Abstract
Procoagulant factor Va (FVa) is inactivated via limited proteolysis at three Arg residues in the A2 domain by the anticoagulant serine protease, activated protein C (APC). Cleavage by APC at Arg306 in FVa causes dissociation of the A2 domain from the heterotrimeric A1:A2:A3 structure and complete loss of procoagulant activity. To help distinguish inactivation mechanisms involving A2 domain dissociation from inactivation mechanisms involving unfavorable changes in factor Xa (FXa) affinity, we used our FVa homology model to engineer recombinant FVa mutants containing an interdomain disulfide bond (Cys609-Cys1691) between the A2 and A3 domains (A2-SS-A3 mutants) in addition to cleavage site mutations, Arg506Gln and Arg679Gln. SDS-PAGE analysis showed that the disulfide bond in A2-SS-A3 mutants prevented dissociation of the A2 domain. In the absence of A2 domain dissociation from the A1:A2:A3 trimer, APC cleavage at Arg306 alone caused a sevenfold decrease in affinity for FXa, whereas APC cleavages at Arg306, Arg506, and Arg679 caused a 70-fold decrease in affinity for FXa and a 10-fold decrease in the kcat of the prothrombinase complex for prothrombin without any effect on the apparent Km for prothrombin. Therefore, for FVa inactivation by APC, dissociation of the A2 domain may provide only a modest final step, whereas the critical events are the cleavages at Arg506 and Arg306, which effectively inactivate FVa before A2 dissociation can take place. Nonetheless, for FVa Leiden (Gln506-FVa) inactivation by APC, A2 domain dissociation may become mechanistically important, depending on the ambient FXa concentration.
Keywords: Factor Va, activated protein C, disulfide, engineered disulfide, coagulation
Blood coagulation proceeds through a complex series of enzymatic reactions that activate protease zymogens via limited proteolysis and culminate in the production of thrombin. Thrombin cleaves fibrinogen, generating fibrin, and activates platelets, thereby contributing to a hemostatic plug or a thrombus (Davie et al. 1991). In addition to its procoagulant activities, thrombin also initiates a physiologic anticoagulant feedback mechanism. When thrombin complexes with thrombomodulin and endothelial cell protein C receptor on the surface of endothelial cells, its specificity changes and it activates protein C, a primary anticoagulant and antiinflammatory protein.
Proper function of the activated protein C (APC) pathway is critical for avoiding thrombosis, as various deficiencies in the APC pathway are major risk factors for thrombosis, especially venous thromboembolism (Esmon 2000). Most notable of these deficiencies is the factor V mutation termed factor V Leiden (Arg506Gln), which is responsible for inherited resistance to APC and is the most common genetic risk factor for venous thrombosis, present in 20%–40% of Caucasian venous thrombosis patients (Dahlback 2000). Additional interest in APC followed the recent report that recombinant APC can reduce death in patients with severe sepsis by 20% (Bernard et al. 2001).
Coagulation factor V (FV) is a 330,000 MW protein composed of six domains of three types in the order A1-A2-B-A3-C1-C2 (Jenny et al. 1987). FV is cleaved by thrombin to remove most of the B domain and produce activated FV (FVa). FVa is composed of a heavy chain (A1-A2, residues 1–709) and a light chain (A3-C1-C2, residues 1546–2196) which form a noncovalent complex (Esmon 1979). The three A domains are thought to form a stable heterotrimer in both FV and FVa (Villoutreix and Dahlback 1998; Pellequer et al. 2000), whereas the C1 and C2 domains each assume a stable β-barrel structure (Villoutreix et al. 1998; Macedo-Ribeiro et al. 1999; Pellequer et al. 2000). FVa is the nonenzymatic cofactor for factor Xa (FXa) in the prothrombinase complex which converts prothrombin to thrombin in the presence of negatively charged phospholipids (Nesheim et al. 1979).
Inactivation of FVa is a complex process involving APC cleavages of FVa at Arg306, Arg506, and Arg679. Cleavage at Arg506 is faster than cleavage at Arg306, but it only partially inactivates FVa. Although it is a slower reaction, cleavage at Arg306 completely inactivates FVa (Kalafatis et al. 1994b; Heeb et al. 1995; Nicolaes et al. 1995). When FVa is cleaved at Arg306, the connection of the A2 domain to the A1 domain is severed and the A2 domain fragments dissociate from the remainder of the FVa molecule, whereas the A1 domain remains associated with the light chain (Mann et al. 1997). The Arg306 cleavage site is in a hydrophilic surface loop of FVa (Villoutreix and Dahlback 1998; Pellequer et al. 2000) such that Arg306 cleavage would not be expected to significantly alter domain interface contacts between the A2 domain and the A1 or A3 domains. This loss of covalent connection is presumably sufficient to cause dissociation of the A2 domain by itself without a large disruption in the tertiary structure of the molecule. This mechanism is proposed to be analogous to the spontaneous dissociation of the A2 domain of FVIIIa, which results in the inactivation of FVIIIa (Fay et al. 1991; Mann et al. 1997).
Although dissociation of the A2 domain correlates with full loss of FVa activity, it has not yet been possible to separate the effects of Arg306 cleavage per se from those of A2 dissociation. Fully inactive FVa loses the ability to bind to FXa (Guinto and Esmon 1984). It was suggested that regions near the Arg506 and Arg306 APC cleavage sites, namely residues 493–506 and 311–325, are involved in the binding of FXa to FVa and that cleavage at either of these sites may disrupt binding of FXa to FVa and thereby diminish FVa activity (Heeb et al. 1996; Kojima et al. 1998). Therefore, to distinguish between inactivation mechanisms involving A2 domain dissociation and inactivation mechanisms involving unfavorable changes in FXa affinity, we decided to engineer into recombinant FVa mutants a disulfide bond between the A2 and the A1 or A3 domains such that the covalent tether provided by the Arg306 surface loop would be alternatively provided by a disulfide bond buried at the domain interface in the A1:A2:A3 trimer. Because of this interdomain disulfide, dissociation of the A2 domain would not occur, permitting us to distinguish the effects of APC cleavages per se from the effects of A2 domain dissociation.
The introduction of engineered disulfide bonds into proteins has been used to stabilize small, well characterized proteins and covalently link multisubunit proteins (Villafranca et al. 1983; Perry and Wetzel 1984; Sauer et al. 1986; Wells and Powers 1986). Several computational methods can analyze known three-dimensional protein structures in order to choose sites for the creation of disulfide bonds by cysteine mutations, and such methods have been applied to proteins with the use of known X-ray crystallographic structures of proteins (Pabo and Suchanek 1986; Sauer et al. 1986; Sowdhamini et al. 1989). However, the crystal structure of FVa has not yet been determined. Nonetheless, we decided to engineer disulfide bonds into FVa by applying the algorithm of Sowdhamini et al. (1989) to our three-dimensional homology model of FVa, which contains over 1200 residues and five protein domains (Pellequer et al. 2000). This program identified potential disulfide bond pairs between the A2 domain and the A1 or A3 domains, and we chose two of these for site-directed mutagenesis. One pair involving Cys609 and Cys1691, designated the A2-SS-A3 mutant, was found to covalently link the A2 domain to the A3 domain in the FVa light chain. We then prepared and purified additional mutants of A2-SS-A3 and used them to characterize mechanisms for FVa inactivation by APC.
Results
The program MODIP (Sowdhamini et al. 1989) used here to identify sites for the introduction of disulfide bridges in our model of FVa provides grades (A, B, C) for each prediction, where grade A disulfide bridges satisfy the defined stereochemical criteria, and grade C disulfides satisfy fewer of the stereochemical constraints. For the FVa structure, no grade A sites were predicted at A1-A2 or A2-A3 interfaces, and a single grade B predicted site was dismissed after further computational graphics analysis. Among the proposed grade C disulfide sites, five potential disulfide sites were constructed into FVa for further evaluation: His609-Glu1691, His253-Asp469, Ala257-Met618, Leu283–618, and Leu238-Gln590. Among them, the site His609-Glu1691, which had the best geometry, with rss = 2.02Å (distance between two sulfur atoms) and χss = 80.9° (dihedral angle between Cβ-S-S-Cβ) and the lowest van der Waals gas phase energy, exhibited improved geometry after refinement, compared to the initial construction. The second best site was Leu238-Gln590, with rss = 2.03Å and χss = −111.6°. Inspection of these two sites in the model of the A domain trimer verified that formation of a disulfide would not significantly alter or disrupt the A domain interfaces with the A trimer. Thus, these two sites were chosen for initial attempts to create disulfide bonds using site-directed mutagenesis. However, preliminary results (see below) demonstrated that the Cys238/Cys590 mutant did not form a disulfide bond. Therefore, this protein was not studied further.
An important issue is whether or not a disulfide bond will maintain the A2 domain in a native-like conformation in complex with the remainder of FVa after cleavage at R306. Although some experimental structural data could help address this issue, it is not possible to do these experiments with the small quantities of recombinant FV that we were able to produce. However, we performed an additional structural analysis with the FVa model to address this question. We determined that the buried surface area of the A2 domain that is in contact with the A1 or A3 domains is 2575 Å2. Figure 1 ▶ shows where the Cys609-Cys1691 disulfide bond is located within this contact surface, and where the exposed Arg306 peptide loop, which maintains the A2 domain covalent attachment to the A1 domain, is located. The Cys609-Cys1691 disulfide bond is located near the solvent-exposed surface of the FVa molecule. From analysis of the model, it is clear that the Cys609-Cys1691 disulfide bond does not cause significant disruption of the A2 domain contact surface and that it is unlikely to introduce strain into the molecule. Similarly, the surface loop in which Arg306 is located is clearly distinct from the A2 domain contact surface and is very exposed to solvent. Therefore, the presence of the Arg306-Asn307 peptide bond is predicted to not alter the interactions of the A2 domain with the A1 and A3 domains apart from providing the necessary covalent link. This, by itself, is sufficient to retain the A2 domain in a native conformation, similarly to what we expect from the Cys609-Cys1691 disulfide bond.
Fig. 1.

Structural model of the A-domains trimer of FVa. Panels A and B show CPK space-filling models shown in two orientations with B rotated approximately 90 degrees relative to A. Panels C and D show ribbon schematic models in the same orientations as A and B, respectively. The A2 domain is highlighted in white. The A1 and A3 domains are gray; Cys609 and Cys1691 are black; Arg306 is white. The surface loop from residue 301 to residue 315, which contains Arg306, is in black. Panels A and B were created with WebLab Viewer Pro and panels C and D were made with Molscript (Kraulis 1991) and rendered with Raster3D (Merritt and Bacon 1997).
For eight recombinant FV species that were produced and purified, yields of pure FV ranged from 5 to 25 μg/L of conditioned media. Based on silver-stained SDS-PAGE, we estimated the purity of the mutants to range from 70% to 90%. Figure 2B ▶ shows representative results for S2183A-FV, A2-SS-A3-FV, and Q506-A2-SS-A3-FV. Each recombinant FV was assayed using prothrombinase activity assays, and the concentrations were determined using ELISA assays. Relative specific activity (activity/antigen) based on these assays was calculated for each FVa (Table 1). The specific activity of normal plasma-derived FVa was defined as 1.0. B-domain-deleted FVa and 2183A B-domain-deleted FVa had somewhat reduced specific activity (Table 1). In contrast, all of the disulfide-crosslinked FVa's had significantly higher specific activity, ranging from 1.6 for A2-SS-A3-FVa to 2.5 for Q506-A2-SS-A3-FVa. APC cleavage site mutants (Q506 and Q506/Q679) also had higher specific activities. This indicates that these engineered FVa's are very active and that our mutations did not cause a loss of activity.
Fig. 2.
Schematic of recombinant B domain-deleted FV molecules and SDS-PAGE analysis of recombinant proteins. (A) The top schematic is of the primary sequence of FVΔB (see Materials and Methods) with the locations of the different domains indicated. The second schematic shows activated FVΔB (FVa), a heterodimer of the N-terminal heavy chain and the C-terminal light chain associated in the presence of Ca2+ ions. Arrows indicate sites of cleavage in FVa by APC. The third schematic shows the fragments produced following cleavage of FVa (FVai) by APC and also shows the sites of the Cys mutations that did or did not result in disulfide bond formation. (B) Silver-stained gel of purified recombinant B-domain-deleted FV and plasma-derived FV. Lane 1, plasma-derived FV; lane 2, 2183A-FV; lane 3, A2-SS-A3-FV; lane 4, Q506-A2-SS-A3-FV. (C) Immunoblot analysis of thrombin-activated FVa with an anti-FV light chain monoclonal antibody. Lane 1, plasma-derived FV; lane 2, thrombin-activated plasma-derived FVa; lane 3, 2183A-FV; lane 4, thrombin-activated 2183A-FVa.
Table 1.
Specific activity of recombinant factor Va
| Relative specific activitya | |
| Wild-type FVΔBa | 0.7 |
| 2183A FVa | 0.6 |
| A2-SS-A3-FVa | 1.6 |
| Q506-A2-SS-A3-FVa | 2.5 |
| Q506-FVa | 2.4 |
| Q506/Q679-A2-SS-A3-FVa | 2.2 |
| Q506/Q679-FVa | 3.2 |
a Specific activity is defined as the functional activity of FVa divided by the antigen level of FVa. Functional activity (Units/mL) was determined by prothrombinase activity assay, and antigen level (μg/mL) was determined by ELISA. Plasma-derived FVa was the standard for each assay and was assigned the specific activity of 1.0. Relative specific activity of the mutants is relative to plasma-derived FVa.
The FVa light chain normally gives a doublet on SDS-PAGE due to heterogeneity created by incomplete glycosylation at Asn2181. Mutation of Ser2183 to Ala eliminates this glycosylation site (Nicolaes et al. 1999). Immunoblots confirmed that all of our recombinant FV molecules had an apparent molecular weight of 188 kD (e.g., see 2183A-FV in Fig. 2C ▶, lane 3), which is consistent with deletion of residues 812 to 1491. Immunoblots also revealed that plasma-derived FVa formed a light chain doublet (Fig. 2C ▶, lane 2), whereas the 2183A-FVa mutant, as predicted, had a single light chain band that comigrated with the smaller light chain of plasma-derived FVa (Fig. 2C ▶, lane 4).
To demonstrate the desired interdomain disulfide bonds in the mutant FV proteins containing two engineered cysteine residues, we immunoblotted FVa and APC-treated FVai (generated by APC cleavages where "i" indicates FVa inactivated by APC cleavages). Figure 2 ▶ shows schemes representing the primary sequences of FVΔB, FVa (formed upon thrombin activation), and FVai.
Immunoblots using a polyclonal anti-FV heavy chain antibody demonstrated that introduction of Cys238/Cys590 mutations into FV or Gln506-FV did not detectably link the A1 and A2 domains, even though these species had normal FVa activity. We therefore concluded that no disulfide bond was formed between these cysteines (data not shown).
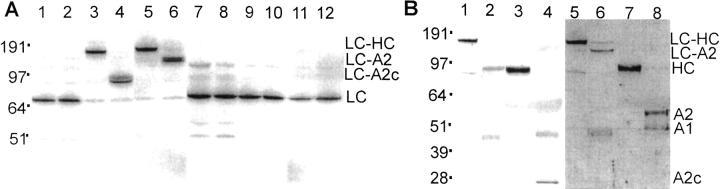
If the Cys609/Cys1691 FV mutations generate a new disulfide bond between the A2 and A3 domains as depicted in Figure 2 ▶, this bond would link the FVa heavy chain (A1-A2) and light chain (A3-C1-C2). In this case, immunoblots of FVa would show a disulfide-bonded species appearing at a molecular weight corresponding to the additive molecular weights of the heavy and light chains. After subsequent APC cleavages at Arg506, Arg306, and Arg679 that normally cause complete FVa inactivation, the light chain of FVai would remain crosslinked to the C-terminal fragment of the A2 domain (A2c, residues 507 to 679). Indeed, such was the case. In immunoblots developed with anti-FV light chain antibodies (Fig. 3A ▶), lanes 1 and 2 containing 2183A-FVa and 2183A-FVai both showed a normal light chain at the expected molecular weight (69 kD), comparable to the published value of 71 kD (Dahlback 1980). As shown in lanes 3 and 5 of the same figure, the mutants containing Cys609 and Cys1691 showed a predominant band predicted for crosslinked light chain and heavy chain (158 kD). Thus, FV mutants containing these two Cys residues are justifiably designated "A2-SS-A3". APC-treated A2-SS-A3-FVai (lane 4) showed a prominent band corresponding to the band predicted for the light chain crosslinked to the A2c fragment (92 kD). A fainter band slightly above this band correlated with a band predicted for heavy chain cleaved at Arg506 but not Arg679, resulting in the fragment 507 to 709 crosslinked to the light chain (101 kD). Lanes 5 and 6 of Figure 3A ▶ contained Q506-A2-SS-A3-FVa and Q506-A2-SS-A3-FVai. In this protein, Arg506 cleavage cannot take place, such that in Q506-A2-SS-A3-FVai (lane 6) the light chain remained crosslinked to the entire A2 domain. Indeed, the observed band in lane 6 corresponded to the light chain crosslinked to the A2 domain (130 kD). Lanes 7 through 12 of Figure 3A ▶ contained samples parallel to those of lanes 1 through 6, which were reduced using DTT. Lanes 7–12 show that, following reduction, the various higher molecular weight crosslinked species disappeared and normal light chain bands appeared, proving that the higher molecular weight light chain-containing species seen in lanes 3–6 (Fig. 3A ▶) were indeed the result of disulfide crosslinks between light and heavy chains.
Fig. 3.
Immunoblots of various FVa and FVai mutants. (A) Immunoblot developed with an anti-FV light chain monoclonal antibody. Samples in lanes 1–6 were not reduced, and those in lanes 7–12 were reduced. Lanes 1 and 7, 2183A-FVa; lanes 2 and 8, 2183A-FVai; lanes 3 and 9, A2-SS-A3-FVa; lanes 4 and 10, A2-SS-A3-FVai; lanes 5 and 11, Q506-A2-SS-A3-FVa,; lanes 6 and 12, Q506-A2-SS-A3-FVai. (B) Immunoblots developed with anti-FV heavy chain polyclonal antibodies. Lane 1, nonreduced A2-SS-A3-FVa; lane 2, nonreduced A2-SS-A3-FVai; lane 3, reduced A2-SS-A3-FVa; lane 4, reduced A2-SS-A3-FVai; lane 5, nonreduced Q506-A2-SS-A3-FVa; lane 6, nonreduced Q506-A2-SS-A3-FVai; lane 7, reduced Q506-A2-SS-A3-FVa; lane 8, reduced Q506-A2-SS-A3-FVai. Band positions for crosslinked and noncrosslinked fragments are indicated at the right side of each blot. LC, light chain; HC, heavy chain; A1, A1 domain; A2, A2 domain; A2c, C-terminal fragment of the A2 domain (residues 507–679). Molecular weight marker positions (kD, Novex SeeBlue standards) are indicated on the left side.
Additional proof for covalent crosslinks between FVa heavy and light chains in A2-SS-A3 mutants containing Cys609/Cys1691 came from immunoblot analyses using anti-FV heavy chain antibodies that showed the same new bands under nonreducing conditions. For example, as seen in Figure 3B ▶, such immunoblots of A2-SS-A3-FVa and A2-SS-A3-FVai, as well as Q506-A2-SS-A3-FVa and Q506-A2-SS-A3-FVai, under nonreducing conditions gave bands predicted to represent the same crosslinked species visualized in immunoblots developed using anti-FV light chain antibodies (Fig. 3A ▶). Lanes 1 and 5 (Fig. 3B ▶) both show bands corresponding to light chain crosslinked to heavy chain that comigrated with that seen in Figure 3A ▶, lane 3 (157 kD). Lane 2 in Figure 3B ▶ shows a band corresponding to the light chain crosslinked to the A2c fragment, comigrating with a band seen in lane 4 of Figure 3A ▶ (102 kD). Lane 6 in Figure 3B ▶ shows a band corresponding to the light chain crosslinked to the A2 domain, equivalent to a band seen in lane 6 of Figure 3A ▶ (132 kD). Finally, free A2-C terminus fragment (24 kD) and A2 (63 kD) fragment were not visible in the nonreduced lanes 2 and 6 of Figure 3B ▶, respectively, but were visible in the reduced lanes 4 and 8, indicating that these fragments were released from the disulfide-linked species upon reduction.
To monitor FVa inactivation time courses for recombinant FVa mutants exposed to APC, we measured residual FVa activity using prothrombinase assays with saturating amounts of FXa (1 nM). As seen in Figure 4A ▶, following APC treatment, 2183A-FVa, the control "wild-type" FVa, was reduced to about 2% activity after 60 min. Under the same conditions, A2-SS-A3-FVa was resistant to full inactivation by APC, but was reduced to approximately 15% activity after 90 min, where it seemed to plateau. The disulfide bond in this mutant crosslinks only the C-terminal fragment of the A2 domain (residues 507–679) to the light chain, such that the A2 domain N-terminal fragment (residues 307 to 506) was potentially free to dissociate. To avoid this possibility and to estimate the importance of Arg506 cleavage, we used various FV mutants containing the Arg506Gln mutation. Q506-FVa appeared somewhat resistant to APC inactivation, but it was reduced to <20% activity after 90 min, whereas the disulfide-linked protein Q506-A2-SS-A3-FVa was highly resistant to APC inactivation, losing only 30% activity after 90 min and essentially plateauing (Fig. 4A ▶). Interestingly, this mutant exhibited most of its activity loss in the first 20 min (Fig. 4A ▶). The mutant Q506/Q679-A2-SS-A3-FVa was even more resistant to APC inactivation and lost only about 15% of its activity at 20 min, whereupon its activity plateaued for up to 90 min. This is illustrated more clearly in Figure 4B ▶, where data for the top three lines of Figure 4A ▶ are expanded to highlight the difference between them. The small but reproducible difference in activity loss between Q506-A2-SS-A3-FVai and Q506/Q679-A2-SS-A3-FVai presumably resulted from cleavage at Arg679 in Q506-A2-SS-A3-FVai.
Fig. 4.

Time course of FVa mutant inactivation by APC. Various recombinant FVa mutants were incubated with APC, and aliquots taken at the indicated timepoints were assayed for remaining FVa activity and also saved for SDS-PAGE analysis. Inactivation reactions were performed with 2.5 nM APC and 4 nM FVa. FVa was assayed in the prothrombinase assay at 20 pM concentration with Xa at 1 nM. (A) 2183A-FVa (▵), A2-SS-A3-FVa (), Q506-FVa (○), Q506-A2-SS-A3-FVa (▪), Q506/Q679-A2-SS-A3-FVa (⋄), and control Q506-A2-SS-A3-FVa without APC (□). (B) Data from A expanded to allow comparison of APC-treated Q506-A2-SS-A3-FVa (▪) and Q506/Q679-A2-SS-A3-FVa (⋄) with control Q506-A2-SS-A3-FVa without APC (□). (C) Immunoblot of aliquots from the timecourse of APC inactivation of Q506-A2-SS-A3-FVa. Lanes are labeled with the time (min) of incubation with APC. Crosslinked and noncrosslinked fragments are indicated on the right side of the blot. LC, light chain; HC, heavy chain; A2, A2 domain. Molecular weight markers are indicated on the left (kD).
Immunoblot analysis of the inactivation reaction of Q506-A2-SS-A3-FVa (Fig. 4C ▶) showed that a majority of the Q506-A2-SS-A3-FVa molecules were cleaved at Arg306 within the first 10 or 20 min, with the result that crosslinked LC-A2 was the predominant observed band. This cleavage correlated well with the initial rapid loss of 20% of the activity. The data in Figure 4 ▶ indicate that FVa cannot be fully or extensively inactivated by cleavage at Arg306 alone, as long as the A2 domain remains attached to the whole FVa. This supports the hypothesis that the mechanism by which FVa is irreversibly inactivated is through dissociation of the A2 domain after cleavage by APC at Arg306.
Immunoblot analyses of Q506-A2-SS-A3-FVa and Q506/Q679-A2-SS-A3-FVa showed that there was a small amount of free light chain that was not crosslinked to heavy chain (Figs. 3, 4C ▶ ▶), indicating that disulfide crosslinking in the A2-SS-A3-FVa mutants was not 100% complete. Densitometry analysis of these nonreduced immunoblots showed that, on average, about 10% of the Q506-A2-SS-A3-FVa molecules lacked disulfide crosslinks, suggesting that at least part of the 30% loss of activity of Q506-A2-SS-A3-FVa caused by APC treatment (Fig. 4A,B ▶) was due to a small subpopulation of FVa mutant molecules lacking a disulfide bond. Similar results were seen on immunoblots of Q506/Q679-A2-SS-A3-FVa, which plateaued at 85% activity upon APC inactivation (data not shown). This suggests that the small subpopulation of FVa lacking a disulfide bond was responsible for virtually all of the 15% loss of activity in APC-treated Q506/Q679-A2-SS-A3-FVai, allowing us to conclude that cleavage at Arg306 alone causes no loss of activity provided that prothrombinase activity was measured under saturating FXa conditions (see below). In Figure 4 ▶, the functional activity of the various FVa species was measured with an excess of FXa (1 nM). Therefore, significant changes in affinity for FXa in the prothrombinase complex might not be apparent in these assays.
To further characterize the functional results of APC cleavages, we determined apparent affinity of various FVa and FVai species for FXa and their apparent kinetic constants for prothrombinase activity using titrations of FXa and prothrombin. To determine whether APC cleavages in A2-SS-A3-FVa, Q506-A2-SS-A3-FVa, or Q506/Q679-A2-SS-A3-FVa altered affinity for FXa, we studied the effect of varying the FXa concentration (5 pM to 600 pM) on prothrombin activation with FVa or FVai species. These FXa titrations were fit to hyperbolic equations (Fig. 5 ▶). The FXa concentration at 50% of maximum prothrombinase activity (K1/2Xa) was calculated for each FVa species and was taken as an apparent dissociation constant for FXa with each FVa/FVai species (Table 2). The K1/2Xa of control 2183A-FVa was 3.5 pM. The K1/2Xa values for the three A2-SS-A3-FVa mutants containing an engineered disulfide ranged from 6.1 to 9.5 pM, not significantly different from one another.
Fig. 5.
Effects of FXa concentration on cofactor activity of intact FVa and APC-cleaved FVai mutants. 2183A-FVa (▵), A2-SS-A3-FVai (•), Q506-A2-SS-A3-FVa (□) and -FVai (▪) and Q506/Q679-A2-SS-A3-FVa (⋄) and -FVai (♦) were assayed for cofactor activity in a prothrombinase assay with variations in FXa. FVa or FVai was at 2 pM except for A2-SS-A3-FVai, which was at 100 pM. FXa was varied from 5 to 600 pM, except with A2-SS-A3-FVai, when it was varied from 300 to 4000 pM. Data are the average of two or more experiments, and the K1/2Xa was derived by best fit to a hyperbolic equation.
Table 2.
Binding and kinetic constants for prothrombinase complex
| Factor Xa | Prothrombin | ||
| K1/2Xa,pMa | Km,app, μMb | kcat,app, sec−1 | |
| 2183A FVa | 3.5 ± 1.4 | 0.11 ± .03 | 41 ± 2 |
| 2183A FVai | NDc | ND | ND |
| A2-SS-A3-FVa | 8.0 ± 1.9 | 0.15 ± .01 | 38 ± 3 |
| A2-SS-A3-FVai | 549 ± 93 | 0.14 ± .01 | 4.1 ± 0.9 |
| Q506-A2-SS-A3-FVa | 6.1 ± 1.5 | 0.11 ± .01 | 42 ± 1 |
| Q506-A2-SS-A3-FVai | 47 ± 10 | 0.14 ± .01 | 31 ± 1 |
| Q506/Q679-A2-SS-A3-FVa | 9.5 ± 2.8 | 0.12 ± .03 | 43 ± 5 |
| Q506/Q679-A2-SS-A3-FVai | 53 ± 12 | 0.12 ± .03 | 39 ± 3 |
a K1/2Xa values (± S.E.) are averages of values calculated by fit to a standard hyperbolic curve of original data sets.
b Km,app and kcat,app values (± S.E.) were similarly obtained.
c ND = not detectable (≤2%).
Although these values for recombinant FVa's are considerably smaller than various published values for the K1/2Xa of plasma-derived FVa for FXa (Rosing et al. 1993; Nicolaes et al. 1995; Mann et al. 1997), prothrombinase complex formation and activity is highly dependent not only on buffer composition but also on the composition and concentration of the phospholipid vesicles used (Rosing et al. 1980, 1993). In addition, we used purified recombinant 2183A-FVa rather than the plasma-derived FVa used in other studies. Therefore, direct comparisons of these data to previously published results cannot be made. However, in control assays with plasma-derived FVa, we obtained values comparable to published K1/2Xa values (25 pM versus 45 or 88 pM; Rosing et al. 1993; Nicolaes et al. 1995).
Following APC treatment of control 2183A-FVa, no FVa activity (≤2%) at up to 600 pM FXa was observed. A2-SS-A3-FVai retained measurable FVa activity but the K1/2Xa for this protein was 540 pM. This is an apparent loss of affinity for FXa of about 70-fold relative to A2-SS-A3-FVa, which is apparently due to cleavages at both Arg506 and Arg306. Q506-A2-SS-A3-FVai and Q506/Q679-A2-SS-A3-FVai had K1/2Xa values of 47 pM and 53 pM, respectively. This sevenfold loss of affinity for FXa is most likely the result of cleavage at Arg306, because it is apparently the same for both proteins.
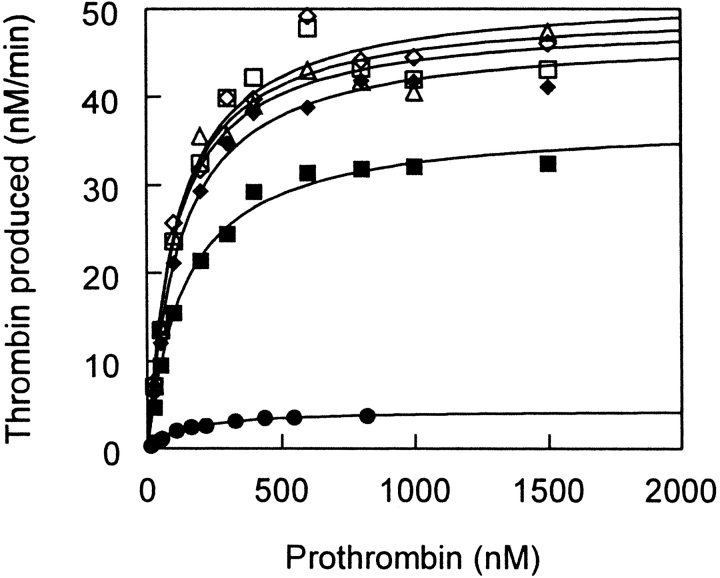
We then determined apparent kinetic constants for prothrombin activation under steady-state conditions for the various FVa and FVai species. These values cannot be considered true steady-state kinetic constants because prothrombin activation can involve cleavage at both Arg273 and Arg323 in prothrombin, both of which may contribute separately to the apparent kinetic constants (Krishnaswamy et al. 1987). Therefore, although detailed mechanistic conclusions cannot be drawn from these kinetic parameters, relative changes in them are informative (Table 2). To define the concentration of active prothrombinase complex (FVa:FXa:phospholipid), the enzyme (FXa) was present in limiting concentration (20 pM) whereas FVa or FVai (1–5 nM) and phospholipid (25 μM) were in excess. Reactions were initiated by addition of prothrombin (25 to 1500 nM) and allowed to proceed for 2.5 min before thrombin production was measured. Figure 6 ▶ shows prothrombin titration curves fit to a hyperbolic equation for various FVa and FVai species, and Table 2 shows the kinetic constants derived from these curves. The prothrombinase complex formed with 2183A-FVa had an apparent Km for prothrombin of 0.11 μM and an apparent kcat of 41 sec−1. Conversion of 2183A-FVa to 2183A-FVai abolished all detectable activity. A2-SS-A3-FVa, Q506-A2-SS-A3-FVa, and Q506/Q679-A2-SS-A3-FVa had kinetic constants in the prothrombinase complex that were similar to that of 2183A-FVa (Table 2). These results, along with the normal K1/2Xa values for these proteins, confirm that these disulfide-linked proteins behaved like normal FVa in functional assays.
Fig. 6.
Determination of kinetic constants of the prothrombinase complex with FVa variants. Prothrombin was titrated into the prothrombinase complex, and Km and kcat were derived by best fit to a hyperbolic equation. Symbols are the same as in Fig. 4 ▶. FXa was at 20 pM, and FVa or FVai was 1 nM except for A2-SS-A3-FVai, which was 5 nM. Prothrombin was varied from 25 to 1500 nM. Data are the average of two or more experiments.
APC cleavage of Q506/Q679-A2-SS-A3-FVa produced no significant change in the kinetic constants for prothrombin activation. APC cleavage of Q506-A2-SS-A3-FVa caused a slight change in kcat,app (42 to 31 sec−1). This change in kcat,app is inferred to be the result of cleavage only at Arg679, because it was not observed for Q506/Q679-A2-SS-A3-FVai and it could explain the small difference in the APC inactivation profiles of Q506-A2-SS-A3-FVa and Q506/Q679-A2-SS-A3-FVa (Fig. 4B ▶). APC cleavages of A2-SS-A3-FVa had a large effect on kcat,app, which decreased from 38 sec−1 to 4.1 sec−1 (Table 2, Fig. 6 ▶). This change is likely due to cleavage at Arg506 because it was not observed in the two mutants containing Gln506.
Discussion
Although disulfide bonds have been engineered into proteins based on X-ray crystallographic structures (Sauer et al. 1986; Wells and Powers 1986), it is unusual to use computational methods for identifying potential disulfide bonds based on a homology model protein structure of the magnitude and complexity of the heterotrimer of A domains of FVa (Pellequer et al. 2000). Additionally, FVa normally contains 18 cysteine residues, which form at least seven disulfide bonds. Western blot analyses showed that the introduction of Cys609 and Cys1691 generated a new disulfide linking the A2 domain to the FVa light chain in >85% of the molecules. This result would seem to validate the model. Activity assays showed that these proteins, containing a novel disulfide, were functionally normal and that they retained significant activity after 100% cleavage by APC compared with control FVa. From a structural point of view, it seems highly unlikely that any FVa that was incorrectly crosslinked would be active at all or that it would retain activity after APC cleavages. Kinetic studies to determine FXa affinity (Fig. 5 ▶) and kinetic constants for the prothrombinase complex (Fig. 6 ▶) also support the assumption that the disulfide-linked FVa species were in a native-like and functional conformation, because every disulfide-linked FVa had typical FXa affinity and normal prothrombinase kinetic constants (Table 2). Thus, we believe it is reasonable to assume that the theoretically designed disulfide linking Cys609 to Cys1691 was formed in each mutant at the A2-A3 interface without any major alteration in FVa activity.
An underlying assumption of this study is that the disulfide-crosslinked FVa will retain native-like structure and that the A2 domain will remain associated in the heterotrimer of the A domains, even after cleavages by APC. The small quantities of recombinant proteins that we obtained make it impractical to prove this by any biophysical technique. However, we believe that this is a reasonable assumption for several reasons. Computational analysis of the FVa model containing the Cys609-Cys1691 disulfide bond showed that the contact surface between the A2 domain and the remainder of the FVa molecule is extensive (2575 Å2) and that the disulfide bond is near the surface and does not disrupt the structure of the protein or the contact surface (Fig. 1 ▶). Introduction of the interdomain disulfide did not reduce FVa activity (Tables 1, 2). This covalent connection is similar to the covalent connection of the surface loop containing Arg306, in that it is exposed and does not have much effect on the contact surface between the A2 domain and the remainder of the FVa molecule. Therefore, given that the exposed connection of the Arg306 surface loop is sufficient to retain the A2 domain in a native conformation, it seems reasonable that the disulfide bond would have the same effect.
When the Arg306-containing sequence that covalently links the A2 domain to the A1 domain in FVa is severed by proteolysis, binding of the A2 domain can be described thermodynamically in terms of a bimolecular association between the A2 domain and the remainder of the FVa molecule, because the A1 domain remains firmly associated with the light chain (A3-C1-C2) (Mann et al. 1997). Furthermore, the two fragments of the A2 domain generated by APC cleavage at Arg506 remain associated with one another, and if FVa is cleaved only at Arg506, the C-terminal A2 domain fragment does not dissociate significantly from the remainder of the FVa molecule (Mann et al. 1997), suggesting that the A2 domain retains a native-like tertiary structure after Arg506 cleavage. Hockin et al. (1999) proposed a lower limit for the dissociation constant of the A2 domain from FVa of 10 μM, clearly a modest affinity. However, the presence of the covalent bond in the A2-SS-A3-FVai mutants changes this A2 domain interaction from an intermolecular interaction to an intramolecular interaction. Therefore, a bimolecular dissociation constant no longer applies to A2-SS-A3-FVai. Instead, A2 domain dissociation can be discussed only in terms of an effective concentration, which is the practical concentration of one component within one molecule relative to another component within the same molecule. The effective concentration that would define the association of the A2 domain with the remainder of the FVa molecule would be expected to be orders of magnitude greater than the dissociation constant for the bimolecular interaction (Creighton 1984). In fact, the covalent association of the A2 domain to the A1 domain in FVa, as provided by the covalent linkage of the Arg306-Asn307 peptide bond, is governed by similar intramolecular interactions and thermodynamic considerations. In this latter case, covalent attachment of the A2 domain is clearly sufficient to maintain the native quaternary structure of FVa, suggesting that our engineered interdomain disulfide link would also maintain the native quaternary structure of FVai.
The highly homologous protein, FVIIIa, provides supporting evidence of the structural integrity of the isolated FVai A2 domain. FVIIIa is cleaved between the A1 and A2 domains by thrombin during its activation. This protein is active following cleavage, but it has poor stability because the A2 domain can subsequently dissociate, resulting in inactive FVIIIa. However, it is possible to purify the dissociated A2 domain in a stable form (Fay et al. 1991), and the A2 domain even retains very low functional activity (Fay and Koshibu 1998). Therefore, the A2 domain of FVIIIa forms a stable tertiary structure even when it is fully dissociated from the remainder of the FVIIIa molecule. Given all this, it is reasonable to assume that, following APC cleavage, the disulfide-crosslinked polypeptides of the FVa A2 domain remain bound to the remainder of the FVa molecule in a native-like conformation.
Our results demonstrate that cleavage at Arg306 in FVa causes a sevenfold decrease in its affinity for FXa and that Arg306 cleavage alone has no affect on the catalytic efficiency (kcat/Km) of the prothrombinase complex towards the substrate prothrombin. We also demonstrated that complete APC cleavage of A2-SS-A3-FVa causes a 70-fold decrease in affinity for FXa and a 10-fold reduction in the kcat of the prothrombinase complex for prothrombin. Our results, combined with previous results demonstrating that APC cleavage at Arg506 without Arg306 cleavage decreases affinity of FVa for FXa (Nicolaes et al. 1995; Mann et al. 1997), demonstrate that each cleavage at either Arg306 or Arg506 causes a significant decrease in the affinity of FVa for FXa, consistent with our previous hypotheses that these cleavages decrease the ability of FVa to bind FXa (Heeb et al. 1996; Kojima et al. 1998).
Previous work established that cleavage at Arg306 readily results in dissociation of the A2 domain from the remainder of the FVai molecule (Mann et al. 1997; Hockin et al. 1999). The A2 domain fragments dissociate with a half-life of 35 sec and a dissociation constant that was not measurable but was estimated to be >10 μM (Hockin et al. 1999). Some residual FVa activity remained after complete cleavage but before dissociation was complete, when measured at saturating FXa levels. The inability to separate the effects of cleavage from the effects of dissociation of A2 domain fragments did not allow separation of the relative activity loss associated with Arg306 cleavage from the relative activity loss associated with dissociation of the fragments of the A2 domain (Mann et al. 1997). Our present results demonstrate that there are significant decreases in FXa affinity upon cleavage at both Arg306 and Arg506 without A2 domain dissociation. This 35-sec half-life of A2 domain bound to FVai (Hockin et al. 1999) may allow significant amounts of cleaved, yet intact, FVai to accumulate during coagulation, and local physiologic influences (e.g., cell receptors for FVa) may prolong this half-life. When coagulation is initiated, FXa levels in circulation are less than 100 pM (Bauer et al. 1989). Therefore, it seems likely that upon cleavage of FVa and before dissociation of the A2 domain can take place, this protein is already effectively inactive because it no longer has sufficient affinity to complex with FXa in the prothrombinase complex under physiologic conditions. Furthermore, whatever small fraction of FXa is in the prothrombinase complex has a 10-fold reduced kcat for its substrate prothrombin. Therefore, although dissociation of the A2 domain is the final and essentially irreversible event in FVa inactivation by APC, the critical inactivation steps are the cleavages at Arg506 and Arg306, which may result in minimally active FVa even before A2 dissociation can take place. The various mechanistic steps for FVa inactivation by APC are summarized in Figure 7 ▶.
Fig. 7.
Model of the mechanism of inactivation of FVa by APC. The schematic representation of the windmill-like structure of factor Va is based on a three-dimensional molecular model of factor Va (Pellequer et al. 2000). APC cleaves FVa at three Arg residues in the heavy chain: Arg306, Arg506, and Arg679. In the model depicted, cleavages at Arg506 and Arg306 are random but cleavage at Arg506 is faster (indicated by the heavier arrow), and therefore cleavage at Arg506 predominantly takes place first. Kd reflects affinity of phospholipid-bound FVa for FXa, and kcat is the value for the prothrombinase complex (FXa:FVa:phospholipid). Cleavage at Arg506 results in a 40-fold loss of affinity for FXa. Cleavage at Arg306 also results in a loss of affinity for FXa. When both cleavages have taken place, the affinity for FXa is reduced by about 100-fold and the kcat for prothrombin is reduced by about 10-fold. Cleavage of Arg679 is likely not significant because it occurs much more slowly than the other cleavages, and it is thus not presented in this scheme. This model is compiled from published work (Kalafatis et al. 1994a; Nicolaes et al. 1995; Mann et al. 1997; Hockin et al. 1999) and the data presented in this paper.
Given our results with highly homologous FVa, we expect that engineering a similar disulfide bond in FVIIIa to link the A2 and A3 domains would stabilize FVIIIa by eliminating spontaneous A2 domain dissociation. Analysis of FVIIIa using MODIP suggested that the FVIII residues 664 and 1826, homologous to FV residues 609 and 1691, are very favorable for this purpose (A. Gale, J.-L. Pellequer, J. Griffin, unpubl.).
In summary, our results with human recombinant FVa mutants suggest that, independent of dissociation of the A2 domain, APC cleavages at Arg506 and Arg306 intrinsically cause substantial loss of FVa activity due to reductions in FXa affinity and, for Arg506 cleavage, a reduction in kcat of the prothrombinase complex for its substrate, prothrombin. We also demonstrate that dissociation of the A2 domain following proteolysis at Arg306 in normal FVa by APC provides only a modest final step in the mechanism for the complete inactivation of FVa by APC. However, if cleavage at Arg506 cannot readily occur, as in FVa Leiden (Gln506-FVa), then A2 domain dissociation may become more mechanistically important, depending on the ambient FXa concentration.
Materials and methods
Purchased materials included: chromogenic substrate CBS 34–47 from American Bioproducts; APC, FXa, and prothrombin from Enzyme Research Laboratories; monoclonal anti-FV light chain antibodies (AHV-5112, AHV-5101) from Haemotologic Technologies; biotinylated donkey-antirabbit-IgG, biotinylated goat-antimouse IgG, streptavidin alkaline phosphatase conjugate, and 1-Step NBT/BCIP from Pierce; polyvinylidene fluoride membrane from Millipore; and Superfect transfection reagent from QIAGEN. Phospholipid vesicles (80% phosphatidylcholine, 20% phosphatidylserine) were prepared as described (Mesters et al. 1991).
Recombinant factor V mutants
The program MODIP (Sowdhamini et al. 1989) was used to identify sites for introduction of disulfide bridges in our molecular model of FVa (Pellequer et al. 2000). Proposed disulfide sites were constructed into FVa using Xfit (McRee 1992) and refined in X-PLOR (Brunger 1992) using the Charmm22 all atom force field. Full-length FV cDNA [a gift from R. Kaufman (Heeb et al. 1999)] in the plasmid pED-FV was removed by digestion with SalI and inserted into a modified pUC119 plasmid. A fragment of the FV cDNA was then created with PCR using a 5` primer that created a BamHI site at nt 4641 (FV cDNA numbering) and a 3` primer that retained the BamHI site at nt 6014, whereas removing the BamHI site at nt 5975. pUC119-FV was digested with BamHI (cutting at nt 2601, 5975, and 6014 in FV cDNA numbering). The new PCR fragment was inserted between the BamHI sites in pUC119-FV between nt 2601 and 6014. These steps resulted in the removal of nt 2602 to 4641 (coding sequence for residues 812 to 1491), creating a construct encoding a B-domainless FV designated FVΔB. This FVΔB gene construct was inserted into the expression vector pcDNA3.1+ from Invitrogen. Then, using the Stratagene Quikchange PCR mutagenesis kit and FVΔB, we mutated Ser2183 to Ala (changing codon AGT to GCC) to prevent glycosylation at Asn2181, yielding the mutant 2183A-FVΔB. This mutation was made to avoid FV heterogeneity due to incomplete glycosylation at Asn2181, which gives two species of FV (FV1 and FV2) that differ in certain functional properties (Rosing et al. 1993; Hoekema et al. 1997). All subsequent mutations in this study were made using this B-domainless, Ser2183A mutant; therefore, mutants based on 2183A-FVΔB are usually denoted as FV mutants, with only additional mutations at Arg cleavages sites or at sites to introduce Cys explicitly noted.
The double mutants that inserted two cysteine residues for creation of a disulfide bond were made with the Quikchange mutagenesis kit by simultaneous addition of four mutagenic primers. The following two pairs were made: Leu238Cys: Gln590Cys (Cys238/Cys590), and His609Cys: Glu1691Cys (A2-SS-A3). Variants were also made with additional mutations of Arg506 and Arg679 to Gln (Q506, Q506/Cys238/Cys590, Q506-A2-SS-A3, and Q506/Q679-A2-SS-A3).
Plasmids containing each mutant were purified with the Qiafilter plasmid midiprep kit from QIAGEN, linearized, and transfected into COS-1 cells using Superfect transfection reagent according to the manufacturer's instructions. Stable clones were selected using 0.8 mg/mL Geneticin (Gibco BRL). Serum-free conditioned media containing 0.05% BSA and 5 mM CaCl2 was collected from COS-1 cells expressing each FV mutant and was precipitated with 16% PEG 6000. The pellet was then redissolved in HBS (50 mM HEPES, 150 mM NaCl, pH 7.4) containing 5 mM CaCl2, 2 mM benzamidine, 5 nM PPACK, and 1 mM PMSF, dialyzed versus the same buffer, and purified using an anti-FV antibody column (Heeb et al. 1999). Fractions containing FV were collected, concentrated, and stored in HBS with 0.1% BSA at −80°C. FVa was quantitated by activity and by ELISA assay after activation by thrombin. The ELISA assays used Nunc Maxisorb plates coated with 10 μg/mL sheep-anti-FV from Affinity Biological and blocked with Superblock from Pierce with antigen detection by mouse anti-FV-light-chain monoclonal antibody (V59).
Functional assays
In the functional assays, the concentration of FV used was a functional concentration determined from activity, assuming that 1 U/mL of FV activity is equivalent to 30 nM FV. FV (40 nM) was activated with thrombin (0.5 nM) in HBS with 0.1% BSA and 5 mM CaCl2 at 37°C for 10 min, and activation was stopped by the addition of 1.1 molar equivalent of hirudin. FVa inactivation assays were performed using FVa at 4 nM and APC at 2.5 nM with 25 μM phospholipid vesicles, with the determination of residual FVa using prothrombinase assays as described (Gale et al. 2000). Briefly, 1-μL aliquots were removed at specified timepoints and added to 40 μL of factor Xa with phospholipid vesicles, followed by 10 μL of prothrombin (final concentrations: 1 nM FXa, 20 pM FVa, 25 μM phospholipid vesicles, and 0.6 μM prothrombin). After 2.5 min, a 15-μL aliquot of this mixture was quenched by addition to 55 μL HBS containing 10 mM EDTA, 0.5% BSA, pH 8.2. Chromogenic substrate CBS 34–47 was added, and the rate of thrombin formation was assessed by measuring the change in absorbance at 405 nm. For some studies, FXa or prothrombin was varied as described in the relevant figure legend.
SDS-PAGE was done with Novex 4%–12% Bis-Tris gradient gels with MOPS buffer (Invitrogen). The proteins were then transferred to Millipore PVDF membranes, and immunoblots were developed with monoclonal anti-FV-light chain antibodies, AHV-5112 or V59, or rabbit polyclonal anti-FV-heavy chain antibodies (Heeb et al. 1999).
Acknowledgments
This study was supported in part by NIH grants HL52246, HL21544, HL07695 and a fellowship from the Leukemia and Lymphoma Society (A.J.G.). We thank A. Tsavaler for technical assistance, Dr. R. Kaufman for the plasmid pED-FV, Drs. J.A. Fernandez and M.J. Heeb for mAb V59, and Dr. P. Schimmel for helpful discussion.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0210002.
References
- Bauer, K.A., Kass, B.L., ten Cate, H., Bednarek, M.A., Hawiger, J.J., and Rosenberg, R.D. 1989. Detection of factor X activation in humans. Blood 742007–2015. [PubMed] [Google Scholar]
- Bernard, G.R., Vincent, J.L., Laterre, P.F., LaRosa, S.P., Dhainaut, J.F., Lopez-Rodriguez, A., Steingrub, J.S., Garber, G.E., Helterbrand, J.D., Ely, E.W., and Fisher, C.J., Jr. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344699–709. [DOI] [PubMed] [Google Scholar]
- Brunger, A.T. 1992. X-PLOR Manual. Version 3.0. Yale University, New Haven.
- Creighton, T.E. 1984. Proteins. W.H. Freeman and Company, New York, pp. 152–157.
- Dahlback, B. 1980. Human coagluation factor V purification and thrombin-catalyzed activation. J. Clin. Invest 66583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlback, B. 2000. Blood coagulation. Lancet 3551627–1632. [DOI] [PubMed] [Google Scholar]
- Davie, E.W., Fujikawa, K., and Kisiel, W. 1991. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry 3010363–10370. [DOI] [PubMed] [Google Scholar]
- Esmon, C.T. 1979. The subunit structure of thrombin-activated factor V: Isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J. Biol. Chem. 254964–973. [PubMed] [Google Scholar]
- Esmon, C.T. 2000. Regulation of blood coagulation. Biochim. Biophys. Acta 1477349–360. [DOI] [PubMed] [Google Scholar]
- Fay, P.J., Haidaris, P.J., and Smudzin, T.M. 1991. Human factor VIIIa subunit structure. Reconstruction of factor VIIIa from the isolated A1/A3-C1-C2 dimer and A2 subunit. J. Biol. Chem. 2668957–8962. [PubMed] [Google Scholar]
- Fay, P.J. and Koshibu, K. 1998. The A2 subunit of factor VIIIa modulates the active site of factor IXa. J. Biol. Chem. 27319049–19054. [DOI] [PubMed] [Google Scholar]
- Gale, A.J., Heeb, M.J., and Griffin, J.H. 2000. The autolysis loop of activated protein C interacts with factor Va and differentiates between the Arg506 and Arg306 cleavage sites. Blood 96585–593. [PubMed] [Google Scholar]
- Guinto, E.R., and Esmon, C.T. 1984. Loss of prothrombin and of factor Xa-factor Va interactions upon inactivation of factor Va by activated protein C. J. Biol. Chem. 25913986–13992. [PubMed] [Google Scholar]
- Heeb, M.J., Kojima, Y., Greengard, J., and Griffin, J.H. 1995. Activated protein C resistance: Molecular mechanisms based on studies using purified Gln506-factor V. Blood 853405–3411. [PubMed] [Google Scholar]
- Heeb, M.J., Kojima, Y., Hackeng, T.M., and Griffin, J.H. 1996. Binding sites for blood coagulation factor Xa and protein S involving residues 493–506 in factor Va. Protein Sci. 51883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeb, M.J., Rehemtulla, A., Moussalli, M., Kojima, Y., and Kaufman, R.J. 1999. Importance of individual activated protein C cleavage site regions in coagulation factor V for factor Va inactivation and for factor Xa activation. Eur. J. Biochem. 26064–75. [DOI] [PubMed] [Google Scholar]
- Hockin, M.F., Cawthern, K.M., Kalafatis, M., and Mann, K.G. 1999. A model describing the inactivation of factor Va by APC: Bond cleavage, fragment dissociation, and product inhibition. