Abstract
Several bacteria use trimethylamine N-oxyde (TMAO) as an exogenous electron acceptor for anaerobic respiration. This metabolic pathway involves expression of the tor operon that codes for a periplasmic molybdopterin-containing reductase of the DMSO/TMAO family, a pentahemic c-type cytochrome, and the TorD cytoplasmic chaperone, possibly required for acquisition of the molybdenum cofactor and translocation of the reductase by the twin-arginine translocation system. In this report, we show that the TorD chaperone from Shewanella massilia forms multiple and stable oligomeric species. The monomeric, dimeric, and trimeric forms were purified to homogeneity and characterized by analytical ultracentrifugation. Small-angle X-ray scattering (SAXS) and preliminary diffraction data indicated that the TorD dimer is made of identical protein modules of similar size to the monomeric species. Interconversion of the native oligomeric forms occurred at acidic pH value. In this condition, ANS fluorescence indicates a non-native conformation of the polypeptide chain in which, according to the circular dichroism spectra, the α-helical content is similar to that of the native species. Surface plasmon resonance showed that both the monomeric and dimeric species bind the mature TorA enzyme, but that the dimer binds its target protein more efficiently. The possible biologic significance of these oligomers is discussed in relation to the chaperone activity of TorD, and to the ability of another member of the TorD family to bind the Twin Arginine leader sequences of the precursor of DMSO/TMAO reductases.
Keywords: TorD, Shewanella massilia, SAXS, oligomers, putative chaperone of the molybdoenzyme TorA, TMAO, Twin Arginine leader sequence
In living organisms such as marine fishes and molluscs, trimethylamine N-oxyde (TMAO) is a widely distributed low molecular weight constituent. It frequently represents more than 1% of the dry weight in the tissues where it is assumed to act as an osmoprotector (Barrett and Kwan 1985). In decaying organisms, the reduction of TMAO to the nauseous trimethylamine (TMA) by bacterial TMAO reductase plays an important role in tissue spoilage. This process occurs because in the absence of oxygen, several bacteria of the fish flora may use TMAO as an exogenous electron acceptor. This respiratory system is encoded by the torCAD and torECAD operons in Escherichia coli and in the marine Shewanella species, respectively. Their transcription is induced in the presence of TMAO or related compounds (Méjean et al. 1994; Dos Santos et al. 1998) and is under the control of the two-component system TorS/TorR.
TorC is a c-type cytochrome anchored to the inner membrane, and it is involved in the electron transfer from the membranous menaquinones to the terminal reductase TorA. TorC is organized into two domains of similar size: the tetrahemic N-terminal domain binds to TorA and transfers the electrons to the monohemic C-terminal domain, which in turn, directly transfers electrons to TorA (Gon et al. 2001). The periplasmic molybdoprotein TorA belongs to the DMSO/TMAO reductase family but, in contrast to the other members of this family, it can only reduce TMAO (Iobbi-Nivol et al. 1996). The structure of the TorA protein from S. massilia showed that the 798 amino acids of the polypeptide chain folds as four domains organized around the molybdenum cofactor (Czjzek et al. 1998). Binding of the molybdenum cofactor is a cytoplasmic event required for the periplasmic translocation of TorA as a folded holoprotein by the Twin Arginin Translocation (TAT) system (Santini et al. 1998; Berks et al. 2000a, 2000b). This process relies on the presence of a characteristic leader peptide containing a crucial twin arginin motif (RRxFL) (Berks 1996).
The torD gene encodes a cytoplasmic TorA chaperone (Pommier et al. 1998), and interestingly, most of the known operons encoding a DMSO/TMAO respiratory system contain a gene coding for a protein homologous to TorD. For example, the dorCDA operon of Rhodobacter species encodes the DMSO/TMAO reductase DorA and the DorD protein, which displays 29.6% sequence identity with TorD from E. coli (Mouncey et al. 1997; Shaw et al. 1999). TorD from E. coli and Shewanella species are 33% identical in sequence (Dos Santos et al. 1998), and searches in the databases did not reveal the presence of known amino acid motives or sequence similarities to proteins of known structures. The presence of short hydrophobic regions at the N- and C-termini regions of TorD initially suggested that the protein might be a b-type cytochrome (Méjean et al. 1994), in line with the finding that this type of cytochrome was implicated for TMAO reduction (Bragg and Hackett 1983). Further studies indicated that TorD from E. coli was neither a membrane-bound protein nor a b-type cytochrome, but instead a cytoplasmic protein that specifically assisted the molybdoenzyme TorA for acquiring functionality in vivo (Pommier et al. 1998).
In this study, we investigated the molecular properties of the 209 residue-long TorD protein from S. massilia. The overexpressed protein was purified as stable monomeric and dimeric species. Interconversion of the two forms occurred at acidic pH value, and involved formation of a non-native conformation according to ANS fluorescence. Biophysical studies using small-angle X-ray scattering and preliminary crystallographic results indicated that the TorD dimer is formed by two identical protein modules of similar size to the monomeric species. Surface plasmon resonance showed that both monomeric and dimeric forms bind the mature terminal reductase TorA, but that the TorD dimer interacts more strongly with its target enzyme. The possible biological significance of these findings with respect to the chaperone properties of TorD on TorA is discussed.
Results
The overexpressed TorD protein of S. massilia accounted for about 20% of the total soluble protein fraction in E. coli. The recombinant protein comprises residues 1–209 of TorD and eight additional residues from the C-terminal histidine tag. The polypeptide chain carries five cysteine residues found to mediate intermolecular disulfide bonds. This process was fully reversible upon addition of a reducing agent, which was therefore added to all buffer solutions throughout the experiments reported here. TorD was eluted in two markedly different peaks from the nickel affinity chromatography column. These peaks corresponded to two stable molecular species of the protein. They had an identical migration pattern on denaturating gels, with an apparent molecular weight around 25 kD, but migrated differently as single bands on non-denaturing PAGE (Fig. 1 ▶). The possibility that these two protein species may correspond to two oligomeric forms of the protein was assessed by sedimentation equilibrium experiments (Fig. 2A, B ▶). Best fits were obtained assuming single molecular species of molecular weights corresponding to the monomeric (24,356 Daltons) and dimeric TorD (48,712 Daltons). The molecular weight of the monomeric species determined by electrospray mass spectrometry (24,354 Daltons) corresponded to the theoretical mass of the recombinant protein devoid of the N-terminal methionine residue.
