Abstract
To probe the role of individual disulfide bonds in the folding kinetics of hen lysozyme, the variants with two mutations, C30A,C115A, C64A,C80A, and C76A,C94A, were constructed. The corresponding proteins, each lacking one disulfide bond, were produced in Escherichia coli as inclusion bodies and solubilized, purified, and renatured/oxidized using original protocols. Their enzymatic, spectral, and hydrodynamic characteristics confirmed that their conformations were very similar to that of native wild-type (WT) lysozyme. Stopped-flow studies on the renaturation of these guanidine-unfolded proteins with their three disulfides intact showed that, for the three variants, the native far-UV ellipticity was regained in a burst phase within the 4-ms instrument dead-time. The transient overshoots of far-UV ellipticity and tryptophan fluorescence that follow the burst phase, as well as the kinetics of transient 8-anilino-1-naphthalene-sulfonic acid (ANS) binding, were diversely affected depending on the variant. Together with previous reports on the folding kinetics of WT lysozyme carboxymethylated on cysteines 6 and 127, detailed analysis of the kinetics showed that (1) none of the disulfide bonds were indispensable for the rapid formation (<4 ms) of the native-like secondary structure; (2) the two intra-α-domain disulfides (C6-C127 and C30-C115) must be simultaneously present to generate the trapped intermediate responsible for the slow folding population observed in WT lysozyme; and (3) the intra-β-domain (C64-C80) and the inter-αβ-domains (C76-C94) disulfides do not affect the kinetics of formation of the trapped intermediate but are involved in its stability.
Keywords: Lysozyme, disulfide, mutants, folding kinetics, secondary structure
Much of our understanding of the basic principles that govern protein folding has been acquired by studying the renaturation of small proteins in vitro. Among these, hen lysozyme has been the most extensively investigated by the use of a wide array of fast-kinetics methods based on a variety of spectroscopic and chemical signals. These include absorption, intrinsic fluorescence of the aromatic residues, dynamic fluorescence quenching, fluorescence of extrinsic probes such as 8-anilino-1-naphthalenesulfonic acid (ANS) or the substrate analog 4-methylumbelliferyl-N,N`-diacetyl-β-chitobiose, circular dichroism, and pulsed proton exchange followed by nuclear magnetic resonance (NMR) or mass spectroscopy (Dobson et al. 1994). These studies, however, failed to reflect the folding of the newly synthesized polypeptide chain, because they deal with a protein in which the four disulfide bonds of the native molecule were kept intact in the denatured state; thus, they were already present at the onset of the renaturation. This contrasts with the physiological folding process, which starts with the reduced protein. This is of importance in view of the marked difference in the rates and efficiencies of renaturation of oxidized and reduced lysozyme in vitro. Although lysozyme with its native disulfides intact refolds in about a second (Kato et al. 1981; Kato et al. 1882) with yields close to 100% even at protein concentrations in the mg/mL range, the reduced molecule was reported to fold and oxidize efficiently into the native conformation only at protein concentrations of a few micrograms per milliliter and with a half-life of several minutes at best (Wetlaufer et al. 1974; Acharya and Taniuchi, 1976; Anderson and Wetlaufer, 1976). Moreover it was shown that rather than just stabilizing the native conformation at the end of the renaturation process, the native disulfides affect very early folding steps. Indeed, whereas the nonreduced polypeptide chain regains most of its native secondary structure in less than 4 ms (Chaffotte et al. 1992), no secondary structure can be detected after 4 ms of renaturation of the reduced protein (Goldberg and Guillou 1994). Thus, the presence of the native disulfide bonds considerably speeds up and facilitates the folding of lysozyme by acting at very early stages of the process. It therefore seemed of interest to investigate the role of the disulfide bonds in the folding of lysozyme.
Several studies have started addressing this problem. Thus, four variant lysozymes, each lacking one of the four native disulfide bonds, were prepared by site-directed mutagenesis and were shown to fold in vitro into an active conformation showing far-UV circular dichroism spectra resembling those of the native, wild-type (WT) molecule (Sawano et al. 1992). This pioneering work indicated that no individual disulfide is indispensable for the correct folding to be possible but failed to provide information on the kinetics and efficiency of folding of each variant. Using original experimental conditions that allowed efficient oxidative refolding of reduced lysozyme at concentrations in which physical chemical studies could be achieved, it was shown (Roux et al. 1999) that the oxidation of the protein proceeds sequentially through intermediates with one, two, and three disulfides, and that significant amounts of secondary structure can be detected only in the population of intermediates with two disulfides. These studies also showed that the molecules with three disulfides, although showing only about 80% of the enzymatic activity of the native protein, have a far-UV ellipticity indicative of a native-like secondary structure. The three SS bond intermediate that accumulates before getting slowly converted into the fully active enzyme was shown to have its Cys76 and Cys94 still reduced and to adopt a native-like conformation (Van den Berg et al. 1999). The 76–94 disulfide is therefore dispensable for the efficient acquisition of the native secondary structure, but a possible role of this disulfide in the collapse resulting in the burst of secondary structure was not investigated. The role of the disulfide bond between Cys6 and Cys127 was investigated using a chemically modified lysozyme in which this disulfide was reduced and the two resulting cysteines carboxymethylated. The modified protein, once unfolded without reduction of the three remaining disulfides, could refold rapidly and showed the collapse and burst of far-UV ellipticity during the dead-time of the stopped-flow apparatus (Eyles et al. 1994). Thus, this disulfide is not required for the rapid formation of secondary structure. Finally, under experimental conditions in which the folding kinetics are very much slowed down (i.e., at 4°C, pH 3 and in the presence of significant concentrations of residual guanidinium chloride), the rate-limiting step in the acquisition of the active conformation, which corresponds to the slowest folding phase in lysozyme, has been investigated for the four variants lacking one of the native disulfide bonds (Yokota et al. 2000). Although the effect of each disulfide bond on the rate-limiting step was characterized in detail, the kinetics of secondary structure regain were not investigated.
To determine whether a specific disulfide, or set of disulfides, is involved in the early acquisition of secondary structure, we investigated systematically the extent and kinetics of secondary structure regain during the early phases of folding of variant lysozyme molecules deprived of one of the four pairs of cysteines involved in native disulfides. Here, we report the construction of these variants, their purification from inclusion bodies, and their oxidative renaturation in vitro. The enzymatic, spectroscopic, and hydrodynamic properties of the three variant lysozymes lacking the C30-C115, C64-C80, or C76-C94 disulfide bond will be reported, and their kinetics of renaturation from the unfolded nonreduced state, as monitored by far-UV CD, intrinsic fluorescence, and ANS binding will be described. The roles of the C30-C115, C64-C80, and C76-C94 disulfide bonds in the renaturation of the WT protein will be discussed in light of these studies and previous reports on the effect of reduction and carboxymethylation of cysteines 6 and 127 (Denton et al. 1994; Eyles et al. 1994).
Results
Expression and initial characterization of recombinant lysozymes
Because the mutants expressing the three disulfide lysozyme variants used in earlier studies (Sawano et al. 1992) were not made available to us, a new set of mutants was prepared by site-directed mutagenesis. The cysteines of our variants were all replaced by alanines. The genes encoding mature WT lysozyme and the doubly mutated variants C6A,C127A (SS6–127− variant), C30A,C115A (SS30–115− variant), C64A,C80A (SS64–80− variant), and C76A,C94A (SS76–94− variant) were placed under the control of a thermoinducible phage λ promotor; the recombinant proteins were produced by induction and growth of the transformed cells at 42°C. Complete cell lysates were analyzed by SDS PAGE and showed a thick band of an overexpressed protein with a molecular weight slightly >15 kD. This band was not observed in control cells that were transfected with the parent vector or visible in cells grown at 32°C, indicating that the expression was under the control of the expected thermoinducible promotor. The overproduced recombinant proteins were all found exclusively in inclusion bodies and could not be detected in any significant amount in the soluble extracts.
The WT, as well as the four variant proteins, showed identical electrophoretic mobilities and migrated slightly, yet significantly, more slowly than natural lysozyme. N-terminal sequencing and mass spectrometry of the purified proteins (see below) together with gene sequencing clearly indicated that the overproduced proteins had the expected amino acid sequence and their slower migration on SDS gels resulted only from the presence of an additional methionine residue at their N terminus.
Purification and renaturation of the WT and variant recombinant lysozymes
The folding kinetics of lysozyme with its C6-C127 disulfide bond disrupted had been studied in detail previously (Denton et al. 1994; Eyles et al. 1994). We therefore focused our attention on the three other variants: SS30–115−, SS64–80−, and SS76–94−.
The variant proteins and the WT recombinant lysozyme (as a control) were overproduced and purified as described under Materials and Methods. As an example, the purification of the SS30–115− variant is illustrated in Figure 1 ▶. Renaturation of the purified recombinant proteins was achieved according to a protocol inspired from previous studies on natural lysozyme (Goldberg et al. 1996), by using low concentrations of either a nondetergent sulfobetaine or urea as adducts for improving the yield in native protein. Using the specific activities published in earlier studies (Sawano et al. 1992), we estimated the renaturation yields to be ∼55% for the WT recombinant protein and over 30% for the three variants. The renaturation/oxidation procedures therefore appeared satisfactory and no attempt was made to optimize them further. Based on the total enzymatic activity for each preparation, 1 L of culture provided about 20 mg of native WT and 10 mg of native variant lysozyme. All variants appeared pure enough except the SS76–94− variant that was further purified by ion exchange chromatography (see Materials and Methods). The pure proteins were freeze-dried for storage. When dissolved and dialyzed in buffer N, some insoluble material could be observed and was removed by centrifugation. SDS PAGE of the resulting supernatants indicated that the proteins were more than 95% pure (Fig. 1 ▶, lane 29).
Fig. 1.
