Abstract
The hydrophobin SC3 belongs to a class of small proteins functioning in the growth and development of fungi. Its unique amphipathic property and remarkable surface activity make it interesting not only for biological studies but also for medical and industrial applications. Biophysical studies have revealed that SC3 possesses at least three distinct conformations, named "soluble-state SC3" for the protein in solution, and "α-helical-state SC3" and "β-sheet-state SC3" for the different states of the protein associated at a hydrophobic-water interface. The present fluorescence study shows that the microenvironment of the dansyl-labeled N terminus of soluble-state SC3 is relatively hydrophobic, whereas it is hydrophilic for α-helical-state and β-sheet-state SC3. Fluorescence collisional quenching indicates that the N terminus of soluble-state SC3 is more solvent-accessible than those of α-helical-state and β-sheet-state SC3, with Stern-Volmer constants for acrylamide of 4.63, 0.02, and 0.2 M−1 for the different states, respectively. Fluorescence resonance energy transfer measurements show that soluble-state SC3 tends to associate in solution but dissociates in TFA. Fluorescence energy transfer was eliminated by conversion of soluble-state SC3 to α-helical-state SC3 on a hydrophobic surface, indicating a spatial separation of the molecules in this state. By inducing the β-sheet state, structural changes were observed, both by CD and by fluorescence, that could be fit to two exponentials with lifetimes of about 10 min and 4 h. Molecules in the β-sheet state also underwent a slow change in spatial proximity on the hydrophobic surface, as revealed by the reappearance of fluorescence resonance energy transfer in time.
Keywords: Hydrophobin, circular dichroism spectroscopy, fluorescence collisional quenching, fluorescence resonance energy transfer
Hydrophobins are a class of small proteins that play an important role in fungal growth and development, for example, the formation of aerial hyphae and the attachment of hyphae to hydrophobic surfaces (Wösten et al. 1993, 1994a; van Wetter et al. 1996, 2000). These proteins are remarkable in that they self-assemble into an amphipathic membrane at hydrophobic-hydrophilic interfaces. This makes them interesting not only from a biological point of view, but also for medical and industrial applications (Wessels 1997; Scholtmeijer et al. 2001). Two different types of hydrophobins, class I and class II, have been distinguished based on the differences in their hydropathy patterns and biophysical properties (Wessels 1994).
SC3, secreted by Schizophyllum commune, is the best characterized class I hydrophobin. It contains eight cysteine residues whose spacings are conserved for all hydrophobins (Wessels 1997). The cysteines form four disulfide bridges, which keep SC3 from spontaneously self-assembling and account for controlled assembly at hydrophilic/hydrophobic interfaces (de Vries et al. 1993; de Vocht et al. 1998, 2000). SC3 is distinguished from other hydrophobins by a long N-terminal sequence proceeding the first cysteine residue. There are 17–22 mannose residues probably attached to the 12 threonine residues in this region, which are exposed at the hydrophilic side of assembled SC3 and, therefore, are expected to determine the surface properties of this side (de Vocht et al. 1998). SC3 exists in three different conformations as elucidated by circular dichroism spectroscopy (CD) and attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR). After the dissociation of assembled SC3 with solvents such as pure trifluoroacetic acid (TFA), SC3 can be resuspended in buffer, resulting in the so-called soluble state (de Vries et al. 1993). CD showed the minimum at 209nm, and ATR-FTIR showed 23% α-helix, 41% β-sheet, 16% β-turn, and 20% random coil structure. Once soluble-state SC3 comes in contact with a hydrophobic surface in water, such as Teflon, or an air-water interface, the secondary structure converts to an α-helical state with minima in the CD spectrum at 205nm and 218nm (de Vocht et al. 1998). The α-helical state is stable on a Teflon surface, but it is only present for about 5 min at the air-water interface; therefore, it is believed to be an intermediate state on the way to forming a β-sheet state. Alpha-helical-state SC3 on Teflon is converted into β-sheet state upon treatment with hot detergent. The CD spectrum of this state shows an ellipticity minimum at 215nm. ATR-FTIR showed that this state contains 65% β-sheet structure compared to about 41% in the soluble state (de Vocht et al. 1998). The β-sheet state is the most interesting state to study, because it is the stable end-structure of all of the class I hydrophobins responsible for a highly insoluble amphipathic membrane on the fungal hyphae surface or the air-water interface. The hydrophobic side of this assembled SC3 is characterized by a mosaic of 5–12 nm-wide parallel rodlets, each of which consists of two tracks of 2–3 protofilaments with a diameter of about 2.5 nm each. (Wösten and de Vocht 2000).
Although class I hydrophobins undergo changes in secondary structure upon coating hydrophobic surfaces, a variety of questions still need to be answered: Is the conformational transition a static or a dynamic process? How do the molecules interact with each other during the conformational transitions? Which parts of the molecules are involved? In the present study, the dynamics of β-sheet formation in SC3 were followed using CD, and the structure of SC3 in different conformations and the interactions of molecules were elucidated using fluorescence spectroscopy.
Results
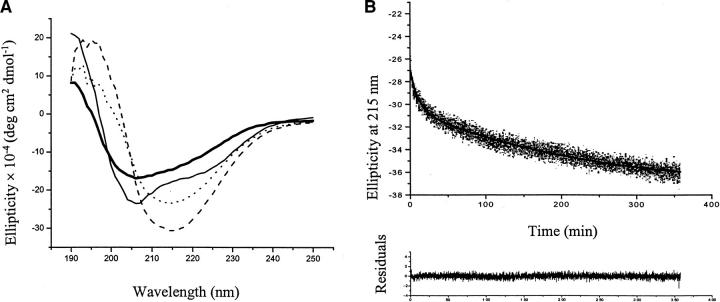
Kinetics of β-sheet-state SC3 formation measured by CD
The secondary structures of SC3 under different conditions have been characterized with CD and ATR-FTIR spectroscopy by de Vocht et al. (1998). Soluble-state SC3 and α-helical-state SC3, the latter of which was achieved by adding Teflon to soluble-state SC3, showed stable CD spectra (Fig. 1A ▶). Induction of β-sheet-state SC3 by heating and adding detergent led to a decrease in time of the ellipticity at 215 nm, reflecting the dynamic process of β-sheet-state formation. The time dependence of the CD measurements in this state could be fit to the sum of two exponentials, thus defining two different kinetic phases. The first phase showed a lifetime of 5–10 min (t1), whereas the second phase showed a lifetime of 1.5–5 h (t2) (Fig. 1B ▶). For all of the experiments in this study with colloidal Teflon, the surface coverage was maintained below 10% to make sure that all the protein is absorbed on the Teflon (de Vocht et al. 1998).
Fig. 1.
