Abstract
Because cytoplasmic dynein plays numerous critical roles in eukaryotic cells, determining the subunit composition and the organization and functions of the subunits within dynein are important goals. This has been difficult partly because of accessory polypeptide heterogeneity of dynein populations. The motor domain containing heavy chains of cytoplasmic dynein are associated with multiple intermediate, light intermediate, and light chain accessory polypeptides. We examined the organization of these subunits within cytoplasmic dynein by separating the molecule into two distinct subcomplexes. These subcomplexes were competent to reassemble into a molecule with dynein-like properties. One subcomplex was composed of the dynein heavy and light intermediate chains whereas the other subcomplex was composed of the intermediate and light chains. The intermediate and light chain subcomplex could be further separated into two pools, only one of which contained dynein light chains. The two pools had distinct intermediate chain compositions, suggesting that intermediate chain isoforms have different light chain–binding properties. When the two intermediate chain pools were characterized by analytical velocity sedimentation, at least four molecular components were seen: intermediate chain monomers, intermediate chain dimers, intermediate chain monomers with bound light chains, and a mixture of intermediate chain dimers with assorted bound light chains. These data provide new insights into the compositional heterogeneity and assembly of the cytoplasmic dynein complex and suggest that individual dynein molecules have distinct molecular compositions in vivo.
Keywords: Cytoplasmic dynein, dynein intermediate chain, microtubule motors, molecular composition and heterogeneity, analytical velocity centrifugation
Dyneins, kinesins, and myosins are the three major classes of cytoskeleton-based motors in eukaryotic cells. While much progress has been made on the biochemistry and mechanochemical cycles of conventional kinesin and myosin II (Vale and Milligan 2000), significantly less is known about cytoplasmic dynein. One reason for this is the complexity of the dynein molecule in comparison to other molecular motors. Both kinesin and myosin motor families comprise a large number of distinct enzymes that owe their heterogeneity to different heavy chains (HCs) (Hirokawa 1998; Sellers 2000). The dynein superfamily, in contrast, derives its heterogeneity from a diverse set of accessory polypeptides that are bound to a limited number of HCs. This has made the study of dynein difficult because it is not yet known how many biochemically distinct dynein isoforms might be present within a given cell.
To date, at least 10 genes encode known polypeptide components of the cytoplasmic dynein motor (Table 1). Each cytoplasmic dynein molecule contains two copies of one of the HC genes along with multiple intermediate, light intermediate, and light chain accessory polypeptides. The ∼4,500 amino-acid HCs are encoded by multiple genes (Vaisberg et al. 1996; Criswell and Asai 1998) and have multiple AAA domains (King 2000a), which may bind ATP (for review, see Neuwald et al. 1999). For cytoplasmic dynein, only the first AAA domain is thought to be capable of ATP hydrolysis, which provides the energy for translocation. The mechanical cycle of microtubule binding and release is thought to utilize, at least in part, a short microtubule-binding domain located within a coiled-coil domain found between the fourth and fifth AAA domains (Gee et al. 1997; Koonce 1997; Koonce and Tikhonenko 2000).
Table 1.
Cytoplasmic dynein polypeptides
| Identitya | Abbreviation | No./molecule | Proteins |
| Heavy chain | HC | 2 | DHC1, DHC2 |
| Intermediate chain | IC | 2 | IC1, IC2 |
| Light intermediate chain | LIC | 2–4 | LIC1, LIC2 |
| Light chain | LC | 4–5 | Tctex-1, rp3, LC8, LC7/robl |
a Data were compiled from the following papers in making this table: King and Patel-King 1995; King et al. 1996a,b, 1998; Benashski et al. 1997; Bowman et al. 1999; Brill and Pfister 2000; Susalka et al. 2000; Tynan et al. 2000.
Of the cytoplasmic dynein accessory polypeptides, the best characterized are the 70–74 kD intermediate chains (ICs). These are encoded by two separate genes in mammals (IC1 and IC2) and are subject to extensive splicing and phosphorylation (Paschal et al. 1992; Dillman III and Pfister 1994; Vaughan and Vallee 1995; Pfister et al. 1996a,b; Nurminsky et al. 1998). The conserved N-terminus of the IC interacts with the dynein activator dynactin (Karki and Holzbaur 1995; Vaughan and Vallee 1995) to allow a variety of dynein-driven functions (for review, see Allan 1996; Schroer 1996; Karki and Holzbaur 1999). The interaction between IC2C, the most ubiquitously expressed IC isoform (Susalka et al. 2000), and dynactin is regulated in part by IC phosphorylation near the N-terminus (Vaughan et al. 2001). The C-terminal part of the IC contains multiple WD-repeat sequences (Paschal et al. 1992; Vaughan and Vallee 1995) that are likely to fold into a β-propeller structure (Neer et al. 1994; Wall et al. 1995; Sondek et al. 1996; ter Haar et al. 1998). This predicted domain is conserved between all dynein ICs (i.e., cytoplasmic and axonemal) and is thought to participate in subunit-subunit interactions within the dynein molecule (Paschal et al. 1992; Ma et al. 1999).
Cytoplasmic dyneins also contain families of light intermediate chains (LICs) (52–61 kD) and light chains (LCs). The role of these subunits may be to interact with various cargoes. The two genes LIC1 and LIC2 encode multiple phosphorylated LIC polypeptides that bind directly to the HC (Gill et al. 1994; Hughes et al. 1995; Tynan et al. 2000). LIC1 polypeptides have been shown to interact directly with the centrosomal protein, pericentrin (Purohit et al. 1999; Tynan et al. 2000), but no binding partners of LIC2 have been identified.
Four cytoplasmic dynein LCs have been identified to date: rp3 (13 kD), Tctex-1 (12.4 kD), LC8 (10.3 kD), and LC7/robl (11 kD) (King and Patel-King 1995; Dick et al. 1996; King et al. 1996a,b, 1998; Bowman et al. 1999; Espindola et al. 2000). Both LC8 and Tctex-1 exist as homodimers in solution and have almost identical three-dimensional structures when examined by X-ray crystallography and/or nuclear magnetic resonance (NMR) (Benashski et al. 1997; Fan et al. 1998, 2001; Liang et al. 1999; Barbar et al. 2001; Lo et al. 2001; Mok et al. 2001). Tctex-1 and LC8 bind to the dynein IC at nonoverlapping sites that share some sequence identity (Lo et al. 2001; Mok et al. 2001). Tctex-1 can interact directly with retinal opsin and is required for correct opsin localization, demonstrating a role in cargo binding (Tai et al. 1999). Overexpression of rp3 displaces Tctex-1 from dynein and blocks the apical delivery of rhodopsin (Tai et al. 2001). LC8 interacts with a wide variety of other polypeptides, however, the identity of any of these as bona fide cytoplasmic dynein cargoes has yet to be determined.
Multiple lines of evidence suggest that a variety of biochemically distinct cytoplasmic dynein isoforms may exist in cells. First, dynein HCs, ICs, and the LCs Tctex-1 and rp3 are differentially expressed in tissues (Vaughan and Vallee 1995; Vaisberg et al. 1996; King et al. 1998; Nurminsky et al. 1998), which suggests that different combinatorial permutations of subunits may exist. In addition, HCs, ICs, and Tctex-1 show distinct subcellular localizations (Vaisberg et al. 1996; King et al. 1998; Nurminsky et al. 1998; Tai et al. 1998). Finally, overexpressed LIC1 and LIC2 appear to be incorporated into distinct dynein subspecies (Tynan et al. 2000). Previous attempts to identify interactions between cytoplasmic dynein components have utilized the chaotropic salt potassium iodide to partially disassemble the dynein molecule. These studies have shown that the dynein HCs and LICs cofractionate away from the ICs (Gill et al. 1994) and two of the LCs, LC8 and Tctex-1 (King et al. 1998). The location of the other two LCs within the cytoplasmic dynein molecule has not been reported.