Fig. 1.
Electrophoretic pattern on SDS-PAGE (lanes 1–3) and on native PAGE (lanes 4, 5). The proteins were stained with Coomassie blue. Lane 1: TorD monomer, lane 2: TorD dimer, lane 3: low range molecular weight markers (BioRAD), lane 4: TorD monomer, lane 5: TorD dimer.
Fig. 2.

Sedimentation equilibrium of the TorD molecular species. (A) Monomeric protein; B) dimeric protein; (C) trimeric protein. The data and the corresponding fitted curves are shown in the lower panel. The top panel represents the goodness of fit of the experimental data.
Previous experiments with the TorD and TorA proteins from E. coli demonstrated that TorD acts as a chaperone of the enzyme (Pommier et al. 1998). The formation of TorD–TorA complexes, possibly illustrating similar properties of the two orthologs in S. massilia was investigated by surface plasmon resonance. Each of the two forms of TorD was immobilized on a dextran matrix over which a purified fraction of TorA was injected. In both cases, a significant increase in the amount of recovered resonance units was observed, indicating that TorD binds TorA (Fig. 3 ▶). Moreover, the results indicated that the dimeric form of TorD binds more efficiently TorA than the monomeric form because at least 70% more resonance units were recovered at the end of TorA injection over immobilized TorD dimers. This finding indicates that TorD dimers (or possibly higher oligomers) have a functional significance with respect to the TorA chaperone function in S. massilia.
Fig. 3.
BIAcore analysis of the interactions between TorD and TorA. The sensorgrams of interactions between TorA and immobilized TorD dimers (—) and immobilized TorD monomers (----) are expressed in resonance units (RU). TorA (120 μL at 0.1 mg/mL ) was injected on a sensor chip with dextran matrix coupled either to TorD monomers, to TorD dimers, or to no protein as a control. The control flow cell (no protein immobilized) was subtracted from the experiment flow cells. The arrow indicates the end of the injection.
The monomer and the dimer were stable over a few weeks, with no evidence of equilibrium between the two states under standard biochemical conditions. Interconversion of the two species was investigated in conditions expected to destabilize the native fold of the TorD protein. Increased temperature only led to protein aggregation, but a significant interconversion between the monomeric and dimeric forms occurred when either species experienced pH 3.0. This process was not observed when the protein was exposed to pH 3.0 for about 30 sec, in fast dilution experiments from pH 8.0 to pH 3.0 and to pH 8.0. Interconversion was very significant when the protein experienced pH 3.0 for longer time scales. Starting from either pure monomeric or dimeric forms, about half of the initial species was converted into the other form (Fig. 4 ▶). In both experiments, higher oligomeric species were also detected. The most abundant of these oligomeric species, purified as a single and stable molecular form according to native gel electrophoresis and analytical gel filtration, was characterized by sedimentation equilibrium experiment to be a trimer of the TorD protein (Fig. 2C ▶).
Fig. 4.
Coomassie blue stained native 6% PAGE of TorD. Lane 1: TorD monomer. Lane 2: TorD dimer. Lane 3: TorD monomer after overnight dialysis against sodium acetate pH 3.0, followed by dialysis against Tris-HCl pH 8.0. Lane 4: TorD dimer after overnight dialysis against sodium acetate pH 3.0, followed by dialysis against Tris-HCl pH 8.0.
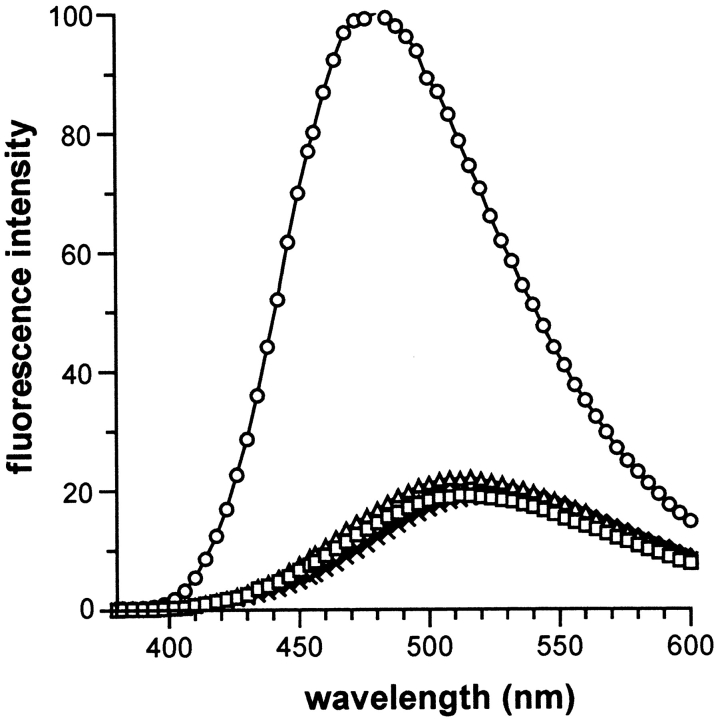
Circular dichroism (CD) spectra at pH 8.0 demonstrated that the content in secondary structures of the native monomeric and dimeric forms were very similar (Fig. 5 ▶). Spectral minima at 208 and 222 nm, which dominate the spectra, are characteristic of α-helices (Adler et al. 1973). The CD spectra of the polypeptide chain at pH 3.0 (Fig. 5 ▶) indicated no major change in the α-helical content, while the mean residue ellipticities were close to zero for the random conformation of the protein denatured with 6 M guanidine hydrochloride (data not shown). The conformation of the polypeptide chain at pH 3.0 and 8.0 was then analyzed using ANS fluorescence. (Semisotnov et al. 1991; Ptitsyn 1992). As shown in Figure 6 ▶, ANS interacts strongly with the protein at pH 3.0. The large increase in ANS fluorescence and the shift of the emission maximum from 530 to 470 nm provide evidence for the presence of exposed hydrophobic surfaces of the protein in this condition. In contrast, the fluorescence of ANS was essentially not affected in the presence of the native monomers and dimers, and in the presence of the GdnHCl-denatured protein.
