Abstract
Hydrophobins self assemble into amphipathic films at hydrophobic–hydrophilic interfaces. These proteins are involved in a broad range of processes in fungal development. We have studied the conformational changes that accompany the self-assembly of the hydrophobin SC3 with polarization-modulation infrared reflection absorption spectroscopy, attenuated total reflection Fourier transform infrared spectroscopy, and circular dichroism, and related them to changes in morphology as observed by electron microcopy. Three states of SC3 have been spectroscopically identified previously as follows: the monomeric state, the α-helical state that is formed upon binding to a hydrophobic solid, and the β-sheet state, which is formed at the air–water interface. Here, we show that the formation of the β-sheet state of SC3 proceeds via two intermediates. The first intermediate has an infrared spectrum indistinguishable from that of the α-helical state of SC3. The second intermediate is rich in β-sheet structure and has a featureless appearance under the electron microscope. The end state has the same secondary structure, but is characterized by the familiar 10-nm-wide rodlets.
Keywords: Hydrophobin, circular dichroism spectroscopy, infrared spectroscopy, electron microscopy, structural changes, interface
Hydrophobins are small secreted proteins that fulfill a broad spectrum of functions in fungal growth and development, all associated with their well-established surface activity (Wessels 1999). For instance, they are involved in the formation of hydrophobic aerial structures (e.g., aerial hyphae, spores, and fruiting bodies such as mushrooms) (Wessels 1997; Wösten et al. 1999; van Wetter et al. 2000); they mediate attachment of hyphae to hydrophobic surfaces (Wösten et al. 1994) and signal the detection of hydrophobic surfaces (Talbot et al. 1996). The most characteristic feature of hydrophobins is that they self-assemble at hydrophobic–hydrophilic interfaces (e.g., the interface between air and water, oil and water, or a hydrophobic solid and water) into amphipathic films (Wösten et al. 1993, 1994, 1995). Upon self-assembly at the hyphal surface, the hydrophilic side of the hydrophobin film faces the hydrophilic cell wall, whereas the hydrophobic side is exposed. Aerial hyphae thus become hydrophobic, whereas hyphae that grow over a hydrophobic substrate can attach themselves to this surface.
SC3 of Schizophyllum commune is the best-characterized hydrophobin and serves as a model for other hydrophobins. During self-assembly, the conformation of SC3 changes and three states of SC3 have been identified (de Vocht et al. 1998) as follows: a monomeric state that is soluble in water, an α-helical state, which is induced in SC3 upon interaction with a hydrophobic solid support such as Teflon, and a β-sheet state, which occurs after self-assembly at the air–water interface. More is known about the β-sheet state, which displays a characteristic rodlet pattern when observed with EM or AFM (Wösten et al. 1993; de Vocht et al. 1998).
To understand the process of self-assembly of SC3, the changes in secondary structure and morphology were studied in more detail with CD, IR, and EM. The conformational changes at the air–water interface were followed directly with PM–IRRAS. This technique allows the recording of IR spectra at the air–water interface directly and avoids the problem of strong water-vapor bands in the area of interest (Cornut et al. 1996; Boncheva and Vogel 1997; Ulrich and Vogel 1999). The amide I band of the IR spectrum is sensitive to the secondary structure of the protein, and in this way, the conformation of SC3 can be followed during self-assembly. The rodlet formation at the air–water interface was followed with EM to correlate secondary structure changes to changes in morphology.