In this study, we performed a thorough biochemical analysis of cytoplasmic dynein subunit organization and heterogeneity. Brain cytoplasmic dynein was separated into two major subcomplexes, one that contained the HCs and LICs, and one that contained the ICs and LCs. The isolated IC+LC subcomplex was further fractionated into two distinct pools and the molecular composition of each was determined. We also found that dynein molecules could be reassembled from mixtures of the HC- and IC- containing subcomplexes. Our data provide the first biochemical evidence of cytoplasmic dynein's compositional heterogeneity, insights as to how the dynein molecules may be assembled, and suggest means to characterize reconstituted dynein molecules with defined subunit composition.
Results
Generation and reassembly of two dynein subcomplexes
Previous studies have shown that treatment of cytoplasmic dynein with the chaotropic salt potassium iodide yields two major species, a subcomplex of the HCs and LICs and a subcomplex of ICs and the LCs (Gill et al. 1994; King et al. 1998). The purified HC and LIC subcomplex has been shown to exhibit microtubule gliding (Gill et al. 1994) and ATPase activity (Kini and Collins 2001), illustrating that the molecules are still functional. While the IC/LC subcomplex is known to contain the LCs Tctex-1 and LC8 (King et al. 1998), the presence of the other LCs (rp3 and LC7/robl) within the cytoplasmic dynein molecule were not examined. Moreover, the details of organization within each subcomplex was not examined. To gain more insight into the fate of all the cytoplasmic dynein LCs and to further explore subunit/subunit organization within the dynein molecule, we extended and refined our previous analysis. We treated purified bovine brain cytoplasmic dynein (Bingham et al. 1998) with 0.7 M KI and separated the resulting protein subcomplexes by gel filtration chromatography in the continued presence of KI. Two slightly overlapping peaks of protein were seen (Fig. 1 ▶). Peak 1 eluted broadly with an apparent Stokes radius of 8.0 nm and consisted of the dynein HCs and the 52–61 kD LICs. Peak 2 eluted with a Stokes radius of ∼5.8 nm and contained predominantly the ICs. For each protein peak, the major visible contaminants were the proteins from the other peak. Examination of the low-molecular-weight components showed that several polypeptides with electrophoretic mobilities ranging from 8 to 16 kD are present in Peak 2 as well as in later gel filtration fractions (Fig. 1C ▶). Three of these polypeptides could be identified as the dynein LCs Tctex-1, rp3, and LC8 in subsequent analyses (see Fig. 5 ▶). An ∼24 kD unidentified polypeptide found in the fractions spanning both Peak 1 and Peak 2 was the only other prominent low-molecular-weight polypeptide (discussed later).
Fig. 1.

Potassium iodide treatment disassembles cytoplasmic dynein into two subcomplexes. (A) A280 profile of Superose 6 gel filtration column. Positions of gel filtration standards (nm Stokes' radius) are shown. (B) Coomassie brilliant blue stained 11% acrylamide gel of disassembled dynein column fractions (indicated by numbers). (C) Silver stained 15% acrylamide gel of disassembled dynein column fractions. (D) Coomassie brilliant blue stained gel of the heavy chain (HC) subcomplex pool subjected to a second round of gel filtration chromatography. For simplicity, only the HC region is shown. (E) Western blot of the HC subcomplex pool as in (D) probed with intermediate chain (IC) and light intermediate chain (LIC) antibodies. (F) Coomassie brilliant blue stained gel of the IC subcomplex pool subjected to a second round of gel filtration chromatography (only the HC region is shown). (G) Western blot of the IC subcomplex pool as in F probed with antibodies to both IC and LIC. L = load. Sizes of dynein components are shown on the right (*, unknown 24-kD polypeptide; bracket, light chains). Molecular weight markers are indicated to the left.
Fig. 5.
Identification and enrichment of dynein accessory chains. (A) Different samples from the intermediate chain purification were analyzed by SDS-PAGE (15% acrylamide) and stained with Coomassie brilliant blue. CD, cytoplasmic dynein; HC, heavy chain peak from Superose 6 column; IC, intermediate chain peak from Superose 6 column; SG, pool of intermediate chain–enriched fractions (8–11) from sucrose gradient; IC–, first intermediate chain peak from Mono Q column, IC/LC, second intermediate chain peak from Mono Q column. The image of the bottom half of the gel has been contrast enhanced to show better the light chain polypeptides. The positions of these four predominant low-molecular-weight bands are indicated to the right. Molecular weight markers are to the left. (B) Samples subjected to SDS-PAGE (15% acrylamide) were either silver-stained (left panel) or immunoblotted with antibodies to rp3, Tctex-1, or LC8. Lanes: 1 is untreated bovine brain dynein; 2 is dynein additionally purified by microtubule affinity, velocity sedimentation, and ion-exchange chromatography. Equal protein amounts were loaded in all lanes. Small hatch marks are shown between panels to indicate positions of the bands seen by silver-staining. Molecular-weight markers are shown on the right.
The slight overlap of the two KI-dissociated peaks might reflect association between the two subcomplexes in the presence of KI. We rechromatographed the separated HC and IC subcomplexes in the presence of KI. When the HC subcomplex pool (fractions 13–15) was analyzed in this manner, the HC and LIC peak broadened (Fig. 1D,E ▶). When the IC subcomplex (fractions 19–22) was rechromatographed, the IC peak eluted as a tighter peak ∼0.5 mL later, which corresponds to a 0.3-nm reduction in the average Stokes radius to ∼5.5 nm (Fig. 1F,G ▶). The slight shifts in elution profiles seen for each subcomplex profile might indicate that some interaction occurred during the original run. However, it also is possible that continued exposure to KI altered the elution profiles of the subcomplexes.
Next, we determined if the IC and HC subcomplexes could reassemble into dynein when KI was removed. Under the conditions used in this experiment, undisrupted dynein sediments at ∼20 S (Fig. 2 ▶, fractions 4–7). Isolated HC subcomplexes sedimented faster than intact dynein and also exhibited a broader range of S values. This suggests that the HC subcomplex may form higher-order assemblies or aggregate in the absence of the IC subcomplex. The IC subcomplex sedimented at ∼5 S, near the top of the gradients. The separated IC and HC pools were combined and dialyzed, and the mixture was examined on sucrose gradients. Because the isolated IC and HC subcomplexes have different sedimentation properties, the reappearance of both IC and HC polypeptides in the same sucrose gradient fractions would indicate that dynein reassembly had occurred. We found that the majority of the HC and IC polypeptides in the mixed pool cosedimented at about 20 S, as expected for undisrupted dynein. Re-association was incomplete, as seen by the broadened HC and IC peaks relative to control, undisrupted dynein, but the majority of both HC and IC did appear to be competent for reassembly.
Fig. 2.


Dynein reassembly after KI treatment. Ten percent to 40% sucrose gradients were loaded with (A) untreated, intact dynein, (B) heavy-chain (HC) subcomplex, (C) intermediate chain (IC) subcomplex, or (D) HC and IC subcomplexes that were mixed and dialyzed to remove KI. Fractions were examined by SDS-PAGE followed by Coomassie brilliant blue staining to visualize HC (top panels A, B, C, and D) or immunoblotting to detect IC (bottom panels A, B, C, and D). Denser fractions are to the left. The positions of the HCs and ICs are shown to the right of each gel strip.