Fig. 5.
Dependence of mean residue ellipticity on wavelength in the peptide region. The CD spectra were recorded at pH 8.0 for the native monomeric (— — —) and dimeric species (——) and for the protein at pH 3.0 (– – – –).
Fig. 6.
ANS fluorescence spectra in the presence of the native monomer (open triangle), native dimer (open square), denatured protein (open diamond) at pH 8.0, and of the protein at pH 3.0 (open circles). ANS spectra alone in buffer (×) is represented for reference.
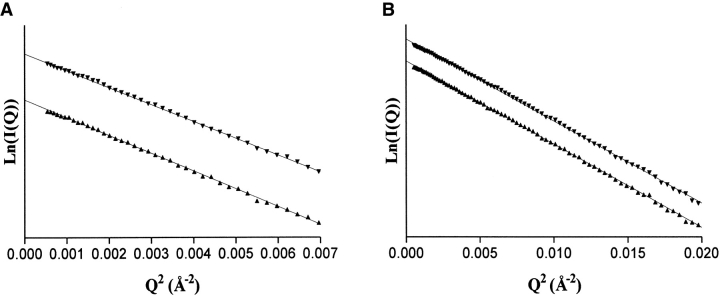
The molecular parameters of the monomeric and dimeric forms of TorD were derived from SAXS experiments using monodispersed protein solutions. The Guinier plots in the small-angle scattering region provided the radii of gyration for the monomeric (Rg = 19.5 ± 0.7 Å) and the dimeric forms (Rg = 27.9 ± 0.8 Å) (Figs. 7A, 8A ▶ ▶). The extrapolated I(0) value, brought to absolute scale, gave estimates of the molecular weights of the TorD species (24.1 ± 1.7 kD and 47.8 ± 1.2 kD for the monomer and the dimer, respectively), in good agreement with the theoretical molecular weights (Table 1).
Fig. 7.
Guinier plots of the X-ray scattering data for the TorD monomer in 20 mM Tris-HCl pH 8.0, 300 mM NaCl, 5 mM DTT at 10.7 mg/mL (filled up triangle) and 14.8 mg/mL (filled down triangle). (A) Guinier representation for Q•Rg < 1.7. (B) Guinier representation including the high scattering angles. For the sake of clarity, the plots have been arbitrarily shifted along the vertical axis.
Fig. 8.
Guinier plots of the X-ray scattering data for the TorD dimer in 20 mM Tris-HCl pH 8.0 under various monodispersed conditions: 204 mM NaCl, 300 mM NaSCN, 5 mM DTT at a protein concentration of 5.1 mg/mL (filled circles); 215 mM NaCl, 200 mM NaSCN, 5 mM DTT at a protein concentration of 2.8 mg/mL (filled down triangles); 215 mM NaCl, 300 mM ammonium sulfate, 5 mM DTT at a protein concentration of 2.1 mg/mL (filled diamonds). (A) Guinier representation for Q•Rg < 1.7. (B) Guinier region including the high scattering angles. For the sake of clarity, the plots have been arbitrarily shifted along the vertical axis.
Table 1.
Molecular parameters for the monomeric and dimeric forms of TorD
| TorD monomer | TorD dimer | |
| Rg (Å) from Guinier analysis | 19.5 ± 0.7 | 27.9 ± 0.8 |
| Rg (Å) from P(r) analysis | 18.9 ± 0.5 | 28.4 ± 1.0 |
| Rgdom (Å) from Ln(I(Q)/Q) vs Q2 | — | 20 ± 0.8 |
| Dmax (Å) | 57 ± 3 | 83 ± 3 |
| Mw (kD) calculated from SAXS data | 24.1 ± 1.7 | 47.8 ± 1.2 |
| Mw (kD) calculated from sequence | 24.4 | 48.7 |
Guinier representations including the scattered intensities at high angles were also examined. The plot remains linear in the whole range of momentum transfer (Q) value for monomeric TorD (Fig. 7B ▶), suggesting a globular single-domain protein (Kataoka et al. 1991). This proposal was also supported by the unimodal distribution of the P(r) function (Fig. 9 ▶) and by the Gaussian distribution of the Kratky plot (Fig. 10 ▶). The Rg values and I(0) values calculated from the P(r) distributions were in good agreement with the corresponding values estimated by the Guinier analysis (Table 1).
Fig. 9.
Distance distribution function for the TorD monomer at 14.8 mg/mL (filled down triangles) and for the TorD dimer at 5.1 mg/mL (filled circles). The symbols correspond to the conditions given in the legends of Figures 6 and 7 ▶ ▶.
Fig. 10.
Kratky plots for monomeric TorD at 14.8 mg/mL (filled down triangles) and for dimeric TorD at 5.1 mg/mL (filled circles). The symbols correspond to the conditions given in the legends of Figures 6 and 7 ▶ ▶.
