at the N terminus of BoNT/G-HCR. An anti–Syt I antibody was used to label all synapses. Stimulation of neurons with high K+ buffer increased the binding of BoNT/G-HCR. BoNT/A served as a control. (D) Rat hippocampal neurons were exposed to 20 nM BoNT/A and 10 nM BoNT/B with (left) or without (right) 400 nM BoNT/G-HCR for 5 min in high K+ buffer. Cells were washed and fixed. Immunostaining was performed using anti-BoNT/A, anti-BoNT/B, and anti-FLAG antibodies. High concentrations of BoNT/G-HCR did not affect the binding of BoNT/A but blocked the binding of BoNT/B to neurons. The experiments described here were performed in at least three independent trials; representative examples of the data are shown. Bars, 20 μm.
Binding and entry of BoNT/G into neurons requires Syts I/II
Next, we determined whether the lack of Syt I and II expression in neurons results in diminished binding of BoNT/G. As shown in Fig. 6 A, BoNT/G-HCR failed to bind Syt I KO neurons. This loss of binding was restored upon the expression of either Syt I or II (Fig. 6 B). We further tested whether loss of Syts I/II blocks the functional entry of BoNT/G into neurons. WT and Syt I KO neurons were exposed to BoNT/G for 5 min in high K+ buffer and were further incubated for 24 h. The level of Syb II was monitored by either immunostaining (Fig. 6 C) or Western blotting (Fig. 6 D). Although Syb II disappeared from most synapses in WT neurons after exposure to BoNT/G, the level of Syb II in Syt I KO neurons remained the same as in neurons that had not been exposed to BoNT/G, demonstrating that BoNT/G failed to enter Syt I KO neurons. Furthermore, entry of BoNT/G was rescued by expressing either Syt I or II in Syt I KO neurons (Fig. 6, C and D). Together, these results provide direct evidence that Syts I and II are the protein receptors that mediate the binding and entry of BoNT/G into neurons.
Figure 6.
Expression of Syt I or II restores the binding and entry of BoNT/G into Syt I KO neurons. (A) WT and Syt I KO neurons were exposed to 100 nM BoNT/G-HCR and 20 nM BoNT/A for 5 min in high K+ buffer. Immunostaining was performed using anti-BoNT/A and anti-FLAG antibodies. BoNT/G-HCR failed to bind Syt I KO neurons. BoNT/A served as a control. (B) Syt I KO neurons were transfected with either Syt I or II. Cells were then exposed to BoNT/A and BoNT/G-HCR, and immunostaining was conducted as described in A. Transfected neurons were visualized by GFP expression. BoNT/G-HCR bound only to neurons that expressed either Syt I or II. (C) WT neurons, Syt I KO neurons, and Syt I KO neurons infected with Syt I or II viruses were exposed to 30 nM BoNT/G for 5 min in high K+ buffer. Neurons were washed and further incubated for 24 h before fixation, and immunostaining was performed using anti–Syb II and antisynapsin antibodies. Cleavage of Syb II by BoNT/G resulted in reduced immunostaining signals for Syb II. The levels of Syb II in Syt I KO neurons remained the same as in neurons that were not exposed to BoNT/G, indicating that BoNT/G failed to enter Syt I KO neurons. Expression of Syt I or II restored the entry of BoNT/G. (D) Experiments were performed as described in C except that neurons were harvested and subjected to Western blot analysis to detect Syt I, Syb II, and Syp. The experiments described here were performed in at least three independent trials; representative examples of the data are shown. Bars, 20 μm.