Results
Structural changes of SC3 at the air–water interface
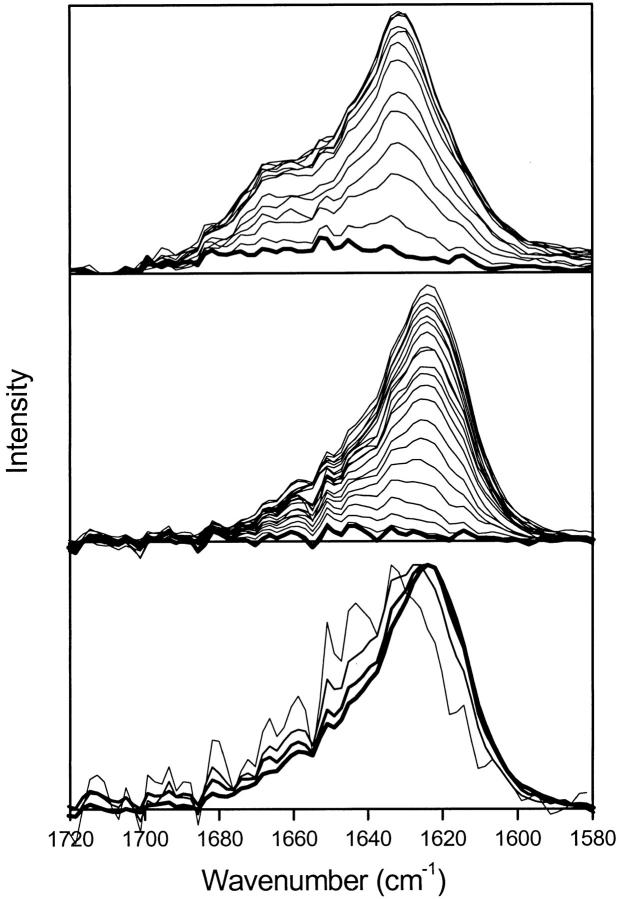
PM–IRRAS was used to follow changes in the secondary structure of SC3 at the air–water interface as a function of time (Fig. 1 ▶). The amide I band was detected 2 min after the start of the incubation of a 2.5-μg/mL solution of SC3 in H2O, indicating a rapid accumulation of SC3 at the air–water interface (top, thick line).
Fig. 1.
PM–IRRAS spectra of SC3 during self-assembly at the air–water interface. PM–IRRAS of the amide I band recorded during self-assembly of SC3 at the air–water interface at 2.5 μg/mL in H2O recorded at 12-min time intervals (top). The first spectrum (thick line) was recorded 2 min after the start of the incubation. (Middle) The result in D2O with 19-min intervals between the spectra; the first spectrum (thick line) was recorded after 10 min of incubation. In H2O, the intermediate is formed within a few minutes, after which β-sheet structure is formed. In D2O, the buildup of the intermediate state is much slower. (Bottom) The amide I′ band in D2O, 48, 86, 124, and 276 min (thin to thick lines) after the start of the incubation, normalized to the maximum intensity.
The shape and intensity of the band did not change in the following 3 min (data not shown). The width of this band (1635–1665 cm−1) indicates the presence of various secondary structure elements. After 5 min, the intensity increased and the shape of the band changed, indicating that the protein adopted a different secondary structure in the later stages of self-assembly. The intensity reached its maximum after 200 min. The peak maximum of the amide I band was located at 1631 cm−1, indicative of the presence of β-sheet; the shoulder at 1660 cm−1 can be explained by α-helical or random coil structure (Goormaghtigh et al. 1994).
The experiment was repeated in D2O to compare the PM–IRRAS with the ATR–FTIR spectra reported previously (de Vocht et al. 1998). The first IR spectrum showed that the amide II band between 1500 and 1590 cm−1 had disappeared completely, indicating complete H/D exchange. The same gradual increase of the amide I′ band was observed as in H2O, but due to the slow buildup of the initial layer, it was impossible to isolate a spectrum of the intermediate state. However, when the spectra were normalized (bottom, Fig. 1 ▶), it became clear that a change in band shape also occurs in D2O. The relative intensity at 1650 cm−1 (indicative of α-helix or random coil structure) decreased in time compared with the peak at 1624 cm−1 (indicative of β-sheet). Thus, self-assembly of SC3 at the air–H2O and air–D2O interface proceeds via a structural intermediate that contains relatively more α-helix or random coil to a final state that is rich in β-sheet.
The α-helical state can be converted to the β-sheet state
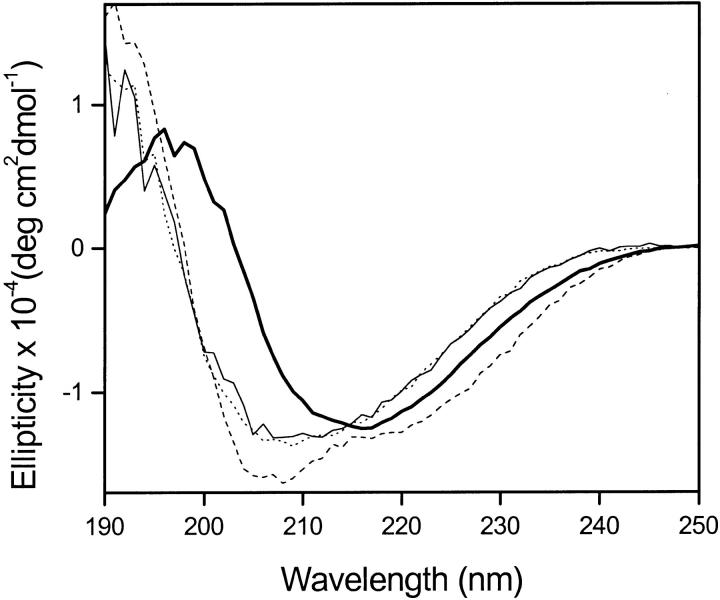
SC3 bound to hydrophobic substrates, such as Teflon, cannot be removed by treatment with 2% SDS at 100°C (Wösten et al. 1994, 1995). Surprisingly, however, SC3 could be removed from the surface by addition of 0.1% Tween-20 at 25°C and a monomeric state of SC3 was observed, which remained in the supernatant after the colloidal Teflon was spun down (Fig. 2 ▶). In contrast, when Tween-20 was added at 85°C, the SC3 remained bound to the Teflon and the structure changed to the β-sheet state.
Fig. 2.
CD spectra of SC3 adsorbed on Teflon before and after addition of detergent. The changes in secondary structure of adsorbed SC3 upon treatment with Tween-20 were followed with CD spectroscopy. The spectra for monomeric SC3 (dotted line) and after adsorption to colloidal Teflon (dashed line) were reported previously (de Vocht et al. 1998). Addition of Tween-20 to the adsorbed SC3 at 25°C results in detachment and monomerization of the protein (thin line). Addition of Tween-20 at 85°C results in a conformational change to the β-sheet state (thick line).
No change in the conformation of monomeric SC3 was detected when it was incubated with hot detergent in solution, nor did the heating of SC3 result in a conformational change in the absence of a detergent. Therefore, the conformational change from the α-helix to the β-sheet state in the presence of a hydrophobic solid is induced by the combination of heat and detergent. Subsequent cooling of the cuvette to 25°C did not affect the β-sheet structure, nor the binding of SC3, even in the presence of Tween-20.
This shows that the β-sheet state of SC3 binds more strongly to the Teflon than the α-helical state (see next section also). In fact, the standard assay for strong binding of hydrophobins, heating for 10 min at 100°C in 2% SDS (Wösten et al. 1994, 1995) also induced formation of β-sheet structure (data not shown). These results support the hypothesis that the α-helical state is an intermediate state induced by interaction with a hydrophobic solid support, which is then converted to the β-sheet state by heat plus detergent.
Binding to a hydrophobic surface after assembly at the air–water interface
SC3 self-assembles at the air–water interface into an amphipathic film with the hydrophobic side exposed to the air (Wösten et al. 1994). We studied whether this film binds to a hydrophobic solid with preservation of the β-sheet structure. To this end, a Teflon sheet was carefully brought in contact with the surface of a solution of 2 μg/mL of 35S-labeled SC3, on top of which the hydrophobin had assembled overnight, and, after 2 min of interaction, the Teflon was retracted. A layer of SC3 had adsorbed to the Teflon that was 85% resistant to extraction with 2% SDS at 100°C, similar to SC3 that had been assembled directly on Teflon (data not shown). In a control experiment in which a fresh SC3 solution was used (i.e., in the absence of an assembled hydrophobin film at the surface of the solution), five times less SC3 adsorbed could be picked up. This procedure clearly allows one to specifically pick up the layer of assembled SC3.