Intermediate chain + light chain subcomplex
To further purify the IC species, IC-containing fractions were dialyzed to remove KI, and the resulting samples were sedimented into a sucrose gradient (Fig. 3 ▶). The ICs sedimented in a broad peak between 4S and 7S that contained little or no HC+LIC subcomplex, but did contain a family of low-molecular-weight polypeptides that were most likely dynein LCs (see below). In an effort to further resolve different IC subpopulations in this pool, IC-containing fractions (8–11) were pooled and fractionated by anion exchange chromatography over a Mono Q column (Fig. 4 ▶). This resolved two protein peaks that differed in LC composition. The first pool (IC–) contained little, if any, of the low-molecular-weight polypeptides. Overdeveloped silver staining of these and other gels revealed only trace amounts of the low-molecular-weight polypeptides in the first IC pool (data not shown). The second pool (IC/LC) contained four low-molecular-weight bands that were most likely LCs. To obtain more information on the low-molecular-weight bands present in the second Mono Q pool, this sample was compared with intact cytoplasmic dynein by SDS-PAGE (Fig. 5A ▶). The same four low-molecular-weight polypeptides were present in the two samples. To establish rigorously the identity of these bands as LCs, samples were probed with antibodies to LC8, Tctex-1, and rp3 (Fig. 5B ▶). As expected (King et al. 1998; Kini and Collins 2001), we found that the epitopes recognized by these antibodies were greatly diminished after KI treatment (data not shown). We therefore limited our analysis to dynein samples that had not been treated with KI. In unfractionated dynein, or in dynein that had been further purified by the combination of microtubule affinity, velocity sedimentation, and Mono Q chromatography, the rp3, Tctex-1, and LC8 antibodies recognized the three largest of the low-molecular-weight bands. Although not conclusive, the remaining low-molecular-weight-polypeptide (Fig. 5 ▶) is most likely LC7/robl.
Fig. 3.
Sucrose density centrifugation of the dynein intermediate chain (IC) subcomplex. Dialyzed IC subcomplex was sedimented into a 5–20% sucrose gradient and 1-mL fractions were collected and analyzed by SDS-PAGE. L = gradient load. Molecular weight markers are on the left. Positions of sucrose gradient standards are shown below the gel. The low-molecular-weight bands are bracketed on the right. Fraction 7 was not used in any further purification steps because of the presence of the dynein heavy chain.
Fig. 4.

Mono Q ion-exchange chromatography separates cytoplasmic dynein intermediate chain (IC) into two biochemically distinct pools. (A) Elution profile of Mono Q column. Left axis = A280, right axis = KCl gradient used for elution. (B) Coomassie brilliant blue stained 15% acrylamide gel of fractions (indicated by numbers). L = column load. The lines on the right indicate positions of presumptive light chains (LCs) described in the text. Molecular weight markers are indicated to the left. (C) Silver-stained, two-dimensional gel analysis of IC polypeptides from the IC– and IC/LC pools. The regions containing the IC polypeptides are shown with the acidic end of the IEF gels to the left. Arrows indicate the positions of the IC2C isoform found in both IC pools and the IC1 isoform found predominantly in the IC/LC pool.
Because the ICs comprise a variety of alternative splicing and phosphorylation isoforms, two-dimensional electrophoresis is the method used most commonly to identify IC isoforms in tissue or motor samples (Brill and Pfister 2000). To determine if the two IC-containing pools exhibited isoform differences, we analyzed them by two-dimensional electrophoresis (Fig. 4 ▶). The first IC pool was almost exclusively composed of the larger, more basic IC2 protein arc (Brill and Pfister 2000), with three predominant isoforms present. The second IC pool contained protein corresponding to the IC1 and IC2 polypeptides, with one isoform of each gene product being more prominent than the others. Careful alignment of the gels with internal control polypeptides showed that the prominent IC2 isoform present in the IC/LC pool is the smallest and most basic isoform, which corresponds to IC2C (Susalka et al. 2000). The disparities in the amounts of the IC isoforms in the two pools suggest that IC1 gene products may bind LCs tighter than IC2 gene products.
These data show clearly that the dynein LCs are present in the IC subcomplex but not the HC subcomplex. The IC subcomplex can be separated into two pools, one that contains LCs and both IC1 and IC2 gene products (IC/LC), and one composed predominantly of IC2 gene products without LCs (IC–) . Both pools were subjected to analytical velocity sedimentation to gain further insight into their molecular compositions. Diffusion-corrected integral distribution plots (G[s*]) for the IC– pool (filled circles in Fig. 6 ▶) show a nearly constant s*20,w of about 3.3 S at low-boundary fractions (low concentrations) but gradually curve to larger s*20,w at high-boundary fractions (higher concentrations). Because this distribution is indicative of self-assembly, we performed finite-element fitting of the data to a monomer-dimer equilibrium model. The fitted molecular weights, s20,w, D20,w, and frictional ratio values for monomer and dimer components are reported in Table 2. The calculated monomer molecular weight agrees well with the molecular weights of ICs predicted from cloned cDNAs from various sources. The best fit value from the data also provided a dimer dissociation constant (Kd) of ∼1.8 μM. Hydrodynamic modeling of the data illustrated that various molecular shapes (long rod, oblate, or prolate spheroids) were possible. However, we favor either the oblate ellipsoid or prolate ellipsoid models because these are the most consistent with solved crystal structures of proteins such as β-G protein transducin (Wall et al. 1995; Sondek et al. 1996) and clathrin (ter Haar et al. 1998), that contain β-propeller structural domains like those predicted to be present in dynein ICs (Paschal et al. 1992; Neer et al. 1994; Vaughan and Vallee 1995; Wilkerson et al. 1995).
Fig. 6.
Analytical velocity sedimentation of intermediate-chain (IC) subcomplexes. Integral distributions of sedimentation coefficients (G[s]) of the IC– (filled circles) and IC/light chain (LC) (open circles) pools are shown extrapolated to infinite time to eliminate the effects of diffusion. The X-axis is sedimentation coefficient and the Y-axis is boundary fraction.
Table 2.
Physical properties of components of the IC + LC subcomplex
| Component identity | M (kD) | S20,w (S) | D20,w (× 107 cm2/sec) | f/f0 |
| IC monomera | 74 | 2.9 | 3.4 | 2.25 |
| IC dimera | 148 | 7.1 | 4.8 | 1.46 |
| IC monomer + LCsb | 121.4 | 6.6 | 4.8 | 1.38 |
| IC dimer + LCsb | ND | 14.4 | ND | <1.0c |
M, molecular weight; ND, not determined. S20,w, sedimentation coefficient corrected for 20°C in water; D20,w, diffusion coefficient corrected for 20°C in water; f/f0, frictional ratio; a determined from the IC-pool; b determined from the IC/LC pool; c physically unrealistic, see discussion in text.
Analytical velocity sedimentation revealed the IC/LC pool to be more complex than the IC– pool, with at least four components present as evidenced by the multi-tiered G(s) distribution in Figure 6 ▶. Our initial fittings showed that the first component had an s20,w of about 3.3 S, which correlated well with the IC monomer. The behavior of the third component suggested it was the IC dimer species described in Table 2. Therefore, we fixed the M, s20,w and D20,w values of these components to those of the previously analyzed IC monomer and IC dimer species and analyzed the data using a finite element model with four independent species. Reiterative fittings of the data then were performed using the G(s*) plot for initial estimates of the second and fourth components. In this way, we were able to improve the global fittings of the data. The second component of the IC/LC pool, comprising nearly 50% of the boundary, has a sedimentation coefficient of 6.6 S. The remainder of the boundary was material with a broad distribution of s20,w values >8.5 S. The fitted parameters for the components are summarized in Table 2. The value obtained for the second component yielded a molecular weight (121.4 kD) consistent with a species containing a single IC (74 kD) plus four LCs (the remaining 47.4 kD). Unfortunately, the known complexity and heterogeneity of the dynein LCs (King and Patel-King 1995; King et al. 1998; Bowman et al. 1999) made it impossible to determine the exact stoichiometry or molecular identity of the bound LCs. As expected from initial fittings, the fourth component had a frictional ratio (f/f0) <1, which indicates that a more complex mixture of sedimenting material is present in this portion of the boundary. Possible components include various mixtures of IC dimer with LCs. Thus, the IC– pool is composed of IC monomer and dimer species while the IC/LC pool contains at least four components: IC monomer, IC monomer with bound LCs, IC dimer, and a mixture of what is probably IC dimer with assorted bound LCs. The large assortment of IC-containing components present in the two IC pools is qualitatively consistent with the relatively broad IC sedimentation peak observed in the sucrose gradient shown in Figure 3 ▶. Quantitative mole-fraction analysis was impossible because of the complexity of the sample.