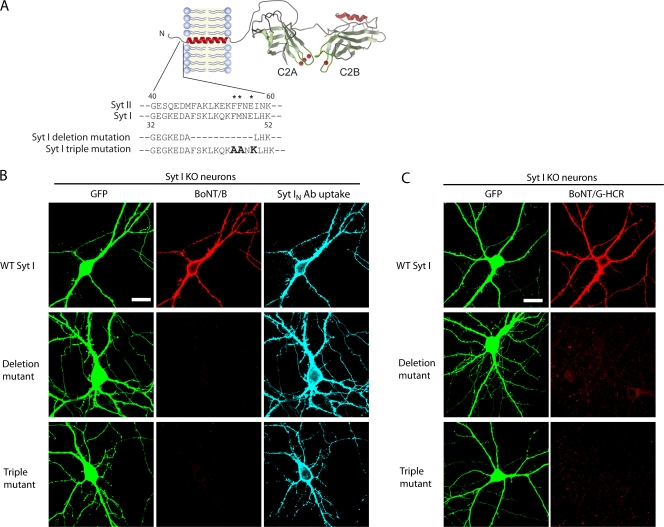
Syt I mutants harboring loss-of-function mutations within the BoNT/B–G-binding domain failed to rescue toxin binding in Syt I KO neurons
The crystal structure of BoNT/B bound to the toxin-binding site of Syt II was recently reported. A hydrophobic groove within the C-terminal domain of BoNT/B interacts with a short segment within the luminal domain of Syt II (Chai et al., 2006; Jin et al., 2006). This hydrophobic groove is conserved in all subtypes of BoNT/B as well as in BoNT/G. The structure also revealed that only residues 45–59 of Syt II interact with BoNT/B. Within this segment, F47, F54, F55, and E57 of Syt II appear to form critical contacts with BoNT/B (Chai et al., 2006; Jin et al., 2006). These structural details enabled us to perform targeted mutagenesis on Syts I/II to selectively disrupt its toxin-binding activity.
To address the concern that mutagenesis within the toxin-binding site may alter the membrane topology or targeting of Syt, we chose to focus this analysis on Syt I instead of Syt II. This is because exposure of the Syt I luminal domain to the cell surface during synaptic vesicle recycling can be conveniently monitored using an antibody that recognizes an epitope in the N terminus of the luminal domain (Syt IN antibody; Fig. 1 A). This provides a simple readout to confirm the correct targeting of mutant forms of Syt I.
As shown in Fig. 7 A, two mutants were designed: a deletion mutant that lacked a critical toxin-binding segment (Δ39–49 in Syt I, corresponding to residues 47–57 of Syt II) and a triple mutant that harbored substitutions of three key residues (F46A, M47A, and E49K of Syt I, corresponding to residues F54, F55, and E57 of Syt II). WT and mutant forms of Syt I were expressed in Syt I KO neurons and exposed to both BoNT/B and Syt IN antibody. The luminal domains of WT and mutant forms of Syt I were all exposed to the surface of cells after stimulation with high K+, as indicated by the binding of Syt IN antibody (Fig. 7 B). However, binding of BoNT/B was observed only on neurons that expressed WT Syt I but not with neurons that expressed the deletion or triple mutant protein (Fig. 7 B). The same results were observed for BoNT/G-HCR (Fig. 7 C). These results demonstrate that BoNT/B and G bind neurons via direct interactions with the luminal domain of Syt I and also validate the crystal structure of the BoNT/B–Syt II complex (which was obtained in the absence of the ganglioside coreceptor using a Syt II fragment that lacked the transmembrane domain of the protein).
Figure 7.
Mutations in the toxin-binding site of Syt I disrupt interactions with BoNT/B and G on the surface of hippocampal neurons. (A) Schematic diagram of the Syt I deletion mutation and triple point mutation. The image was generated using the crystal structure of the cytoplasmic domain of Syt III (Sutton et al., 1999); the remaining regions were added with a drawing program. Asterisks indicate the three residues that are mutated in Syt I. Bold letters indicate amino acids that were used to replace the original amino acids in Syt I triple mutation. (B) WT, deletion mutant, and the triple mutant version of Syt I were expressed in Syt I KO neurons, and neurons were exposed to both BoNT/B and Syt IN antibody for 5 min in high K+ buffer. Cells were fixed and imaged. Binding of BoNT/B was visualized using an anti-BoNT/B antibody. The Syt IN antibody bound to neurons expressing WT, deletion, or triple mutant versions of Syt I; BoNT/B bound only to neurons that expressed WT Syt I. (C) Experiments were performed as described in B except that neurons were exposed to BoNT/G-HCR. Binding of BoNT/G-HCR was observed only for neurons that expressed WT Syt I. The experiments described here were performed in at least three independent trials; representative examples of the data are shown. Bars, 20 μm.