In contrast, Guinier representations for the TorD dimer, when including the scattered intensities at high angles, displayed a biphasic distribution (Fig. 8B ▶) (Fujisawa 1987; Kataoka et al. 1989). The slope at the smallest angles (Q2 < 0.004, Fig. 8A ▶) reflects the contribution of the entire molecule (Rg = 27.9 ± 0.8 Å), whereas the data at higher angles reflect the contribution of the domains within the molecule (Fujisawa 1987). The value of the average radius of gyration of these domains (Rgdom) was evaluated from the slope of the ln(I(Q)/Q) versus Q2 plot (Fig. 11 ▶) (Fujisawa 1987). This value (20 ± 0.8 Å) was very similar to the Rg value of the TorD monomer (Rg = 19.5 ± 0.7 Å). The distance between the centers of monomers in the dimer (rnc) was evaluated by the relation Rg2 = (Rgdom)2 +r2nc/4 to be 40 Å. The P(r) function gives a maximum dimension (Dmax) value of 83 Å and displays a shoulder at about 58 Å (Fig. 9 ▶). The distribution is not Gaussian and also expresses a bimodal distribution of atoms within the protein (Kataoka et al. 1991; Shi et al. 1996). The Kratky plot (Fig. 10 ▶) leads to a similar conclusion (Kataoka et al. 1995).
Fig. 11.
Ln(I(Q)/Q) versus Q2 plot for the TorD dimer at 5.1 mg/mL (filled circles), 2.8 mg/mL (filled down triangles), and 2.1 mg/mL (filled diamonds) providing the Rgdom value of 20 ± 0.8 Å. The symbols correspond to the conditions given in the legend of Figure 7 ▶.
A further characterization of the TorD dimer was given by a self-rotation function calculated from the diffracted intensities of a native TorD dimer crystal (Table 2). These crystals belong to the orthorhombic space group with cell parameters a = 64.3 Å, b = 95.9 Å, c = 97.3 Å, and contain one dimer per asymmetric unit assuming a solvent content of 60% (Matthews 1968). A self-rotation function calculated using the program POLARRFN (CCP4 1994) with the native data set revealed the presence of a single molecular twofold axis (κ = 180°) for polar angles values of ϕ = 90° and ξ = 45° (Fig. 12 ▶).
Table 2.
Data collection and statistics
| Native | |
| Unit cell parameters | a = 64.3 Å, b = 95.9 Å, c = 97.3 Å α = β =γ = 90a |
| Resolution Å (outer shell) | 19.61–2.42 (2.48–2.42) |
| Reflections Measured/unique | 108921/21290 |
| Completeness % (outer shell) | 90.3 (81.6) |
| I/σ Overall/outer shell | 11/2.8 |
| Rsyma (outer shell) | 0.040 (0.368) |
aRsym = ΣΣ |〈I〉 − Ii|/ΣΣIi
Fig. 12.
The κ = 180° section of the self-rotation function. Peaks are scaled from 0 to 100% (100% corresponds to the origin peak). ϕ angles and the position of the crystallographic axis are marked on the circumference. Contour lines correspond to 2% increment, and were plotted for peaks whose intensities are at least 15% to that of the origin peak.
Discussion
The SAXS data and the preliminary crystallographic informations indicate that dimeric TorD is organized into two identical domains, of similar size to the TorD monomeric species. These biophysical data suggest that dimer formation may involve a monomer–monomer association through a molecular twofold symmetry axis. However, the absence of equilibrium between the monomeric and dimeric species of TorD may indicate a different conformation of the monomer within the dimer, stabilized by the association with the other monomer. According to the biochemical experiments, this conformation only occurs in significant amounts when the protein experiences acidic pH values. The CD spectra of the monomeric and dimeric species were, however, very similar (Fig. 5 ▶), suggesting that there are no major differences in secondary structure of the TorD polypeptide chain, whether it is associated to another subunit in the dimer or folded as a monomeric species. The two conformations might, therefore, only differ in solvent-exposed loop regions, and the acidic pH transition would affect the conformation of these loops and favor dissociation of the dimer by disrupting polar interactions at the intersubunit interface.
The pH transition also produced higher oligomeric species of the TorD protein, and the most abundant form was purified. This species, the monomeric and the dimeric forms, were stable molecular species that differed in both their electrophoretic pattern on native gels and elution profiles from analytical gel filtration. Each single form was analyzed by analytical ultracentrifugation, and the sedimentation equilibrium data fitted to monomeric, dimeric, and trimeric species of the TorD protein. This finding raised the possibility that the TorD protein may undergo a cooperative assembly process.
CD spectroscopy and ANS fluorescence provided some insights into the conformation of the polypeptide chain. The CD spectrum of the protein at pH 3.0 indicated that the α-helical content seems unaltered compared to that of the native monomeric and dimeric forms at pH 8.0 (Fig. 5 ▶). Upon addition of 6 M guanidine hydrochloride, the polypeptide chain was denatured and the CD spectrum showed no indication of remaining secondary structure (data not shown). ANS fluorescence was unaffected in the presence of native monomeric and dimeric forms, and in the presence of GdnHCl-denatured protein. In contrast, a strong increase in fluorescence intensity, and an emission maximum shift of ANS were observed in the presence of TorD at pH 3.0 (Fig. 6 ▶). These features are characteristic of the binding of ANS to hydrated hydrophobic surfaces or clusters in the protein at pH 3.0 that are absent in the monomeric and dimeric native states.
These experiments suggest that the polypeptide chain at pH 3.0 experiences a significant structural transition into a non-native conformation in which the polypeptide chain contains most of the α-helical elements present in the native monomeric and dimeric forms. Alternate folding pathways from this non-native conformation and 3D domain swapping could explain the occurrence of multiple oligomeric species of the protein. Completion of the native fold may either proceed within a single polypeptide chain leading to monomeric TorD or through association with other partially folded monomers leading to oligomeric species. The biophysical characterizations do not argue against this possibility, and the biochemical data would be in line with the general features documented for proteins known to undergo 3D domain swapping (Schlunegger et al. 1997), such as diphteria toxin (Bennett et al. 1994), mutated calbindin D(9K) (Hakansson et al. 2001), RNase A (Richards and Vithayathil 1959) and p13suc1 (Birck et al. 1996; Rousseau et al. 2001).