Heavy chain + light intermediate chain subcomplex
To further characterize the dynein HC+LIC subcomplex, the HC-containing peak from the Superose 6 column (Fig. 1 ▶, fractions 15–16) was subjected to sucrose density centrifugation. The dynein HCs and LICs cosedimented in a broad peak within the sucrose gradient (Fig. 7 ▶), which further supports the conclusion that these subunits are tightly associated with each other. A small amount of the 74 kD intermediate chain was present throughout the HC+LIC peak, which may indicate that a small fraction of dynein molecules were not completely dissociated by KI treatment. To determine if the broad peak seen for the HC+LIC subcomplex in the sucrose gradient actually represented discrete subpopulations of the subcomplex, we analyzed four sequential HC+LIC sucrose-gradient samples (fractions 4–7) by analytical velocity sedimentation. In each fraction, a broad distribution of S values existed that ranged from 15 S to almost 80 S with a weighted mean of 25 S (data not shown). Clearly, a large number of components are present in these samples, likely ranging from monomeric HCs (Kini and Collins 2001) to higher-order structures. Previous analysis of the HC+LIC subcomplex has shown that ATPase activity is retained (Kini and Collins 2001) although microtubule sliding activity is weak (Gill et al. 1994). Our attempts to further purify/fractionate the HC-containing species were unsuccessful because the protein was extremely unstable; we were not able to study it further.
Fig. 7.
Components of the dynein heavy-chain–containing subcomplex. (A) The dynein heavy-chain pool from KI disruption was sedimented into a sucrose gradient as shown. Fractions were collected and analyzed by 11% acrylamide SDS-PAGE. L = load; P, resuspended pellet. Numbers correspond to sucrose gradient fractions. Sizes of dynein components are shown on the right (HC, heavy chain; IC, intermediate chain; LIC, light intermediate chain; 24, unknown 24 kD polypeptide). Positions of sucrose gradient standards are shown below the gel. (B) Immunoblots of purified dynein samples probed with antibodies to the intermediate chain (top panels), αB-crystallin (bottom left panel) or reticulon (bottom right panel). Lanes: 1 is dynein from bovine brain (Bingham et al. 1998); 2 is ATP release dynein purified further over a sucrose gradient and Mono Q column.
As seen in Figures 1 and 5 ▶ ▶, the intact dynein sample contained a 24-kD band that did not react with any of the LC antibodies we tested (see also [King et al. 1996b]). This polypeptide appeared to remain associated with the HC+LIC subcomplex after KI dissociation and subsequent velocity sedimentation (Figs. 1, 4, and 7 ▶ ▶ ▶). We microsequenced the 24-kD polypeptide to determine its identity. Sequences corresponding to the proteins αB-crystallin (Carver et al. 1990) and reticulon (Roebroek et al. 1996; Senden et al. 1996) were obtained. To determine if these proteins were bona fide components of cytoplasmic dynein, we compared by immunoblotting the starting dynein preparation to dynein that had been purified further by the combination of ATP-dependent microtubule affinity and Mono Q chromatography (Fig. 7B ▶). Whereas both αB-crystallin and reticulon were present in the unfractionated dynein sample, neither protein could be detected in the most highly purified sample. Therefore, αB-crystallin and reticulon appear to be contaminants of the starting sample rather than bona fide cytoplasmic dynein subunits.
Discussion
An understanding of cytoplasmic dynein's accessory polypeptide composition and organization is becoming ever more important as the myriad functions of cellular dynein are elucidated. In this study, we analyzed the composition of two subcomplexes of cytoplasmic dynein. The larger subcomplex consisted of dynein HCs and LICs whereas the smaller subcomplex was composed of the dynein ICs and LCs. Interestingly, the IC plus LC subcomplex could be separated into two pools. One contained little, if any, light chains and was composed almost exclusively of IC2 gene products (the IC– pool) and the other contained four LCs and ∼equal amounts of IC1 and IC2 (the IC/LC pool). That we could isolate IC-containing complexes of distinct composition suggests that individual cytoplasmic dynein molecules may have different IC and LC compositions in vivo. Analytical velocity sedimentation was used to characterize the IC subcomplexes further. This analysis revealed that the IC is capable of dimerization in the absence of LCs and that a single IC monomer can bind several LCs.
There are several important similarities and differences in the compositions of cytoplasmic and outer-arm flagellar dynein (reviewed in [King 2000b]). In both molecules, the heavy chains and the intermediate chains can be fractionated into separate subcomplexes (Mitchell and Rosenbaum 1986; King et al. 1998). Flagellar dynein HCs (two or three per molecule) have LCs attached, whereas cytoplasmic dynein HCs (two per molecule) are in complex with the LICs. In each dynein class, LC8, LC7/robl, and the remaining LCs (different ones in cytoplasmic or flagellar dynein) are found in the IC subcomplex. The major difference between the two complexes is the polypeptide complexity of individual molecules. Flagellar outer arm dyneins are homogeneous in composition, whereas cytoplasmic dyneins appear to vary with respect to IC, LIC, and LC composition. This may reflect the distinct cargoes carried by each molecule. Flagellar dyneins bind their "cargo," the A-tubule, directly via the dynein IC subcomplex, which interacts directly with the microtubule and indirectly with a 7S docking complex (King et al. 1991; Takada and Kamiya 1994). Cytoplasmic dynein has the capacity to interact with multiple proteins and protein complexes, including the dynein activator dynactin, several known cargoes, and multiple other proteins that may be bona fide dynein cargoes. It does this using a complex structure that contains the IC+LC subcomplex and the LIC polypeptides. Thus, the existence of the cytoplasmic dynein heterogeneity described here and elsewhere may be a result of cytoplasmic dynein's requirement and ability to bind a wide array of cargoes.
Cytoplasmic dynein compositional heterogeneity has been characterized in three ways: analysis of the biochemical characteristics of the native protein (this study); examination of cell type–specific expression pattern, and subcellular localization of different subunits (Vaughan and Vallee 1995; Vaisberg et al. 1996; King et al. 1998; Nurminsky et al. 1998; Tai et al. 1998); and overexpression studies (Tynan et al. 2000; Tai et al. 2001). Compositional complexity is expected to play a role in dynein function and may be an essential mechanism in targeting dynein to its various cargoes. For some time, cytoplasmic dynein has been believed to function exclusively as a motor in conjunction with dynactin, whose only role was thought to be a cargo-binding adapter. Recently, however, this division of labor has been shown to be less clear-cut. Besides serving as a cargo-binding adapter protein, dynactin augments cytoplasmic dynein motor activity by increasing its processivity (King and Schroer 2000). Dynactin appears to provide a second weak contact site with the microtubule, similar to what has been found in the motor or neck domains of monomeric kinesin KIF1A (Okada and Hirokawa 1999) or dimeric conventional kinesin (Thorn et al. 2000).
The existence of two biochemically distinct IC pools appears to be the direct result of stronger binding of LCs to particular IC isoforms. Although many tissues express only IC2C (Susalka et al. 2000), brain, the source of dynein used in this study, expresses multiple isoforms of both IC1 and IC2 (Dillman and Pfister 1994; Vaughan and Vallee 1995; Pfister et al. 1996a; Brill and Pfister 2000). Two-dimensional gel analysis identified the predominant IC isoforms in each pool. The IC– pool contains roughly equal amounts of three IC2 isoforms and almost no IC1 isoforms. In contrast, the IC/LC pool contained roughly equal amounts of a single IC2 (most likely IC2C, [Susalka et al. 2000]) and a single IC1 species with lesser amounts of other isoforms present. Therefore, the LCs appear to be more tightly bound to IC1 isoforms than to IC2 isoforms. How this may impact LC composition within dynein molecules, especially because IC2C is the ubiquitous IC isoform, remains to be determined.