CD measurements on SC3 in different conformations. (A) CD spectrum of SC3 in different conformations. Thick solid line: soluble-state SC3 in buffer. Thin solid line: α-helical-state SC3 on Teflon. Dotted line: β-sheet -state SC3 on Teflon after 30-min incubation at 65°C in the presence of 0.1% (v/v) Tween 80. Dashed line: β-sheet-state SC3 on Teflon after 300-min incubation at 65°C in the presence of 0.1% (v/v) Tween 80. (B) The kinetics of β-sheet-state conformational change. Ellipticity was measured at 215 nm, 65°C in the presence of 0.1% (v/v) Tween 80. The surface coverage of the Teflon by SC3 was about 5%. The decrease of ellipticity can be fit to two exponentials with a lifetime, t1 of 10 min and long-lifetime, t2 of 223 min. The residuals are plotted below the decay curve.
Fluorescence measurements of dansyl-SC3 in different conformational states
Labeling of SC3 with dansyl succinimidyl ester
Because the eight cysteines in SC3 form four intramolecular disulfide bridges and lysine residues are not present, the N terminus can, in principle, be labeled specifically with the amine selective reagent dansyl succinimidyl ester. The labeling was performed according to the procedure described in the Materials and Methods section. After reaction and purification, the labeled protein showed fluorescence spectra different from those of the control samples, that is, free dansyl reagent in solution or dansyl-labeled angiotensin I under the same condition, confirming the success of labeling (Fig. 2 ▶). MALDI-TOF spectra showed that 50%–60% of SC3 was labeled. To make sure that the incomplete labeling yield was not caused by the experimental conditions, a rapid, sensitive assay for the detection of primary amines in proteins using fluorescamine was performed on SC3 (McCaman and Robins 1962). With two small peptides as controls, the accessibility of the N terminus of SC3 was found to be 60%, which was consistent with the yield of labeling with dansyl. A possible reason for the less than stoichiometric labeling is the self-association of molecules in solution, which is also suggested by the FRET measurements (see the following section). To make sure that nonspecific labeling was not contributing to the fluorescence, another control experiment was performed. The N terminus of SC3 was specifically blocked by an excess of acetic anhydride, an amine-reactive reagent, followed by the standard dansyl labeling procedure. The reacted protein was subjected to the same purification protocol and MALDI-TOF measurements were performed. No dansyl-labeled SC3 mass could be assigned in the MALDI-TOF spectrum, confirming that the fluorescent label in dansyl-SC3 was situated at the N terminus, and all that unreacted dansyl is removed after the purification. After the induction of the various secondary structures, dansyl-labeled SC3 showed the same CD spectra as unlabeled SC3, indicating that labeling did not alter the ability of the protein to undergo the changes in secondary structure (data not shown).
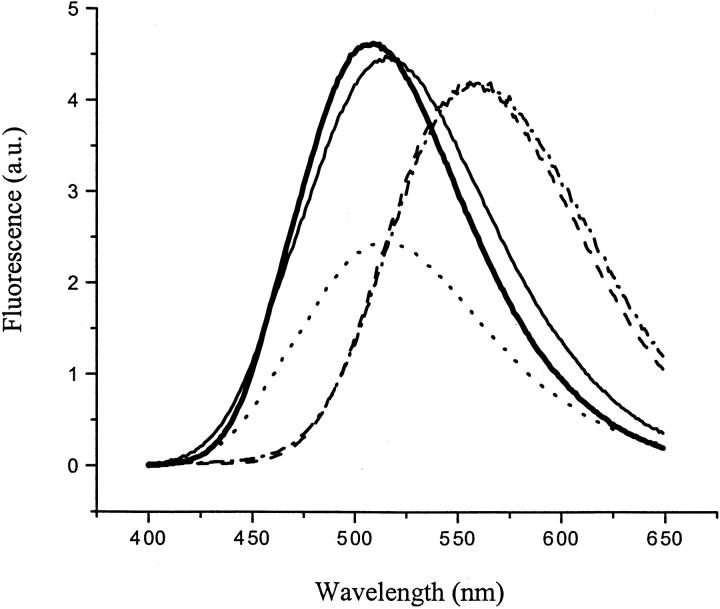
Fig. 2.
Fluorescence spectra of dansyl-SC3 in different conformations. Thick solid line: soluble-state dansyl-SC3 in buffer. Thin solid line: the α-helical-state dansyl-SC3 on Teflon. Dotted line: the β-sheet-state dansyl-SC3 on Teflon after 1-h incubation at 65°C in the presence of 0.1% (v/v) Tween 80. Dashed line: free dansyl reagent in buffer. Dashed-dotted line: dansyl-labeled angiotensin I. The spectra were corrected for the background and the volume changes.
Fluorescence measurements
The dansyl moiety is sensitive to environmental changes, and therefore the fluorescence spectra of dansyl-SC3 should report the microenvironments around the N terminus of the three conformational states of the protein. As shown in Figure 2 ▶, soluble-state dansyl-SC3 showed a fluorescence emission maximum at 510 nm, versus 560 nm for the unreacted reagent and 556 nm for the dansyl-labeled angiotensin I. The large blue shift for the dansyl-labeled SC3, compared to the dansyl-labeled peptide, indicates that the N terminus of soluble-state SC3 is situated in a hydrophobic, possibly buried, environment. Upon binding to Teflon spheres, the emission maximum shifted to 520 nm for α-helical-state SC3 and shifted even further to 526 nm for the β-sheet-state SC3, suggesting that the N terminus of both forms of the protein on Teflon is located in a more hydrophilic environment than in soluble SC3.
Collisional quenching properties of dansyl-SC3 in different conformational states
Fluorescence collisional quenching is a typical approach to determine the polarity of the fluorophore environment and the degree of its exposure to the solvent. Acrylamide appeared to be the best collisional quencher for soluble-state dansyl-SC3. The quenching constant Ksv of acrylamide for a 10 μg/mL solution of soluble-state dansyl-SC3 was 4.6 M−1. I− and Cs+ were poorer quenchers with Ksv of 1.7 and 2.1 M−1, respectively, at the same protein concentration (Fig.3 ▶, Table 1). The ionic quenchers appear to have less access to the N terminus, possibly because it is buried inside the protein matrix, but also charge residues around the fluorophore could have affected the accessibility. Surprisingly, when 2 μg/mL labeled protein was used instead of 10 μg/mL, higher quenching constants were obtained, especially for acrylamide (Table 1). This could indicate that soluble-state SC3 undergoes self-association with more of the dissociated species at lower concentrations.
Fig. 3.