Gangliosides are essential for the binding of BoNT/A, B, and G to neurons
In addition to protein receptors, it has been proposed that gangliosides may serve as coreceptors for BoNTs (Montecucco, 1986). A role for gangliosides in vivo has been previously demonstrated for BoNT/A and B by the finding that mice lacking complex gangliosides (PSGs) are less sensitive to these toxins (Kitamura et al., 1999). However, it has not been determined whether gangliosides are required for the toxicity of BoNT/G in vivo. Thus, we extended a previous study to assess whether gangliosides are necessary for the toxicity of BoNT/G in vivo using a KO mouse line that lacks PSGs as a result of the disruption of GM2/GD2 synthase (GalNac-T−/−, denoted as ganglioside KO hereafter), an enzyme required to synthesize complex gangliosides (Liu et al., 1999). The sensitivity of ganglioside KO mice to BoNT/G was compared with WT mice using an established rapid time to death assay (Dong et al., 2003). Identical amounts of BoNT/G were injected into WT and ganglioside KO littermate mice, and the survival time (time to death) of each mouse was determined. The survival time was converted to units of LD50 as previously described (Dong et al., 2003). As shown in Fig. 8 A, ganglioside KO mice survived a mean of 132 min, whereas WT mice survived only 51 min. The difference in the survival time corresponds to a 20-fold difference in the apparent LD50 value (i.e., although the same amount of toxin was injected into WT and ganglioside KO mice, the toxicity displayed in ganglioside KO mice is 20 times lower than that of WT mice). These results indicate that gangliosides are important for the toxicity of BoNT/G in vivo.
Figure 8.
Binding and entry of BoNT/A, B, and G is reduced in neurons lacking gangliosides. (A) 5-wk-old littermate WT and ganglioside KO mice were injected with the same amount of BoNT/G. The survival time of each mouse (time to death, given in minutes) was determined, and the apparent lethal dose (LD50) of toxin was calculated based on their survival time as described previously (Dong et al., 2003). Data from five representative pairs are shown. (B) Cultured littermate WT and ganglioside KO neurons were exposed to 20 nM BoNT/A, 10 nM BoNT/B, or 100 nM BoNT/G-HCR (right) for 5 min in high K+ buffer. Ganglioside KO neurons preloaded with exogenous gangliosides (250 mg/ml ganglioside mixture for 12 h) were also tested in parallel. Cells were washed and fixed. Immunostaining was performed using antibodies against BoNT/A, BoNT/B, and FLAG tag. vGlut-1 was also labeled to visualize synapses. (C) Cultured WT and ganglioside KO neurons were exposed to 5 nM BoNT/A and 10 nM BoNT/B simultaneously (left) or to 30 nM BoNT/G alone (right) for 5 min in high K+ buffer. Ganglioside KO neurons preloaded with exogenous gangliosides were also tested in parallel. Neurons were washed and incubated for 24 h. Cells were harvested and subjected to Western blot analysis. Syt I was assayed as an internal control for equal loading of lysates. The experiments described here were performed in at least three independent trials; representative examples of the data are shown. Bar, 20 μm.
The data from the in vivo experiments demonstrate the physiological importance of PSGs for the toxicity of BoNT/A, B, and G. However, whether this reduction in toxicity is caused by a decrease in toxin binding to neurons or by changes in other steps in the action of these toxins has not been addressed. Therefore, we examined the binding of BoNT/A and B and BoNT/G-HCR to hippocampal neurons cultured from ganglioside KO and WT mice. As shown in Fig. 8 B, BoNT/A and B failed to bind neurons from ganglioside KO mice. Binding of BoNT/G-HCR to the ganglioside KO neurons was greatly diminished but was not completely abolished. The loss of binding in these experiments was caused by the absence of gangliosides because binding was restored by loading the KO neurons with exogenous gangliosides (Fig. 8 B). These data suggest that gangliosides are coreceptors that are required for the binding of BoNT/A, B, and G to neurons.