The LCs, rp3 and LC7/robl, which had not previously been localized within the cytoplasmic dynein molecule, are clearly associated with ICs and not the HC+LIC subcomplex. Previous studies have suggested that Tctex-1 and rp3 are expressed in different tissues and may be associated with distinct cytoplasmic dynein molecules by means of competition for a single binding site on the IC (Roux et al. 1994; Kai et al. 1997; King et al. 1998; Tai et al. 1998, 2001). Our data add a further level of complexity to the possible ways in which cytoplasmic dynein LCs might be distributed between different dynein molecules. In particular, LC compositions may be dependent on the IC composition of a particular dynein molecule. We and others have noted that the immunoreactivity of LCs appears to be significantly reduced after KI treatment. This has been suggested to involve iodination of the hydroxyl moieties of tyrosine residues (King et al. 1998). We find that inclusion of 10 mM DTT, which may reduce KI-generated free radicals, during KI extraction increased the reactivity of LC8 with its antibody (data not shown).
The combination of two IC genes, each yielding products with multiple phosphorylation and alternative splice sites (Dillman III and Pfister 1994; Vaughan and Vallee 1995; Pfister et al. 1996a,b), and multiple possible LC compositions (King and Patel-King 1995; Dick et al. 1996; King et al. 1996a,b, 1998; Bowman et al. 1999), might well provide the IC+LC subcomplex enough heterogeneity to allow different dynein molecules to interact specifically with a wide range of cargoes. The interactions of Tctex-1 with retinal opsin and of LIC1 with pericentrin are two examples of direct binding between dynein cargoes and dynein accessory polypeptides that are required for correct subcellular localization (Tai et al. 1999, 2001; Purohit et al. 1999; Tynan et al. 2000). Multiple dynein– and dynactin-mediated cargo interactions may constitute a system of individual interactions that in combination allow a large number of distinct cargoes to be specified by a much smaller number of polypeptides, with additional functional control at the level of dynein activation and processivity.
The dynein LCs have been the subject of extensive three-dimensional structural analysis. LC8 and Tctex-1 each form homodimers in solution and have strikingly similar three-dimensional structures (Benashski et al. 1997; Fan et al. 1998, 2001; Liang et al. 1999; Barbar et al. 2001; Lo et al. 2001; Mok et al. 2001). Tctex-1 and LC8 bind to the dynein IC at adjacent sites (Lo et al. 2001; Mok et al. 2001) just N-terminal to the WD-repeats at the C-terminus of the IC. The symmetric LC8 dimer has been shown to bind target peptides from dynein IC, neuronal nitric oxide synthase, and the proapoptotic factor Bim via peptide-binding channels located on opposite faces of the LC8 dimer (Liang et al. 1999; Fan et al. 2001; Lo et al. 2001). The peptide-binding channels could be used to link dynein to its cargo as long as one face of the LC8 dimer binds dynein and the other face binds cargo. To date, there is evidence that Myosin V and the neuronal scaffold protein GKAP may "share" an LC8 dimer in this manner (Naisbitt et al. 2000). However, this has yet to be shown for the dynein IC and other putative LC8 cargoes.
In addition to playing possible roles in cargo binding, the ICs and LCs may serve to stabilize and/or regulate the dynein molecule. Isolated brain HC+LIC subcomplex has increased ATPase activity (Kini and Collins 2001) but is unstable and exhibits reduced microtubule gliding activity (Gill et al. 1994). Our results of the analytical velocity sedimentation analysis of the HC subcomplex and the sucrose density analysis of the single- and mixed-dynein subcomplexes show that the HC subcomplex is unstable and can assume multiple conformations, some of which behave as vastly larger molecules. Our dynein reassembly experiments showed that the addition of the IC subcomplex appears to prevent the formation of these larger species and help maintain the HC subcomplex in a more native configuration. One feature of the IC likely to contribute to structural stability is the β-propeller structure predicted from the IC C-terminal sequence. β-propellers have a large surface area that can undergo extensive protein-protein interactions (Wall et al. 1995; Sondek et al. 1996). This predicted structural motif is conserved between axonemal and cytoplasmic dyneins (Paschal et al. 1992), suggesting that it participates in inter-subunit interactions within the dynein molecule, most likely with the heavy chain (Ma et al. 1999), rather than dynein-cargo interactions. Another polypeptide that may act to stabilize the dynein structure is the LC8 polypeptide, whose association with an array of cytosolic factors suggests it might play a broad role as a structural building block or molecular chaperone. Understanding how the IC+LC subcomplex stabilizes the HC subcomplex will provide clues into how cytoplasmic dynein assembles. Furthermore, the ability to dissociate and reassociate cytoplasmic dynein opens the door to future experiments that examine the enzymatic and motility properties of chimeric dynein molecules with HC and IC subcomplexes from different native or recombinant sources.
Materials and methods
Protein purification
Most experiments utilized bovine brain cytoplasmic dynein purified to ∼94% homogeneity as described (Bingham et al. 1998). For some experiments, bovine brain dynein was purified further by ATP-dependent binding and release from microtubules followed by sucrose density centrifugation and Mono Q anion exchange chromatography, as previously described (Schroer and Sheetz 1991). Bovine brain tubulin was twice cycled and purified over a phosphocellulose column (Sloboda and Rosenbaum 1982), frozen dropwise in liquid nitrogen, and stored at –80°C until later use.
Gels, blots, and antibodies
One-dimensional SDS-PAGE (Laemmli 1970) and immunoblotting (Towbin et al. 1979) were performed as described. Two-dimensional SDS-PAGE was performed according to the method of O'Farrell (O'Farrell 1975) by Kendrick Labs, Inc. The first-dimension gel included 2% pH 3.5–10 ampholines (Amersham Pharmacia Biotech) and the second dimension was an 8% acrylamide slab gel. Primary antibodies were used to the following proteins: dynein IC (70.1 [Steuer et al. 1990], 70.2, 70.3 [Steuer 1991], and 74.1 [Chemicon International, Inc.]); dynein light intermediate chains (DLIC) (Gill et al. 1994); αB-crystallin (StressGen Biotechnologies Corp.); reticulon (RNL-4) (Senden et al. 1996); and dynein LCs, LC 8 (PIN/LC8 [Jaffrey and Snyder 1996]), rp3, and Tctex-1 (Tai et al., 1998). Appropriate alkaline phosphatase-conjugated secondary antibodies were detected by chemiluminescence (Tropix, Inc.).
Isolation of dynein subcomplexes
Fresh bovine dynein (5.5 mg) was disrupted by addition of freshly prepared 6 M KI in water to a final concentration of 0.7 M. The sample was incubated on ice for 30 min. then loaded on a Superose 6 HR 10/30 gel filtration column (Pharmacia LKB Biotechnology, Inc.) preequilibrated in column buffer (2 mM Tris-Cl, pH 8.0, 0.2 mM ATP, 0.2 mM CaCl2, 0.5 mM DTT, 0.7 M KI) at 0.35 mL/min. Fractions of 0.5 mL were collected, aliquots were removed for SDS-PAGE, and the remainder of the samples was dialyzed immediately (2 X 30 min.) against TKAM buffer (25 mM Tris-Cl, pH 8.0, 100 mM KCl, 5 μM ATP, 1 μM β-mercaptoethanol) at 4°C to remove KI. Estimated Stokes radii for molecular standards were determined using the method of Bloom et al. (1988) using a Superose 6 column run in 0.7 M KI identical to the dynein samples. Two rounds of dynein disruption were performed and appropriate HC- and IC-containing peak fractions were pooled after dialysis. The HC-containing peak was layered onto 10–40% sucrose gradients in TKAM buffer with a 1-mL, 60% sucrose cushion. The IC peak was layered onto 5–20% sucrose gradients in TKAM buffer. All samples were centrifuged 11 h at 37,600 rpm at 4°C in a SW-41 rotor. Sucrose gradient fractions of 1-mL were collected and analyzed by SDS-PAGE.