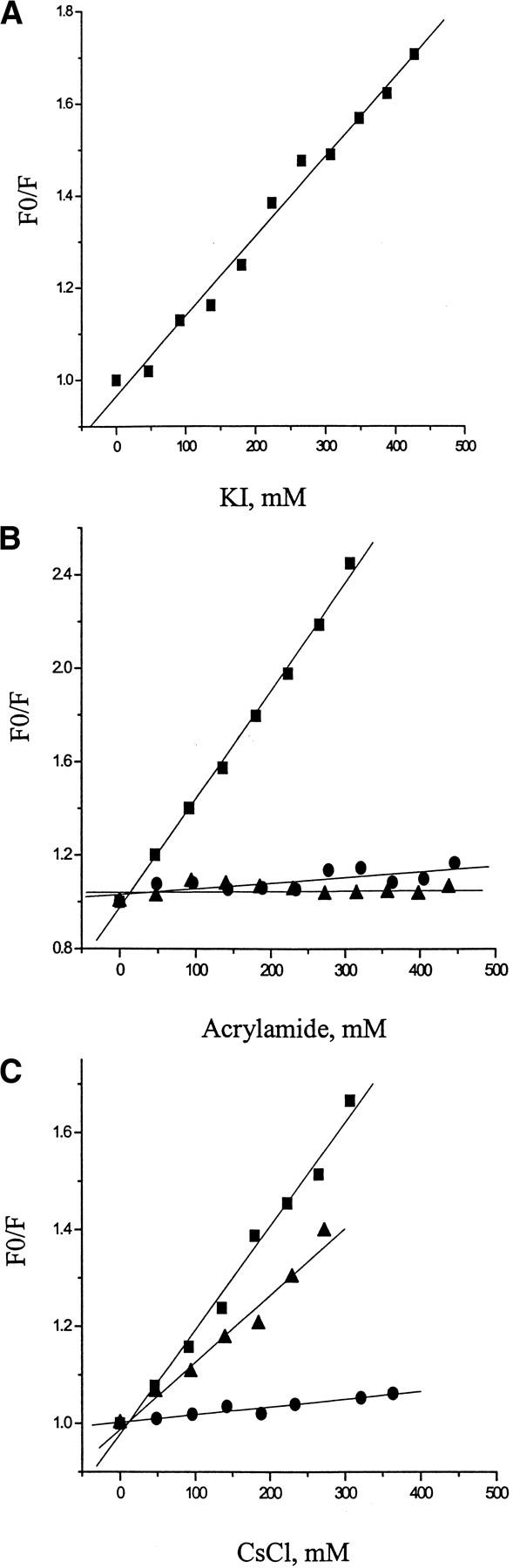
Fluorescence collisional quenching properties of dansyl-SC3 in different conformations. Black squares: collisional quenching on soluble-state dansyl-SC3. Black triangles: collisional quenching on α-helical-state dansyl-SC3 on Teflon. Black dots: β-sheet-state dansyl-SC3 on Teflon after 6 h of incubation at 65°C in the presence of 0.1% (v/v) Tween 80. Straight lines: linear fits of quenching data. For the KI quenching experiment, Teflon congeals upon adding KI and therefore the reliable quenching on α-helical- and β-sheet-state SC3 could not be determined. The protein concentration used here was maintained at 10 μg/mL.
Table 1.
Stern-Volmer constants for collisional quenching of dansyl-labeled SC3 in different conformations
| Ksv (I+)(M−1) | Ksv (Cs+)(M−1) | Ksv (acrylamide)(M−1) | |||
| Conformation | 10 μg/mL | 2 μg/mL | 10 μg/mL | 10 μg/mL | 2 μm/mLa |
| Soluble-state | 1.73 | 4.64 | 2.14 | 4.64 | 9.91 |
| α-helical-state | no resultb | 1.38 | 0.02 | ||
| β-sheet-state, 15-min incubationc | no result | 0.13 | 0.27 | ||
| β-sheet-state, 30-min incubationc | no result | 0.10 | 0.60 | ||
| β-sheet-state, 6-h incubationc | no result | 0.15 | 0.23 | ||
a Different concentrations of dansyl-SC3.
b Teflon spheres tended to congeal under the experimental condition, and therefore no reliable results were obtained.
c β-sheet-state formation was stopped at the indicated times by cooling down the samples from 65°C to 25°C (de Vocht et al., unpublished).
When SC3 is in the α-helical or β-sheet state on a Teflon surface, the quenching constants were lower than for soluble state (Fig.3 ▶, Table 1), indicating that, in these two conformations, the N terminus is considerably less accessible from the solvent. Reliable results for KI quenching on β-sheet-state SC3 could not be obtained, because Teflon spheres tended to congeal under the experimental condition.
Fluorescence resonance energy transfer studies
Information on changes in the aggregation states and spatial proximity of SC3 molecules in their various conformations was collected using N-terminal-labeled dansyl-SC3 as donor and N-terminal-labeled dabcyl-SC3 as acceptor. Because dabcyl does not fluoresce, FRET can only be determined by the fluorescence quenching of donor. The Förster distance (R0) of this energy transfer pair has been reported to be 3–4nm (Miki and Remedios 1990; Adair and Engelman 1994). The SC3 concentration dependence in the acrylamide quenching data suggests that aggregation of soluble-state SC3 has influenced the accessibility of the N terminus of the protein. Based on the assumption that SC3 molecules associate in solution but dissociate in TFA, two different ways to prepare the samples for FRET measurements were used. In the first method, stock solutions of 100 μg/mL soluble dansyl-SC3 and 100 μg/mL soluble dabcyl-SC3 were prepared separately in 10 mM sodium phosphate, pH 7.0. The solutions were then mixed to achieve a specified molar ratio and a total protein concentration of 10 μg/mL. This preparation method is called TFA/dansyl-SC3 + TFA/dabcyl-SC3. In the second method, the same amount of labeled protein and the same molar ratios were used, but after mixing, the solution was freeze-dried, treated with TFA, dried with nitrogen gas, and finally dissolved in buffer to a total protein concentration of 10 μg/mL. This preparation method is called TFA/dansyl-SC3/dabcyl-SC3. Fluorescence was measured immediately after preparing the samples unless stated otherwise. A correction was made for the background signal of α-helical-state and β-sheet-state SC3 by measuring buffer containing the same amount of Teflon alone under the same experimental conditions. For time-dependent measurements, the fluorescence at 520 nm was recorded continuously at a rate of 5 min per data point in the first 1 h and at 15 min in the following 14 h.