Control by SDS-PAGE of the purification of the SS30–115− variant. The purification described in this figure was conducted with the cells harvested from a 4-L culture, that is, twice the usual volume. Hence, two DEAE-Sephacel columns were run in parallel for the last purification step. At each purification step, 15 μL-samples were loaded onto the gels, submitted to electrophoresis, and stained with Coomassie blue. Lanes 1, 11, and 19 molecular mass markers (in kD): 19.4, 28.3, 33.4, 48.3, 82, and 104. Soluble extract after sonication (lane 2). Wash of pelleted inclusion bodies (lane 3). Inclusion bodies solubilized in 10 M urea, 100 mM DTT before centrifugation (lane 4), and after centrifugation (pellet, lane 5; supernatant, lane 6). Sample after dialysis in 8 M urea, 0.1 M acetic acid, and centrifugation (pellet, lane 7; supernatant, lane 8). After dilution in 4 M urea, 0.1 M acetic acid and centrifugation (pellet, lane 9; supernatant lane 10 duplicated in lane 12). Sample after dialysis against buffer C and centrifugation (supernatant, lane 13 and pellet, lane 14). Flow-through fractions of the two DEAE-Sephacel columns (lanes 15–16). First washes of each column with 3 mL of buffer C (lanes 17–18, duplicated in lanes 20–21). Second washes of each column with 8 mL of buffer C (lanes 22–23). First washes of the columns with 3 mL (lanes 24–25) and second washes with 8 mL (lanes 26–27) of buffer C supplemented with 0.5 M NaCl. Recombinant WT lysozyme from a similar purification (lane 28). Renatured variant protein after freeze-drying, dialysis, and centrifugation (lane 29). The samples in lanes 5, 7, 9, and 14 were centrifugation pellets resuspended with difficulty in 50 mM TrisCl, 5 mM 2-mercaptoethanol, and 0.1 mM PMSF (volumes corresponding to 1/2 or 1/10 of the sample before centrifugation). The fractions shown in lanes 15 and 16 were pooled and processed for storage and renaturation.
It is noteworthy that the need to introduce an additional purification step for two of the mutants became apparent only on N-terminal sequencing of the "purified" variants, which showed significant contamination by the DNA binding protein HU and the cold shock protein CSPC. Indeed, these contaminants were not detected on SDS PAGE using Coomassie blue staining, and their very low content in aromatic amino acids made them undetectable by near-UV absorbance measurements. It should also be noted that the amount of contamination varied from culture to culture and that the contaminants were not present in any significant amount in our preparations of WT and SS30–115− variants.
Characterization of the renatured WT and variant lysozymes
N-terminal sequencing of the purified proteins showed a unique sequence starting with the methionine encoded by the initiation codon and followed by the first residues of natural lysozyme (Met-Lys-Val-Phe-Glu). Furthermore, the absorbance at 280 nm and the method of Bradford provided identical values for the protein concentrations (which was not the case for the SS64–80− and SS76–94− variants before introduction of the additional purification steps), confirming that the non-UV-absorbing HU and CSPC proteins (see above) had been eliminated.
Mass spectrometry revealed an essentially unique peak at a molecular mass of 14,441 ± 0.5 Da for the WT. For the variants, the molecular masses ranged between 14,374.2 ± 1.2 and 14,374.9 ± 1.25 Da, which, compared with the predicted value of 14,374.2 Da, ascertained that no unwanted mutation was introduced, the proteins did not undergo proteolytic truncation, their chemical integrity was maintained throughout the preparation procedure, and, in particular, no cyanylation occurred during the incubations with urea at high pH.
The purified proteins were assayed for lysozyme activity. Their specific activities are reported in Table 1. When compared with that of natural lysozyme (38,400 ± 2000 units/mg), these values are in the same ratio as those reported previously (Sawano et al. 1992) for pure natural lysozyme and the purified variants. This confirmed that the proteins were pure and native.
Table 1.
Hydrodynamic and spectroscopic characteristics of the recombinant lysozymes
| Wild type | SS30–115 | SS64–80 | SS76–94 | |
| Specific activity | 38,600 ± 2100 | 27,200 ± 2400 | 32,000 ± 900 | 50,100 ± 2700 |
| S20,w | 1.84 ± 0.1 | 1.88 ± 0.1 | 1.62 ± 0.15 | 1.78 ± 0.1 |
| 106 × D20,W | 1.23 ± 0.15 | 1.38 ± 0.15 | 1.73 ± 0.2a | 1.45 ± 0.15 |
| M | 13,400 ± 2200 | 12,400 ± 1800 | 8400 ± 2000a | 11,400 ± 1800 |
| Fluorescence | 100 | 145 | 75 | 145 |
Specific activities are expressed in units/mg, S20,w is in Svedberg units, D20,W is expressed in cm2s−1 and multiplied by 106, the relative molecular weight M is calculated from the sedimentation and diffusion coefficients and expressed in Daltons, and the fluorescence is the ratio of the fluorescence emitted by the native protein at 345 nm (excitation at 280 nm) expressed in percent relative to the emission of natural lysozyme at the same concentration.
a The high value of the apparent diffusion coefficient of this variant is attributable to the slow formation of aggregates during the centrifugation and results in a severe artificial underestimate of the apparent molecular weight.
The refolded recombinant proteins were further characterized by analytical centrifugation. The values obtained for their standard sedimentation and diffusion coefficients are reported in Table 1. The close similarity of these sedimentation coefficients to that determined for natural lysozyme (1.98 ± 0.1 S) indicated that no difference in the degree of compactness of folding could be detected between the four proteins and the native lysozyme. Determination of their molecular weights from their sedimentation and diffusion coefficients ascertained that the WT and variant recombinant lysozymes were monomeric. It should be noted that for the SS64–80− variant, the absorbance plateau on the leading side of the boundary decreased more rapidly than expected from the radial dilution effect, and that the apparent diffusion coefficient was abnormally high, strongly indicating that some heavy aggregates were formed and sedimented rapidly during the centrifugation. This propensity of the SS64–80− variant to aggregate was always observed at 20°C, but could be circumvented by keeping the protein at 4°C. The abnormal boundary spreading observed for SS64–80− explains the low value obtained for the apparent molecular weight of this variant.
The absorption spectra (not shown), fluorescence emission spectra (Fig. 2 ▶), and both the far and near-UV circular dichroism spectra (not shown) of the recombinant WT lysozyme were identical to those obtained for natural lysozyme. Thus, the enzymatic, hydrodynamic, and spectral properties of the natural and recombinant WT lysozymes were identical, indicating that the renatured/oxidized recombinant protein had regained a conformation indistinguishable from that of native natural lysozyme and confirming that the N-terminal methionine of the recombinant protein did not alter significantly the conformation of the polypeptide chain.
Fig. 2.
Emission fluorescence spectra of wild-type (WT) and variant lysozymes. The spectra of the unfolded WT protein (••••) and of native WT (solid squares), SS30–115− (+), SS64–80− (open circles), and SS76–94− (solid triangles) lysozymes were recorded as indicated in the Materials and Methods section. The final protein concentrations were between 19 and 43 μg/mL. The spectra shown have been normalized to 30 μg/mL. The excitation wavelength was 280 nm. The fluorescence is in arbitrary units.
The absorption spectra of the three recombinant variant proteins were identical to that of WT lysozyme, both in the native and guanidinium chloride unfolded states (data not shown). Similarly, in the denatured state, their fluorescence emission spectra (Fig. 2 ▶) were superimposable to that of WT lysozyme (either natural or recombinant, not shown). However, the fluorescence emission spectra of the native variant proteins showed distinct features. Variants SS30–115− and SS76–94− both showed a strong increase, whereas variant SS64–80− showed a marked decrease in the quantum yield compared with the native WT protein (Fig. 2 ▶). The lower quantum yield of WT lysozyme compared with SS30–115− was likely to be caused by the quenching of the tryptophan residues 111 and 123 by the sulfur atom(s) of half-cystines 115 and 30, respectively. Indeed, in the native conformation, the indole ring of tryptophan 111 is extremely close to the sulfur of cysteine 115, and the indole ring of tryptophan 123 is in contact with cysteine 30. Similarly, the lower intrinsic fluorescence of native WT lysozyme as compared with the SS76–94− variant was likely attributable to a quenching of the fluorescence of tryptophan 63 by the sulfurs of the C76-C94 disulfide bond that, in the WT, are very close to the indole ring of tryptophan 63. The reduced quantum yield of variant SS64–80− compared with WT lysozyme was more indicative of a difference in the environment of some tryptophan residue(s) that might result from a significant local conformational difference between the variant and the WT protein.
The near-UV CD spectra of the WT and variant proteins (not shown) were similar to those reported previously (Yokota et al. 2000). In line with the observed differences in intrinsic fluorescence, this indicated slight differences in the environment of some aromatic residues. The far-UV CD spectra of the variants were also similar to those previously reported (Sawano et al. 1992). Compared with the WT enzyme, they showed some slight differences in the 220–240 nm range (data not shown). Ellipticity differences in this spectral region might result from differences in the secondary structures of the WT and variant proteins. This seemed, however, unlikely because whereas the amplitude of a positive peak of ellipticity in the 180–200-nm region is highly sensitive to changes in protein secondary structure, particularly α-helix contents, the peaks centered at 192 nm had similar amplitudes for the WT and the variant proteins. This indicated that the secondary structures of the WT and variant proteins were similar, and that the ellipticity difference observed in the 200–240-nm region was due essentially to the removal of an optically active disulfide chromophore in each variant.
In conclusion, the fluorescence as well as near-UV CD spectra of the WT and variant proteins showed some differences that were likely to reflect mostly local changes in the immediate environment of the mutated residues. Yet, more profound effects of the mutations on the tertiary structure and/or the dynamics of the protein cannot be entirely ruled out. The close similarity of the hydrodynamic parameters of the proteins, their catalytic properties, and their far-UV CD spectra in the 180–200-nm region indicate that they have similar conformations and, most importantly, secondary structures. This corroborates the conclusions reached by unpublished NMR studies quoted by Yokota and coworkers (Yokota et al. 2000).
Folding kinetics of WT and recombinant lysozymes
The results just reported confirmed that, as concluded previously (Sawano et al. 1992), removal of any single disulfide bond did not prevent the lysozyme polypeptide chain from folding into its native conformation. The effects of the individual disulfide bonds on the folding kinetics, primarily on the kinetics of secondary structure formation, were then investigated.
First, it was verified that the denaturation of the native oxidized WT and variant proteins with their disulfide bonds intact was fully reversible. The WT protein and the three variants were unfolded in 6 M GuHCl without reduction of the native disulfide bonds. Their refolding was initiated by an 80-fold dilution in renaturation buffer N, and the enzymatic activity was assayed within minutes after the dilution. For the recombinant WT, as well as the three variant proteins, the specific activity of the refolded protein was identical to that of the initial native protein, and all the enzymatic activity present before unfolding was recovered after the renaturation.
Comparison of the folding kinetics of natural and recombinant WT lysozymes
Preliminary far-UV CD stopped-flow studies performed at 228 nm indicated that the folding kinetics of the natural mature lysozyme and recombinant WT protein were both very similar to those previously reported (Chaffotte et al. 1992). The rate constants for the fast and slow folding phases, obtained by fitting to two exponentials, were 38 ± 20 s−1 and 2.7 ± 1 s−1 for the recombinant protein and 101 ± 65 s−1 and 2.3 ± 0.6 s−1 for the natural enzyme. The large error on the fast phase rate constants was caused by the large scattering of the experimental points resulting from the small number of data accumulations. In view of this imprecision, the observed rate constants did not differ significantly. To improve the comparison between the natural and the recombinant WT proteins, similar stopped-flow refolding experiments were performed while monitoring the intrinsic fluorescence of their aromatic residues for which the signal to noise ratio (hence, the precision of the measurements) was far better than for circular dichroism. For each protein, two sets of data were collected, one with a 1-ms sampling interval to get a precise value of the fast rate constant; the second with a 5-ms sampling interval to get a better value of the slow rate constant. The values obtained by fitting for the fast phase rate constant (with the 1-ms sampling interval) were 72 ± 10 s−1 and 73 ± 10 s−1; those for the slow phase rate constants (with the 5-ms sampling interval) were 3.6 ± 0.5 s−1 and 3.5 ± 0.5 s−1 for the natural and recombinant WT proteins, respectively. The quasi-identity of these sets of values clearly indicated that the presence of the additional N-terminal methionine in the recombinant protein did not noticeably affect any of the observable phases in the folding kinetics, as already reported for the slowest phase (Yokota et al. 2000). The folding kinetics of the natural mature protein, available in much larger amounts than the recombinant enzyme, were therefore used from then on as a control.
SS30–115− folding kinetics
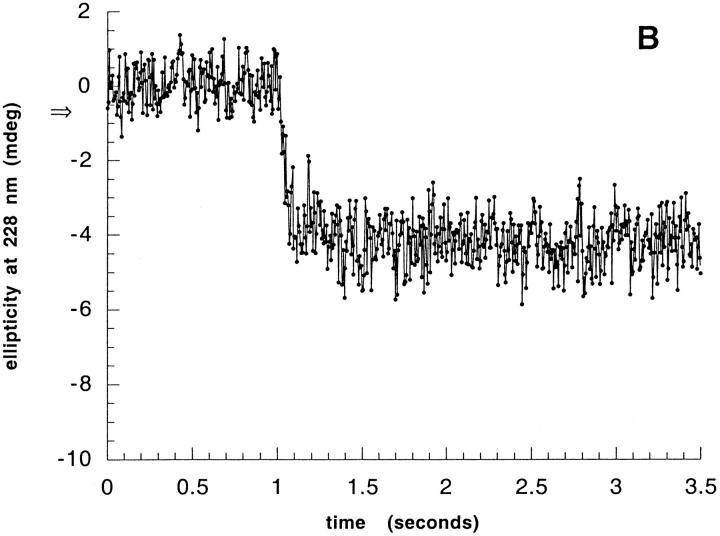
To determine as precisely as possible the amount of ellipticity regained within the stopped-flow dead-time, the continuous/stopped-flow method previously designed for analyzing the secondary structure formation was used. When applied to the recombinant WT protein, this method confirmed the existence of an ellipticity burst and of kinetics very similar to those already reported (Chaffotte et al. 1992). At 222 nm, where the ellipticity reflects the secondary structure and is hardly affected by the SS bonds in natural lysozyme, most of the ellipticity of the native conformation was reached within the 4-ms dead-time of the apparatus, and only a very small ellipticity change could be observed at later stages, as previously reported for natural lysozyme (Chaffotte et al. 1992; Radford et al. 1992). At 228 nm, where CD changes reflect constraints on SS bonds in natural lysozyme, the kinetics showed three phases: A burst phase within the dead-time, then a rapid decrease to reach an overshoot of the negative ellipticity, and a subsequent slow increase to reach the final, native-like ellipticity (Fig. 3A ▶). The rate constants determined by fitting a double exponential model to the experimental data were 37 ± 7 s−1 and 3.2 ± 0.2 s−1, respectively, for the rapid and slow phases. These values are reasonably close to those previously reported for the slow (2.6 ± 0.1 s−1) and rapid (62 ± 11 s−1) phases observed at 228 nm (Chaffotte et al. 1992), taking into account that the rate constant we determined for the rapid phase is quite certainly underestimated. Indeed, to improve the signal to noise ratio without using much larger amounts of proteins, we used a 5-ms sampling time in our CD stopped-flow measurements. This sampling time is too long for kinetics with a rate constant of 50–100 ms because too few data points can be acquired and a dumping of the kinetics is artificially introduced. Thus, the far-UV CD kinetics obtained for the natural and recombinant WT lysozymes appear similar.
Fig. 3.
Kinetics of ellipticity at 228 nm recovery during the folding of nonreduced natural and SS3–115− variant lysozymes. Natural WT or the recombinant variant lysozyme was subjected to a continuous flow-stopped flow experiment. In a first phase, 390 μL of buffer N were injected from each large syringe over 100 ms and recording of the ellipticity was started. After 1 sec (first plateau on the diagram), a second phase consisted of injecting 30 μL of unfolded nonreduced lysozyme (6 mg/mL in buffer N containing 6 M GuHCl) from the small syringe together with 1.185 mL of buffer N from each large syringe over a 300-ms period, resulting in an 80-fold dilution of the protein and guanidinium chloride and a 4-ms dead-time. In a third phase, the flow was stopped and the ellipticity was recorded during 2.2 additional seconds. At the end of the second phase (continuous flow) a plateau was reached, as shown in a control experiment in which natural lysozyme was injected during a 500-ms period at the same flow rate. During these three phases, the ellipticity at 228 nm was continuously recorded as a function of time and is shown in mdeg. The sampling interval was 5 ms. For each sample, successive kinetics were accumulated as indicated below and averaged. The arrow on the ordinate scale indicates the ellipticity of the unfolded protein determined by a similar experiment in which the unfolded protein was diluted into 6 M GuHCl instead of buffer N. (A) Natural lysozyme, average of 15 kinetics; (B) SS30–115−, average of 15 kinetics.
The folding kinetics of the SS30–115− variant were investigated next. When this variant protein was subjected to the same continuous/stopped-flow experiment, the kinetics showed flat traces both at 222 (not shown) and 228 nm (Fig. 3B ▶). The ellipticities at these two wavelengths did not change with time after the burst and corresponded to those of the native protein. According to the interpretation of the far-UV CD kinetics previously proposed on the basis of a detailed analysis of the time-resolved complete far-UV CD spectra during the folding of lysozyme, the ellipticity at 220–222 nm reflects only the secondary structure contents, whereas the ellipticity changes observed at 228 nm after the dead-time reflect changes in the configuration of a disulfide bond(s) imposed by tertiary structure changes. Based on this interpretation, the results obtained with the variant indicated that, within the precision of far-UV CD measurements, the secondary structure content of the native variant protein was regained in less than 4 ms, and that either the native tertiary structure was already achieved within the dead-time or the ellipticity at 228 nm of the SS30–115− variant had become insensitive to the tertiary structure rearrangements involved in the rapid and slow phases observed with the WT protein. The latter possibility would be easily attributed to the possibility that, in WT lysozyme, the ellipticity changes observed at 228 nm originate only from the configuration of the C30-C115 disulfide bond. Thus, because it may lack the corresponding "optical probe," the SS30–115− variant may fail to show ellipticity changes at 228 nm during the last phases of its folding.
To distinguish between the two possible interpretations of the kinetics (accelerated folding or optically silent phases), the folding kinetics were investigated with a different optical probe—the intrinsic fluorescence. Figure 4A ▶ shows the results obtained with the recombinant WT and SS30–115− variant proteins. The kinetics obtained with the WT protein very much resembled those reported earlier for the folding of lysozyme under slightly different conditions (Itzhaki et al. 1994). They were characterized by a burst phase, followed by a rapid decrease in fluorescence emission (rate constant of 87 ± 10 s−1), and by a slow increase (3.2 ± 1 s−1) to reach the fluorescence intensity of the native protein. With the variant, only two phases were observed. A burst phase and a slight increase in fluorescence that could be fitted to a single exponential process with a rate constant of 9.2 ± 0.5 s−1, intermediate between those of the rapid and slow phases of the WT. Moreover, the amplitudes of the phases markedly differed for the WT and variant proteins. Taking as a reference the fluorescence of the unfolded proteins (which were identical for the WT and the variant), the burst phases represented a variation of −65% and −27% for the WT and the variant, respectively. This strongly indicates that the C30-C115 disulfide bond is directly involved in the quenching of the tryptophan fluorescence that occurs in less than 4 ms during the initial collapse of the WT polypeptide chain. The relative amplitudes of the rapid and slow phases in the WT were −12% and +20%, respectively, of the unfolded protein fluorescence, whereas the amplitude of the single observable phase for the variant was only +5%. The detection of a fluorescence change in the 4–200-ms time interval ruled out that the folding of the variant might have been completed within the 4-ms dead-time of the far-UV CD stopped-flow experiments. The kinetics observed could, however, still be interpreted in two ways. One would be that the folding of the variant, although incomplete after 4 ms, would be terminated in about 200 ms, that is, significantly faster than for the WT protein. Alternatively, because the sulfur atoms of the C30-C115 disulfide bond are clearly involved in the quenching of the protein fluorescence (see above), the fluorescence changes observed during the rapid and slow folding phases for the WT might reflect changes in the quenching efficiency of these atoms associated with tertiary rearrangements within the molecule. As discussed above for the ellipticity at 228 nm, removal of the two cysteines in the SS30–115− variant might then result in the rapid and slow folding phases becoming undetectable.
Fig. 4.
Kinetics of intrinsic fluorescence regain during the folding of nonreduced natural and variant lysozymes. Fifteen microliters of unfolded nonreduced lysozyme (2 mg/mL in buffer N containing 6 M GuHCl) were injected together with 592 μL of buffer N from each large syringe over 150 ms, resulting in an 80-fold dilution of the protein and GuHCl and a 4-ms dead-time. The flow was stopped and the fluorescence intensity was recorded during a total of 2 sec through a low-frequency pass filter with a cutoff at 350 nm (A and B) or 320 nm (C) and is shown in arbitrary units. The excitation wavelength was 294 nm. The sampling interval and filtering time constants were 2 ms. Ten successive kinetics were accumulated and averaged for each sample, and the background signal of buffer alone was subtracted. In each panel, the thick line corresponds to the variant and the thin line to the natural protein. The open diamonds correspond to the fluorescence of the fully unfolded natural lysozyme obtained by diluting the unfolded protein in buffer N containing 6 M GuHCl. The same fluorescence intensity value was obtained for the unfolded variant. (A) SS30–115−; (B) SS64–80−; (C) SS76–94−. The diluted proteins were recovered from the stopped-flow and found to be fully active, indicating perfect renaturation.
To solve this alternative, the folding process was monitored with an optical probe that should be sensitive to the folding state of the polypeptide chain but not to the removal of the disulfide bond. ANS seemed appropriate for that purpose. Although susceptible to affect the folding kinetics (Engelhard and Evans 1995), this hydrophobic probe was shown to reduce the folding rate of lysozyme only slightly (Itzhaki et al. 1994), an effect we tried to minimize by using lower ANS concentrations (30 μM) in our experiments. Because ANS binds transiently to hydrophobic areas that are solvent exposed in folding intermediates but inaccessible in the native conformation, the release of ANS is thought to reflect the completion of the folding process (Semisotnov et al. 1991). In WT lysozyme, the burst, rapid and slow folding phases detected by various methods are also detected by ANS (Itzhaki et al. 1994). One could therefore predict that if the folding of the variant were faster than that of the WT, the release of ANS from the intermediates should also be faster for the variant than for the WT. Conversely, if the folding of the variant included a rapid and slow phase occurring after the initial burst, these phases should be detectable with ANS. Figure 5A ▶ shows the kinetics of ANS fluorescence changes during the folding of the WT and SS30–115− variant proteins. For the WT, after a very rapid increase occurring during the instrument dead-time, the fluorescence decreased to a level close to that of free ANS. Fitting of this decrease was achieved using either a mono or a double-exponential model. The χ2 value obtained with a single exponential was 8.8 × 10−7, compared with 2.1 × 10−8 with two exponentials. Moreover, the residuals obtained with a one-exponential model showed a nonrandom distribution, whereas the distribution of the residuals obtained with two exponentials was random. These results indicated that a one-exponential model was clearly inadequate, whereas two exponentials sufficed to describe the kinetics. Thus, three significant phases were observed. The burst (completed in 4 ms), a rapid phase (k1 = 40 ± 6 s−1), and a slow phase (k2 = 3.4 ± 0.8 s−1). An additional very slow phase, identified as being caused by photobleaching (Engelhard et al. 1995), could also be detected in our experiments. Comparing these kinetics with those of the SS30–115− variant showed three important features. First, the fluorescence at the end of the burst phase was the same for the WT and variant proteins, indicating that both polypeptide chains had reached similar folding states. This ruled out that the variant protein might have progressed further along the folding pathway than the WT during the burst phase and that folding events corresponding to the rapid and/or slow phases observed with the WT protein might have been hidden in the burst phase for the variant. Second, in the case of the variant protein, only one significant phase rather than two for the WT was observed with ANS (fitting the fluorescence decrease with a single exponential provided random residuals and χ2 = 2.4 × 10−8). Its rate constant was k = 5.6 ± 1 s−1. The third striking difference between the WT and SS30–115− proteins (Fig. 5A ▶) was that the final ANS fluorescence values they reached were widely different. Although the final ANS fluorescence was quite low for the WT protein, it remained at about half the burst value for the variant. Because, in some cases, ANS had been shown to dramatically slow down the folding process (Engelhard and Evans 1995), one could argue that the high ANS fluorescence observed with the variant after 2 sec of folding might very slowly decrease to ultimately reach the same level as that reached with the WT. Alternatively, one could envisage that ANS binding would not slow down significantly the folding of the variant, but that even in the absence of ANS, the final conformation reached by the polypeptide chain would not provide a complete shielding of the hydrophobic core and still allow the binding of significant amounts of ANS. The first possibility was ruled out by adding ANS to the native variant protein and recording the fluorescence of the mixture. Panel D in Figure 5 ▶ represents the fluorescence emission spectra of ANS bound to the WT and variant proteins. These spectra clearly show that ANS can bind to the native variant protein in such a way that its fluorescence is indeed much larger than that observed with the WT. Although the total quantum yield, reflected by the area under each spectrum in Figure 5D ▶, and the fluorescence collected through a filter with a cutoff at 450 nm, reflected by the voltage measured at the end of the kinetics (Fig. 5A ▶), cannot be directly correlated, the ratio of the quantum yields of ANS bound to the WT and the variant proteins are similar to the ratio of the final intensities determined in the stopped-flow. This clearly indicates that the difference in the final fluorescence observed in the ANS binding kinetics results from a difference between the conformations or molecular dynamics of the native WT and variant proteins rather than from a specific effect of ANS on their folding kinetics. This increased binding of ANS by the variant as compared with the WT is very likely attributable to the much lower stability and increased flexibility of the 3SS variants previously reported (Tachibana et al. 1994, Yokota et al. 2000).
Fig. 5.
Kinetics of ANS binding during the folding of nonreduced lysozyme. Fifteen microliters of unfolded nonreduced lysozyme (2 mg/mL in buffer N containing 6 M GuHCl) were injected over 150 ms together with 592 μL from each large syringe of buffer N supplemented with 30 μM ANS, resulting in an 80-fold dilution of the protein and GuHCl and a 4-ms dead-time. The flow was stopped and the fluorescence intensity was recorded during a total of 2 sec through a low-frequency pass filter with a cutoff at 450 nm and is shown in arbitrary units. The excitation wavelength was 366 nm. The sampling interval and filtering time constants were 2 ms. For each sample, 10 successive kinetic profiles were accumulated and averaged. In each panel, the thick line corresponds to the variant, the thin continuous line to natural lysozyme, and the thin dotted line to the fluorescence of free ANS obtained by injecting only buffer N containing 30 μM ANS. (A) SS30–115−; (B) SS64–80−; (C) SS76–94−; (D) fluorescence (in arbitrary units) spectra of ANS bound to the native natural WT (closed circles) or the native SS30–115− variant (open circles) lysozymes (25 μg/mL) obtained by subtracting the spectrum of free ANS from that of the corresponding protein in buffer N containing ANS (30 μM). The excitation wavelength was 366 nm.
SS64–80− folding kinetics
The folding kinetics of the SS64–80− variant were investigated in the same way as reported above for SS30–115−. A flow/stopped-flow experiment in which the refolding was monitored at 228 nm showed the same plateau of ellipticity as in the control with WT lysozyme during the flow phase, indicating that the burst of ellipticity still existed with this variant and that suppression of the C64-C80 disulfide bond did not affect the amount of secondary structure regained during the burst. A slight barely detectable overshoot of negative ellipticity could be detected after the burst, but its amplitude was too small (relative to the noise in the recording) to enable us to determine reliable rate constants for the appearance and disappearance of the corresponding intermediate.
The kinetics of intrinsic fluorescence regain during the folding of the SS64–80− variant (Fig. 4B ▶) were qualitatively similar to those observed for the WT protein: a very rapid signal decrease occurred during the 4-ms dead-time of the apparatus and was followed by a transient fluorescence overshoot. The amplitude of the fluorescence decrease during the burst phase was the same for the WT and the variant proteins, indicating that the C64-C80 disulfide bond is not involved in the quenching of the tryptophan fluorescence that occurs during the initial collapse of the WT and variant polypeptide chains. Yet, several features of the kinetics appeared quantitatively different. As expected from the emission spectra of the native proteins, the final fluorescence signal was significantly smaller for the SS64–80− variant than for the WT protein. The amplitude of the signal overshoot was very much smaller for the variant than for the WT. Although the rate constant of the rapid observable phase in the variant (85 sec−1) was found to be similar to that in the WT, the rate constant of the slow phase (11 sec−1) was significantly faster than in the WT (3 sec−1).
Finally, the folding kinetics of SS64–80− were studied in the presence of ANS. The kinetics of ANS binding and release (Fig. 5B ▶) were again qualitatively similar to, but quantitatively different from, those observed with the WT protein. ANS binding occurred in a burst during the 4-ms dead-time and resulted in a fluorescence signal similar to that observed with the WT protein. It was followed by a biphasic fluorescence decrease. However, as reported above for the SS30–115− variant, the final fluorescence was much higher (approximately fivefold) than for the WT, indicating that the SS64–80− variant is less compact or more flexible than WT lysozyme. Furthermore, the rate constant of the rapid observable phase of ANS fluorescence decrease was slower for the variant (17 sec−1) than for the WT protein (38 sec−1), whereas the rate constant of the slow phase of ANS release (4 sec−1) was about the same in the variant as in the WT (3 sec−1).
SS76–94− folding kinetics
Finally, the folding kinetics of SS76–94− were investigated using the same methods as used for the two other variants. Far-UV CD flow/stopped-flow experiments were conducted at 222 and 228 nm. Both showed the same plateau of ellipticity as in the controls with WT lysozyme during the flow phase, indicating that the burst of ellipticity, and hence the very rapid appearance of the native-like secondary structure, was not affected by the suppression of the C76-C94 disulfide bond. At 222 nm, no ellipticity change could be detected after the initial burst (not shown). At 228 nm, a small transient decrease in ellipticity could be detected, but its amplitude was too small to allow for any reliable quantitative analysis of its kinetics. Yet, examination of the kinetic traces indicated that the time range of appearance of the corresponding intermediate was of the same order of magnitude as for the WT protein, whereas the rate of its disappearance seemed distinctly slower (the final plateau was not yet reached at the end of the recording).
The SS76–94− folding kinetics monitored by intrinsic fluorescence showed a very rapid signal decrease, with the same amplitude as in the WT, that occurred during the 4-ms dead-time of the apparatus and was followed by a transient fluorescence overshoot (Fig. 4C ▶). That the amplitude of the initial fluorescence burst was the same in the variant as in the WT protein indicated that the C76-C80 disulfide bond is not involved in the tryptophan quenching that occurs as a result of the initial collapse. Although qualitatively similar to those obtained with the wild-type protein, the kinetics appeared quantitatively different. As expected from the emission spectra of the native proteins, the final fluorescence signal was significantly higher for the variant than for the WT protein. As observed previously for the SS64–80− variant, the amplitude of the rapid fluorescence decrease that follows the burst was smaller for the SS76–94− variant than for the WT. However, unlike what was observed in the SS64–80− variant, the amplitude of the subsequent slow fluorescence increase was comparable for the WT and SS76–94− variants. Finally, although the rate constant of the rapid observable phase in the variant (81 sec−1) was found to be similar to that in the WT, the rate constant of the slow phase (0.7 sec−1) was considerably smaller than in the WT (3 sec−1).
The kinetics of ANS binding and release during the folding of the SS76–94− variant were again qualitatively similar to, but quantitatively different from, those observed with the WT protein (Fig. 5C ▶). ANS binding occurred in a burst during the 4-ms dead-time and resulted in a fluorescence signal lower by about 25% than observed for the burst of ANS binding in the WT protein. It was followed by a biphasic fluorescence decrease. As opposed to the SS30–115− and SS64–80−, the final fluorescence of bound ANS was only slightly higher (30%) than for the WT, indicating that the SS76–94− variant is nearly as compact and rigid as the WT lysozyme. Furthermore, the rate constant of the first phase of ANS fluorescence decrease in this variant (58 sec−1) was slightly higher than in the WT protein (38 sec−1), whereas the rate constant of the slow phase of ANS release was considerably smaller in the variant (0.6 sec−1) compared with the WT (3 sec−1).
The characteristics of the kinetics observed for the wild-type and the three variant proteins are summarized in Table 2 together with a comparison of their burst signals for the far-UV CD, intrinsic fluorescence, and ANS binding.
Table 2.
Wild type and variant lysozymes kinetic folding phases
| Wild type | R6,127-Cxa | SS30–115 | SS64–80 | SS76–94 | ||
| Tryp Fluo | burst | 100 ± 5 | 90 | 45 ± 3 | 100 ± 6 | 100 ± 4 |
| k1 | 80 ± 10 | >5 | 9 ± 1 | 85 ± 9 | 81 ± 11 | |
| k2 | 3 ± 1 | n.o. | n.o. | 11 ± 1.5 | 0.7 ± 0.1 | |
| ANS Fluo | burst | 100 ± 9 | n.s. | 100 ± 8 | 105 ± 10 | 80 ± 7 |
| k1 | 38 ± 13 | n.s. | 6 ± 1.5 | 17 ± 9 | 58 ± 10 | |
| k2 | 3 ± 1 | n.s. | n.o. | 4 ± 0.8 | 0.6 ± 0.2 | |
| Θ228 nm | burst | 100 ± 3 | 100 | 100 ± 4 | 100 ± 6 | 100 ± 5 |
| k1 | 37 ± 12 (62 ± 11)b | n.o. | n.o. | n.d. | n.o. | |
| k2 | 3 ± 1 | n.o. | n.o. | n.d. | n.o. |
The amplitudes of the burst of tryptophan fluorescence (Tryp Fluo), bound ANS fluorescence (ANS Fluo), and ellipticity at 228 nm (Θ228 nm) are expressed in percent of the difference between the signals observed for the protein in 6 M GuHCl and for natural lysozyme during the flow phase (i.e., for the burst intermediate). k1 is the rate constant determined for the rapid (or unique) phase observed after the burst; k2 is that determined for the slow phase. Both are expressed in seconds−1; n.o., not observed; n.d., the rate constant could not be determined; n.s., the corresponding signal was not studied.
a Estimated from published results (Denton et al. 1994; Eyles et al. 1994).
b The value obtained in these experiments was underestimated (see text). The value in parentheses is the more precise one obtained under the same experimental conditions (Chaffotte et al. 1992).
Discussion
The results reported above will be discussed in terms of a model that, in addition to the widely accepted "triangular" mechanism (Wildegger and Kiefhaber 1997) for the folding of lysozyme with its four disulfide bonds intact, includes a collapsed intermediate with native-like, but relatively unstable, secondary structure formed in less than 4 ms (Chaffotte et al. 1992; Denton et al. 1994). From this "burst" intermediate, the folding proceeds through the triangular pathway in which one branch leads directly to the native state, whereas the other branch includes an inactive, energetically "trapped" intermediate (Wildegger and Kiefhaber, 1997). Under conditions that strongly favor folding, such as those used in our studies, this model can be schematized as follows,
 |
where U represents the unfolded molecule, B the burst intermediate, IT the trapped intermediate, and N the native enzyme. The burst intermediate is characterized by a native-like secondary structure, partial quenching of the intrinsic fluorescence, and transient ANS binding. The IT intermediate is responsible for the overshoots in far-UV CD and intrinsic fluorescence signals, and its conversion to N is rate limiting.
Previous studies on natural lysozyme in which the C6-C127 disulfide bond had been reduced and the resulting cysteines carboxymethylated (R6127-Cx lysozyme) showed that disruption of this disulfide bond did not affect the appearance of the burst intermediate. Morever, R6127-Cx lysozyme folded more rapidly than the natural enzyme with its four disulfide bonds intact (Denton et al. 1994; Eyles et al. 1994). Furthermore, it was shown that the R6127-Cx protein no longer proceeded through the intermediate IT, indicating that the presence of the C6-C127 disulfide bond was needed to give rise to this intermediate (Denton et al. 1994). Together with these observations, the results we obtained shed some light on the role of each disulfide bond in the formation of the burst and the trapped intermediates.
The burst intermediate
Several pieces of evidence, summarized in Table 2, indicate that suppression of any one of the four disulfide bonds in lysozyme does not noticeably affect the formation of the burst intermediate. Indeed, all our 3-disulfide variants, as well as R6127-Cx lysozyme, showed the same burst of far-UV ellipticity as the natural protein, which shows their ability to regain their native-like secondary structure content in less than 4 ms. The ability to bind ANS was either the same (variants SS30–115− and SS64–80−) or only slightly smaller (variant SS76–94−) when compared with the natural protein. The extent of fluorescence quenching during the collapse was the same for the natural protein and for the proteins lacking the C6-C127, C64-C80, and C76-C94 disulfide bonds. Moreover, from data reported in the literature (Denton et al. 1994; Eyles et al. 1994) it can be calculated that the amplitude of the fluorescence burst for R6127-Cx lysozyme should be about 90% of that observed for natural lysozyme. This indicates very similar environments for the tryptophan residues in the corresponding burst intermediates. That the burst intermediate shows a much higher fluorescence in the SS30–115− variant when compared with the natural and other variant lysozymes strongly indicates that a major contribution to the quenching in the burst intermediate of natural lysozyme results from the formation of the C-terminal α-helix that brings the sulfur of half-cystine 115 and the indole ring of tryptophan-111 into close contact. This tandem of cysteinyl-tryptophanyl residues is the single one in lysozyme to be four residues apart in the sequence, which spontaneously brings their side chains together on formation of the α-helix. For the other tryptophanyl and cysteinyl residues involved in the fluorescence quenching, a stable native-like tertiary conformation of the polypeptide chain is required to maintain the quenching half-cystine in close contact with the indole of the quenched tryptophan. Because the burst intermediate was shown by near-UV CD stopped-flow studies to have no fixed tertiary structure (Chaffotte et al. 1992; Radford et al. 1992; Denton et al. 1994), it is therefore not surprising that all the lysozyme variants or derivative lacking one disulfide bond, except SS30–115−, have burst amplitudes that are comparable to that of the natural molecule.
Thus, despite the difference observed in the intrinsic fluorescence of the SS30–115− burst intermediate, which can be considered a "local" effect, it can be concluded that the four molecules lacking one disulfide are able to give rise to similar burst intermediates in less than 4 ms, with native-like secondary structure, compactness (as reflected by ANS binding), and internal polarity (as evidenced by intrinsic fluorescence). Hence, none of the four disulfides of lysozyme seem indispensable for the formation of the burst intermediate.
The trapped intermediate
The results obtained by monitoring the intrinsic fluorescence and transient ANS binding showed that suppression of any single disulfide bond profoundly affected the properties of the trapped intermediate. Thus, suppression of the intra-α-domain disulfide bond in variant SS30–115− results in folding kinetics that closely resemble those reported for R6127-Cx lysozyme in that, for both proteins, a unique phase was observed after the burst in intrinsic and ANS fluorescence, and both proteins folded significantly faster than the protein with four disulfides (Table 2). Thus, suppression of any one of the two intra-α-domain disulfide bonds (6–127 or 30–115) alters the folding pathway in the same way. Suppression of the C6-C127 disulfide was shown to result in the disappearance of the trapped intermediate, thus letting all the molecules use the more rapid "direct" folding pathway (Denton et al. 1994). In view of the similar effects of the two intra-α-domain disulfide bonds on the folding kinetics, it can be concluded that the simultaneous presence of these two disulfide bonds is requested for the formation of the trapped intermediate. Unlike the intra-α-domain ones, neither of the other two disulfide bonds appears crucial for the formation of the trapped intermediate because suppression of either of them does not eliminate the presence of this intermediate.
From the rate constants obtained from intrinsic fluorescence (the interpretation of which is far more reliable than that of ANS fluorescence), it appears that suppression of the intra-β-domain disulfide C64-C80 or of the inter-αβ-domain disulfide C76-C94 does not affect the rate of appearance of the trapped intermediate. Rather, these disulfides significantly modify the rate of conversion of this intermediate into the native state. Suppression of the intra-β-domain disulfide accelerates the conversion by a factor four, indicating that it stabilizes the trapped intermediate in natural lysozyme. Conversely, suppression of the inter-αβ-domain disulfide slows down the conversion by a factor four, indicating that this bond, when present, destabilizes the trapped intermediate. These observations constitute striking examples of how individual long-range interactions may affect the stability of transiently trapped intermediates during protein folding. In this respect, they complement recent observations made by modifying the energy landscape (Wolynes et al. 1996) of lysozyme through the addition of salts (Kulkarni et al. 1999).
Our results also complement published studies on the role of each disulfide bond in lysozyme folding kinetics (Yokota et al. 2000). Indeed, these studies deal with the effect of individual disulfides on the stability of the transition state in the folding-unfolding reaction, which was shown (Wildegger and Kiefhaber 1997) to have the same free energy and, therefore, to probably be the same on the direct and indirect folding pathways. Our studies do not deal with this transition state. They are focused on the role of individual SS bonds in the formation of the burst intermediate, in the partitioning between the two possible pathways leading from the trapped intermediate to the native state, and in controlling the stability of the trapped intermediate that exists on the "slow" pathway. In summary, we show that (1) no individual disulfide is required to give rise to the burst, (2) both intra-α-domain disulfides must be formed to orient the polypeptide chain toward the trapped intermediate, and (3) whereas the intra-β-domain and inter-αβ-domain disulfides do not appear to be involved in the partitioning between the two pathways, the former one stabilizes the trapped intermediate, as compared with the transition state, and the latter destabilizes it.
The initial question that prompted our investigations was to find out which and how many disulfide bonds are needed to elicit the extremely rapid formation of secondary structure observed for lysozyme with its four disulfide intact. The studies reported here leave this question incompletely answered. It shows that any combination of three disulfides is enough for the burst of secondary structure to be observed. It has been reported that none of the four 1 SS variants (variants in which three disulfides were suppressed by conversion of the six corresponding cysteines to alanines) was able to form a native-like secondary structure, even at low temperature, in nondenaturing buffer (Tachibana 2000). It is therefore clear that no single disulfide would suffice to give rise to the secondary structure burst. However, two 1 SS variants, those with an intra-α-domain disulfide, show a significant propensity to form some secondary structure. The next step in understanding the origin of the burst would therefore be to find out whether or not some 2 SS variants are able to fold into a native-like conformation. Some 2 SS variants were indeed constructed and that containing the two intra-α-domain disulfides (6–127 and 30–115) was shown to contain a large amount of helical structure (Tachibana et al. 2001). However, the far-UV CD spectrum of this variant differs significantly from that of native WT lysozyme, which should prompt further characterization of its conformation. Moreover, no study was made on the rate of the far-UV ellipticity regain in the variant.
Performing such studies on the rate of refolding of variants with a limited number of intact disulfides is of considerable interest in understanding the relative roles of noncovalent interactions and disulfide bond formation during the natural process of lysozyme oxidative folding. Indeed, it has long been recognized that there exists a thermodynamic coupling between disulfides and secondary-tertiary structure. However, the question of which is the kinetically important phenomenon is still debated. Two pieces of evidence indicate that, for lysozyme, the kinetically limiting factor is the formation of appropriate disulfides. The first is that at least two disulfides must be formed before significant amounts of secondary structure can be detected (Roux et al. 1999). The second is that the kinetics of secondary structure formation in lysozyme with a limited number of native disulfides is extremely fast (see above) as compared with oxidation of disulfides and secondary structure formation during the oxidative folding (Roux et al. 1997 and 1999).
Understanding the relative roles of noncovalent interactions versus disulfide bonds in initiating the folding process may be of particular physiological significance in the case of secreted disulfide-containing proteins like lysozyme. Indeed, for such proteins, one can hypothesize that a major role of SS bonds may be to control the state of folding of the polypeptide chain so that the protein can fold only after its translocation on the luminal side of the endoplasmic reticulum. Thus, if SS bonds were critical for fast folding, the protein would remain essentially unfolded in the cytoplasm (where SS bonds hardly form) and hence would remain ready for transmembrane transport. On the contrary, once transported across the membrane, the polypeptide chain may be oxidized and only then would it be able to fold into its stable, native conformation. Moreover, that the secondary-tertiary structures of lysozyme cannot form in the absence of SS bonds but appear extremely rapidly when some of the proper SS bonds are present, leads to speculation about a likely coupling between the catalytic efficiency of the protein disulfide isomerase involved in the formation of the SS bonds and the folding of its substrate protein. Indeed, three of the four disulfide bonds of native lysozyme are deeply buried inside the core of the polypeptide chain, and the fourth one (6–127) is hardly solvent accessible. If the reduced polypeptide chains were able to fold into an approximately native conformation before oxidation of their disulfides, the disulfide isomerase active site would never be able to reach the cysteines and catalyze their oxidation. On the other hand, if the polypeptide chains would remain unfolded for a significant time after oxidation of proper cysteine pairs, the disulfide isomerase would catalyze disulfide exchange back to wrong disulfides. A rapid secondary structure formation and collapse of the polypeptide chain occurring only after, immediately after, the formation of some critical disulfide bonds (as we report for lysozyme) enables the disulfide isomerase to rapidly catalyze the oxidation/exchange leading to such critical disulfides but prevents the isomerase from reshuffling these critical bonds. One can therefore imagine that the natural folding pathway of lysozyme may well be controlled by the very rapid folding events that immediately follow the formation of critical disulfides. In this respect, deciphering the protein disulfide isomerase catalyzed oxidation pathway of lysozyme would be of crucial importance in understanding its natural folding process.
Materials and methods
Chemicals and protein preparations
Hen egg white (referred to as "natural") lysozyme and the oxidized and reduced forms of glutathione were purchased from Boehringer Mannheim GmbH. Reduced dithiothreitol (DTT) came from Research Organics Inc. Micrococcus lysodeikticus, for activity tests, and ANS were from Sigma. Guanidine hydrochloride (GuHCl) and urea were purchased from ICN Biomedical Inc. For purification by chromatography, Biorad Polyprep columns were used, DEAE Sephacel, and activated (with the thiopyridyl group) Thiopropyl Sepharose 6B were purchased from Amersham Pharmacia Biotech. Prestained molecular mass markers came from Biorad. The SB256 nondetergent sulfobetain (Goldberg et al. 1996) was generously provided by Dr. Thierry Rabilloud (CEN-Grenoble, France).
For all experiments with native natural or recombinant lysozyme, the freeze-dried protein was dissolved and dialyzed overnight at 4°C against buffer N (see below) and centrifuged before use for 10 min at 10,000 rpm in a bench centrifuge. For the preparation of denatured, nonreduced proteins, the freeze-dried proteins were dissolved and dialyzed for 3 h in buffer N, submitted to centrifugation as above, and dialyzed overnight at 4°C against 6 M GuHCl in buffer N. Buffer A: 0.1 M NaH2PO4, 1 mM EDTA, pH6; Buffer C: 8 M urea, 20 mM DTT adjusted to pH 8.8 with 1 M diethanolamine; and Buffer N: 0.01 M potassium phosphate pH7.8.
Bacterial strains, parental plasmid, and plasmid constructions
Mutation of the eight different cysteines into alanines were performed by site-directed mutagenesis (Kunkel et al. 1987; Messing and Vieira 1982). The lysS gene, obtained by excision from the parental plasmid pKSP-lys (Stueber et al. 1984), was introduced between the MscII and HindIII sites of the plasmid pET22b(+) (www.novagen.com). The recombinant plasmid served for the generation of single-strand DNA in the Escherichia coli strain RZ1032, using M13-K07 as a helper phage (Kunkel et al. 1987; Messing and Vieira 1982). The orientation of the lysS ORF relative to f1 ORI in the pET22b(+) resulted in the generation of a single-strand DNA homologous to the coding strand of the lysS ORF; therefore, all the primers used for substituting the different cysteines to alanines were designed to be homologous to the noncoding strand of the lysS gene. Whenever possible, a new restriction enzyme site was generated as a direct result of the Cys to Ala codon replacement and was used to control the mutations by analysis of the corresponding restriction fragments. For each pair of cysteines engaged in a given disulfide bond, one of the mutations was introduced first and the resulting variant was submitted to a second round of mutagenesis to replace the second cysteine. Whenever possible, all the steps were first verified by analysis of the restriction fragments. Clones with the correct restriction pattern were subjected to automated sequence analysis in an Applied Biosystem sequencer, using T7 and pET reverse primers and the dye termination method.
To obtain the best expression of recombinant proteins, the DNA fragments coding for WT and variant lysozymes were excised and amplified by PCR. The DNA fragments were inserted between the NdeI and the BamHI sites of the expression plasmid pTRAC which uses the principle of two-cistrons translational coupling (Schoner 1997). High-level expression of the cloned DNA is controlled by the strong heat-inducible PR promoter of phage λ and the T7 phage gene 10 translation initiation region (Ray et al. 1992). All the constructs were verified by sequencing and shown to have the expected sequences. The names pTRAClys, pTRAC8 (C6A,C127A), pTRAC6 (C76A,C94A), pTRAC9 (C30A,C115A), and pTRAC19 (C64A,C80A) were given to the plasmids encoding WT lysozyme and the variants carrying the two mutations indicated in parentheses, respectively. The E. coli strain BLR (Novagen) was transformed with each construct using the CaCl2 method (Sambrook et al. 1989).
Protein production
For the production, strains BLR(pTRAClys), BLR(pTRAC6), BLR(pTRAC8), BLR(pTRAC9), and BLR(pTRAC19) were grown in 2 L of LB medium (Sambrook et al. 1989) supplemented with ampicillin (100 μg/mL) and glucose 0.2% at 30°C until A600 = 2. Expression of the lysS derivative was induced by transfer of the culture vial in an agitated water bath at 42°C for 10 min followed by incubation in a hot room under agitation for 3 h. The cultures were then centrifuged (20 min, 4°C, 4000g). The pellets were weighed and resuspended into 10 times their weight of buffer A. The cell suspensions were sonicated (Branson sonicator, 1-cm diameter probe) and centrifuged (13,000g, 30 min, 4°C). The pellets, which contained inclusions bodies, were washed by suspension in buffer A followed by centrifugation as above.
Solubilization and purification of lysozymes from inclusions bodies
All the purification steps described below were valid for all the recombinant lysozymes. The pellet (see above) was weighed and suspended in 10-fold its weight of 10 M urea and 100 mM DTT adjusted at pH 10. Solubilization of the inclusion bodies was achieved by incubation for 2 h at 25°C. After centrifugation (13,000g, 20 min), the soluble extract was dialyzed against 8 M urea and 0.1 M acetic acid overnight. Insoluble contaminant proteins were removed by centrifugation (13 000g, 20 min, 4°C). An equivalent volume of 0.1 M acetic acid was added to the supernatant and the acidified solution was incubated for 1 h at room temperature. Insoluble proteins were again removed by centrifugation as above. The supernatant was dialyzed against buffer C (8 M urea, 20 mM DTT adjusted to pH 8.8 with 1 M diethanolamine); 2-mL DEAE-Sephacel columns (Pharmacia) were washed with 15 mL of buffer C and loaded with the dialyzed protein. The fraction flowing through the column was collected and dialyzed overnight against 0.01 M HCl at 4°C. All the fractions at each purification step were analyzed by electrophoresis on tricine SDS-polyacrylamide gels (Schägger and Von Jagow, 1987).
An additional purification step was introduced at this stage for the SS64–80− variant. The reduced-denatured purified protein obtained from the DEAE-Sephacel step was dialyzed extensively against 8 M urea, 100 mM NaCl, and 1 mM EDTA in 10 mM dihydrogen-potassium phosphate acidified to pH 3.6 by addition of 1 M HCl. The pH of the dialyzate (70 mL) was raised to 7.5 by the addition of ∼7 mL of 1 M potassium phosphate at pH 7.5. The protein solution was then immediately mixed with 7 g of Thiopropyl Sepharose 6B (Amersham Pharmacia Biotech) previously washed and equilibrated (by three buffer changes) in 30 mL of 8 M urea, 100 mM NaCl, and 1 mM EDTA in 100 mM dihydrogen-potassium phosphate at pH 7.5. The suspension was incubated under permanent agitation for 18 h. The resin was then packed in 10 small columns. Each column was washed with 8 M urea, 100 mM NaCl, and 1 mM EDTA in 100 mM dihydrogen-potassium phosphate at pH 7.5, and the adsorbed protein was released, washed out with the same buffer supplemented with 20 mM reduced DTT, and dialyzed against 0.01 M HCl at 4°C with three buffer changes.
Refolding of reduced denatured lysozyme
The concentration of the reduced, denatured, and purified lysozyme was estimated from the UV absorbance at 280 nm by using the specific extinction coefficient of unfolded, pure WT lysozyme, ɛ = 2.37 cm2/mg (Wetlaufer et al. 1974). The refolding was initiated by dilution under vigorous agitation (Goldberg et al. 1991) of the purified protein to reach 0.1–0.2 mg/mL in the final renaturation buffer (70 mM TrisHCl pH 8.2, 0.7 mM EDTA, 2 mM oxidized glutathione, 2 mM reduced glutathione, and the desired concentration of the SB256 nondetergent sulfobetaine). Preliminary tests were performed to determine, for each variant, the optimal SB256 concentration, which was found to be 0.25 M for the variants and 0.6 M for the WT protein, respectively. Renaturation/oxidation was complete after a 1-h incubation at 20°C. In the case of the SS64–80− variant, although the renaturation yield was quite satisfactory, complete removal of the sulfobetaine SB256 required a large number of long dialyses during which an important loss of activity was observed. SB256 was therefore replaced with urea, for which the optimal concentration was found to be 0.75 M, as an adduct for minimizing aggregation during the renaturation (Goldberg et al. 1996). The renatured protein was dialyzed extensively against 50 mM ammonium hydrogen carbonate with various buffer changes until complete elimination of the sulfobetaine or urea was achieved. For SB256, this could be ascertained by the disappearance of the absorption peak in the 250–260-nm region. This dialysis induced precipitation of significant amounts of contaminating proteins or misfolded lysozyme. The precipitate was removed by centrifugation at 13,000g, 20 min, 4°C, and the resulting supernatant was freeze-dried and stored at −20°C.
The renaturation yield was estimated by dividing the total enzymatic activity recovered after renaturation by the total amount of protein determined before renaturation from absorbance measurements (see above), taking as 100% either the specific activity of native WT lysozyme or that of the pure variant protein (Sawano et al. 1992).
For the SS76–94− variant, an additional purification step was added to the "standard" protocol. After the renaturation and freeze-drying steps described above, the protein was dissolved (about 2 mg/mL) in buffer N, dialyzed against 5 mM potassium phosphate at pH 7.8, centrifuged to remove aggregates, and applied (∼2 mg per run) onto a Poros-20HS ion exchange column (Boehringer Mannheim) equilibrated with the same buffer. The column was washed with 5 mL of the same buffer and the protein was eluted by means of a 15-mL linear potassium chloride gradient (0–1 M) in 5 mM potassium phosphate pH 7.8 at a flow rate of 1 mL/min. The main absorbance (280 nm) peak was collected. The fractions obtained from successive runs were pooled, dialyzed against 0.01 M ammonium bicarbonate, freeze-dried, and stored at −20°C.
Lysozyme activity was measured as described (Roux et al. 1997). The protein concentration in the assay was between 0.05 mg/mL and 0.2 mg/mL. The concentration of native lysozyme was determined by measuring the UV absorbance at 280 nm by using the published specific extinction coefficient of ɛ = 2.63 cm/mg (Wetlaufer et al. 1974).
Analysis of recombinant lysozymes by mass spectrometry and by microsequencing
Purified recombinant WT and variant lysozymes were dialyzed against distilled water, lyophilized, and analyzed by mass spectrometry. Samples were dissolved in water : methanol : formic acid (50 : 50 : 5) and introduced into an API365(Perkin-Elmer-Sciex) triple-quadrupole mass spectrometer at 5 mL/min by means of a syringe pump (Harvard Apparatus). The device was equipped with an atmospheric pressure ion source used to sample positive ions produced from a pneumatically assisted electrospray interface. The ionspray probe tip was held at 4.5 kV, and the orifice voltage was set at 45 V. The mass spectrum was scanned continuously from m/z 700–1800 with a scan step of 0.1 and a dwell time per step of 2 ms, resulting in a scan duration of 22 sec. Ten scans were averaged for each analysis. Mass calibration of the instrument was accomplished by matching ions of polypropylene glycol to known reference masses stored in the mass calibration table of the instrument. Data were collected on a Power Macintosh 8600/200 and processed through the Biotoolbox 2.2 software from Sciex.
For the amino acid sequence analysis, the proteins were submitted to SDS gel electrophoresis, colored by amido schwartz, and transferred onto an Immobilon P membrane (Millipore). The band corresponding to lysozyme was cut out and analyzed on an Applied Biosystems Procise sequencer by Mr. Jacques d'Alayer at the protein microsequencing laboratory (Institut Pasteur, Paris). The sequence of the 5 N-terminal residues was determined for each sample.
Analytical ultracentrifugation
Analytical ultracentrifugation was performed in a Beckman Optima-XLA ultracentrifuge, using standard double sector cells with 1.2-mm thick aluminium centerpieces. Natural lysozyme, WT recombinant lysozyme, and the recombinant variant proteins were dialyzed against buffer N and diluted until A280 = 0.7. Proteins were centrifuged at 58,000 rpm and 20°C. Radial scans at 280 nm were recorded at 15 min intervals. The apparent sedimentation coefficient, diffusion coefficient, and apparent molecular mass were calculated for each protein by using the second moment method of the Origin-based Optima XLA Data Analysis Software (Beckman Instruments).
Fluorimetric measurements
All fluorimetric measurements were performed at 20°C using a Perkin Elmer LS5B double monochromator spectrofluorometer. The scanning speed was 60 nm/min. For intrinsic fluorescence spectra, the band widths used were 2.5 nm for both excitation and emission, and the response setting (filtering constant) was 2. For ANS-binding experiments (30 μM total ANS), the band widths were 2.5 nm for excitation and 10 nm for emission, and the response setting was 4. The signal of the solvent was always subtracted from the signal of the protein sample.
Circular dichroism spectra
Circular dichroism spectra were recorded in a CD6 spectropolarimeter (Jobin-Yvon-Instrument SA) equipped with a thermostated (20°C) cell holder. The optical path of the cells was 0.2 mm for the far-UV CD region and 10 mm for the near-UV region. The scanning interval was 0.5 nm, the integration time was 2 sec, and the bandwidth was kept constant at 2 nm. Three (respectively, five) successive scans of each solution were averaged for the far-(respectively, near-) UV. The spectrum of the buffer alone was subtracted from that of the sample to obtain the CD contribution of the protein to the spectrum.
Stopped-flow experiments
Stopped-flow experiments were performed with the SFM-3 stopped-flow module (BioLogic) equipped with one small (4.5 mL) and two large (18 mL) syringes. The syringes, mixing lines, and observation cell were temperature regulated at 20°C. The injection flow-rates, volumes injected, sampling interval, and filtering time constants were as indicated in the Results section for each experiment. Unless otherwise stated, lysozyme unfolded in 6 M GuHCl in buffer N contained in the small syringe was diluted 80-fold with buffer N contained in the two large syringes at a flow rate resulting in a 4-ms dead-time.
For fluorescence measurements, the observations were made in a black-wall F15 (1.5 mm × 1.5 mm cross-section) fluorescence cell combined with the optical bench and detection modules of BioLogic. The excitation wavelengths were 294 nm and 366 nm for intrinsic and ANS fluorescences, respectively. The filters used for detection were high wavelength-pass filters with a cutoff at 350 nm for intrinsic fluorescence and at 450 nm for ANS fluorescence, except for the intrinsic fluorescence of the SS76–94− variant that was monitored through a high-pass filter with a cutoff at 320 nm. The data were analyzed by means of the Biokine software supplied by BioLogic.
For circular dichroism measurements, the observations were made in a conventional F15 (1.5 mm × 1.5-mm cross-section) fluorescence cell combined with a CD6 spectrodichrograph (Jobin-Yvon/Instruments S.A.), as described previously (Chaffotte et al. 1992). Experiments were performed at two different wavelengths, 222 and 228 nm. For each experiment, 15 kinetics were accumulated and averaged. The data files were converted into ASCII files by means of the conversion subroutine of the CD-Max software (Jobin-Yvon/Instruments S.A.), and fitting was performed by means of the Kaleidagraph software.
Acknowledgments
This research was supported by funds from the Institut Pasteur and the Centre National de la Recherche Scientifique (URA 1129). Pascale Roux benefited from a fellowship of the M.E.N.R.T. (Ministry of National Education, Research and Technology). The authors thank Abdelkader Namane for performing the mass spectrometry measurements and Jacques d'Alayer for the microsequencing experiments. The generosity of Dr. Thierry Rabilloud in providing the SB256 nondetergent sulfobetaine is gratefully acknowledged.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
ANS, 8-anilino-1-naphthalene-sulfonic acid
CD, circular dichroism
GuHCl, guanidine hydrochloride
R6127-Cx lysozyme, lysozyme with the disulfide bond between cysteines 6 and 127 reduced and these two cysteines carboxymethylated
SB 256, the nondetergent sulfobetaine benzyldimethyl(propyl-3-sulfonate) ammonium
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.3960102.
References
- Acharya, A.S. and Taniuchi, H. 1976. A study of renaturation of reduced hen egg white lysozyme. J. Biol. Chem. 251 6934–6946. [PubMed] [Google Scholar]
- Anderson, W.L. and Wetlaufer, D.B. 1976. The folding pathway of reduced lysozyme. J. Biol. Chem. 251 3147–3153. [PubMed] [Google Scholar]
- Chaffotte, A.-F., Guillou, Y., and Goldberg, M.E. 1992. Kinetic resolution of peptide bond and side chain far-UV circular dichroism during the folding of hen egg white lysozyme. Biochemistry 31 9694–9702. [DOI] [PubMed] [Google Scholar]
- Denton, M.E., Rothwarf, D.M., and Scheraga, H.A. 1994. Kinetics of folding of guanidine-denatured hen egg white lysozyme and carboxymethyl (Cys6-Cys127)-lysozyme: A stopped-flow absorbance and fluorescence study. Biochemistry 33 11225–11236. [DOI] [PubMed] [Google Scholar]
- Dobson, C.M., Evans, P.A. and Radford, S.E. 1994. Understanding how proteins fold: The lysozyme story so far. TIBS 19 31–37. [DOI] [PubMed] [Google Scholar]
- Engelhard, M and Evans, P.A. 1995. Kinetics of interaction of partially folded proteins with a hydrophobic dye: Evidence that molten globule character is maximal in early folding intermediates. Protein Sci. 4 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles, S.J., Radford, S.E., Robinson, C.V., and Dobson, C.M. 1994. Kinetic consequences of the removal of a disulfide bridge on the folding of hen lysozyme. Biochemistry 33 13038–13048. [DOI] [PubMed] [Google Scholar]
- Goldberg, M.E., Rudolph, R., and Jaenicke, R. 1991. A kinetic study of the competition between renaturation and aggregation during the refolding of denatured-reduced egg white lysozyme. Biochemistry 30 2790–2797. [DOI] [PubMed] [Google Scholar]
- Goldberg, M.E. and Guillou, Y. 1994. Native disulphide bonds greatly accelerate secondary structure formation in the folding of lysozyme. Protein Sci. 3 883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, M.E., Expert-Bezançon, N., Vuillard, L., and Rabilloud, T. 1996. Non-detergent sulphobetaines: A new class of molecules that facilitate in vitro protein renaturation. Fold. Des. 1 21–27. [DOI] [PubMed] [Google Scholar]
- Itzhaki, L.S., Evans, P.A., Dobson, C.M., and Radford, S.E. 1994. Tertiary interactions in the folding pathway of hen lysozyme: Kinetic studies using fluorescent probes. Biochemistry 3 5212–5220. [DOI] [PubMed] [Google Scholar]
- Kato, S., Okamura, M., Shimamoto, N., and Utiyama, H. 1981. Identification and characterization of the direct folding process of hen egg-white lysozyme. Biochemistry 20 1080–1085. [DOI] [PubMed] [Google Scholar]
- Kato, S., Shimamoto, N., and Utiyama, H. 1982. Spectral evidence for a rapidly formed structural intermediate in the refolding kinetics of hen egg-white lysozyme. Biochemistry 21 38–43. [DOI] [PubMed] [Google Scholar]
- Kulkarni, S.K., Ashcroft, A.E., Carey, M., Robinson, C.V., and Radford, S.E. (1999) A near-native state on the slow refolding pathway of hen lysozyme. Protein Sci. 8 35–44. [DOI] [PMC free article] [PubMed]
- Kunkel, T.A., Roberts, J.D., and Zakour, R.A. 1987. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 154 367–382. [DOI] [PubMed] [Google Scholar]
- Messing, J. and Vieira, J. 1982. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene 19 269–276. [DOI] [PubMed] [Google Scholar]
- Radford, S.E., Dobson, C.M., and Evans, P.A. 1992. The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature 385 302–307. [DOI] [PubMed] [Google Scholar]
- Ray, S., Zozulya, S., Niemi, G.A., Flaherty, K.M., Brolley, D., Dizhoor, A.M., McKay, D.B., and Stryer, L. 1992. Cloning, expression, and crystallization of recoverin, a calcium sensor in vision. Proc. Natl. Acad. Sci. 89 5705–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, P., Delepierre, M., Goldberg, M.E., and Chaffotte, A.-F. 1997. Kinetics of secondary structure recovery during the refolding of reduced hen egg white lysozyme. J. Biol. Chem. 272 24843–24849. [DOI] [PubMed] [Google Scholar]
- Roux, P., Ruoppolo, M., Chaffotte, A.-F., and Goldberg, M.E. 1999. Comparison of the kinetics of S-S bond, secondary structure and active site formation during refolding of reduced denatured hen egg white lysozyme. Protein Sci. 8 2751–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. 1989. Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sawano, H., Kuomoto, Y., Ohta, K., Sasaki, Y., Segawa, S.-I., and Tachibana, H. 1992. Efficient in vitro folding of the three-disulphide derivatives of hen lysozyme in the presence of glycerol. FEBS Lett. 303 11–14. [DOI] [PubMed] [Google Scholar]
- Schägger, H. and Von Jagow, G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166 368–379. [DOI] [PubMed] [Google Scholar]
- Schoner, B.E. 1997. Synthetic two-cistron expression system. Methods Mol. Biol. 62 89–97. [DOI] [PubMed] [Google Scholar]
- Semisotnov, G.V., Rodionova, N.A., Razgulyaev, O.I., Uversky, V.N., Gripas, A.F., and Gilmanshin, R.I. 1991. Study of the "molten globule" intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers 31 119–128. [DOI] [PubMed] [Google Scholar]
- Stueber, D., Ibrahimi, I., Cutler, D., Dobberstein, B., and Bujard, H. 1984. A novel in vitro transcription-translation system: Accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 3 3143–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana, H. 2000. Propensities for the formation of individual disulfide bonds in hen lysozyme and the size and stability of disulfide-associated submolecular structures. FEBS Lett. 480 175–178. [DOI] [PubMed] [Google Scholar]
- Tachibana, H., Ohta, K., Sawano, H., Koumoto, Y., and Segawa, S. 1994. Relationship between the optimal temperature for oxidative refolding and the thermal stability of refolded state of hen lysozyme three-disulfide derivatives. Biochemistry 33 15008–15016. [DOI] [PubMed] [Google Scholar]
- Tachibana, H., Oka, T., and Akasaka K. 2001. Native-like tertiary structure formation in the alpha-domain of a hen lysozyme two-disulfide variant. J. Mol. Biol. 314311–320. [DOI] [PubMed] [Google Scholar]
- Van den Berg, B., Chung, E.W., Robinson, C. V., and Dobson, C.M. 1999. Characterization of the dominant oxidative folding intermediate of hen lysozyme. J. Mol. Biol. 290 781–796. [DOI] [PubMed] [Google Scholar]
- Wetlaufer, D.B., Johnson, E.R., and Clauss, L.M. 1974. Rapid nonenzymic regeneration of reduced human leukemia lysozyme. In Lysozyme (eds. E.F. Ossermann, R.E. Canfield, and S. Beychock), pp 269–280, Academic Press, New York and London.
- Wildegger, G. and Kiefhaber T. 1997. Three-state model for lysozyme folding: Triangular folding mechanism with an energetically trapped intermediate. J. Mol. Biol. 270 294–304. [DOI] [PubMed] [Google Scholar]
- Wolynes, P.G., Luthey-Schulten, Z., and Onuchic, J.N. 1996. Fast folding experiments and the topography of protein folding energy landscapes. Chem. Biol. 3 425–432. [DOI] [PubMed] [Google Scholar]
- Yokota, A., Izutani, K., Takai, M., Kubo, Y., Noda, Y., Koumoto. Y., Tachibana, H., and Segawa, S.-I. 2000. The transition state in the folding-unfolding reaction of four species of three-disulfide variant of hen lysozyme: the role of each disulfide bridge. J. Mol. Biol. 295 1275–1288. [DOI] [PubMed] [Google Scholar]