The multiple oligomeric species for the TorD protein provide new insights into the function of the chaperone. The fact that the TorD dimers interact more strongly with TorA than the TorD monomers supports the idea that oligomerization of TorD is physiologically relevant. This property may possibly be related to the chaperone activity of TorD that is required for insertion of the molybdopterin cofactor into the precursor TorA protein (Pommier et al. 1998). On the other hand, a recent report showed that DmsD, an E. coli protein displaying 21% of sequence identity with TorD from S. massilia, binds the twin-arginine leader sequences (RRxFL motif) of the precursors of TorA and DmsA, the dimethyl sulphoxide (DMSO) reductase subunit that belongs to the same family as TorA (Oresnik et al. 2001). It will therefore be of great interest to test whether TorD can also bind the TorA leader sequence, and whether DmsD can interact with the processed DmsA enzyme. If this is the case, then it raises the possibility that proteins of the TorD family can play a role in both the folding of metalloenzymes and their addressing to the TAT machinery.
Materials and methods
Cloning of torD gene
The torD gene of S. massilia was amplified from chromosomal DNA by PCR using the primers SMD1: 5` TTT CCA TAT GAG TCA AGT CGA TAT CAA CCA CGC 3` and SMD2: 5` TTT CTC GAG GCT AAT TAT CGC CAC AGC GGG TTC 3` that incorporate the NdeI and XhoI restriction sites at the 5` and 3` ends, respectively. The purified PCR product was ligated to NdeI- and XhoI-digested pET-22b vector expression (NOVAGEN) to form the plasmid, pet-TorD. The sequence was confirmed by DNA sequencing.
Protein expression and purification
The recombinant vector was transformed into Escherichia coli strain BL21(DE3)pLysS. Bacteria were grown at 37°C in Lenox broth medium supplemented with 100 μg mL−1 ampicillin and 35 μg mL−1 chloramphenicol to an OD600nm of 0.7. Expression of TorD protein was induced by addition of 0.5 mM IPTG (isopropyl β-d-thiogalactopyranoside) for 4 h to an OD600nm of about 1.5. Cells were harvested by centrifugation at 4500 × g for 20 min, resuspended in lysis buffer (20 mM sodium phosphate pH 7.6, 500 mM NaCl, 1 mM DTT, 150 U benzonase (Merck), 10 μg mL−1 leupeptin, pepstatin, and TPCK, 0.1 mM PMSF) and disrupted by sonication at 0°C. The insoluble material was removed by centrifugation at 9000 × g for 2 h.
All protein purification procedures were carried out at 4°C. The supernatant was loaded onto a 5 mL Ni-sepharose column equilibrated with 20 mM sodium phosphate pH 7.6, 500 mM NaCl (buffer A). The column was washed with 10 mL of buffer A followed by 10 mL of buffer A implemented with 5 mM imidazole. The bound proteins were eluted with 70 mL of a 0.1–0.25 M imidazole linear gradient in buffer A. TorD eluted in two peaks at 160 mM (major peak, monomeric form) and 240 mM imidazole (minor peak, dimeric form), which were purified independently. After dialysis against 10 mM sodium phosphate pH 7.6, 10 mM DTT, each protein fraction was applied to an UnoQ6 anion exchange column (Biorad) equilibrated with 20 mM Tris-HCl pH 8.0, 10 mM DTT eluted with a 0.075–0.4 M NaCl linear gradient. The monomeric TorD fractions were eluted at 150 mM NaCl and the dimeric form at 220 mM NaCl. Monomeric TorD was finally purified on a Superdex 75 column (Hiload 16/60, Pharmacia) equilibrated with 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 10 mM DTT. The TorD monomers (in 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 10 mM DTT) and dimers (in 20 mM Tris-HCl pH 8.0, 220 mM NaCl, 10 mM DTT) were concentrated to 10 mg/mL and 2.5 mg/mL, respectively, using Centricon 10 filter units (Amicon-Millipore) and stored at 4°C. The protein samples were analyzed on 15% SDS-PAGE and on 6% PAGE.
Interconversion of the molecular species
The monomeric and dimeric TorD species were dialysed overnight against 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 10 mM DTT. The concentration of each protein sample was 0.8 mg/mL . The temperature and the pH effects were investigated. For the temperature effect, three 20-μl samples of TorD monomer were incubated for 1 h at 20, 40, and 60°C, respectively, and then brought to 4°C in 30 min. For the pH effect, the temperature was set to 4°C and two buffers were used: 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 10 mM DTT and 20 mM sodium acetate pH 3.0, 150 mM NaCl, 10 mM DTT. The monomeric and dimeric proteins were handled in three different conditions: (a) fast dilution of the 20 μL protein sample in 100 μL of pH 3.0 buffer followed by a fast dilution in 500 μL of pH 8.0 buffer. The protein solution was then concentrated to 50 μL on a microcon 10 filter unit (Amicon, Millipore); (b) fast dilution of the 20 μL protein sample in 100 μL of pH 3.0 buffer followed by overnight dialysis against 100 mL of pH 8.0 buffer; (c) overnight dialysis of the 20 μL protein sample against 100 mL of pH 3.0 buffer followed by a 4-h dialysis against 100 mL of pH 8.0 buffer. The protein samples were analysed on 6% PAGE.
