Abstract
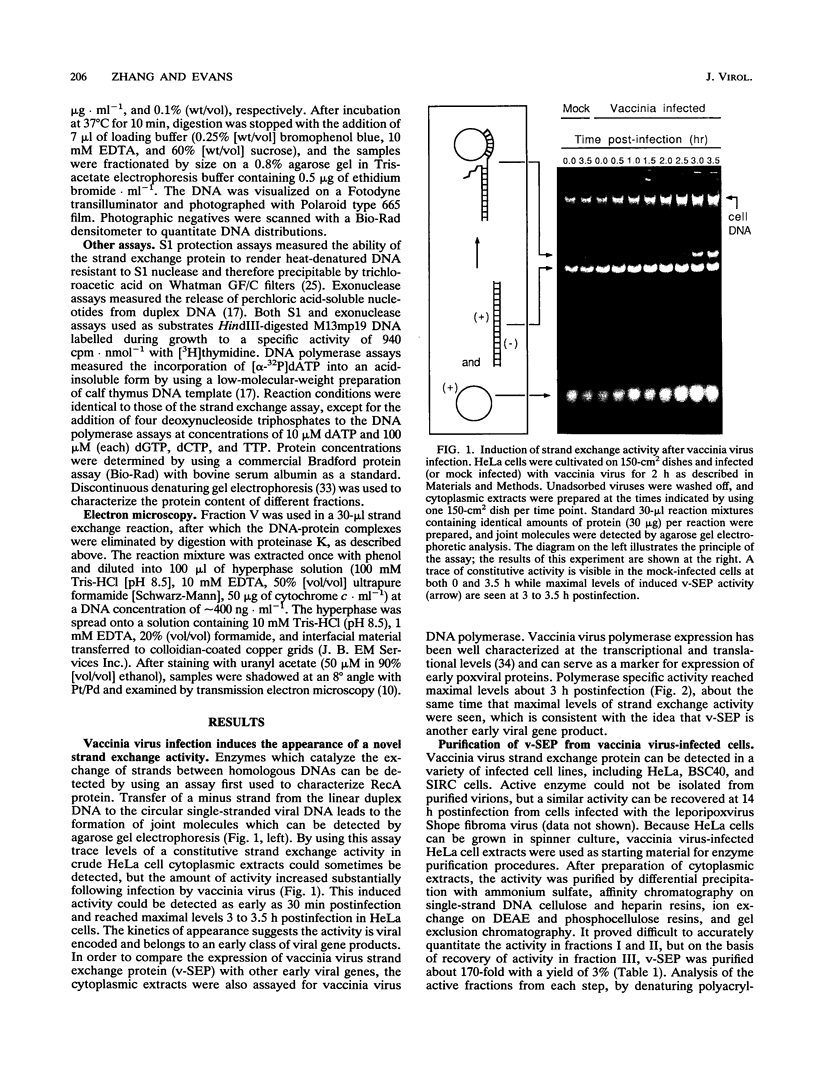
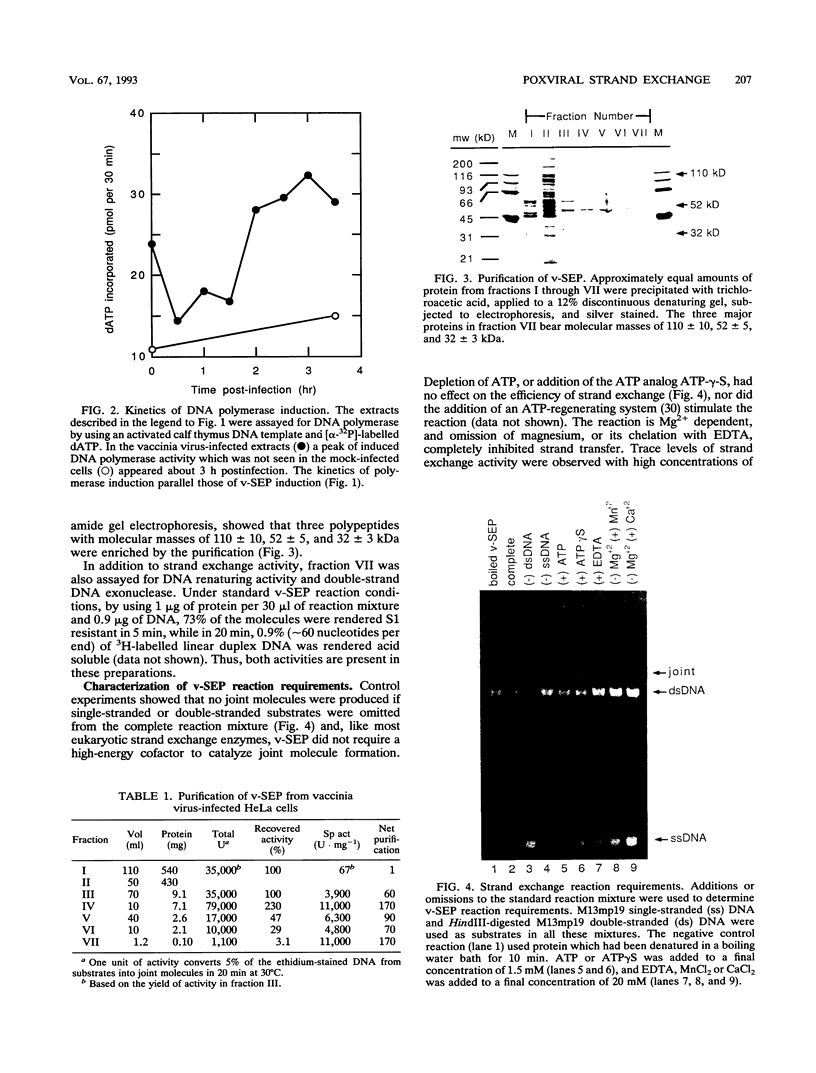
Vaccinia virus infection induces expression of a protein which can catalyze joint molecule formation between a single-stranded circular DNA and a homologous linear duplex. The kinetics of appearance of the enzyme parallels that of vaccinia virus DNA polymerase and suggests it is an early viral gene product. Extracts were prepared from vaccinia virus-infected HeLa cells, and the strand exchange assay was used to follow purification of this activity through five chromatographic steps. The most highly purified fraction contained three major polypeptides of 110 +/- 10, 52 +/- 5, and 32 +/- 3 kDa. The purified protein requires Mg2+ for activity, and this requirement cannot be satisfied by Mn2+ or Ca2+. One end of the linear duplex substrate must share homology with the single-stranded circle, although this homology requirement is not very high, as 10% base substitutions had no effect on the overall efficiency of pairing. As with many other eukaryotic strand exchange proteins, there was no requirement for ATP, and ATP analogs were not inhibitors. Electron microscopy was used to show that the joint molecules formed in these reactions were composed of a partially duplex circle of DNA bearing a displaced single-strand and a duplex linear tail. The recovery of these structures shows that the enzyme catalyzes true strand exchange. There is also a unique polarity to the strand exchange reaction. The enzyme pairs the 3' end of the duplex minus strand with the plus-stranded homolog, thus extending hybrid DNA in a 3'-to-5' direction with respect to the minus strand. Which viral gene (if any) encodes the enzyme is not yet known, but analysis of temperature-sensitive mutants shows that activity does not require the D5R gene product. Curiously, v-SEP appears to copurify with vaccinia virus DNA polymerase, although the activities can be partially resolved on phosphocellulose columns.
Full text
PDF


Images in this article
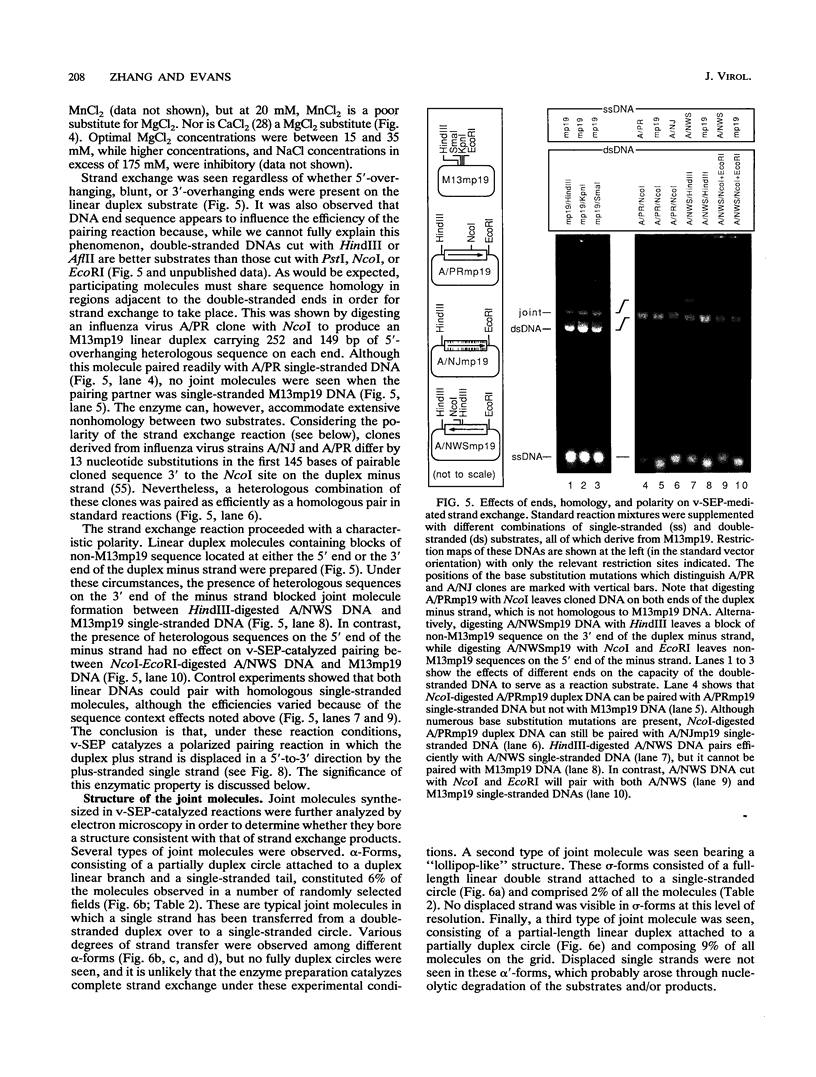
Selected References
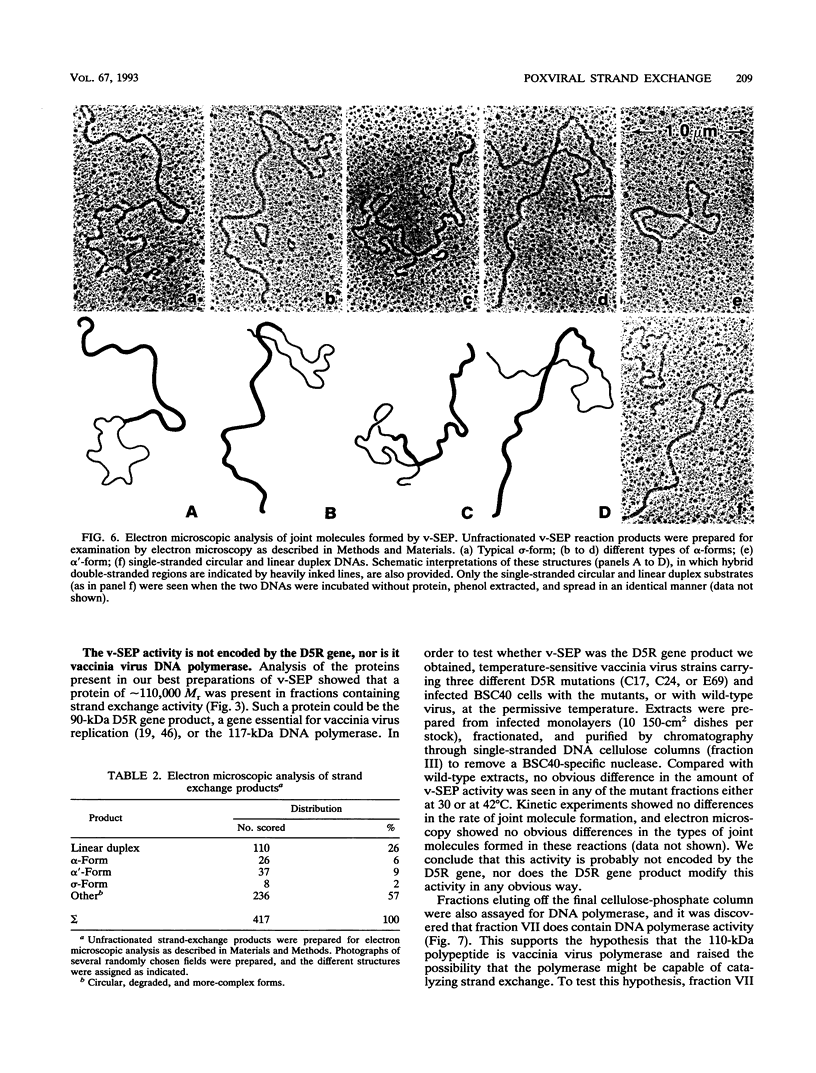
These references are in PubMed. This may not be the complete list of references from this article.
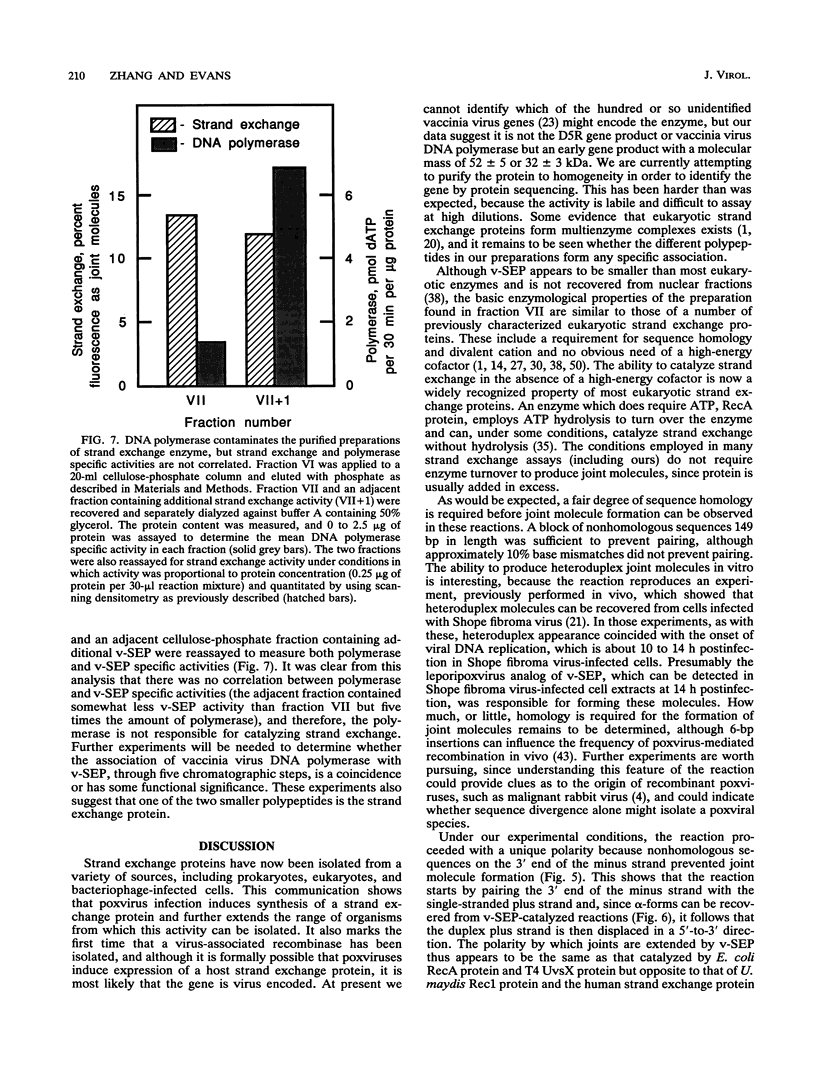
- Arai N., Kawasaki K., Shibata T. A multicomponent protein of a fission yeast that promotes joint molecule formation from homologous DNAs. J Biol Chem. 1992 Feb 15;267(5):3514–3522. [PubMed] [Google Scholar]
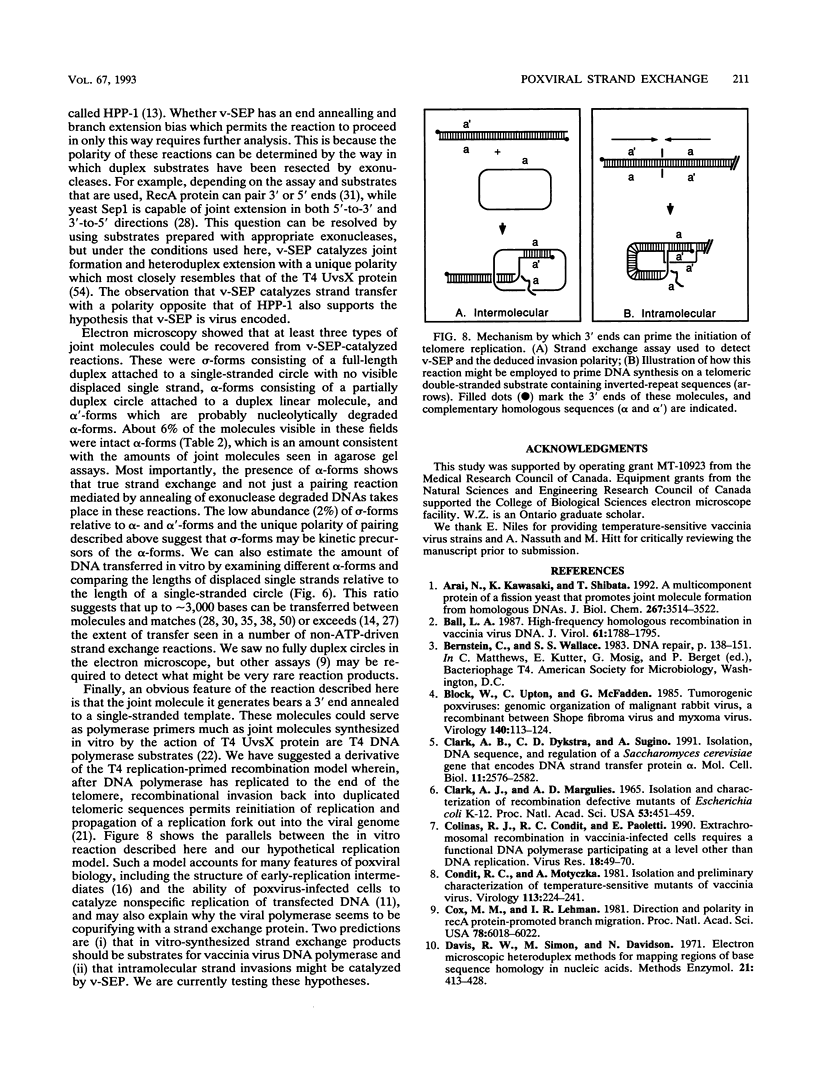
- Ball L. A. High-frequency homologous recombination in vaccinia virus DNA. J Virol. 1987 Jun;61(6):1788–1795. doi: 10.1128/jvi.61.6.1788-1795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block W., Upton C., McFadden G. Tumorigenic poxviruses: genomic organization of malignant rabbit virus, a recombinant between Shope fibroma virus and myxoma virus. Virology. 1985 Jan 15;140(1):113–124. doi: 10.1016/0042-6822(85)90450-7. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. B., Dykstra C. C., Sugino A. Isolation, DNA sequence, and regulation of a Saccharomyces cerevisiae gene that encodes DNA strand transfer protein alpha. Mol Cell Biol. 1991 May;11(5):2576–2582. doi: 10.1128/mcb.11.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinas R. J., Condit R. C., Paoletti E. Extrachromosomal recombination in vaccinia-infected cells requires a functional DNA polymerase participating at a level other than DNA replication. Virus Res. 1990 Dec;18(1):49–70. doi: 10.1016/0168-1702(90)90089-t. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. Directionality and polarity in recA protein-promoted branch migration. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6018–6022. doi: 10.1073/pnas.78.10.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange A. M., McFadden G. Sequence-nonspecific replication of transfected plasmid DNA in poxvirus-infected cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):614–618. doi: 10.1073/pnas.83.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra C. C., Kitada K., Clark A. B., Hamatake R. K., Sugino A. Cloning and characterization of DST2, the gene for DNA strand transfer protein beta from Saccharomyces cerevisiae. Mol Cell Biol. 1991 May;11(5):2583–2592. doi: 10.1128/mcb.11.5.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleston A. K., Kowalczykowski S. C. An overview of homologous pairing and DNA strand exchange proteins. Biochimie. 1991 Feb-Mar;73(2-3):163–176. doi: 10.1016/0300-9084(91)90199-b. [DOI] [PubMed] [Google Scholar]
- Eisen A., Camerini-Otero R. D. A recombinase from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7481–7485. doi: 10.1073/pnas.85.20.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J. Isolation and genetic characterization of temperature-sensitive mutants of vaccinia virus WR. J Virol. 1982 Sep;43(3):778–790. doi: 10.1128/jvi.43.3.778-790.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban M., Flores L., Holowczak J. A. Model for vaccinia virus DNA replication. Virology. 1977 Dec;83(2):467–473. doi: 10.1016/0042-6822(77)90197-0. [DOI] [PubMed] [Google Scholar]
- Evans D. H., Linn S. Excision repair of pyrimidine dimers from simian virus 40 minichromosomes in vitro. J Biol Chem. 1984 Aug 25;259(16):10252–10259. [PubMed] [Google Scholar]
- Evans D. H., Stuart D., McFadden G. High levels of genetic recombination among cotransfected plasmid DNAs in poxvirus-infected mammalian cells. J Virol. 1988 Feb;62(2):367–375. doi: 10.1128/jvi.62.2.367-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Traktman P. Molecular genetic analysis of a vaccinia virus gene with an essential role in DNA replication. J Virol. 1987 Oct;61(10):3152–3162. doi: 10.1128/jvi.61.10.3152-3162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel R., Derbyshire M. K., Moore S. P., Young C. S. Biochemical studies of homologous and nonhomologous recombination in human cells. Biochimie. 1991 Feb-Mar;73(2-3):257–267. doi: 10.1016/0300-9084(91)90211-i. [DOI] [PubMed] [Google Scholar]
- Fisher C., Parks R. J., Lauzon M. L., Evans D. H. Heteroduplex DNA formation is associated with replication and recombination in poxvirus-infected cells. Genetics. 1991 Sep;129(1):7–18. doi: 10.1093/genetics/129.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa T., Alberts B. M. DNA synthesis dependent on genetic recombination: characterization of a reaction catalyzed by purified bacteriophage T4 proteins. Cell. 1986 Dec 5;47(5):793–806. doi: 10.1016/0092-8674(86)90522-2. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Halbrook J., McEntee K. Purification and characterization of a DNA-pairing and strand transfer activity from mitotic Saccharomyces cerevisiae. J Biol Chem. 1989 Dec 15;264(35):21403–21412. [PubMed] [Google Scholar]
- Heyer W. D., Evans D. H., Kolodner R. D. Renaturation of DNA by a Saccharomyces cerevisiae protein that catalyzes homologous pairing and strand exchange. J Biol Chem. 1988 Oct 15;263(29):15189–15195. [PubMed] [Google Scholar]
- Hsieh P., Meyn M. S., Camerini-Otero R. D. Partial purification and characterization of a recombinase from human cells. Cell. 1986 Mar 28;44(6):885–894. doi: 10.1016/0092-8674(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Johnson A. W., Kolodner R. D. Strand exchange protein 1 from Saccharomyces cerevisiae. A novel multifunctional protein that contains DNA strand exchange and exonuclease activities. J Biol Chem. 1991 Jul 25;266(21):14046–14054. [PubMed] [Google Scholar]
- Kmiec E., Holloman W. K. Homologous pairing of DNA molecules promoted by a protein from Ustilago. Cell. 1982 Jun;29(2):367–374. doi: 10.1016/0092-8674(82)90153-2. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Evans D. H., Morrison P. T. Purification and characterization of an activity from Saccharomyces cerevisiae that catalyzes homologous pairing and strand exchange. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5560–5564. doi: 10.1073/pnas.84.16.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konforti B. B., Davis R. W. DNA substrate requirements for stable joint molecule formation by the RecA and single-stranded DNA-binding proteins of Escherichia coli. J Biol Chem. 1991 Jun 5;266(16):10112–10121. [PubMed] [Google Scholar]
- Kowalczykowski S. C. Biochemistry of genetic recombination: energetics and mechanism of DNA strand exchange. Annu Rev Biophys Biophys Chem. 1991;20:539–575. doi: 10.1146/annurev.bb.20.060191.002543. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W. F., Crozel-Goudot V., Traktman P. Transient expression of the vaccinia virus DNA polymerase is an intrinsic feature of the early phase of infection and is unlinked to DNA replication and late gene expression. J Virol. 1992 Jan;66(1):534–547. doi: 10.1128/jvi.66.1.534-547.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetski J. P., Bear D. G., Kowalczykowski S. C. Stable DNA heteroduplex formation catalyzed by the Escherichia coli RecA protein in the absence of ATP hydrolysis. Proc Natl Acad Sci U S A. 1990 Jan;87(1):21–25. doi: 10.1073/pnas.87.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchlinsky M. Intramolecular homologous recombination in cells infected with temperature-sensitive mutants of vaccinia virus. J Virol. 1989 May;63(5):2030–2035. doi: 10.1128/jvi.63.5.2030-2035.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. P., Fishel R. Purification and characterization of a protein from human cells which promotes homologous pairing of DNA. J Biol Chem. 1990 Jul 5;265(19):11108–11117. [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Isolation and properties of the vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1977 Oct 10;252(19):6930–6938. [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Fungal recombination. Microbiol Rev. 1985 Mar;49(1):33–58. doi: 10.1128/mr.49.1.33-58.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks R. J., Evans D. H. Effect of marker distance and orientation on recombinant formation in poxvirus-infected cells. J Virol. 1991 Mar;65(3):1263–1272. doi: 10.1128/jvi.65.3.1263-1272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks R. J., Evans D. H. Enhanced recombination associated with the presence of insertion and deletion mutations in poxvirus-infected cells. Virology. 1991 Sep;184(1):299–309. doi: 10.1016/0042-6822(91)90846-4. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Helical interactions in homologous pairing and strand exchange driven by RecA protein. J Biol Chem. 1991 Mar 25;266(9):5355–5358. [PubMed] [Google Scholar]
- Roseman N. A., Hruby D. E. Nucleotide sequence and transcript organization of a region of the vaccinia virus genome which encodes a constitutively expressed gene required for DNA replication. J Virol. 1987 May;61(5):1398–1406. doi: 10.1128/jvi.61.5.1398-1406.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M., Lowenhaupt K., Lane W. S., Rich A. Cloning and characterization of Rrp1, the gene encoding Drosophila strand transferase: carboxy-terminal homology to DNA repair endo/exonucleases. Nucleic Acids Res. 1991 Aug 25;19(16):4523–4529. doi: 10.1093/nar/19.16.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto J., Celenza L. M., Condit R. C., Niles E. G. Genetic map of the vaccinia virus HindIII D Fragment. Virology. 1987 Sep;160(1):110–119. doi: 10.1016/0042-6822(87)90051-1. [DOI] [PubMed] [Google Scholar]
- Spyropoulos D. D., Roberts B. E., Panicali D. L., Cohen L. K. Delineation of the viral products of recombination in vaccinia virus-infected cells. J Virol. 1988 Mar;62(3):1046–1054. doi: 10.1128/jvi.62.3.1046-1054.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Nitiss J., Resnick M. A. ATP-independent DNA strand transfer catalyzed by protein(s) from meiotic cells of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3683–3687. doi: 10.1073/pnas.85.11.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff D. X., Johnson A. W., Kolodner R. D. Molecular and genetic analysis of the gene encoding the Saccharomyces cerevisiae strand exchange protein Sep1. Mol Cell Biol. 1991 May;11(5):2593–2608. doi: 10.1128/mcb.11.5.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktman P. The enzymology of poxvirus DNA replication. Curr Top Microbiol Immunol. 1990;163:93–123. doi: 10.1007/978-3-642-75605-4_4. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonesaki T., Minagawa T. T4 phage gene uvsX product catalyzes homologous DNA pairing. EMBO J. 1985 Dec 1;4(12):3321–3327. doi: 10.1002/j.1460-2075.1985.tb04083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. D., Evans D. H. Detection and identification of human influenza viruses by the polymerase chain reaction. J Virol Methods. 1991 Jun;33(1-2):165–189. doi: 10.1016/0166-0934(91)90017-t. [DOI] [PubMed] [Google Scholar]