We further assayed whether the loss of binding in the aforementioned experiments corresponded to a decrease in functional entry of the toxins. WT neurons, ganglioside KO neurons, and ganglioside KO neurons that had been loaded with exogenous gangliosides were exposed to either BoNT/A and B simultaneously (Fig. 8 C, left) or to BoNT/G (Fig. 8 C, right) for 5 min in high K+ buffer; neurons were then further incubated in the absence of toxin for 24 h. Cell lysates were analyzed by Western blotting. SNAP-25 and Syb II were protected from BoNT/A and BoNT/B, respectively, in ganglioside KO neurons, whereas they were cleaved in KO neurons that had been loaded with exogenous gangliosides (Fig. 8 C, left). We observed only partial protection of Syb II from BoNT/G in ganglioside KO neurons. Together with the observation that there is a low level of binding of BoNT/G-HCR to ganglioside KO neurons (Fig. 8 B, right), these data suggest that BoNT/G is less dependent on the presence of gangliosides than BoNT/A and B in hippocampal neurons under our experimental conditions.
Discussion
Femtomolar levels of BoNTs in the bloodstream of humans and other animals can cause paralysis. The extreme efficacy of BoNTs in vivo is caused not only by their enzymatic activity (cleavage of SNARE proteins) but also involves their ability to selectively target neurons. Thus, a major focus is to identify the receptors and entry pathways for each BoNT. In this study, we performed experiments to address the questions of whether Syts I and II function as the protein receptors for BoNT/B and G in neurons and whether gangliosides are required for the binding of BoNT/A, B, and G to neurons.
To address the role of Syts I/II, we used cultured hippocampal neurons from Syt I KO mice as our model system. We first observed that BoNT/B and G are unable to bind or enter Syt I KO hippocampal neurons, which lack both Syts I and II; binding and entry was restored by expressing Syt I or II. The lack of binding and entry of BoNT/B and G using Syt I KO neurons was not the result of impaired synaptic vesicle recycling caused by the loss of Syt I because BoNT/A, which also enters neurons via recycling synaptic vesicles, efficiently bound and entered these neurons. To further explore this question, we designed two Syt I mutants that harbored loss of function mutations within the toxin-binding site (a deletion mutant and a triple mutant) based on the crystal structure of the BoNT/B–Syt II complex. Once expressed in Syt I KO neurons, these mutant forms of Syt maintained the correct membrane topology, as indicated by the binding of Syt IN antibody to their luminal domain. However, both mutants failed to rescue the binding of BoNT/B and G. These data further establish Syt as a protein receptor for BoNT/B and G and also validate the physiological relevance of the crystal structure of the BoNT/B–Syt II complex. These experiments provide the first cell-based data showing that specific mutations within the toxin-binding site of Syt render neurons resistant to BoNT/B and G.
To address the role of gangliosides in the action of the BoNTs, we used ganglioside KO mice as a model system. We found that ganglioside KO mice displayed a lower sensitivity to BoNT/G in vivo, as reported previously for BoNT/A and B (Kitamura et al., 1999), indicating that gangliosides are important for the toxicity of BoNT/G in intact animals. Moreover, we demonstrate that binding and entry of BoNT/A, B, and G was reduced using neurons that lacked gangliosides. This defect was caused by the lack of gangliosides because binding and entry was rescued by adding back exogenous gangliosides. Together with the previous finding that SV2 is the protein receptor for BoNT/A (Dong et al., 2006), the current data support a double-receptor model for BoNT/A, B, and G, which predicted that the receptors for BoNTs are composed of both gangliosides and proteins (Montecucco, 1986).
In contrast to the loss of binding of BoNT/A and B to hippocampal neurons cultured from ganglioside KO mice, a low level of BoNT/G binding activity persisted. This low level of BoNT/G binding was confirmed by the fact that there was only partial protection of Syb II cleavage by BoNT/G in neurons that lacked gangliosides. These findings suggest that BoNT/G is less dependent on gangliosides under our experimental conditions, although the presence of gangliosides clearly enhances the binding and entry of BoNT/G into hippocampal neurons.
It was recently reported that protease treatment or boiling of purified rat brain synaptosomes did not affect the binding of two other members of the BoNT family, BoNT/C and D, suggesting that these toxins might not have proteinaceous receptors (Tsukamoto et al., 2005). Furthermore, although gangliosides are essential for the entry of BoNT/C, they are not required for the entry of BoNT/D in vivo (Tsukamoto et al., 2005). Thus, the double-receptor model may not apply to all BoNTs.