Circular dichroism
Circular dichroism (CD) spectra were measured from 195 to 280 nm at a rate of 0.2 nm•sec−1 with a 1-mm pathlength cuvette at 4°C by using a Jobin-Yvon CD6 spectropolarimeter. Each spectrum was an average of five scans. TorD monomers (16 μM) and dimers (12 μM) were prepared in 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM DTT, and in 20 mM Tris-HCl pH 8.0, 240 mM NaCl, 1 mM DTT, respectively. TorD (16 μM) at pH 3.0 was obtained after dialysis of the protein against 20 mM sodium acetate, 150 mM NaCl, 1 mM DTT. Unfolding conditions of the protein (16 μM) were provided by the addition of 6 M GdnHCl in 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM DTT.
ANS binding fluorescence assays
Emission spectra were collected at 4°C from 380–600 nm using a P.T.I. (Photon Technology International) fluorescence spectrophotometer. The excitation wavelength was 365 nm (Semisotnov et al. 1991; Ptitsyn 1992). Monomeric TorD (5 μM) was mixed with 500 μM 1-anilino naphtalene-8-sulfonic acid (ANS) in 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM DTT (supplemented with 6 M GdnHCl when studying the unfolding conditions), and in 20 mM sodium acetate pH 3.0, 150 mM NaCl, 1 mM DTT. Dimeric TorD (2.5 μM) was mixed with 500 μM ANS in 20 mM Tris-HCl pH 8.0, 240 mM NaCl, 1 mM DTT.
Interaction experiments
Partially purified TorA was obtained from a periplasmic fraction of 20 g of S. massilia cells anaerobically grown in rich medium supplemented with 50 mM TMAO. Purification involved chromatographic steps on a Q-sepharose Hiload 16/10 column, followed by a monoQ HR5/5 and by heparin affinity chromatography. The TorA protein was followed spectrophotometrically by measuring the TMAO reductase activity and by SDS-PAGE. It represented more than 80% of total proteins in the fraction used for the interaction experiments. Surface plasmon resonance (BIAcore, Pharmacia Biosensor) was used to analyze, in real time, the binding between TorA and monomer or dimer of TorD. All experiments were carried out at 25°C. The TorD monomer and dimer stock solutions were quickly diluted to 0.1 mg/mL in 10 mM sodium acetate buffer (pH 3.0) and their stability after dilution was checked by native PAGE. Each molecular form of TorD was then immobilized on a sensor chip CM5 (BIAcore) through amine coupling. Almost the same resonance units were recovered in both cases (2621 and 2691 RU for the monomer and the dimer, respectively). TorA (0.1 mg/mL ) in 20 mM phosphate buffer (pH 7.0) was injected over the test and control (no protein immobilized) surfaces at a constant flow rate of 10 μL/min. The resulting sensorgrams were evaluated using the biomolecular interaction analysis evaluation software (BIAcore).
Sedimentation equilibrium
Sedimentation equilibrium experiments were carried out at 20°C with a Beckman Optima XL-A analytical ultracentrifuge equipped with a four-hole An-55 rotor and standard 1.2-cm hexa-sector cells. Monomeric TorD (0.6 mg/mL) and trimeric TorD (0.33 mg/mL) were dialyzed against 10 mM Tris-HCl pH 7.8, 150 mM NaCl, 10 mM TCEP (tris-[2 carboxyethyl] phosphine hydrochloride). Dimeric TorD (0.5 mg/mL) was dialyzed against 10 mM Tris-HCl pH 7.8, 220 mM NaCl, 10 mM TCEP. Samples were run at 15,000, 12,000, and 11,000 rpm for the monomeric, dimeric, and trimeric form, respectively. Protein concentration distributions were determined at 280 nm. Data were collected over a 22-h period. After centrifugation for 12 h, scans were compared at 2-h intervals to ensure that equilibrium had been reached. Sedimentation equilibrium data and protein distribution were analyzed and evaluated using a nonlinear least-squares curve-fitting algorithm by MicroCal Origin-based Optima XL-A software (Beckman). The data, absorbance versus radial distance, were analyzed using the fitting models for a single homogeneous species. A partial specific volume of 0.735 mL g−1 for the recombinant TorD protein was calculated from the amino acid composition. The solvent density was set to 1.0.
Small-angle X-ray scattering (SAXS)
These experiments were performed on monomeric and dimeric TorD. The conditions of monodispersity are given in the legend of Figures 6 and 7 ▶ ▶. X-ray scattering data were collected on the small angle instrument D24 (Depautex et al. 1987) using synchrotron radiation from a bending magnet of the DCI storage ring at LURE (Orsay, France). The data acquisition system has been described (Boulin et al. 1986). The wavelength of the X-rays was 1.488 Å (Ni K-absorption edge), and the sample to detector distance was set to 1639.5 mm. Scattered intensities were recorded in the angular range 0.0155 Å−1 < Q < 0.2000 Å−1, where Q is the scattering vector (4πsinθ/λ), and 2θ and λ are the scattering angle and wavelength of the X-rays, respectively. All experiments were performed at 4°C by using a temperature-controlled cell (Dubuisson et al. 1997). Eight successive frames of 200 sec were collected for each sample. The scattering intensity of a reference sample of carbon black, recorded immediately before and after each experiment, was used to normalize all data to the transmitted intensity. The scattering contribution of the buffer was substracted before further analysis.
The radius of gyration (Rg) was estimated by the Guinier approximation (Guinier 1939): I(Q) =I(0) exp(−Rg2Q2/3), where I(Q) is the scattered intensity and I(0) the extrapolated scattered intensity at zero scattering angle (Guinier and Fournet 1955). For monodisperse solutions, the Guinier plot of lnI(Q) versus Q2 approximates a straight line with a slope proportional to Rg in the small-angle region. After normalization to protein concentration (c), the value of I(0)/c is proportional to the molecular weight of the scattering species. To calibrate the I(0) value, we measured the scattered intensities of a monodisperse solution of lyzozyme (Mw = 14,300 Daltons) in 50 mM sodium acetate pH 4.6, 150 mM NaCl and 5 mM DTT, using identical experimental settings.