The entire procedure was repeated, but with silanized germanium (contact angle >100°; for review, see de Vocht et al. 1998) as the hydrophobic substrate. After picking up a layer of self-assembled SC3, the ATR–FTIR spectrum was determined, before (Fig. 3 ▶, thick line) and after (Fig. 3 ▶, thin line) SDS extraction at 100°C. Both spectra are typical for β-sheet structure with a maximum at 1630 cm−1. Heating in SDS reduced the intensity of the amide I′ band by 20%, but did not affect the shape, showing that the protein can bind strongly to a hydrophobic substrate in the β-sheet state and that the secondary structure is not affected by the SDS treatment. The spectra are essentially the same as the spectrum of SC3 after vortexing and drying down on a germanium crystal (Fig. 3 ▶, dashed line) and the spectrum of SC3 at the air–water interface as determined by PM–IRRAS (Fig. 3 ▶, dotted line), although the peak maximum is shifted by 6 cm−1. Careful examination of the amide II band showed that in the ATR–FTIR experiments, the H2O was not completely exchanged for D2O after flushing with D2O-saturated Nitrogen gas. This can explain the difference in the position of the peak maximum of the ATR–FTIR spectra compared with the PM–IRRAS spectrum.
Fig. 3.
Comparison of the amide I′ band recorded by different methods. Amide I′ band of SC3 self-assembled at the air–water interface in the β-sheet conformation, after vortexing (dashed line, from reference de Vocht et al. 1998) or directly on the air–water interface (PM–IRRAS, dotted line). After picking up on hydrophobic germanium, the SC3 layer is still in the β-sheet conformation (thick line) and 80% resistant to hot SDS extraction (thin line). All spectra show predominantly β-sheet structure. Different experimental setups (see Results) can explain the differences in shape.
Changes in morphology during self-assembly
The property of SC3 to self-assemble at the air–water interface into a rodlet layer is well documented (Wösten et al. 1993, 1994). The self-assembled layer was picked up on a holey carbon grid at different times of incubation at 10, 25, 50, and 100 μg/mL. After negative staining, the layers were studied with EM. The images in Figure 4 ▶ and the data in Table 1 show that the self-assembly occurs in at least two stages. Initially, no protein film could be transferred to the holey grid (Table 1), indicating the absence of a coherent film. In the absence of protein, no film was observed. At later stages of assembly, a featureless, but mechanically stable layer was formed (Fig. 4A ▶). The stability of this layer is higher than that of a lipid monolayer, because the latter cannot withstand the stress of the transfer when picked up on the EM grid. The familiar rodlets, with a width on the order of 10 nm, were observed after overnight incubation at 50 μg/mL or after 2 h incubation at 100 μg/mL (Fig. 4B ▶; Table 1).
Fig. 4.

SC3 assembled at the air–water interface investigated by EM. (A) A negatively stained image of an amorphous layer SC3 initially formed at the air–water interface when a 10-μg/L protein solution was incubated overnight and picked up on a holey carbon film. The layer has no distinct morphology, but its presence indicates structural integrity. (B) A negatively stained image of the rodlets that SC3 formed at the later stages of the assembly process when a 50-μg/Ll protein solution was incubated overnight and then picked up on a holey carbon film. The rodlet layer is clearly visible. (Rodlet width is on the order of 10 nm; image magnification: ×∼250,000).
Table 1.
Time course of rodlet formation as followed with EM
| SC3 concentration (μg/mL) | Incubation time | Result |
| 10 | Overnight | Some materiala |
| 25 | Overnight | Some material. Featureless film |
| 50 | 15 min | Transfer not successfulb |
| 30 min | Transfer not successful | |
| 60 min | Transfer not successful | |
| 120 min | Transfer not successful | |
| 240 min | Some material. Featureless film | |
| Overnight | Featureless film + rodlets | |
| 100 | 15 min | Featureless film |
| 30 min | Featureless film | |
| 60 min | Featureless film | |
| 120 min | Featureless film + rodlets | |
| 240 min | Featureless film + rodlet | |
| Overnight | Rodlets |
a Predominantly empty holes were found on the grid, but holes covered with the protein film were also present. No rodlets were found in this condition.
b The protein film at the air–water interface, if formed at all, was not stable enough to withstand the stress of the transfer to the holey grid.