SC3 in soluble state
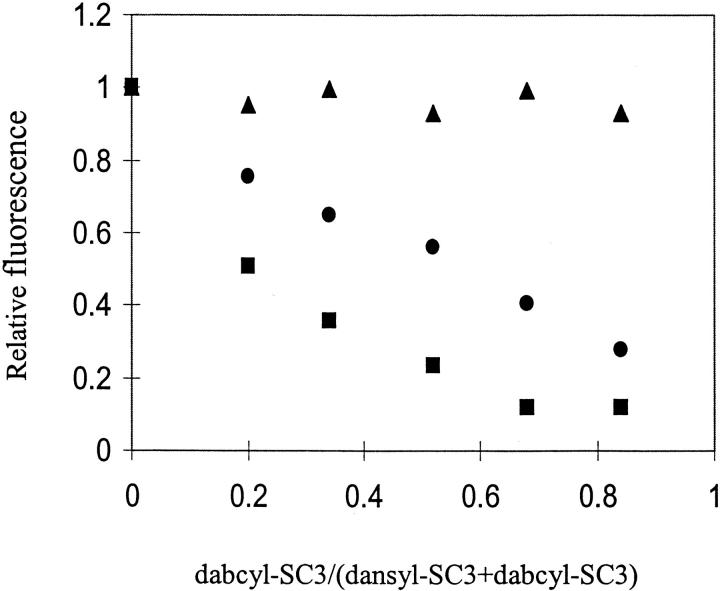
TFA/dansyl-SC3 was mixed with the same amount of TFA/dabcyl-SC3 in buffer, and the fluorescence spectrum was recorded immediately after mixing. The total protein concentration was maintained below 20 μg/mL, which was low enough to avoid the effect of molecular diffusion in solution. The presence of TFA/dabcyl-SC3 resulted in about 20%–30% fluorescence quenching (Fig. 4A ▶). When the same experiment was repeated but with samples treated with TFA after mixing (the second method), a more dramatic FRET was observed with about 60%–70% of the fluorescence being quenched (Fig. 4B ▶). When dansyl-SC3 was mixed with dabcyl-SC3 in different molar ratios, for both preparation methods, FRET increased with increasing amounts of dabcyl-SC3 (Fig. 5 ▶). These data confirm that SC3 self-associates in the soluble state and, under these conditions, only a limited amount of exchange between associated populations occurs. Treatment of the mixed populations with TFA initiates monomerization and randomization. Subsequent self-association after removal of TFA results in much higher levels of FRET.
Fig. 4.
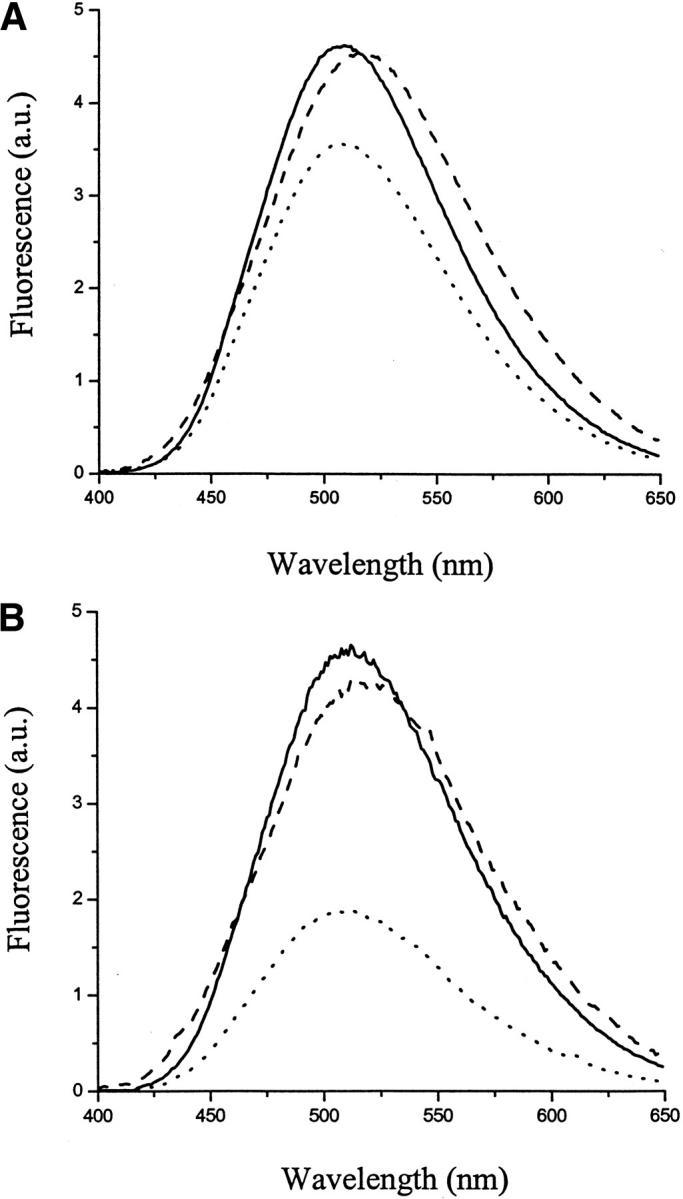
Fluorescence resonance energy transfer study on soluble-state and α-helical-state dansyl-SC3. (A) Solid line: fluorescence spectrum of soluble-state TFA/dansyl-SC3 in buffer. Dotted line: TFA/dansyl-SC3+TFA/dabcyl-SC3 in buffer (molar ratio 1:1). Dashed line: the same sample as dotted line but supplemented with Teflon spheres. (B) Solid line: fluorescence spectrum of soluble TFA/dansyl-SC3 in buffer. Dotted line: TFA/dansyl-SC3/dabcyl-SC3 in buffer (molar ratio 1:1). Dashed line: the same sample as dotted line but supplemented with Teflon spheres. The spectra were corrected for the background signal and the volume changes.
Fig. 5.
Concentration-dependent FRET on soluble-state and α-helical-state dansyl-SC3. Black dots: relative fluorescence intensities of TFA/dansyl-SC3 in the presence of different amounts of TFA/dabcyl-SC3. Black squares: normalized fluorescence intensities of the same samples as black dots but with freeze-drying, TFA treatment, and being dissolved in buffer. Black triangles: normalized fluorescence intensities of the same samples as black squares but supplemented with Teflon spheres.
SC3 in α-helical state
Figure 4 ▶ depicts the change of FRET upon attachment of SC3 to a Teflon surface. Irrespective of the preparation method, the FRET observed with soluble-state SC3 disappeared and donor fluorescence returned to its original level. Similar observations were made in the concentration dependence when the surface coverage of the Teflon sphere was kept below 10% (Fig. 5 ▶). Apparently, upon binding to Teflon, SC3 molecules dissociate, convert to the α-helical state, and keep a certain distance from each other. Time-dependent fluorescence measurements on α-helical-state SC3 showed that, in contrast to β-sheet-state SC3 (see next section), the fluorescence remained stable on a time scale of more than 10 h (Fig. 6B ▶). Apparently, upon attaching to a Teflon surface, molecules of SC3 in the α-helical state are separated and immobilized, and, in agreement with the CD observations, undergo no further conformational change.
Fig. 6.
