Abstract
Background
Recent evidence indicates that individuals who are homozygous for the short (s) allele in the promoter region of the serotonin transporter gene have higher rates of depression and other psychiatric disorders as a function of exposure to increasing levels of stressful life events than do individuals who have one or two copies of the long (l) allele. Despite the reliability of this association, the mechanism by which this polymorphism confers risk for psychopathology in the presence of stress is not understood. The present study was designed to examine the formulation that individuals who are homozygous for the s allele are characterized by a greater biological reactivity to stress than are their counterparts who have one or two copies of the l allele.
Methods
Girls at high (n = 25) and low (n = 42) risk for depression by virtue of the presence or absence of a family history of this disorder were genotyped and exposed to a standardized laboratory stress task. Cortisol levels were assessed before the stressor, after the stressor, and during an extended recovery period.
Results
Girls who were homozygous for the s-allele produced higher and more prolonged levels of cortisol in response to the stressor than did girls with an l allele.
Conclusions
These findings indicate that the 5-HTTLPR polymorphism is associated with biological stress reactivity, which may increase susceptibility to depression in the face of stressful life events.
Major Depressive Disorder (MDD) is one of the most common and debilitating of all psychiatric disorders (1). The high chronicity and recurrence of depression, combined with its significant prevalence, personal loss, and societal costs, make it imperative that we identify factors that are involved in the onset of this disorder. Consistent findings that a family history of depression is one of the strongest predictors of the development of this disorder have led investigators to examine the heritability of depression. Indeed, there is now considerable evidence from twin, adoption, and pedigree investigations, and from genome-wide linkage studies, indicating that there is a significant genetic contribution to MDD (2). It is important to note, however, that the majority of individuals with a positive family history of depression do not develop the disorder. Thus, most contemporary theories concerning the role of genes in the onset of depression do not postulate that genes affect depression directly; rather, they are explicitly diathesis-stress theories, positing that a genetic vulnerability interacts with major life stressors to produce depression (3). Given the importance of the serotonin system in depression and the effectiveness of selective serotonin reuptake inhibitors in the treatment of this disorder, it is not surprising that investigators examining this formulation have focused on the serotonin transporter (5-HTT) gene (SLC6A4) and, in particular, on a polymorphism in the promoter region of this gene (5-HTTLPR). The short and long alleles in the 5-HTTLPR have been shown to affect transcriptional rates of the 5-HTT gene (4). Perhaps most notably, Caspi et al. (5) recently found that individuals with one or two copies of the short allele of the 5-HTTLPR polymorphism exhibited higher levels of depressive symptoms, higher rates of diagnosable depression, and more suicidality as a function of exposure to increasing levels of stressful life events than did individuals who were homozygous for the long allele.
Several investigators have now replicated Caspi et al.’s (5) results (6,7,8). Despite growing evidence that the 5-HTT gene moderates the association between life stress and depression, however, the mechanisms underlying this moderation are not well understood. Findings from animal research suggest that one possible mechanism involves the construct of stress reactivity. Li et al. (9), for example, found that mice with diminished or absent function of the 5-HTT gene exhibited greater increases in the stress hormone adrenocorticotropin (ACTH) in response to stress than did their control littermates. Indirect support for the involvement of stress reactivity also comes from studies with humans. Kendler et al. (10) found that individuals with two short alleles in the 5-HTTLPR were more likely to become depressed in response to “common, low-threat events,” and hypothesized that this genetic polymorphism produces an increased sensitivity to the impact of stressful events. Perhaps reflecting this “sensitivity,” investigators have found that, compared to individuals who have at least one long allele in the promoter region of the 5-HTT gene, individuals homozygous for the short allele exhibit greater amygdala activation in response to fearful stimuli (11,12).