Materials and methods
Antibodies, materials, cell lines, and mouse lines
Monoclonal antibodies directed against Syb II (CI 69.1), Syt I (α–Syt IN, CI 604.4; α–Syt I C2AB, CI 41.1), SV2 (pan-SV2), synaptophysin (Syp; CI 7.2), and SNAP-25 (CI 71.2) were provided by R. Jahn (Max-Planck-Institute for Biophysical Chemistry, Gottingen, Germany). A human anti-BoNT/A (RAZ-1) was provided by J. Lou and J. Marks (University of California, San Francisco, San Francisco, CA). Rabbit anti-BoNT/B antibodies were generated using purified neurotoxin. The following antibodies were purchased from the indicated companies: mouse monoclonal anti–Syt II (BD Biosciences), mouse monoclonal anti–SNAP-25-C (Research & Diagnostic Antibodies), rabbit polyclonal antisynapsin and guinea pig anti–vesicular glutamate transporter 1 (Chemicon), chicken polyclonal anti-GFP (Abcam), and mouse monoclonal anti-FLAG (M2; Sigma-Aldrich). Bovine brain gangliosides were obtained from Matreya LLC. BoNT/G was obtained from Metabiologics. Rat brain detergent extracts were prepared from crude synaptosomes using 1% Triton X-100 as described previously (Lewis et al., 2001).
A Syt I KO mouse line has been described previously (Geppert et al., 1994) and was obtained from The Jackson Laboratory. Ganglioside KO mice lack the gene encoding GM2/GD2 synthase (gene symbol Galgt1). This mouse line was described previously (Liu et al., 1999) and was obtained from the Consortium for Functional Glycomics. Genotypes were determined by PCR.
cDNA and expression constructs
The following cDNA were provided by the indicated groups. Rat Syt I was provided by T.C. Sudhof (University of Texas Southwestern Medical Center, Dallas, TX), and mouse Syt II was provided by M. Fukuda (RIKEN Institute of Physical and Chemical Research, Ibaraki, Japan). Syt I C2AB and Syt II C2AB were described previously (Hui et al., 2005). To express Syts I/II in neurons, full-length Syts I and II were subcloned into the Lox-Syn-Syn lentivirus vector, which has been described previously (Dong et al., 2006). This vector contains two separate neuronal-specific promoters (synapsin promoter). One promoter controls the expression of Syt, and the other promoter controls the expression of GFP to detect transfected cells.
BoNT/G-HCR recombinant protein was provided by J. Barbieri (Medical College of Wisconsin, Milwaukee, WI). This fragment corresponds to residues 863–1,297 of BoNT/G with a His6 tag and 3xFLAG tag at the N terminus. Vector construction and protein purification procedures were performed as described previously (Baldwin and Barbieri, 2007).
Neuronal cell cultures, transfection, cell lysates, viral infection, and loading gangliosides
Hippocampal neurons were prepared from embryonic day 18–19 rats. Cultured Syt I KO and ganglioside KO hippocampal neurons were prepared from postnatal day 1 mice. Neurons were plated on 12-mm poly-d-lysine–coated glass coverslips at a density of 50,000/cm2 and cultured in neurobasal medium supplemented with 2% B-27 and 2 mM glutamax. Transfections were performed on neurons at 5 d in vitro (DIV) using Lipofectamine 2000 (Invitrogen) and were analyzed at 14 DIV.
Lysates from cultured neurons (14 DIV) were prepared by first washing cells with PBS. Then, 100 μl of lysis buffer (PBS with 1% Triton X-100, 0.05% SDS, and protease cocktails) was added per coverslip. Lentiviral particles were generated by cotransfecting HEK293 cells with the virus packaging vectors (vesicular stomatitis virus G glycoprotein and Δ8.9) and Syt I or II in Lox-Syn-Syn lentivirus vector. Viruses were added to neurons at 5 DIV, and these neurons were analyzed at 14 DIV. To load cells with exogenous gangliosides, ganglioside KO neurons were incubated in media plus 250 μg/ml gangliosides for 12 h.