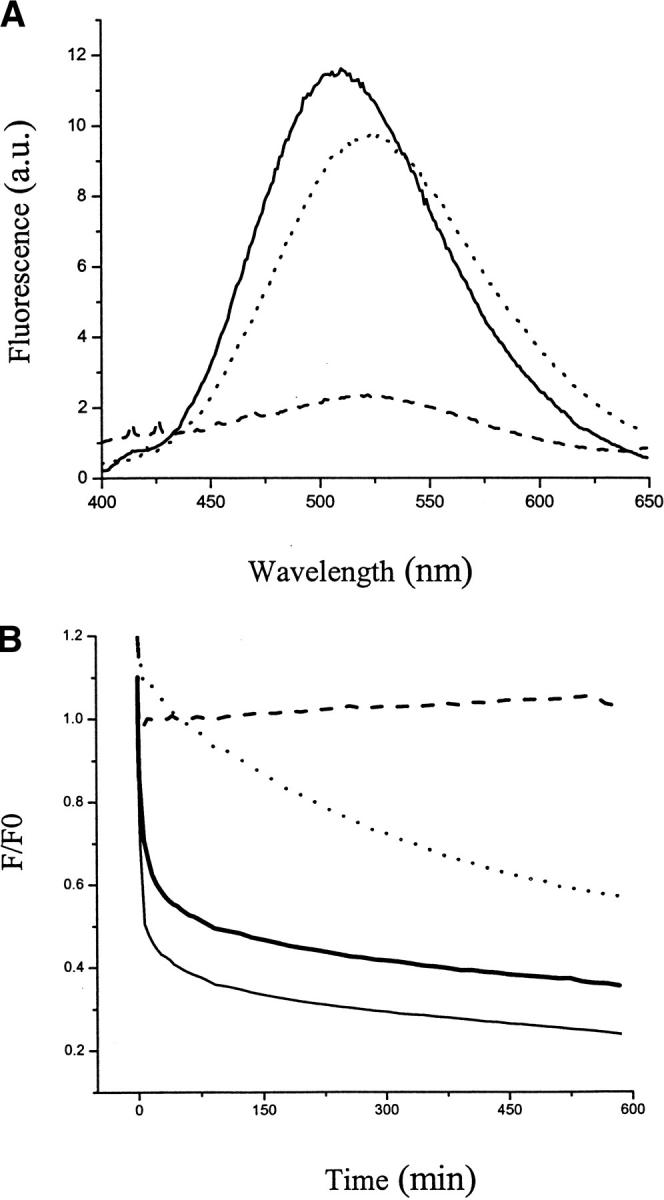
FRET on the β-sheet-state dansyl-SC3. (A) Solid line: fluorescence spectrum of TFA/dansyl-SC3 + TFA/dabcyl-SC3 (molar ratio 1:1) in buffer. Dotted line: the same sample supplemented with Teflon spheres and heated up to 65°C. Dashed line: the same sample supplemented with 0.1% (v/v) Tween 80 and incubated to 15 h. The spectra were corrected for the background signal and the changes of volumes. (B) The kinetic fluorescence recorded at 520nm. Thick solid line: fluorescence change of β-sheet-state TFA/dansyl-SC3 + TFA/dabcyl-SC3 (molar ratio 1:1) on Teflon. Thin solid line: β-sheet-state TFA/dansyl-SC3/dabcyl-SC3 (molar ratio 1:1) on Teflon. Dotted line: β-sheet-state dansyl-SC3 on Teflon. The decrease in fluorescence could be fit to two exponentials, with a lifetime t1 value of 2.5 min and a lifetime t2 of 278 min. Beta-sheet-state SC3 was induced by heating to 65°C in the presence of 0.1% (v/v) Tween 80. Dashed line: α-helical-state dansyl-SC3 on Teflon induced by adding Teflon spheres. F0 indicates the initial fluorescence recorded at time 0.
SC3 in β-sheet state
The spatial proximity of SC3 molecules on a hydrophobic surface changes when molecules convert to the β-sheet state. This state was induced by heating the α-helicalstate SC3 on Teflon up to 65°C in the presence of 0.1% (v/v) Tween 80. As shown in Figure 6A ▶, once the β-sheet state was induced in the mixture of TFA/dansyl-SC3 + TFA/dabcyl-SC3, the fluorescence intensity decreased dramatically, with more than 80% of the fluorescence being quenched after 15 h. This result suggests that FRET occurs due to the clustering of molecules on the surface. The fluorescence intensity of the molecules on the surface changes as a function of time, as reported in Figure 6B ▶. For both TFA/dansyl-SC3 + TFA/dabcyl-SC3 and TFA/dansyl-SC3/dabcyl-SC3 samples, the fluorescence underwent a rapid increase, followed by a fast decrease within 10 min and, finally, a very slow decrease over a period of 10 h. Although these two decay curves could not be fit to two exponentials very well, two kinetic components can still be estimated. The fast component has a lifetime of 2.2 min with more than 40% of contribution to the total decrease. The contribution of the fast component in fluorescence decrease was larger for TFA/dansyl-SC3/dabcyl-SC3 than for TFA/dansyl-SC3 + TFA/dabcyl-SC3, because prior TFA treatment makes the solution more homogeneous and, therefore, facilitates the energy transfer between molecules. The surface coverage of the Teflon spheres by SC3 was maintained at 5%–10% in both cases, which rules out the possibility that difference in different diffusion rates caused the different levels of FRET.
TFA/dansyl-SC3 in the absence of dabcyl-SC3 was also induced to form β-sheet state on Teflon as a control (Fig. 6B ▶). The fluorescence also underwent a fast increase, similar to the sample in the presence of dabcyl-SC3, followed by a slow decrease. This decay curve could be fit to two exponentials with t1 of 2.5 min and t2 of 278 min, which are in the same range as the two kinetic lifetimes obtained from CD measurements, 10 min and 223 min, respectively. The contribution of the fast component to the total fluorescence decrease is about 10%. Therefore, we suggest that the fluorescence decrease observed with β-sheet-state dansyl-SC3 in the absence of FRET is mainly caused by conformational changes in SC3 molecules, and the fluorescence decrease observed in the presence of FRET is the combination of conformational changes and molecular proximity.
Discussion
Purification of SC3
For many years, SC3 has been purified by a method based on electrobubbling precipitation, TFA treatment, and ethanol precipitation (Wösten et al. 1993; Wessels 1997). There are some disadvantages to this method, especially the low purification yield. A new procedure with higher yield and shorter labor time was needed to produce SC3 on a large scale. It was reported that SC3 can bind to hydrophobic resins such as phenyl sepharose in the presence of a high concentration of (NH4)2SO4 and can be eluted with a lower concentration of (NH4)2SO4 (Martin et al. 2000). We have combined the elements of the two methods, electrobubbling, TFA treatment, and hydrophobic interaction chromatography to purify SC3 on a large scale, yielding about 120 mg of pure SC3 from a 10 L culture.
The effect of conformational changes on the fluorescence
Compared to free dansyl reagent in solution and dansyl-labeled angiotensin I, soluble-state dansyl-SC3 showed a large blue-shifted emission maximum, indicating a more hydrophobic microenvironment around the N terminus. The emission maximum of dansyl-SC3 varied from 508 to 512 nm, depending on the batch of purified protein being used. This variation might reflect the relatively unstable structure of soluble-state dansyl-SC3, which could also be observed in the CD spectra with the ellipticity minimum shifting between 208 and 211 nm. However, variation in the degree and/or nature of the association of molecules in solution may also cause the variations in the microenvironment of the N terminus. In contrast to soluble-state SC3, α-helical-state and β-sheet-state SC3 showed stable fluorescence emission maxima at 520 nm and 526 nm, respectively. The red-shifted emission maximum, compared to that of soluble-state SC3, suggests that the N terminus of the molecules in the α-helical state and β-sheet state is located in a more hydrophilic environment. This finding is consistent with the presence of multiple O-glycosylation sites at the N terminus, which are believed to be responsible for the high hydrophilicity of the hydrophilic side of assembled SC3 (Wösten et al. 1994b; Scholtmeijer et al. 2001).
Solvent accessibility of the N terminus of dansyl-SC3
It is evident from collisional quenching that the N terminus of soluble-state dansyl-SC3 is more accessible to the solvent than the α-helical-state and β-sheet-state SC3. This is not surprising because soluble-state SC3 is less structured than the other forms, making the buried N terminus more accessible to quenchers. It is very likely that steric hindrance caused a lower accessibility of N terminus in the α-helical- and β-sheet-state SC3. The increase in secondary structure in α-helical-state SC3 and β-sheet-state SC3 probably results in a more compact structure and a more buried N terminus.
There was an increased quencher accessibility upon lowering the concentration of dansyl-SC3 from 10 to 2 μg/mL. Upon raising the protein concentration above 10 μg/mL, the accessibility did not decrease much further, suggesting the existence of a critical concentration for the association of molecules in solution in the low μg/mL range. SC3 associates above this critical concentration but tends to dissociate below it. Similar results were obtained when thioflavine T (ThT) fluorescence was used to monitor the self-assembly of SC3 at the air-water interface; in those experiments a critical concentration of 3.7 μg/mL was determined (M.L. de Vocht et al., unpubl.).
Association of soluble-state SC3 in solution
FRET has been widely used as a spectroscopic ruler for long-range distance determination in macromolecules. It has also been used to detect actin filament assembly and Alzheimer Aβ peptide assembly (Huang et al. 1997). Until the present study, it was assumed that soluble-state SC3 exists in the monomeric form, and only self-assembled at hydrophilic/hydrophobic interfaces. However, when we mixed soluble TFA/dansyl-SC3 with TFA/dabcyl-SC3, FRET occurred spontaneously, indicative of association of molecules in solution. An increased degree of FRET was achieved by enabling complete mixing of the two species by treatment with TFA to dissociate the aggregates. A detailed study to determine the size and equilibrium of the multimers in solution is in progress.
Dissociation of SC3 in α-helical state
Alpha-helical-state SC3 is an intermediate state that is formed in the presence of a water/hydrophobic surface interface. If the hydrophobic surface is air, this intermediate state is relatively short lived (minutes) on the way to a stable self-assembled β-sheet state. If the hydrophobic surface is a solid such as Teflon, the protein is trapped in the α-helical intermediate state and structural rearrangements cannot progress further unless heat and detergent are applied (M.L. de Vocht et al., unpubl.). The present data show that the SC3 molecules in this intermediate state are far enough separated from each other as to prevent FRET. This is the first time that the conformational change of SC3 on a hydrophobic surface has been seen to be accompanied by a spatial separation of the molecules. The kinetic measurement on dansyl-SC3 in α-helical state showed that this intermediate state was stable on the time-scale of hours (Fig. 6B ▶). Residues 76–86 are believed to form an amphipathic α-helix which can act as a sensor that triggers subsequent assembly process (Wösten et al. 2000). The spatial separation of molecules, whether transient at the air/water interface or more stable on a hydrophobic surface, is probably significant for the coming formation of the β-sheet state in that it could offer more conformational freedom, especially for residues 74–86, to initiate the structural change.
Kinetic clustering of molecules in β-sheet form
Induction of the β-sheet state of labeled SC3 on the Teflon surface resulted in a dramatic increase in FRET between dansyl-SC3 and dabcyl-SC3. As shown in Figure 6B ▶, FRET observed in this state is a dynamic process with two kinetic phases, a fast and a slow one. Thorough mixing of dansyl-SC3 and dabcyl-SC3 molecules in TFA gave rise to more FRET than when the species were mixed in buffer, confirming the dissociation of molecules in TFA. However, for both preparation methods, TFA/dansyl-SC3 + TFA/dabcyl-SC3 and TFA/dansyl-SC3/dabcyl-SC3, the donor fluorescence showed similar decay curves, suggesting that the FRET observed was caused by the close spatial proximity of molecules. Therefore, SC3 seems to undergo a dynamic change in the spatial proximity of molecules on the hydrophobic surface during the β-sheet state formation. It is unlikely that the spatial proximity of the N-termini rather than that of the protein molecules causes the dynamic FRET during the β-sheet formation, because the distance between two molecules on the Teflon surface is more than 15 nm for a surface coverage of less than 10%. The radius of SC3 is estimated to be less than 1.5 nm, indicating that FRET can only take place when the intact molecules move to each other.
Inducing β-sheet state formation of dansyl-SC3 in the absence of dabcyl-SC3 also led to a donor fluorescence decrease which has two characteristic kinetic components. The similarity of the lifetimes between the fluorescence data and CD data confirms the notion that the conformational change associated with the conversion from α-helical state to β-sheet state causes this slow fluorescence decrease when dabcyl-SC3 is absent. At present, the nature of the quenching of the N-terminal dansyl is unclear. One possibility would be collisional quenching by one of the four disulfides in SC3. Self-energy transfer between dansyl fluorophores can be ruled out because there is no spectral overlap of the absorbance and fluorescence of dansyl-SC3 (data not shown).
The data for dansyl-SC3 both in the presence and in the absence of dabcyl-SC3 could be fit to two exponentials, although the fit of the latter is better than that of the latter. In these two kinetic components, the first and fast one contributes 40% to the total fluorescence decrease for the case of FRET, versus only 10% for the case of only dansyl-SC3. The difference of the contribution between these two cases is caused by FRET. However, the lifetime t1 values of the first component for both cases are comparable, in the time range of 2–3 min. This might indicate that the conformational change and FRET take place simultaneously when the β-sheet state is induced.
Rodlets of SC3 form from the β-sheet state without further distinguishable secondary structure changes, both at the air-water interface and on a hydrophobic surface (Wösten et al. 1993, 1994c; de Vocht et al. 2000). The formation of the β-sheet-state at the air-water interface was followed as a function of time with infrared spectroscopy; electron microscopy was used simultaneously to monitor the formation of a membranous structure (M.L. de Vocht et al. unpubl.). Two β-sheet states have been distinguished, "β-sheet1" and "β-sheet2". They have similar infrared spectra but can be distinguished in their rate of formation and the microscopic structure to which they contribute. Beta-sheet1 state contributes to a mechanically stable but amorphous protein layer formed at the beginning of the self-assembly process, whereas β-sheet2 state is responsible for the rodlet layer formed after overnight incubation. We suggest that the time dependence of the formation of these two states correlates with two kinetic components reported here in the CD and fluorescence measurements.
Materials and methods
Purification of hydrophobin SC3
SC3 was purified from the culture medium of strain 4–40 of S. commune using a method that combined the electrobubbling precipitation and TFA treatment as described (Wösten et al. 1993; Wessels 1997) with hydrophobic interaction chromatography (Martin et al. 2000). After being separated from the mycelium by Nylon gauze (200 μm mesh), the 7-d cultured medium was moved to a container in which a Pt cathode was positioned at the bottom, and the anode was placed underneath the medium surface. By applying a current of 150 mA, hydrogen bubbles were generated around which SC3 assembled, resulting in foam on the top of the medium. The foam was collected, freeze-dried, treated with TFA, and dried again in a stream of nitrogen. The protein was dissolved in 0.8M sodium sulfate, pH 7.0 and subsequently applied onto a phenyl sepharose column (Pharmacia) equilibrated with the same buffer. After being washed with 2 volumes 0.8M sodium sulfate, pH 7.0, the column was eluted with water. Fractions were collected and immediately measured by CD and SDS-PAGE. Those fractions containing SC3 were pooled and dialyzed exhaustively against water. Pure SC3 was finally obtained after freeze-drying. The purity was assessed by SDS-PAGE and by comparing the weight of dried SC3 and the exact amount of protein determined by CD (de Vocht et al. 1998).
Induction of secondary structures of SC3
The purified and freeze-dried SC3 was treated with TFA and dried with nitrogen gas, and soluble-state SC3 was then obtained by dissolving the dried material in a desired buffer. Alpha-helical-state SC3 was obtained by addition of colloidal Teflon to the solution containing soluble-state SC3. SC3 converts to the α-helical state spontaneously upon coating of the solid hydrophobic surface. For the experiments reported here, the calculated surface coverage of the Teflon spheres by SC3 was maintained below 10%, given the fact that 1 mg of SC3 can coat 1.5m2 hydrophobic surface (de Vocht et al. 1998). To obtain β-sheet-state SC3, the solution containing α-helical-state SC3 on Teflon was heated to 65°C, followed by the addition of Tween 80 to a final concentration of 0.1%. The β-sheet state forms immediately after the addition of detergent (M.L. de Vocht et al., unpubl.).
Circular dichroism spectroscopy (CD)
Different secondary structures of SC3 and the dynamic process of β-sheet structure formation were determined by CD. The spectra were recorded over the wavelength range from 190 to 250 nm on an Aviv 62A DS CD spectrometer, using a 5-mm quartz cuvette. The temperature was kept at 25°C, and the sample compartment was continuously flushed with nitrogen gas. The final spectra were obtained by averaging 5 scans, using a bandwidth of 1 nm, a stepwidth of 1 nm, and a 5-sec averaging per point. A correction was made for the background signal using a reference solution without the protein. For the kinetic measurement of β-sheet-state SC3 formation, the wavelength was fixed at 215 nm and the temperature was kept at 65°C. After Tween 80 was added to the solution to a final concentration of 0.1%, the measurement was started immediately and the ellipticity data were collected over 5 h with sampling every 3 sec.
Fluorescent labeling of SC3
The N terminus of SC3 was specifically labeled with 6-((5-dimethylaminonaphthalene-1-sulfonyl)amino)hexanoic acid, succinimidyl ester (dansyl) and 4-((4-(dimethylamino)azo) benzoic acid, succinimidyl ester (dabcyl) (Molecular Probes), both of which are amine-reactive. Five mg/mL soluble-state SC3 was prepared as described above. The buffer used for the reaction contained 0.1M NaHCO3, pH 7.2 for dansyl labeling and 0.1M NaHCO3, pH 8.3 plus 0.1% (v/v) Empigen for dabcyl labeling. Concentrated fluorescence dye in DMF was added slowly to a stirred solution of soluble-state SC3 until the molar ratio of fluorescence dye to SC3 reached 20:1; the volume added was less than 10% of the total volume. The reaction mixture was stirred slowly overnight in the dark. Subsequently, a PD-10 desalting column (Pharmacia) was used to separate the labeled SC3 and free fluorescence dye. The reaction mixture was first centrifuged at 10,000g for 15 min, and 0.5 mL of supernatant was then applied to water-equilibrated PD-10, directly followed by elution with water. The pellet from the centrifugation step was washed twice with water and then freeze-dried. The freeze-dried material was treated with TFA, resuspended in 0.5 mL water, and applied to PD-10 as described above. The eluants were collected and measured by CD. Those fractions showing both high absorbance and correct SC3 CD spectra were pooled and freeze-dried. MALDI-TOF mass spectrometry was mainly used to determine the stoichiometry of labeling with dansyl (de Vocht et al. 1998). After the labeling reaction, the spectrum showed a mixture of unlabeled SC3 and 50%–60% labeled SC3 with a 346 D increased mass. For the labeling of angiotensin I with dansyl succinimidyl ester, the same conditions of reaction were used as that for SC3, and the labeled peptide was subsequently separated from the free dansyl by HPLC. The labeled angiotensin I after HPLC was freeze-dried and then dissolved in 10 mM sodium phosphate, pH 7.0 before being applied to the fluorescence measurements. In the case of dabcyl SC3, MALDI-TOF spectra could not be achieved after the reaction and isolation, probably due to the presence of residual detergent. Therefore, the stoichiometry of the reaction was based on the absorbance of dabcyl at 453 nm using an extinction coefficient of 32,000 M−1cm−1. The labeling yield was about 90%–100%, assuming that one dabcyl molecule coupled to one SC3 molecule. The reason that a higher labeling yield was obtained here compared to that of dansyl labeling is probably because of the different labeling conditions and/or the deviation of the extinction coefficient being used. Dabcyl-labeled SC3 in the soluble, α-helical, and β-sheet states also showed CD spectra similar to those of the unlabeled protein.
Fluorescence spectroscopy
An SPF-500C spectrofluorometer (SLM Aminco) with a 300 watt Xenon lamp type, 300 UV was used to measure the fluorescence of dansyl-labeled protein. Soluble-state dansyl-SC3 was prepared in 10mM sodium phosphate, pH7.0. The fluorescence spectra were then recorded with the excitation wavelength of 340 nm. Spectra were corrected for buffer and instrumental distortion. For the kinetic measurements of β-sheet-state formation, fluorescence intensities were recorded at 520 nm at a rate of 5 min per data point in the first 1 h and 15 min in the following 14 h.