Consistent with this “stress reactivity” formulation, investigators have found cortisol, a reliable indicator of hypothalamic-pituitary-adrenocortical (HPA) axis functioning and stress reactivity, not only to have a hereditary component (13), but further, to be elevated in 40–60% of adults diagnosed with MDD (14). Indeed, hypercortisolemia has been postulated to lead to hippocampal neuronal loss, which in turn has been posited to be involved in the pathogenesis of depression (15). Given the research described above, it is possible that depressed individuals, many of whom are likely to be 5-HTTLPR “s-carriers,”5 are characterized by hypercortisolemia not only because they have been exposed to a greater number of stressful life events than are nondepressed persons (16), but also because they are biologically more reactive to stressors. Indeed, the results of a recent meta-analysis indicate that MDD patients have higher cortisol levels following exposure to a stressor than do nondepressed individuals (17). The formulation that s-carriers are more biologically reactive to stress than are individuals who are homozygous for the long allele may explain both Caspi et al.’s (5) finding of an increased likelihood of developing depression in response to stressful events among s-carriers and Kendler et al.’s (10) finding of the importance of low-threat events in predicting the onset of depression.
The present study was designed, in part, to examine this formulation. To ensure that we had participants in this study who might go on to develop an episode of depression, we assessed genotype and stress reactivity in young girls at high and low risk for this disorder by virtue of a the presence or absence of a family history of recurrent depression (18). Based on the literatures reviewed above, we predicted that girls who were homozygous for the short allele 5-HTTLPR polymorphism would exhibit greater and more prolonged cortisol production in response to a laboratory stressor than would girls with one or two long alleles.
Method
Participants
Participants were 67 girls between the ages of 9 and 14 with no current or past Axis I disorder. Forty-two girls had biological mothers with no current or past Axis I disorder (low-risk daughters), and 25 girls had biological mothers with a history of recurrent episodes of Major Depressive Disorder (MDD) during their daughter’s lifetime (high-risk daughters). Participants were recruited through advertisements posted in numerous locations within the local community (e.g., internet bulletin boards, university kiosks, supermarkets) and through the Department of Psychiatry and Behavioral Sciences at Stanford University. The mothers’ responses to a telephone interview provided initial selection information. This phone screen established that both mothers and daughters were fluent in English and that daughters were between 9 and 14 years of age. This telephone interview was also used to identify mothers who were likely either to have no psychiatric history or to meet criteria for recurrent depression during their daughter’s lifetime, and daughters who were likely to have no past or current psychiatric disorder. Those mother and daughter pairs who were considered likely to be eligible for participation were invited to come to the laboratory for more extensive interviews.
Assessment of Depression and Psychopathology
Interviews
All mothers and daughters were administered structured clinical interviews by different trained interviewers to diagnose the presence of at least two distinct episodes of depression in the MDD mothers since the birth of their daughters (Structured Clinical Interview for DSM-IV-TR [SCID]) (19) and a lifetime absence of psychopathology in the daughters (Kiddie Schedule for Affective Disorders and Schizophrenia [K-SADS]) (20) and in the control mothers (SCID). Both the daughters and the mothers were administered the K-SADS to assess the daughters’ functioning, and both informants had to report an absence of any past or current Axis-I disorder in the daughter. To assess inter-rater reliability, an independent trained rater who was blind to group membership randomly evaluated 10% of the SCID and K-SAD-PL interviews. In all cases, diagnoses of former depressive episodes in mothers, no history of depressive episodes in mothers, and absence of any current or previous Axis-I disorder in the girls matched the diagnosis made by the original interviewer, κ=1.00. Eligible participants were invited to come back to the lab within one week of their interview session to take part in the stress task, and saliva samples for the DNA analyses were taken.
Questionnaires
Daughters completed the 10-item version of the Children’s Depression Inventory (CDI-S) (21), a self-report measure of depressive symptomatology for children between the ages of 8 and 17. Mothers completed the Beck Depression Inventory-II (BDI) (22), a 21-item self-report measure of the severity of depressive symptoms.
Verbal Intelligence
The vocabulary section of the verbal subtest of the Wechsler Intelligence Scale for Children-III (23) was administered to the daughters to ensure that any group differences in response to the stressor are not a function of differences in intellectual ability.