Binding of BoNTs to hippocampal neurons and immunocytochemistry
The buffers used in Fig. 1 A were control buffer (140 mM NaCl, 3 mM KCl, 1.5 mM KH2PO4, 8 mM Na2HPO4, and 0.5 mM MgCl2) and high K+ (same as control buffer but adjusted to 56 mM KCl and 87 mM NaCl and contains 1 mM CaCl2). Unless specified in the text, hippocampal neurons were generally exposed to toxins in high K+ buffer for 5 min. All images were collected using a confocal microscope (FV1000; Olympus) with a 60× NA 1.10 water immersion objective (Olympus) at 25°C. Immunofluorescence was visualized using secondary antibodies conjugated with Cy2, Cy3 (Jackson ImmunoResearch Laboratories), or AlexaFluor647 (Invitrogen). Images were acquired using Fluoview software version 1.6 (Olympus). The only image parameters that were adjusted were the brightness and contrast, which were processed using Photoshop version CS (Adobe).
Rapid BoNT toxicity assay in mice
BoNT/G effective toxicity in mice was estimated using the intravenous method described previously (Dong et al., 2003). In brief, each mouse was injected intravenously (lateral tail vein) with 0.1 ml BoNT/G (10 μg/ml), and their time to death was determined. The time to death values were converted to intraperitoneal LD50/milliliter using a standard curve (Dong et al., 2003).
Acknowledgments
We acknowledge the Consortium for Functional Glycomics (grant GM62116) for providing ganglioside KO mice. We thank C. Dean and other members of the Chapman laboratory for discussions.
This work was supported by a grant from the National Institutes of Health (National Institute of Allergy and Infectious Diseases grant R01 AI057744) to E.R. Chapman. E.R. Chapman and E.A. Johnson acknowledge membership and support from the Region V Great Lakes Regional Center of Excellence in Biodefense and Emerging Infectious Diseases (National Institutes of Health award 1-U54-AI-057153). E.R. Chapman is an Investigator of the Howard Hughes Medical Institute.
Abbreviations used in this paper: BoNT, botulinum neurotoxin; DIV, days in vitro; KO, knockout; PSG, polysialioganglioside; Syb, synaptobrevin; Syp, synaptophysin; Syt, synaptotagmin; TeNT, tetanus neurotoxin; WT, wild type.
References
- Arnon, S.S., R. Schechter, T.V. Inglesby, D.A. Henderson, J.G. Bartlett, M.S. Ascher, E. Eitzen, A.D. Fine, J. Hauer, M. Layton, et al. 2001. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 285:1059–1070. [DOI] [PubMed] [Google Scholar]
- Baldwin, M.R., and J.T. Barbieri. 2007. Association of botulinum neurotoxin serotypes a and B with synaptic vesicle protein complexes. Biochemistry. 46:3200–3210. [DOI] [PubMed] [Google Scholar]
- Baumert, M., P.R. Maycox, F. Navone, P. De Camilli, and R. Jahn. 1989. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 8:379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi, J., E.R. Chapman, E. Link, T. Binz, S. Yamasaki, P. De Camilli, T.C. Sudhof, H. Niemann, and R. Jahn. 1993. a. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 365:160–163. [DOI] [PubMed] [Google Scholar]
- Blasi, J., E.R. Chapman, S. Yamasaki, T. Binz, H. Niemann, and R. Jahn. 1993. b. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 12:4821–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose, N., A.G. Petrenko, T.C. Sudhof, and R. Jahn. 1992. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 256:1021–1025. [DOI] [PubMed] [Google Scholar]
- Chai, Q., J.W. Arndt, M. Dong, W.H. Tepp, E.A. Johnson, E.R. Chapman, and R.C. Stevens. 2006. Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature. 444:1096–1100. [DOI] [PubMed] [Google Scholar]
- Chapman, E.R. 2002. Synaptotagmin: a Ca(2+) sensor that triggers exocytosis? Nat. Rev. Mol. Cell Biol. 3:498–508. [DOI] [PubMed] [Google Scholar]