Discussion
Upon interaction with a hydrophobic–hydrophilic interface, SC3 self-assembles into rodlets that are also found on the outside of aerial hyphae (Wösten et al. 1993, 1998). Three different states in the self-assembly process have been identified previously. These states are referred to as monomeric for the state that is soluble in water, the α-helical state after binding to a hydrophobic substrate, and the β-sheet state after self-assembly at the air–water interface (de Vocht et al. 1998).
Four different pieces of evidence indicate that the α-helical state is actually an intermediate in the assembly process. (1) The intermediate state observed with PM–IRRAS during the self-assembly shows a spectrum indistinguishable from that of the α-helical state of SC3 (see Fig. 1 ▶). (2) During the spontaneous self-assembly of alkylated SC3 (see de Vocht et al. 2000), the α-helical state was also observed as an intermediate. In this case, no interface is present. (3) After picking up a layer of assembled protein on hydrophobic Germanium, the β-sheet end-state was observed (see Fig. 3 ▶), which shows that SC3 can also bind to a hydrophobic solid in the β-sheet state. (4) The α-helical state can change to the β-sheet state after interaction with detergent, but not vise versa (see Fig. 2 ▶). From these results, we conclude that the α-helical state, found on a hydrophobic solid, is, in fact, an intermediate state in the self-assembly process of SC3 and that the hydrophobin is arrested in this state at a hydrophobic solid.
Recently, it was shown that the hydrophobin rodlets show many similarities with amyloid fibrils (de Vocht et al. 2000; Butko et al. 2001; Mackay et al. 2001). In general, amyloid fibril formation is induced by conditions that destabilize the native fold of a protein (Chiti et al. 1999). Therefore, it is not surprising that heat also induces a conformational change to the amyloid-like β-sheet state in SC3. The presence of detergent is required for the conformational transition at the Teflon surface. Possibly, the detergent gives the protein some flexibility and/or mobility, permitting the transition to the β-sheet state. The resistance of the binding of SC3 to treatment with hot detergent shows that the β-sheet state binds strongly to the hydrophobic solid. Interestingly, SC3 in the α-helical state can be extracted from the Teflon (in the monomeric state) by incubation in 0.1% Tween-20 at 25°C (Fig. 2 ▶). In contrast, SC3 in the β-sheet state is not affected by these conditions and can only be removed by use of TFA or formic acid (de Vries et al. 1993; de Vocht et al. 1998). This shows that the β-sheet state is the most stable state.
ATR–FTIR-spectroscopy on a Germanium crystal showed that the β-sheet layer formed at the air–water interface binds strongly to a hydrophobic solid support with preservation of the β-sheet state (Fig. 3 ▶). Furthermore, this layer is resistant to heating in SDS. This supports the hypothesis that this β-sheet state is identical to that of SC3 bound to the Teflon surface after treatment with detergent at 85°C (Fig. 2 ▶). It also confirms that the β-sheet state is the final state of self-assembly both on a hydrophobic support and on the air–water interface.
The changes in morphology associated with the assembly of SC3 at the air–water interface were followed by EM after transferring the protein film onto perforated carbon film grids, which is known to be the least perturbative transfer method (Brisson et al. 1999). The first identifiable intermediate observed by TEM was a mechanically stable, but amorphous protein layer (Fig. 4A ▶). On the basis of PM–IRRAS experiments, this intermediate would correspond to the β-sheet state and is now called the β-sheet1 state. After a few hours of incubation, the typical rodlet layer was observed. Because no changes in secondary structure were observed during the rodlet formation, this state is called the β-sheet2 state (Fig. 5 ▶). However, it is possible that a reorientation occurs of the molecules within the layer during the rodlet formation. Hence, the accumulation of SC3 in the β-sheet state at the air–water interface is required before rodlet formation can commence. This is consistent with our observation that self-assembly of SC3 can only occur above a critical concentration of SC3 in the monomeric state (M.L. de Vocht, unpubl.).
Fig. 5.