The IC sucrose gradient pool (fractions 8–11) was loaded onto a Mono Q HR 5/5 anion exchange column (Pharmacia LKB Biotechnology, Inc.) at 0.5 mL/min with 35 mM Tris-Cl, pH 7.2, 5 mM MgSO4 as the column buffer. Proteins were eluted using a three-component gradient: 0 to 250 mM KCl in 10 mL, 250 to 350 mM KCl in 10 mL, and 350 to 500 mM KCl in 5 mL, all in 35 mM Tris-Cl, pH 7.2, 5 mM MgSO4. Fractions (0.5 mL) were collected and analyzed by SDS-PAGE. The two pools of IC protein were concentrated by running additional Mono Q runs with discontinuous step gradients from 300 to 350 mM KCl for the first IC pool and from 380 to 450 mM KCl for the second IC pool.
Dynein subcomplex reassembly was performed by combining equal molar amounts of the HC (fractions 13–15) and IC (fractions 19–22) subcomplexes prior to dialysis. Intact dynein, HC subcomplex, and IC subcomplex samples were also dialyzed. All samples were loaded onto 10–40% sucrose gradients in TKAM buffer (10 mL volume) and spun and analyzed as above.
Protein sequencing
The HC/LIC peak polypeptides from the Superose 6 column were separated by SDS-PAGE and Coomassie stained. After brief destain, the 24-kD band was excised, washed twice in 50% acetonitrile, and frozen at −80°C. Tryptic fragments were sequenced at the Harvard Microchemistry Facility by microcapillary reverse-phase HPLC nano-electrospray tandem mass spectrometry (mLC/MS/MS) on a Finnigan LCQ quadrupole ion trap mass spectrometer.
Analytical velocity sedimentation
Sedimentation velocity experiments were performed using a Beckman XL-I analytical ultracentrifuge. Samples (0.43 mL) were loaded into cells with 12-mm double-sector, charcoal-filled Epon centerpieces and quartz windows. Velocity runs were carried out at 4°C in an An-60 Ti rotor at 45,000 rpm for the IC subcomplexes and at 25,000 rpm for HC subcomplexes. Data scans were recorded continuously throughout the experiment using the absorption optical system at either 280 nm or 231 nm depending on the protein concentration of samples; the nominal radial step size was 0.001 cm. All data were analyzed initially using the method of van Holde and Weischet (van Holde and Weischet 1978). Extrapolation plots were made and used to generate diffusion-corrected integral distribution G(s*) plots. Following preliminary analysis, data were further fitted to the Lamm equation using the finite-element method as implemented in the program Ultrascan (Demeler and Saber 1998). Initial estimates for the fitting parameters were obtained from the van Holde-Weischet analysis.
Protein samples were dialyzed against 25 mM Tris-Cl, pH 8.0, 100 mM KCl, 1 μM β-mercaptoethanol overnight. The partial specific volume ( )was calculated from the amino-acid composition using the method of Cohn and Edsall (1943). The molar extinction coefficient (ɛ280) was calculated from the amino-acid composition using the method of Edelhoch as adapted by Pace and coworkers (Edelhoch 1967; Pace et al. 1995). For dynein polypeptides for which there is no bovine sequence in the database (i.e., all dynein components except for Tctex-1), we used the human amino-acid compositions for
)was calculated from the amino-acid composition using the method of Cohn and Edsall (1943). The molar extinction coefficient (ɛ280) was calculated from the amino-acid composition using the method of Edelhoch as adapted by Pace and coworkers (Edelhoch 1967; Pace et al. 1995). For dynein polypeptides for which there is no bovine sequence in the database (i.e., all dynein components except for Tctex-1), we used the human amino-acid compositions for 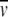 and ɛ280 calculations. The best fits of the data (described in the text) had randomly distributed residuals and a variance of 3.69 × 10−5 for the IC– pool and 5.73 × 10−5 for IC/LC pool. The solvent density (ρ) was calculated from buffer composition as described by Laue et al. (1992). These calculations, along with corrections for temperature, were calculated using either the program Sednterp (Laue et al. 1992) or Ultrascan. We estimate that the dimer species in the IC– pool had a dissociation constant (Kd ) of 1.87 OD. We determined the best fit Kd using a molar extinction coefficient (ɛ280nm = 1 × 105) calculated from the sequence of human IC and the measured A231/A280 = 4.8 for this sample. Monte Carlo analysis (1725 iterations) was used to estimate the confidence intervals of the fitted parameters based on the original noise level of the data. The 95% confidence interval of the Kd was found to be 1.7–2.0 × 10−6 M. The actual error is likely larger as a result of our inability to address the possibility that one or more forms of systematic error may have been present in the data set.
and ɛ280 calculations. The best fits of the data (described in the text) had randomly distributed residuals and a variance of 3.69 × 10−5 for the IC– pool and 5.73 × 10−5 for IC/LC pool. The solvent density (ρ) was calculated from buffer composition as described by Laue et al. (1992). These calculations, along with corrections for temperature, were calculated using either the program Sednterp (Laue et al. 1992) or Ultrascan. We estimate that the dimer species in the IC– pool had a dissociation constant (Kd ) of 1.87 OD. We determined the best fit Kd using a molar extinction coefficient (ɛ280nm = 1 × 105) calculated from the sequence of human IC and the measured A231/A280 = 4.8 for this sample. Monte Carlo analysis (1725 iterations) was used to estimate the confidence intervals of the fitted parameters based on the original noise level of the data. The 95% confidence interval of the Kd was found to be 1.7–2.0 × 10−6 M. The actual error is likely larger as a result of our inability to address the possibility that one or more forms of systematic error may have been present in the data set.
Acknowledgments
We thank Dr. Ching-Hwa Sung for Tctex-1 and rp3 antibodies, Dr. Frank Ramaekers for reticulon antibody, Dr. Solomon Snyder for PIN/LC8 antibody, and Drs. Linda Ehler and Emily Hildebrandt and members of the Schroer laboratory for critical reading of the manuscript. This work was supported by a Lucile and David Packard Fellowship for Science and Engineering and NIH award GM44589 (T.A.S.). S.J.K. was supported by NRSA GM 19061. Instrumentation support was provided by NSF award DBI-9871456 (E.N. Moudrianakis, P.I.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.2520102.
References
- Allan, V. 1996. Motor proteins: A dynamic duo. Curr. Biol. 6 630–633. [DOI] [PubMed] [Google Scholar]
- Barbar, E., Kleinman, B., Imhoff, D., Li, M., Hays, T.S., and Hare, M. 2001. Dimerization and folding of LC8, a highly conserved light chain of cytoplasmic dynein. Biochemistry 40 1596–1605. [DOI] [PubMed] [Google Scholar]
- Benashski, S.E., Harrison, A., Patel-King, R.S., and King, S.M. 1997. Dimerization of the highly conserved light chain shared by dynein and myosin V. J. Biol. Chem. 272 20929–20935. [DOI] [PubMed] [Google Scholar]
- Bingham, J.B., King, S.J., and Schroer, T.A. 1998. Purification of dynactin and dynein from brain tissue. Meth. Enzymol. 298 171–184. [DOI] [PubMed] [Google Scholar]
- Bloom, G.S., Wagner, M.C., Pfister, K.K., and Brady, S.T. 1988. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry 27 3409–3416. [DOI] [PubMed] [Google Scholar]
- Bowman, A.B., Patel-King, R.S., Benashski, S.E., McCaffery, J.M., Goldstein, L.S.B., and King, S.M. 1999. Drosophila roadblock and Chlamydomonas LC7: A conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J. Cell Biol. 146 165–179. [PMC free article] [PubMed] [Google Scholar]
- Brill II, L.B. and Pfister, K.K. 2000. Biochemical and molecular analysis of the mammalian cytoplasmic dynein intermediate chain. Methods 22 307–316. [DOI] [PubMed] [Google Scholar]
- Carver, J.A., Aquilina, J.A., Cooper, P.G., Williams, G.A., and Truscott, R.J.W. 1990. Alpha-crystallin: Molecular chaperone and protein surfactant. Biochem. Biophys. Acta. 1204 195–206. [DOI] [PubMed] [Google Scholar]
- Cohn, E.J. and Edsall, J.T. 1943. Density and apparent specific volume of protein. In Proteins, Amino Acids and Peptides as Ions and Dipolar Ions, pp. 370–377. Rheinhold, New York.