- Dong, M., D.A. Richards, M.C. Goodnough, W.H. Tepp, E.A. Johnson, and E.R. Chapman. 2003. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J. Cell Biol. 162:1293–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, M., F. Yeh, W.H. Tepp, C. Dean, E.A. Johnson, R. Janz, and E.R. Chapman. 2006. SV2 is the protein receptor for botulinum neurotoxin A. Science. 312:592–596. [DOI] [PubMed] [Google Scholar]
- Fischer, A., and M. Montal. 2007. Single molecule detection of intermediates during botulinum neurotoxin translocation across membranes. Proc. Natl. Acad. Sci. USA. 104:10447–10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M.A., and J.R. Sanes. 2007. Synaptotagmin I and II are present in distinct subsets of central synapses. J. Comp. Neurol. 503:280–296. [DOI] [PubMed] [Google Scholar]
- Geppert, M., Y. Goda, R.E. Hammer, C. Li, T.W. Rosahl, C.F. Stevens, and T.C. Sudhof. 1994. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 79:717–727. [DOI] [PubMed] [Google Scholar]
- Hui, E., J. Bai, P. Wang, M. Sugimori, R.R. Llinas, and E.R. Chapman. 2005. Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc. Natl. Acad. Sci. USA. 102:5210–5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn, R., and R.H. Scheller. 2006. SNAREs–engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7:631–643. [DOI] [PubMed] [Google Scholar]
- Jin, R., A. Rummel, T. Binz, and A.T. Brunger. 2006. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature. 444:1092–1095. [DOI] [PubMed] [Google Scholar]
- Kitamura, M., M. Iwamori, and Y. Nagai. 1980. Interaction between Clostridium botulinum neurotoxin and gangliosides. Biochim. Biophys. Acta. 628:328–335. [DOI] [PubMed] [Google Scholar]
- Kitamura, M., K. Takamiya, S. Aizawa, and K. Furukawa. 1999. Gangliosides are the binding substances in neural cells for tetanus and botulinum toxins in mice. Biochim. Biophys. Acta. 1441:1–3. [DOI] [PubMed] [Google Scholar]
- Koriazova, L.K., and M. Montal. 2003. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat. Struct. Biol. 10:13–18. [DOI] [PubMed] [Google Scholar]
- Kozaki, S., Y. Kamata, S. Watarai, T. Nishiki, and S. Mochida. 1998. Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb. Pathog. 25:91–99. [DOI] [PubMed] [Google Scholar]
- Lewis, J.L., M. Dong, C.A. Earles, and E.R. Chapman. 2001. The transmembrane domain of syntaxin 1A is critical for cytoplasmic domain protein-protein interactions. J. Biol. Chem. 276:15458–15465. [DOI] [PubMed] [Google Scholar]
- Liu, Y., R. Wada, H. Kawai, K. Sango, C. Deng, T. Tai, M.P. McDonald, K. Araujo, J.N. Crawley, U. Bierfreund, et al. 1999. A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J. Clin. Invest. 103:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrhold, S., A. Rummel, H. Bigalke, B. Davletov, and T. Binz. 2006. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 580:2011–2014. [DOI] [PubMed] [Google Scholar]
- Matthew, W.D., L. Tsavaler, and L.F. Reichardt. 1981. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J. Cell Biol. 91:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco, C. 1986. How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem. Sci. 11:314–317. [Google Scholar]
- Montecucco, C., and J. Molgo. 2005. Botulinal neurotoxins: revival of an old killer. Curr. Opin. Pharmacol. 5:274–279. [DOI] [PubMed] [Google Scholar]
- Nicholson-Tomishima, K., and T.A. Ryan. 2004. Kinetic efficiency of endocytosis at mammalian CNS synapses requires synaptotagmin I. Proc. Natl. Acad. Sci. USA. 101:16648–16652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiki, T., and G.J. Augustine. 2004. Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J. Neurosci. 24:6127–6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiki, T., Y. Kamata, Y. Nemoto, A. Omori, T. Ito, M. Takahashi, and S. Kozaki. 1994. Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J. Biol. Chem. 269:10498–10503. [PubMed] [Google Scholar]