Model for the self-assembly of SC3 on the interface between water (light area) and a hydrophobic phase (dark area), such as air, oil, or a hydrophobic solid. In the monomeric state, the protein is soluble in water. Interaction with air or a hydrophobic solid rapidly induces the intermediate α-helical state. On a hydrophobic solid, this state is arrested and only in the presence of heat and detergent is the β-sheet state formed. On the air–water interface, the β-sheet state is formed spontaneously. Electron microscopy showed that after formation of the β-sheets, first an amorphous layer is formed, after which rodlets appear.
PM–IRRAS showed that the final structure formed at the air–water interface is the previously described β-sheet state that is rich in β-sheet structure. As mentioned, SC3 can also adopt this structure at a hydrophobic solid by treating the surface at 85°C with 0.1% Tween-20 or 2% SDS. An AFM study on another class I hydrophobin, ABH1 (HYPA), adsorbed to a hydrophobic substrate, has shown that washing with hot SDS can lead to a structural change and formation of rodlets (Gunning et al. 1998). Because the rodlets are rich in β-sheet structure (see below), this is consistent with our result. The PM–IRRAS spectrum can also provide information on the orientation of the protein with respect to the surface plane. The amide I band is positive, which means for this angle of incidence (75°) the transition moment is oriented preferentially parallel to the plane of the surface (Ulrich and Vogel 1999). As the transition moment for the amide I band is oriented perpendicular to the β-strands, close to the direction of the backbone hydrogen bonds (Goormaghtigh et al. 1994; Marsh 1997), the hydrogen bonds of the β-sheets are also oriented preferentially parallel to the air–water interface.
The induction of the β-sheet state is also important from a technological perspective. For many applications of hydrophobins (e.g., coating of implants, binding to biosensors) a strong interaction with the surface is required. Because the protein binds strongest to surfaces in the β-sheet state, the induction of this state is important to obtain good binding. We conclude that self-assembly on the air–water interface and on a hydrophobic solid are, in principle, the same; however, in the latter case, the intermediate α-helical state is trapped on the surface, and to be converted to the β-sheet state, must be exposed to detergent at elevated temperature (Fig. 5 ▶).
Materials and methods
Protein
The hydrophobin SC3 was purified from the culture medium of strain 4–40 of S. commune (CBS 340.81) as described (Lugones et al. 1998). Before use, the freeze-dried SC3 was disassembled with pure TFA and dried in a stream of nitrogen (de Vries et al. 1993). The monomeric protein was then dissolved in the desired buffer.
Polarization-modulated infrared spectroscopy
PM–IRRAS was used to follow the secondary structure directly at the air–water interface. A total of 16 mL of 2.5 μg/mL SC3 in 50 mM phosphate (pH 7) in H2O or D2O was put into a homemade glass trough, and spectrum recording was started within 30 sec. For D2O measurements, the trough was enclosed in a gas-tight chamber that had been flushed with nitrogen for 1 h before introduction of the sample. The spectra were recorded on a Vector 22 Spectrometer (Bruker, Karlsruhe, Germany) equipped with an external polarization modulation setup. All other conditions were the same as described (Ulrich and Vogel 1999). A total of 2500 scans were recorded at 4-cm−1 resolution. The spectra were apodized with a triangular function and Fourier transformed with one level of zero filling. The spectra were not smoothed. The same solution without protein was used for the background spectra.
Circular dichroism spectroscopy
The secondary structure of SC3 was studied with CD spectroscopy. The CD spectra were recorded over the wavelength region 190–250 nm on an Aviv 62A DS CD spectrometer, using a 1-mm quartz cuvette and 1-sec averaging per point with other conditions as described (de Vocht et al. 1998). To follow the changes in the secondary structure at a hydrophobic solid support, SC3 was added to colloidal Teflon and the CD spectrum was determined (surface coverage 9%; for review, see de Vocht et al. 1998). Concentrated Tween-20 was then added to this mixture at 25 or 85°C to a final concentration of 0.1% Tween-20.