Collisional fluorescence quenching
Stock solutions of 5M acrylamide, 5M KI, and 5M CsCl in10 mM sodium phosphate, pH 7.0 were added in 5 μL aliquots to 0.5 mL of 10 μg/mL or 2 μg/mL soluble-state dansyl-SC3 in the same buffer. All the quencher solutions were prepared fresh, and 0.1 mM Na2S2O3 was added to the KI stock solution to prevent I3− formation. Fluorescence intensities were corrected for dilution and scattering and, in control measurements, KCl was added at the same concentrations to correct for any ionic strength effects. The absorbance of 10 μg/mL dansyl-SC3 was lower than 0.02 at 340 nm so that possible interference from inner filter effects could be avoided under the experimental conditions. The quenching data were analyzed using the Stern-Volmer equation (Weller et al. 1966).
Acknowledgments
We thank Dr. Jaap Broos for the valuable discussion about the fluorescence studies. The research was supported by a grant from the Ubbo-Emmius Foundation of the University of Groningen.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
CD, circular dichroism spectroscopy
ATR-FTIR, attenuated total reflection-Fourier transform infrared spectroscopy
TFA, trifluoroacetic acid
FRET, fluorescence resonance energy transfer
dansyl, 6-((5-dimethylaminonaphthalene-1-sulfonyl)amino)hexanoic acid, succinimidyl ester
dabcyl, 4-((4-(dimethylamino)azo)benzoic acid, succinimidyl ester
MALDI-TOF, matrix-assisted laser desorption ionization-time of flight
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.4530102.
References
- Adair, B.D. and Engelman, D.M. 1994. Glycophorin A helical transmembrane domains dimerize in phospholipid bilayers: A resonance energy transfer study. Biochemistry 33 5539–5544. [DOI] [PubMed] [Google Scholar]
- de Vocht, M.L., Reviakine, I.R., Wösten, H.A.B., Brisson, A., Wessels, J.G.H., and Robillard, G.T. 2000. Structural and functional role of the disulfide bridges in the hydrophobin SC3. J. Biol. Chem. 275 28428–28432. [DOI] [PubMed] [Google Scholar]
- de Vocht, M.L., Scholtmeijer, K., van der Vegte, E.W., de Vries O.M.H., Sonveaux, N., Wösten, H.A.B., Ruysschaert, J.M., Hadziioannou, G., Wessels, J.G.H., and Robillard, G.T. 1998. Structural characterization of the hydrophobin SC3, as a monomer and after self-assembly at hydrophobic/hydrophilic interfaces. Biophys. J. 74 2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, O.M.H., Fekkes, M.P., Wösten, H.A.B., and Wessels, J.G.H. 1993. Insoluble hydrophobin complexes in the walls of Schizophyllum commune and other filamentous fungi. Arch. Microbiol. 159 330–335. [Google Scholar]
- Huang, T.H.J., Fraser, P.E., and Chakrabartty, A. 1997. Fibrillogenesis of Alzheimer Aβ peptides studied by fluorescence energy transfer. J. Mol. Biol. 269 214–224. [DOI] [PubMed] [Google Scholar]
- Martin, G.G., Cannon, G.C., and McCormick, C.L. 2000. SC3P Hydrophobin organization in aqueous media and assembly onto surfaces as mediated by the associated polysaccharide schizophyllan. Biomacromolecules 1 49–60. [DOI] [PubMed] [Google Scholar]
- McCaman, M.W. and Robins, E. 1962. Fluorimetric method for the determination of phenyl-alanine in serum. J. Lab. Clin. Med. 59 885–890. [Google Scholar]
- Miki, M. and Remedios, C.G. 1990. A determination of the radial coordinate of Tyr-69 in F-action using fluorescence energy transfer. Biochem. Int. 22 125–132. [PubMed] [Google Scholar]
- Scholtmeijer, K., Wessels, J.G.H., and Wösten, H.A.B. 2001. Fungal hydrophobins in medical and technical applications. Appl. Microbiol. Biotechnol. In press. [DOI] [PubMed]
- van Wetter, M.A., Schuren, F.H.J., Schuurs, T.A., and Wessels, J.G.H. 1996. Targeted mutation of the SC3 hydrophobin gene of Schizophyllum commune addects formation of aerial hyphae. FEMS Microbiol. Lett. 140 265–269. [Google Scholar]
- van Wetter, M.A., Wösten, H.A.B., and Wessels, J.G.H. 2000. SC3 and SC4 hydrophobins have distinct roles in formation of aerial structures in dikaryons of Schizophyllum commune. Mol. Microbiol. 36 201–210. [DOI] [PubMed] [Google Scholar]
- Ware, W.R. and Novros, J.S. 1966. Kinetics of diffusion-controlled reactions. An experimental test of the theory as applied to fluorescence quenching. J. Phys. Chem. 70 3246–3253. [Google Scholar]
- Wessels, J.G.H. 1994. Developmental regulation of fungal cell wall formation. Ann. Rev. Phytopathol. 32 413–437. [Google Scholar]
- Wessels, J.G.H. 1997. Hydrophobins: Proteins that change the nature of a fungal surface. Adv. Microb. Phys. 38 1–45. [DOI] [PubMed] [Google Scholar]
- Wösten, H.A.B., Asgeirsdottir, S.A., Krook, J.H., Drenth, J.H.H., and Wessels, J.G.H. 1994c. The SC3p hydrophobin self-assembles at the surface of aerial hyophae as a protein membrane constituting the hydrophobic rodlet layer. Eur. J. Cell Biol. 63 122–129. [PubMed] [Google Scholar]
- Wösten, H.A.B. and de Vocht, M.L. 2000. Hydrophobins, the fungal coat unraveled. Biochim. Biophys. Acta 1469 79–86. [DOI] [PubMed] [Google Scholar]
- Wösten, H.A.B., de Vries, O.M.H., and Wessels, J.G.H. 1993. Interfacial self-assembly of a fungal hydrophobin into a hydrophobic rodlet layer. Plant Cell 5 1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten, H.A.B., de Vries, O.M.H., van der Mei, H.C., Busscher, H.J., and Wessels, J.G.H. 1994b. Atomic composition of the hydrophobic and hydrophilic sides of self-assembled SC3p hydrophobin. J. Bacteriol. 176 7085–7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten, H.A.B., Schuren, F.H.J., and Wessels, J.G.H. 1994a. Interfacial self-assembly of a hydrophobin into an amphipathic membrane mediates fungal attachment to hydrophobic surfaces. EMBO J. 13 5848–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]