- Nishiki, T., Y. Tokuyama, Y. Kamata, Y. Nemoto, A. Yoshida, K. Sato, M. Sekiguchi, M. Takahashi, and S. Kozaki. 1996. The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett. 378:253–257. [DOI] [PubMed] [Google Scholar]
- Pang, Z.P., E. Melicoff, D. Padgett, Y. Liu, A.F. Teich, B.F. Dickey, W. Lin, R. Adachi, and T.C. Sudhof. 2006. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J. Neurosci. 26:13493–13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J.E., and G. Warren. 1994. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr. Biol. 4:220–233. [DOI] [PubMed] [Google Scholar]
- Rummel, A., T. Karnath, T. Henke, H. Bigalke, and T. Binz. 2004. a. Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J. Biol. Chem. 279:30865–30870. [DOI] [PubMed] [Google Scholar]
- Rummel, A., S. Mahrhold, H. Bigalke, and T. Binz. 2004. b. The HCC-domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol. Microbiol. 51:631–643. [DOI] [PubMed] [Google Scholar]
- Rummel, A., T. Eichner, T. Weil, T. Karnath, A. Gutcaits, S. Mahrhold, K. Sandhoff, R.L. Proia, K.R. Acharya, H. Bigalke, and T. Binz. 2007. Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proc. Natl. Acad. Sci. USA. 104:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo, G., F. Benfenati, B. Poulain, O. Rossetto, P. Polverino de Laureto, B.R. DasGupta, and C. Montecucco. 1992. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 359:832–835. [DOI] [PubMed] [Google Scholar]
- Schiavo, G., O. Rossetto, S. Catsicas, P. Polverino de Laureto, B.R. DasGupta, F. Benfenati, and C. Montecucco. 1993. a. Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J. Biol. Chem. 268:23784–23787. [PubMed] [Google Scholar]
- Schiavo, G., C.C. Shone, O. Rossetto, F.C. Alexander, and C. Montecucco. 1993. b. Botulinum neurotoxin serotype F is a zinc endopeptidase specific for VAMP/synaptobrevin. J. Biol. Chem. 268:11516–11519. [PubMed] [Google Scholar]
- Schiavo, G., C. Malizio, W.S. Trimble, P. Polverino de Laureto, G. Milan, H. Sugiyama, E.A. Johnson, and C. Montecucco. 1994. Botulinum G neurotoxin cleaves VAMP/synaptobrevin at a single Ala-Ala peptide bond. J. Biol. Chem. 269:20213–20216. [PubMed] [Google Scholar]
- Schiavo, G., M. Matteoli, and C. Montecucco. 2000. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 80:717–766. [DOI] [PubMed] [Google Scholar]
- Simpson, L.L. 2004. Identification of the major steps in botulinum toxin action. Annu. Rev. Pharmacol. Toxicol. 44:167–193. [DOI] [PubMed] [Google Scholar]
- Sudhof, T.C. 2004. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27:509–547. [DOI] [PubMed] [Google Scholar]
- Sutton, R.B., J.A. Ernst, and A.T. Brunger. 1999. Crystal structure of the cytosolic C2A-C2B domains of synaptotagmin III. Implications for Ca(+2)-independent snare complex interaction. J. Cell Biol. 147:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto, K., T. Kohda, M. Mukamoto, K. Takeuchi, H. Ihara, M. Saito, and S. Kozaki. 2005. Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J. Biol. Chem. 280:35164–35171. [DOI] [PubMed] [Google Scholar]
- Ullrich, B., C. Li, J.Z. Zhang, H. McMahon, R.G. Anderson, M. Geppert, and T.C. Sudhof. 1994. Functional properties of multiple synaptotagmins in brain. Neuron. 13:1281–1291. [DOI] [PubMed] [Google Scholar]
- Verderio, C., S. Coco, O. Rossetto, C. Montecucco, and M. Matteoli. 1999. Internalization and proteolytic action of botulinum toxins in CNS neurons and astrocytes. J. Neurochem. 73:372–379. [DOI] [PubMed] [Google Scholar]
- Verderio, C., O. Rossetto, C. Grumelli, C. Frassoni, C. Montecucco, and M. Matteoli. 2006. Entering neurons: botulinum toxins and synaptic vesicle recycling. EMBO Rep. 7:995–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yowler, B.C., and C.L. Schengrund. 2004. Botulinum neurotoxin A changes conformation upon binding to ganglioside GT1b. Biochemistry. 43:9725–9731. [DOI] [PubMed] [Google Scholar]