Binding of self-assembled SC3
The possibility of selectively picking up a layer of SC3 assembled at the air–water interface was assessed with Teflon-binding experiments, following the procedure described (Wösten et al. 1994). A glass compartment was filled with 2 μg/mL 35S-labeled SC3. After 5 min or 16 h of incubation, the air–water interface was approached carefully with a Teflon sheet of 1.5 cm2 and allowed to contact the water surface for 2 min. The Teflon was retracted and the amount of SC3 bound to the Teflon was determined by counting the sheet in scintillation liquid, before and after hot extraction for 10 min with 2% SDS at 100°C.
To study the secondary structure of SC3 self-assembled at the air–water interface after adsorption to a solid hydrophobic surface, the same procedure was used, but with unlabeled SC3 and a silanized Germanium crystal as the hydrophobic substrate (for review, see de Vocht et al. 1998). The IR spectrum was determined with ATR–FTIR, before and after hot-SDS extraction, following the same procedure as described (de Vocht et al. 1998).
Transmission electron microscopy
For transmission electron microscopy, 17-μL Teflon wells were filled with 10–100-μg/mL SC3 solutions. Holey carbon film grids (Sjöstrand and Rhodin 1957) were applied to the surface of the wells after assembly periods ranging from a few minutes to 16 h. Assembly was performed in a constant humidity chamber. The specimens were stained with 1% uranyl acetate and observed in a Philips CM120 electron microscope equipped with a LaB6 cathode operated at 120 kV.
Acknowledgments
This research is supported financially by the Netherlands Technology Foundation (STW) and is coordinated by the Life Sciences Foundation (SLW). We thank N. Sonveaux and J.M. Ruysschaert for assistance in the recording of the ATR–FTIR spectra. We thank A.J. Schouten and E.J. Vorenkamp for assistance with the initial IRRAS experiments.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
S. commune, Schizophyllum commune
EM, electron microscopy
AFM, atomic force microscopy
IR, infrared
ATR-FTIR, attenuated total reflection-Fourier transform infrared spectroscopy
CD, circular dichroism spectroscopy
TFA, trifluoroacetic acid
PM-IRRAS, polarization-modulation infraRed reflection absorption spectroscopy
SDS, sodium dodecyl sulfate
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.4540102.
References
- Boncheva, M. and Vogel, H. 1997. Formation of stable polypeptide monolayers at interfaces: Controlling molecular conformation and orientation. Biophys. J. 73 1056–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson, A., Bergsma-Schutter, W., Oling, F., Lambert, O., and Reviakine, I. 1999. Two-dimensional crystallization of proteins on lipid monolayers at the air-water interface and transfer to an electron microscopy grid. J. Cryst. Growth 196 456–470. [Google Scholar]
- Butko, P., Buford, J.P., Goodwin, J.S., Stroud, P.A., McCormick, C.L., and Cannon, G.C. 2001. Spectroscopic evidence for amyloid-like interfacial self-assembly of hydrophobin Sc3. Biochem. Biophys. Res. Commun. 280 212–215. [DOI] [PubMed] [Google Scholar]
- Chiti, F., Webster, P., Taddei, N., Clark, A., Stefani, M., Ramponi, G., and Dobson, C.M. 1999. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc. Natl. Acad. Sci. 96 3590–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornut, I., Desbat, B., Turlet, J.M., and Dufourcq, J. 1996. In situ study by polarization modulated Fourier transform infrared spectroscopy of the structure and orientation of lipids and amphipathic peptides at the air-water interface. Biophys. J. 70 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vocht, M.L., Scholtmeijer, K., van der Vegte, E.W., de Vries, O.M.H., Sonveaux, N., Wösten, H.A.B., Ruysschaert, J.M., Hadziioannou, G., Wessels, J.G.H., and Robillard, G.T. 1998. Structural characterization of the hydrophobin SC3, as a monomer and after self-assembly at hydrophobic/hydrophilic interfaces. Biophys. J. 74 2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vocht, M.L., Reviakine, I., Wösten, H.A.B., Brisson, A., Wessels, J.G.H., and Robillard, G.T. 2000. Structural and functional role of the disulfide bridges in the hydrophobin SC3. J. Biol. Chem. 275 28428–28432. [DOI] [PubMed] [Google Scholar]
- de Vries, O.M.H., Fekkes, M.P., Wösten, H.A.B., and Wessels, J.G.H. 1993. Insoluble hydrophobin complexes in the walls of Schizophyllum commune and other filamentous fungi. Arch. Microbiol. 159 330–335. [Google Scholar]
- Goormaghtigh, E., Cabiaux, V., and Ruysschaert, J.M. 1994. Determination of soluble and membrane protein structure by Fourier transform infrared spectroscopy. Subcell. Biochem. 23 329–450. [DOI] [PubMed] [Google Scholar]
- Gunning, A.P., de Groot, P.W.J., Visser, J., and Morris, V.J. 1998. Atomic force microscopy of a hydrophobin protein from the edible mushroom Agaricus bisporus.J. Coll. Interface Sci. 201 118–126. [Google Scholar]
- Lugones, L.G., Wösten, H.A.B., and Wessels, J.G.H. 1998. A hydrophobin (ABH3) specifically secreted by vegetatively growing hyphae of Agaricus bisporus (common white button mushroom). Microbiology 144 2345– 2353. [DOI] [PubMed] [Google Scholar]
- Mackay, J.P., Matthews, J.M., Winefield, R.D., Mackay, L.G., Haverkamp, R.G., and Templeton, M.D. 2001. The hydrophobin eas is largely unstructured in solution and functions by forming amyloid-like structures. Structure 9 83–91. [DOI] [PubMed] [Google Scholar]
- Marsh, D. 1997. Dichroic ratios in polarized Fourier transform infrared for nonexial symmetry of β-sheet structures. Biophys. J. 72 2710–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstrand, F.S. and Rhodin, J. 1957. Electron microscopy. In the Proceedings of the Stockholm conference. Almqvist and Wiksell, Stockholm.
- Talbot, N.J., Kershaw, M., Wakley, G.E., de Vries, O.M.H., Wessels, J.G.H., and Hamer, J.E. 1996. MPG1 encodes a fungal hydrophobin involved in surface interactions during infection-related development of the rice blast fungus Magnaporte grisea.Plant Cell 8 985–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, W.P. and Vogel, H. 1999. Polarization-modulated FTIR spectroscopy of lipid/gramicidin monolayers at the air/water interface. Biophys. J. 76 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wetter, M.-A., Wösten, H.A.B., and Wessels, J.G.H. 2000. SC3 and SC4 hydrophobins have distinct roles in formation of aerial structures in dikaryons of Schizophyllum commune.Mol. Microbiol. 36 201–210. [DOI] [PubMed] [Google Scholar]
- Wessels, J.G.H. 1997. Hydrophobins: Proteins that change the nature of the fungal surface. Adv. Microb. Physiol. 38 1–45. [DOI] [PubMed] [Google Scholar]
- ———. 1999. Fungi in their own right. Fungal Genet. Biol. 27 134–145. [DOI] [PubMed] [Google Scholar]
- Wösten, H.A.B., de Vries, O.M.H., and Wessels, J.G.H. 1993. Interfacial self-assembly of a fungal hydrophobin into a hydrophobic rodlet layer. Plant Cell 5 1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten, H.A.B., Schuren, F.H.J., and Wessels, J.G.H. 1994. Interfacial self-assembly of a hydrophobin into an amphipathic protein membrane mediates fungal attachment to hydrophobic surfaces. EMBO J. 13 5848–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wösten, H.A.B., Ruardy, T.G., van der Mei, H.C., Busscher, H.J., and Wessels, J.G.H. 1995. Interfacial self-assembly of a Schizophyllum commune hydrophobin into an insoluble amphipathic membrane depends on surface hydrophobicity. Colloids Surf B: Biointerfaces 5 189–195. [Google Scholar]
- Wösten, H.A.B., van Wetter, M.-A., Lugones, L.G., van der Mei, H.C., Busscher, H.J., and Wessels, J.G.H. 1999. How a fungus escapes the water to grow into the air. Curr. Biol. 9 85–88. [DOI] [PubMed] [Google Scholar]