Cortisol collection and stress task
Participants refrained from eating and drinking (except water) one hour prior to arrival at the laboratory and during the experimental procedures. Participants were first instructed to rest and relax for 30 min. They were allowed to read magazines and listen to music, and saliva samples were collected just before they were given instructions for the task. They then underwent a 15-minute laboratory session during which they were stressed by an experimenter and salivary cortisol was collected at regular intervals. More specifically, daughters completed a three-minute serial subtraction task in which they were instructed to begin at 400 and count backwards by 7’s as quickly and accurately as possible. If they made a mistake they were interrupted by the experimenter and were told to start over. Daughters who moved quickly and easily through this procedure were stopped and told to start over at 4000 and count backwards by 17’s. Following this task, daughters were administered the 12-minute Ewart Social Competence Interview (24), a semi-structured interview developed to induce emotional stress and arousal in adolescents by discussing details of stressful life situations.
Four saliva samples were collected from each girl using salivettes (Sarstedt, Germany) during the laboratory stress paradigm: one sample immediately before task instructions and three samples at 15-, 30-, and 45-minutes post-onset of stressor. These collection times are based on meta-analytic findings indicating that peak cortisol response occurs 21–40 minutes following the onset of an acute stressor and that complete recovery to baseline values occurs within 41–60 minutes (25). Following the laboratory stressor (i.e., during collection of the final two samples), participants watched a neutral videotape about Denali National Park. On average, the first sample was collected at 12:15 h; groups did not differ in their collection times, t(65) < 1. Saliva samples were stored in a freezer chest until they were transferred to a −20° freezer located at the General Clinical Research Center at Stanford University, where they were maintained until radioimmunoassays were performed. Samples were assayed together to control for inter-assay error, and controls were included to evaluate variability.
A minimum of 0.2 ml of liquid saliva was absorbed into a small cotton roll and expressed through a plastic tube into a sterile vial (Salivette Sarstedt without additives device). Cortisol was assayed by luminescence immunoassay reagents using a commercial kit from Immuno-Biological Laboratories, Inc (Hamburg, Germany). The sensitivity of the assay was set at 0.015 mg/dl (or 0.414 nmol/l). The intra-assay variation on three saliva pools of the low, medium, and high controls were averaged 2.78%, 10.45%, and 4.79%, respectively. The mean values of the low, medium, and high controls were 0.054, 0.228, and 0.863 mg/dl, respectively. The inter-assay coefficients of the variations of the low, medium, and high controls were 10.9, 10.5 and 5.5%, respectively.
Genotyping
To genotype the daughters, saliva was collected using the Oragene Kit (DNA Genotek, Inc. Ottawa, Ontario, Canada), an all-in-one system for the collection, preservation, transportation, and purification of DNA from saliva. DNA extracted by this method is of high quality and allows for a high success rate of genotyping (26); indeed, the DNA yield in our laboratory is 20 ug and higher. Oligonucleotide primers flanking the 5-HTT-linked polymorphic region (27) and corresponding to the nucleotide positions −1416 to −1397 (stpr5, 5′-GGC GTT GCC GCT CTG AAT GC) and −910 to −888 (stpr3, 5′-GAG GGA CTG AGC TGG ACA ACC AC) of the 5-HTT gene 5′-flanking regulatory region were used to generate 484bp or 528bp fragments. The PCR products were electrophoresed through 5% Polyacrylamide gel (Acrylamide/bis-Acrylamide ratio 19:1) at 60 V for 60 min.
Results
Participant Characteristics
Demographic and clinical characteristics of the high-risk (RSK) and low-risk (CTL) groups are presented in Table 1. There were no significant group differences in age, t(65) < 1, proportion of girls who were post-menarcheal, χ2(1,67) <1, WISC vocabulary scores, t(57) = 1.56, ethnicity, χ2(1,67) < 1, or mothers’ education, χ2(1,66) = 1.76, all ps > .05; the CTL group had a higher percentage of married mothers, χ2(1,66) = 12.58, p<.01. The RSK girls obtained slightly but significantly higher scores on the CDI-S than did the CTL girls, t(61) = 3.87, p < .01; it is important to note, however, that both groups were far below the recommended cut-off score of 8 (21). In addition, although they did not meet diagnostic criteria for current MDD, the mothers of the RSK girls obtained significantly higher scores on the BDI-II than did the mothers of the CTL girls, t(62) = 4.17, p < .01. Finally, although none of the mothers had a current diagnosis of an Axis-I disorder, six of the mothers in the high-risk group were diagnosed with a past disorder besides MDD: two with obsessive-compulsive disorder, one with post-traumatic stress disorder, one with panic disorder/social phobia, and one with specific phobia/bulimia nervosa.
Table 1.
Demographic information for the low-risk and high-risk participants
| Low Risk | High Risk | |
|---|---|---|
| Daughter age M (SD) | 11.76 (1.51) | 12.16 (1.70) |
| Post-pubertal % | 43 | 55 |
| Mother age M (SD) | 44.10 (5.22) | 43.52 (7.19) |
| Caucasian % | 81 | 72 |
| Married % | 85 | 44 |
| College education % | 85 | 72 |
| Daughter CDI M (SD) | 1.13 (1.45) | 3.04 (2.45) |
| Mother BDI M (SD) | 2.41 (3.51) | 9.08 (9.01) |
| Daughter WISC M (SD) | 48.23 (8.01) | 51.29 (6.35) |
Notes. CDI = Child Depression Inventory; BDI = Beck Depression Inventory; WISC = Wechsler Intelligence Scale for Children.
Genotype and Risk for Depression
The 5-HTTLPR allele frequencies for the low-risk and high-risk groups are presented in Table 2. The frequency of the s-allele in Caucasian populations is approximately .40; the genotype frequencies are in Hardy-Weinberg equilibrium. Although it appears that a higher proportion of the high-risk than the low-risk girls have at least one copy of the s-allele, the two groups of daughters do not differ significantly in their genotype distribution, χ2(2)=1.91 p>.05. Consequently, we combined participants in the low- and high-risk groups in examining the effects of genotype on stress reactivity.
Table 2.
Genotype counts in the low-risk and high-risk groups
| N | N | N | |
|---|---|---|---|
| Genotype | Full Sample | Low Risk | High Risk |
| S/s | 19 | 11 | 8 |
| S/l | 34 | 20 | 14 |
| L/l | 14 | 11 | 3 |
Genotype and Stress Reactivity
Several outlier cortisol samples at baseline and during the stress session positively skewed the data, even after logarithmic and other types of transformations. We therefore excluded these outlying samples from the analysis. Outliers were defined as values that were above 1.5 times the inter-quartile range for a given collection point. The data were analyzed using mixed-design analyses of variance with group and collection time as the main factors. We controlled for age effects and effects of time of day that the stress task was administered, as well as for demographic and clinical variables1. Additional analyses were conducted using the incremental area under the curve (AUCi), an index measuring increase in hormone levels from baseline within a series of measurements (31).
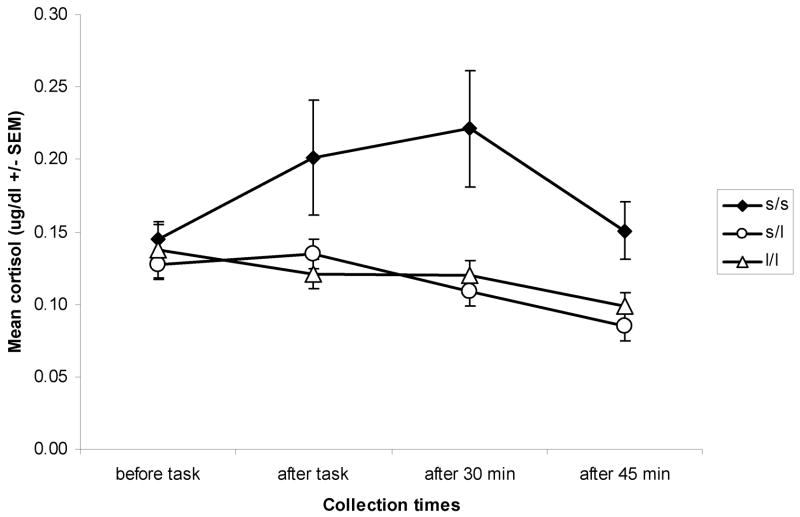
We examined patterns of stress reactivity as a function of genotype (Figure 1). Daughters who are homozygous for the s-allele showed a marked increase in cortisol production during and following exposure to the stressor. In contrast, daughters with at least one copy of the long allele exhibited a slight decrease in cortisol production over the course of the stress session. This differential pattern of responding is reflected in a significant interaction of genotype and time in the linear trend, F(2,63)=4.30, p<.05, and in the quadratic trend, F(2,63)=3.51, p<.05. Importantly, this interaction remained significant after controlling for mothers’ marital status and BDI scores, participants’ ages and CDI scores, and time of day at which the stress task was administered. These genotype-associated differences in cortisol response and recovery are further supported by a significant difference in incremental area under the curve (AUCi), an index measuring increase in cortisol levels from baseline within a series of measurements (31), F(2,63)=4.26, p<.05, reflecting the elevated cortisol production in response to stress in daughters who were homozygous for the s-allele2.
Figure 1.
Stress reactivity by genotype
Discussion
The pattern of findings reported here both provides an explanation for the results of studies documenting associations among genotype, exposure to stress, and probability of depression, and extends our understanding of the relation between the 5-HTTLPR polymorphism and stress reactivity. Investigators have known for decades that exposure to severe life stressors increases the likelihood of subsequent depression (35). We also know, however, that many individuals who experience life stress do not develop depression. Addressing this issue, there is now a growing literature demonstrating higher rates of depression among individuals with at least one copy of the short allele of the 5-HTTLPR polymorphism with increasing exposure to stressful life events than among individuals who are homozygous for the long allele. In particular, Kendler et al. (10) found that individuals with two copies of the short allele were more likely than were their counterparts with two long alleles to become depressed in response to “common, low-threat events.” Although Kendler et al. hypothesized, based on these data, that this genetic polymorphism produces an increased sensitivity to the impact of stressful events that in turn increases the likelihood of depression, they could not explicitly examine this formulation with their data.
The findings presented in this paper demonstrate for the first time in a sample of young individuals that biological stress reactivity may be a critical mechanism underlying the association between the serotonin transporter gene and exposure to stressful events in increasing risk for depression. Although investigators have recognized the relation between the serotonin system and depression, only recently have they focused on the link between serotonin and stress. In fact, several lines of evidence now suggest that the serotonin system plays an important role in regulating HPA-axis activity. For example, researchers have now documented involvement of serotonin neurotransmission in both activation and feedback control of the HPA axis (36). More specifically, investigators have demonstrated in animals that serotonin activates the HPA axis by stimulating CRF release, triggering ACTH release, and stimulating corticosteroid secretion (37). Indeed, serotonin has been found to enhance the negative feedback control of cortisol (38). Moreover, as we noted earlier, mice with the serotonin transporter gene knocked out have been found to exhibit increased HPA-axis response to acute stress (9). Finally, Barr et al. (39) examined the interactive influence of variation in the serotonin transporter gene promoter region and rearing condition on endocrine responses to stress in infant rhesus macaques. These investigators found that animals with one copy of the s allele raised by peers had higher levels of ACTH during separation than did both l/l animals and animals with an s allele that were raised maternally, indicating that serotonin transporter gene variation affects HPA-axis activity, and that the influence of 5-HTTLPR on hormonal responses during stress is modulated by early experience.
It is noteworthy that while daughters with s/l and l/l allele polymorphisms in this study were both less reactive to stress than were homozygous s-allele carriers, they did not differ significantly from each other; that is, the s/l daughters were not more reactive than were their l/l counterparts. Studies of the interaction of genotype with a history of severe life stress have generally found a step function, in which having one short allele is associated with greater risk for depression than is having two long alleles, and being homozygous for the short allele confers the highest risk (5). This is the first study to examine cortisol levels in response to a laboratory stressor as a function of genotype. It may be that, in contrast to cumulative severe life events, a single laboratory stressor is too mild, transient, and circumscribed to elicit cortisol production in l/l and s/l individuals, but sufficient to provoke a cortisol response in s/s participants. Thus, individuals who are homozygous for the short allele may have a lower threshold for cortisol production in response to stress than do their peers. Indeed, this formulation is consistent with Kendler et al.’s (10) finding that individuals with two copies of the short allele were more likely than were their counterparts with two long alleles to become depressed in response to what they referred to as “common, low-threat events.” Studies designed to manipulate stress level parametrically would be valuable in examining this formulation more explicitly.
In closing, we have demonstrated in this study that biological stress reactivity is a plausible mechanism underlying the association between genotype and exposure to life stress in predicting the onset of depression. A notable strength of this study is that the results are not confounded with a history of depression; indeed, none of the participants had experienced any previous Axis-I disorder. It will be important to follow this sample to examine the utility of genotype, family history, and stress reactivity in predicting the development of disorder. Our results underscore the importance of the relation between the 5-HTTLPR polymorphism and HPA-axis functioning in leading to emotional disturbance. It is critical, therefore, that investigators continue to work to elucidate the nature of this association so that we can develop programs and procedures that may prevent the occurrence of psychiatric disorders in high-risk individuals.
Acknowledgments
This research was supported by grants from the National Alliance for Research in Schizophrenia and Affective Disorders and the National Institute of Mental Health (MH74849). The authors thank Kirsten Gilbert for her help in recruiting and running the participants in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
The three genotype groups did not differ with respect to demographic variables. While we did not match groups specifically with respect to time during the menstrual cycle at which cortisol was assessed, it is important to note both that the three genotype groups did not differ in the proportion of girls who were pre- and post-menarche, χ2(4,67) <1, and that results of studies with humans are mixed with respect to the possible association of cortisol reactivity with phase of the menstrual cycle (28–30). Given that the three genotype groups also did not differ with respect to time of day at which the cortisol was collected, F(2,63)<1, it is unlikely that time of assessment during the menstrual cycle could explain differences in cortisol reactivity among the three genotype groups.
Recently, functional variants in the L allele, designated as LA and LG, have been found to confer different levels of transporter expression: the LG and S alleles appear to have comparable levels of serotonin transporter expression, and both have lower levels than that of the LA allele (32,33). We chose to conduct our main analysis classifying participants into three genetic groups based on their S- and L-alleles, irrespective of the LA and LG subtypes: (i.e., L/L, L/S, and S/S), because few studies to date have investigated subtypes of L, and this analysis therefore permits comparisons to a larger body of published work. Nevertheless, we also classified participants into the three groups that are more tightly coupled to 5-HTT expression levels: (i) individuals who are heterozygous for the long allele that confers the greatest 5-HT transporter expression (high-expressing: LA/LA); (ii) individuals who have one copy of either the S allele or the reduced long allele and one copy of the LA allele (LA/S or LA/LG); and (iii) individuals who have two copies of the S allele or one S and one reduced-expression long allele (low-expressing: S/S or S/LG). The LG allele has been reported to behave comparably to the low-expressing S allele (34). The results of the stress reactivity analysis with this grouping are comparable to those reported in this paper: a significant group by time interaction in the linear trend, F(2,61)=5.93, p<.01, and in the quadratic trend, F(2,61)=3.69, p<.05. A graph of this interaction is available from the first author.
References
- 1.Gotlib IH, Hammen CL, editors. Handbook of depression. New York, NY: Guilford Press; 2002. [Google Scholar]
- 2.Levinson DF. The genetics of depression: A review. Biol Psychiat. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Firk C, Markus CR. Serotonin by stress interaction: A susceptibility factor for the development of depression? J Psychopharmacol OnlineFirst. 2007 April 19; doi: 10.1177/0269881106075588. [DOI] [PubMed] [Google Scholar]
- 4.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg DB, Petri S. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 5.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 6.Kim J-M, Stewart R, Kim S-W, Yang S-J, Shin I-S, Kim Y-H, Yoon J-S. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol Psychiat. 2007 doi: 10.1016/j.biopsych.2006.11.020. online. [DOI] [PubMed] [Google Scholar]
- 7.Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Wichems C, Heils A, Van de Dar LD, Lesch K-P, Murphy DL. Reduction of 5-HT1A binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- 10.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 11.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 12.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 13.Federenko IS, Nagamine M, Hellhammer DH, Wadhwa PD, Wüst S. The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. J Clin Endocrinology & Metabolism. 2004;89:6244–6250. doi: 10.1210/jc.2004-0981. [DOI] [PubMed] [Google Scholar]
- 14.Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Hormones & Behavior. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 15.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 16.Hammen CL. Stress and depression. Ann Rev Clin Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 17.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2006;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Williamson D, Birmaher B, Axelson D, Ryan N, Dahl R. First episode of depression in children at low and high familial risk for depression. J Am Academy of Child Psychiatry. 2004;43:291–297. doi: 10.1097/00004583-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Clinician Version. Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 20.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. K-SADS-PL: Initial Reliability and Validity Data. J Am Academy of Child and Adol Psychiatry. 2000;39:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs M. The Children’s Depression Inventory manual. New York: Multi-Health Systems; 1992. [Google Scholar]
- 22.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 23.Wechsler D. WISC-III: Wechsler Intelligence Scale for Children-3rd Edition Manual. San Antonio TX: Psychological Corporation; 1991. [Google Scholar]
- 24.Ewart CK, Jorgensen RS, Suchday S, Chen E, Matthews KA. Measuring stress resilience and coping in vulnerable youth: The social competence interview. Psychological Assessment. 2002;14:339–352. doi: 10.1037//1040-3590.14.3.339. [DOI] [PubMed] [Google Scholar]
- 25.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 26.Rylander-Rudqvist T, Hakansson N, Tybring G, Wolk A. Quality and quantity of saliva DNA obtained from the self-administrated oragene method: A pilot study on the cohort of Swedish men. Cancer Epidemiol Biomarkers Prev. 2006;15:1742–1745. doi: 10.1158/1055-9965.EPI-05-0706. [DOI] [PubMed] [Google Scholar]
- 27.Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 28.Marinari KT, Leshner AI, Doyle MP. Menstrual cycle status and adrenocortical reactivity to psychological stress. Psychoneuroendocrinology. 1976;1:213–8. doi: 10.1016/0306-4530(76)90011-1. [DOI] [PubMed] [Google Scholar]
- 29.Abplanalp JM, Livingston L, Rose RM, Sandwisch D. Cortisol and growth hormone responses to psychological stress during the menstrual cycle. Psychosom Med. 1977;39:158–77. doi: 10.1097/00006842-197705000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 32.Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry. 2006;63:978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- 33.Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 34.Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk: Neurobiological, behavioral, and environmental relations to drinking. Alcoholism: Clinical & Experimental Research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 35.Brown G, Harris T. Social origins of depression. London: Tavistock Publications; 1978. [Google Scholar]
- 36.O’Hara R, Schroder CM, Mahadevan R, Schatzberg AF, Lindley S, Fox S, et al. Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: Association and interaction with cortisol. Mol Psychiatry. 2007;12:544–555. doi: 10.1038/sj.mp.4001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuller RW. Serotonin receptors involved in regulation of pituitary-adrenocortical function in rats. Behav Brain Res. 1996;73:215–219. doi: 10.1016/0166-4328(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 38.Porter RJ, Gallagher P, Watson S, Young AH. Corticosteroid-serotonin interactions in depression: A review of the human evidence. Psychopharmacology. 2004;173:1–17. doi: 10.1007/s00213-004-1774-1. [DOI] [PubMed] [Google Scholar]
- 39.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]