- Criswell, P.S. and Asai, D.J. 1998. Evidence for four cytoplasmic dynein heavy chain isoforms in rat testis. Mol. Biol. Cell. 9 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeler, B. and Saber, H. 1998. Determination of molecular parameters by fitting sedimentation data to finite-element solutions of the Lamm equation. Biophys. J. 74 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, T., Ray, K., Salz, H.K., and Chia, W. 1996. Cytoplasmic dynein (ddlc1) mutations cause morphogenetic defects and apoptotic cell death in Drosophila melanogaster. Mol. Cell Biol. 16 1966–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman III, J.F., and Pfister, K.K. 1994. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J. Cell Biol. 127 1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch, H. 1967. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6 1948–1954. [DOI] [PubMed] [Google Scholar]
- Fan, J.-S., Zhang, Q., Li, M., Tochio, H., Yamazaki, T., Shimizu, M., and Zhang, M. 1998. Protein inhibitor of neuronal nitric-oxide synthase, PIN, binds to a 17-amino acid residue fragment of the enzyme. J. Biol. Chem. 273 33472–33481. [DOI] [PubMed] [Google Scholar]
- Fan, J.-S., Zhang, Q., Tochio, H., Li, M., and Zhang, M. 2001. Structural basis of diverse sequence-dependent target recognition by the 8 kDa dynein light chain. J. Mol. Biol. 306 97–108. [DOI] [PubMed] [Google Scholar]
- Gee, M.A., Heuser, J.A., and Vallee, R.B. 1997. An extended microtubule binding domain structure within the dynein motor domain. Nature 390 636–639. [DOI] [PubMed] [Google Scholar]
- Gill, S.R., Cleveland, D.W., and Schroer, T.A. 1994. Characterization of DLC-A and DLC-B, two families of cytoplasmic dynein light chain subunits. Mol. Biol. Cell. 5 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa, N. 1998. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279 519–526. [DOI] [PubMed] [Google Scholar]
- Hughes, S.M., Vaughan, K.T., Herskovits, J.S., and Vallee, R.B. 1995. Molecular analysis of a cytoplasmic dynein light intermediate chain reveals homology to a family of ATPases. J. Cell Sci. 108 17–24. [DOI] [PubMed] [Google Scholar]
- Jaffrey, S.R. and Snyder, S.H. 1996. PIN: An associated protein inhibitor of neuronal nitric oxide synthase. Science 274 774–777. [DOI] [PubMed] [Google Scholar]
- Kai, N., Mishina, M., and Yagi, T. 1997. Molecular cloning of Fyn-associated molecules in the mouse central nervous system. J. Neurosci. Res. 48 407–424. [PubMed] [Google Scholar]
- Karki, S. and Holzbaur, E.L.F. 1995. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J. Biol. Chem. 270 28806–28811. [DOI] [PubMed] [Google Scholar]
- Karki, S. and Holzbaur, E.L.F. 1999. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 11 45–53. [DOI] [PubMed] [Google Scholar]
- King, S.J. and Schroer, T.A. 2000. Dynactin increases the processivity of the cytoplasmic dynein motor. Nature Cell Biol 2 20–24. [DOI] [PubMed] [Google Scholar]
- King, S.M. 2000a. AAA domains and organization of the dynein motor unit. J. Cell Sci. 113 2521–2526. [DOI] [PubMed] [Google Scholar]
- King, S.M. 2000b. The dynein microtubule motor. Biochim. Biophys. Acta. 1496 60–75. [DOI] [PubMed] [Google Scholar]
- King, S.M., Barbarese, E., Dillman III, J.F., Benashski, S.E., Do, K.T., Patel-King, R.S., and Pfister, K.K. 1998. Cytoplasmic dynein contains a family of differentially expressed light chains. Biochemistry 37 15033–15041. [DOI] [PubMed] [Google Scholar]
- King, S.M., Barbarese, E., Dillman, III, J.F., Patel-King, R.S., Carson, J.H., and Pfister, K.K. 1996a. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J. Biol. Chem. 271 19358–19366. [DOI] [PubMed] [Google Scholar]
- King, S.M., Dillman III, J.F., Benashski, S.E., Lye, R.J., Patel-King, R.S., and Pfister, K.K. 1996b. The mouse t-complex-encoded protein Tctex-1 is a light chain of brain cytoplasmic dynein. J. Biol. Chem. 271 32281–32287. [DOI] [PubMed] [Google Scholar]
- King, S.M., and Patel-King, R.S. 1995. The M(R) = 8,000 and 11,000 outer arm dynein light chains from Chlamydomonas flagella have cytoplasmic homologues. J. Biol. Chem. 270 11445–11452. [DOI] [PubMed] [Google Scholar]
- King, S.M., Wilkerson, C.G., and Witman, G.B. 1991. The Mr 78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with α-tubulin in situ. J. Biol. Chem. 266 8401–8407. [PubMed] [Google Scholar]
- Kini, A.M. and Collins, C.A. 2001. Modulation of cytoplasmic dynein ATPase activity by the accessory subunits. Cell Motil. Cytoskel. 48 52–60. [DOI] [PubMed] [Google Scholar]
- Koonce, M.P. 1997. Identification of a microtubule-binding domain in a cytoplasmic dynein heavy chain. J. Biol. Chem. 272 19714–19718. [DOI] [PubMed] [Google Scholar]
- Koonce, M.P. and Tikhonenko, I. 2000. Functional elements within the dynein microtubule-binding domain. Mol. Biol. Cell. 11 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227 680–685. [DOI] [PubMed] [Google Scholar]
- Laue, T.M., Shah, B.D., Ridgeway, T.M., and Pellitier, S.L. 1992. Computer-aided interpretation of analytical sedimentation data for proteins. In Analytical ultracentrifugation in biochemistry and polymer science (eds. S. Harding and A. Rowe), pp. 90–125. Royal Society of Chemistry, Cambridge, England.