The distance distribution function, P(r), shows the frequency of vector r, relating any two volume elements within the entire volume of the scattering particle. It was calculated using the indirect Fourier transform method (Glatter 1977) implemented in the GNOM program (Svergun 1992, 1993), and provided the maximum particle dimension, Dmax. Calculation of I(0) and of Rg values were obtained from the zeroth and the second moment of the P(r) function, respectively, according to the equations: P(r) = and
 |
and
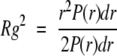 |
Crystallization and data collection
Crystallization of the dimeric protein was achieved using the hanging-drop vapor-diffusion method at 4°C. One microliter of protein solution (1.2 mg/ml in 20 mM Tris-HCl pH 8.0, 220 mM NaCl, 10 mM DTT) was mixed with an equal volume of the reservoir solution (1.6 M ammonium sulfate in 100 mM MES pH 6.4). Crystals appeared in 4 to 6 days. A 2.42 Å native data set was collected at 100 K on the BM30 beamline at the European Synchrotron Radiation Facility (ESRF, Grenoble). Prior to flash cooling in a stream of gaseous nitrogen, the crystal was cryoprotected by soaking for a few seconds in a solution of the reservoir complemented with 15% (w/v) ethylene glycol. Data were collected on a Mar345 detector. Intensities were processed and scaled with DENZO and SCALEPACK from the HKL suite of software (Otwinowski 1991; Otwinowski and Minor 1997). Data collection statistics are given in Table 2. Subsequent calculations were performed using the CCP4 suite of Programs (CCP4 1994).
Acknowledgments
We thank the scientific staff of beam lines D24 at LURE (Orsay) and BM30 at the European Synchrotron Radiation Facility (ESRF, Grenoble) for excellent data collection facilities, Odile Burlet-Schiltz and Valérie Guillet (IPBS, Toulouse) for their valuable contributions in mass spectrometry and spectroscopy experiments, respectively. The financial support was provided by CNRS, the Université of Toulouse and the Université de la Méditerannée.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0202902.
References
- Adler, A.J., Greenfield, N.J., and Fasman, G.D. 1973. Circular dichroism and optical rotatory dispersion of proteins and polypeptides. Methods Enzymol. 27 675–735. [DOI] [PubMed] [Google Scholar]
- Barrett, E.L. and Kwan, H.S. 1985. Bacterial reduction of trimethylamine oxide. Annu. Rev. Microbiol. 39 131–149. [DOI] [PubMed] [Google Scholar]
- Bennett, M.J., Choe, S., and Eisenberg, D. 1994. Domain swapping: Entangling alliances between proteins. Proc. Natl. Acad. Sci. 91 3127–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berks, B.C. 1996. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 22 393–404. [DOI] [PubMed] [Google Scholar]
- Berks, B.C., Sargent, F., De Leeuw, E., Hinsley, A.P., Stanley, N.R., Jack, R.L., Buchanan, G., and Palmer, T. 2000a. A novel protein transport system involved in the biogenesis of bacterial electron transfer chains. Biochim. Biophys. Acta 1459 325–330. [DOI] [PubMed] [Google Scholar]
- Berks, B.C., Sargent, F., and Palmer, T. 2000b. The Tat protein export pathway. Mol. Microbiol. 35 260–274. [DOI] [PubMed] [Google Scholar]
- Birck, C., Vachette, P., Welch, M., Swaren, P., and Samama, J.P. 1996. Is the function of the cdc2 kinase subunit proteins tuned by their propensities to oligomerize? Conformational states in solution of the cdc2 kinase partners p13suc1 and p9cksphy. Biochemistry 35 5577–5585. [DOI] [PubMed] [Google Scholar]
- Boulin, C., Kempf, R., Koch, M.H.J., and McLaughlin, S.M. 1986. Data appraisal, evaluation and display for synchrotron radiation experiments: Hardware and software. Nucl. Instr. Methods A249 399–407. [Google Scholar]
- Bragg, P.D. and Hackett, N.R. 1983. Cytochromes of the trimethylamine N-oxide anaerobic respiratory pathway of Escherichia coli. Biochim. Biophys. Acta 725 168–177. [DOI] [PubMed] [Google Scholar]
- CCP4. 1994. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50 760–763. [DOI] [PubMed] [Google Scholar]
- Czjzek, M., Dos Santos, J.P., Pommier, J., Giordano, G., Méjean, V., and Haser, R. 1998. Crystal structure of oxidized trimethylamine N-oxide reductase from Shewanella massilia at 2.5 A resolution. J. Mol. Biol. 284 435–447. [DOI] [PubMed] [Google Scholar]
- Depautex, C., Desvignes, C., Leboucher, P., Lemonnier, M., Dagneaux, D., Benoit, J.P., and Vachette, P. 1987. LURE annual report 1985–1987. Documentation CEN Saclay.
- Dos Santos, J.P., Iobbi-Nivol, C., Couillault, C., Giordano, G., and Méjean, V. 1998. Molecular analysis of the trimethylamine N-oxide (TMAO) reductase respiratory system from a Shewanella species. J. Mol. Biol. 284 421–433. [DOI] [PubMed] [Google Scholar]
- Dubuisson, J.M., Decamps, T., and Vachette, P. 1997. Improved signal-to-background ratio in small-angle X-ray scattering experiments with synchrotron radiation using an evacuated cell for solutions. J. Appl. Crystallogr. 30 49–54. [Google Scholar]
- Fujisawa, T., Ueki, T., Inoko, Y., and Kataoka, M. 1987. X-ray scattering from a Troponin C solution and its interpretation with a dumbbell-shaped-molecule model. J. Appl. Crystallogr. 20 349–355. [Google Scholar]
- Glatter, O. 1977. A new method for the evaluation of small-angle scattering data. J. Appl. Crystallogr. 10 415–421. [Google Scholar]
- Gon, S., Giudici-Orticoni, M.T., Méjean, V., and Iobbi-Nivol, C. 2001. Electron transfer and binding of the c-type cytochrome TorC to the trimethylamine N-oxide reductase in Escherichia coli. J. Biol. Chem. 276 11545–11551. [DOI] [PubMed] [Google Scholar]
- Guinier, A. 1939. La diffraction des rayons X aux très petits angles; application á l'étude de phénomènes ultramicroscopiques. Ann. Phys. 12 161–237. [Google Scholar]
- Guinier, A. and Fournet, G. 1955. Small angle X-ray scattering. John Wiley & Sons Inc., New York.