- Liang, J., Jaffrey, S.R., Guo, W., Snyder, S.H., and Clardy, J. 1999. Structure of the PIN/LC8 dimer with a bound peptide. Nat. Struct. Biol. 6 735–740. [DOI] [PubMed] [Google Scholar]
- Lo, K.W.-H., Naisbitt, S., Fan, J.S., Sheng, M., and Zhang, M. 2001. The 8 kDa dynein light chain binds to its targets via a conserved "K/R-X-T-Q-T" motif. J. Biol. Chem. 276 14059–14066. [DOI] [PubMed] [Google Scholar]
- Ma, S., Trivinos-Lagos, L., Graf, R., and Chisholm, R.L. 1999. Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J. Cell Biol. 147 1261–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, D.R. and Rosenbaum, J.L. 1986. Protein-protein interactions in the 18S ATPase of Chlamydomonas outer dynein arms. Cell Motil. Cytoskel. 6 510–520. [DOI] [PubMed] [Google Scholar]
- Mok, Y.-K., Lo, K.W.-H., and Zhang, M. 2001. Structure of Tctex-1 and its interaction with cytoplasmic dynein intermediate chain. J. Biol. Chem. 276 14067–14074. [DOI] [PubMed] [Google Scholar]
- Naisbitt, S., Valtschanoff, J., Allison, D.W., Sala, C., Kim, E., Craig, A.M., Weinberg, R.J., and Sheng, M. 2000. Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin-V and dynein. J. Neurosci. 20 4524–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer, E.J., Schmidt, C.J., Nambudripad, R., and Smith, T.F. 1994. The ancient regulatory-protein family of WD-repeat proteins. Nature 371 297–300. [DOI] [PubMed] [Google Scholar]
- Neuwald, A.F., Aravind, L., Spouge, J.L., and Koonin, E.V. 1999. AAA+: A class of cheperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9 27–43. [PubMed] [Google Scholar]
- Nurminsky, D.I., Nurminskaya, M.V., Benevolenskaya, E.V., Shevelyov, Y.Y., Hartl, D.L., and Gvozdev, V.A. 1998. Cytoplasmic dynein intermediate-chain isoforms with different targeting properties created by tissue-specific alternative splicing. Mol. Cel. Biol. 18 6816–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell, P.H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250 4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okada, Y. and Hirokawa, N. 1999. A processive single-headed motor: kinesin superfamily protein KIF1A. Science 283 1152–1157. [DOI] [PubMed] [Google Scholar]
- Pace, C.N., Vajdos, F., Fee, L., Grimsley, G., and Gray, T. 1995. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 4 2411–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal, B.M., Mikami, A., Pfister, K.K., and Vallee, R.B. 1992. Homology of the 74-kD cytoplasmic dynein subunit with a flagellar dynein polypeptide suggests an intracellular targeting function. J. Cell Biol. 118 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister, K.K., Salata, M.W., Dillman III, J.F., Torre, E., and Lye, R.J. 1996a. Identification and developmental regulation of a neuron-specific subunit of cytoplasmic dynein. Mol. Biol. Cell. 7 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister, K.K., Salata, M.W., Dillman III, J.F., Vaughan, K.T., Vallee, R.B., Torre, E., and Lye, R.J. 1996b. Differential expression and phosphorylation of the 74-kDa intermediate chains of cytoplasmic dynein in cultured neurons and glia. J. Biol. Chem. 271 1687–1694. [DOI] [PubMed] [Google Scholar]
- Purohit, A., Tynan, S.H., Vallee, R., and Doxsey, S.J. 1999. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 147 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroek, A.J., Ayoubi, T.A., Van de Velde, H.J., Schoenmakers, E.F., Pauli, I.G., and Van de Ven, W.J. 1996. Genomic organization of the human NSP gene, prototype of a novel gene family encoding reticulons. Genomics 32 191–199. [DOI] [PubMed] [Google Scholar]
- Roux, A.-F., Rommens, J., McDowell, C., Anson-Cartwright, L., Bell, S., Schappert, K., Fishman, G.A., and Musarella, M. 1994. Identification of a gene from Xp21 with similarity to the tctex-1 gene of the murine t complex. Hum. Mol. Genet. 3 257–263. [DOI] [PubMed] [Google Scholar]
- Schroer, T.A. 1996. Structure and function of dynactin. Semin. Cell Devel. Biol. 7 321–328. [Google Scholar]
- Schroer, T.A. and Sheetz, M.P. 1991. Two activators of microtubule-based vesicle transport. J. Cell Biol. 115 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers, J.R. 2000. Myosins: A diverse superfamily. Biochim. Biophys. Acta. 1496 3–22. [DOI] [PubMed] [Google Scholar]
- Senden, N.H., Timmer, E.D., Boers, J.E., van de Velde, H.J., Roebroek, A.J., Van de Ven, W.J., Broers, J.L., and Ramaekers, F.C. 1996. Neuroendicrine-specific protein C (NSP-C): Suncellular localization and differential expression in relation to NSP-A. Eur. J. Cell Biol. 69 197–213. [PubMed] [Google Scholar]
- Sloboda, R.D. and Rosenbaum, J.L. 1982. Purification and assay of microtubule-associated proteins (MAPs). Meth. Enzymol. 85 409–416. [DOI] [PubMed] [Google Scholar]
- Sondek, J., Bohm, A., Lambright, D.G., Hamm, H.E., and Sigler, P.B. 1996. Crystal structure of a GA protein βγ dimer at 2.1 angstrom resolution. Nature 379 369–374. [DOI] [PubMed] [Google Scholar]
- Steuer, E.R. 1991. Characterization of cytoplasmic dynein and its immunolocalization in dividing cells. In Ph. D. Thesis: Department of Cell Biology. Washington University, St. Louis, MO. p. 138.
- Steuer, E.R., Schroer, T.A., Wordeman, L., and Sheetz, M.P. 1990. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature 345 266–268. [DOI] [PubMed] [Google Scholar]
- Susalka, S.J., Hancock, W.O., and Pfister, K.K. 2000. Distinct cytoplasmic dynein complexes are transported by different mechanisms in axons. Biochim. Biophys. Acta. 1496 76–88. [DOI] [PubMed] [Google Scholar]
- Tai, A.W., Chuang, J.-Z., Bode, C., Wolfrum, U., and Sung, C.-H. 1999. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex1. Cell 97 877–887. [DOI] [PubMed] [Google Scholar]
- Tai, A.W., Chuang, J.-Z., and Sung, C.-H. 1998. Localization of Tctex-1, a cytoplasmic dynein light chain, to the golgi apparatus and evidence for dynein complex heterogeneity. J. Biol. Chem. 273 19639–19649. [DOI] [PubMed] [Google Scholar]
- Tai, A.W., Chuang, J.-Z., and Sung, C.-H. 2001. Cytoplasmic dynein regulation by subunit heterogeneity and its role in apical transport. J. Cell Biol. 153 1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada, S. and Kamiya, R. 1994. Functional reconstitution of Chlamydomonas outer dynein arms from α-β and γ subunits: Requirement of a third factor. J. Cell Biol. 126 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar, E., Musacchio, A., Harrison, S.C., and Kirchhausen, T. 1998. Atomic structure of clathrin: A β propeller terminal domain joins an α zigzag linker. Cell 95 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn, K.S., Ubersax, J.A., and Vale, R.D. 2000. Engineering the processive run length of the kinesin motor. J. Cell Biol. 151 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin, H., Staehelin, T., and Gordon, J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Pro. Natl. Acad. Sci. 76 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan, S.H., Gee, M.A., and Vallee, R.B. 2000. Light Intermediate chain 1 defines a functional subfraction of cytoplasmic dynein which binds to pericentrin. J. Biol. Chem. 275 32763–32768. [DOI] [PubMed] [Google Scholar]
- Vaisberg, E.A., Grissom, P.M., and McIntosh, J.R. 1996. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J. Cell Biol. 133 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale, R.D. and Milligan, R.A. 2000. The way things move: Looking under the hood of molecular motor proteins. Science 288 88–95. [DOI] [PubMed] [Google Scholar]
- van Holde, K.E. and Weischet, W.O. 1978. Boundary analysis of sedimentation velocity experiments with monodisperse and paucidisperse solutions. Biopolymers 17 1387–1403. [Google Scholar]
- Vaughan, K.T. and Vallee, R.B. 1995. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J. Cell Biol. 131 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, P.S., Leszyk, J.D., and Vaughan, K.T. 2001. Cytoplasmic dynein intermediate chain phosphorylation regulates binding to dynactin. J. Biol. Chem. 276 26171–26179. [DOI] [PubMed] [Google Scholar]
- Wall, M.A., Coleman, D.E., Lee, E., Iniguez-Lluhi, J.A., Posner, B.A., Gilman, A.G., and Sprang, S.R. 1995. The structure of the G protein heterotrimer Giα1β1γ2. Cell 83 1047–1058. [DOI] [PubMed] [Google Scholar]
- Wilkerson, C.G., King, S.M., Koutoulis, A., Pazour, G.J., and Witman, G.B. 1995. The 78,000 Mr intermediate chain of Chlamydomonas outer arm dynein is a WD-repeat protein required for arm assembly. J. Cell Biol. 129 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]