- Hakansson, M., Svensson, A., Fast, J., and Linse, S. 2001. An extended hydrophobic core induces EF-hand swapping. Protein Sci. 10 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iobbi-Nivol, C., Pommier, J., Simala-Grant, J., Méjean, V., and Giordano, G. 1996. High substrate specificity and induction characteristics of trimethylamine-N-oxide reductase of Escherichia coli. Biochim. Biophys, Acta 1294 77–82. [DOI] [PubMed] [Google Scholar]
- Kataoka, M., Head, J.F., Seaton, B.A., and Engelman, D.M. 1989. Melittin binding causes a large calcium-dependent conformational change in calmodulin. Proc. Natl. Acad. Sci. 86 6944–6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka, M., Head, J.F., Vorherr, T., Krebs, J., and Carafoli, E. 1991. Small-angle X-ray scattering study of calmodulin bound to two peptides corresponding to parts of the calmodulin-binding domain of the plasma membrane Ca2+ pump. Biochemistry 30 6247–6251. [DOI] [PubMed] [Google Scholar]
- Kataoka, M., Nishii, I., Fujisawa, T., Ueki, T., Tokunaga, F., and Goto, Y. 1995. Structural characterization of the molten globule and native states of apomyoglobin by solution X-ray scattering. J. Mol. Biol. 249 215–228. [DOI] [PubMed] [Google Scholar]
- Matthews, B.W. 1968. Solvent content of protein crystals. J. Mol. Biol. 33 491–497. [DOI] [PubMed] [Google Scholar]
- Méjean, V., Iobbi-Nivol, C., Lepelletier, M., Giordano, G., Chippaux, M., and Pascal, M.C. 1994. TMAO anaerobic respiration in Escherichia coli: Involvement of the tor operon. Mol. Microbiol. 11 1169–1179. [DOI] [PubMed] [Google Scholar]
- Mouncey, N.J., Choudhary, M., and Kaplan, S. 1997. Characterization of genes encoding dimethyl sulfoxide reductase of Rhodobacter sphaeroides 2.4.1T: An essential metabolic gene function encoded on chromosome II. J. Bacteriol. 179 7617–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oresnik, I.J., Ladner, C.L., and Turner, R.J. 2001. Identification of a twin-arginine leader-binding protein. Mol. Microbiol. 40 323–331. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. 1993. Oscillation data reduction program. In Proceedings of CCP4 study weekend. (eds. L. Sawyer, N. Isaacs, and S. Bailey), pp. 56–62. SERC Daresbury Laboratory, Warrington, UK.
- Otwinowski, Z.O. and Minor, W. 1997. Processing of X-ray diffraction data collected in oscillation mode. In Methods in enzymology (eds. C.W. Carter and R.M. Sweet), pp. 307–326. Academic Press, New York. [DOI] [PubMed]
- Pommier, J., Méjean, V., Giordano, G., and Iobbi-Nivol, C. 1998. TorD, a cytoplasmic chaperone that interacts with the unfolded trimethylamine N-oxide reductase enzyme (TorA) in Escherichia coli. J. Biol. Chem. 273 16615–16620. [DOI] [PubMed] [Google Scholar]
- Ptitsyn, O.B. 1992. The molten globule state. In Protein folding (ed. T.E. Creighton), pp. 243–300. W.H. Freeman, New York.
- Richards, F.M. and Vithayathil, P.J. 1959. The preparation of subtilisin-modified ribonuclease and the separation of the peptide and protein components. J. Biol. Chem. 234 1459–1465. [PubMed] [Google Scholar]
- Rousseau, F., Schymkowitz, J.W., Wilkinson, H.R., and Itzhaki, L.S. 2001. Three-dimensional domain swapping in p13suc1 occurs in the unfolded state and is controlled by conserved proline residues. Proc. Natl. Acad. Sci. 98 5596–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini, C.L., Ize, B., Chanal, A., Muller, M., Giordano, G., and Wu, L.F. 1998. A novel sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J. 17 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlunegger, M.P., Bennett, M.J., and Eisenberg, D. 1997. Oligomer formation by 3D domain swapping: A model for protein assembly and misassembly. Adv. Protein Chem. 50 61–122. [DOI] [PubMed] [Google Scholar]
- Semisotnov, G.V., Rodionova, N.A., Razgulyaev, O.I., Uversky, V.N., Gripas, A.F., and Gilmanshin, R.I. 1991. Study of the "molten globule" intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers 31 119–128. [DOI] [PubMed] [Google Scholar]
- Shaw, A.L., Leimkuhler, S., Klipp, W., Hanson, G.R., and McEwan, A.G. 1999. Mutational analysis of the dimethylsulfoxide respiratory (dor) operon of Rhodobacter capsulatus. Microbiology 145 1409–1420. [DOI] [PubMed] [Google Scholar]
- Shi, L., Kataoka, M., and Fink, A.L. 1996. Conformational characterization of DnaK and its complexes by small-angle X-ray scattering. Biochemistry 35 3297–3308. [DOI] [PubMed] [Google Scholar]
- Svergun, D.I. 1992. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 25 495–503. [Google Scholar]
- Svergun, D.I. 1993. A direct indirect method of small-angle scattering data treatment. J. Appl. Crystallogr. 26 258–267. [Google Scholar]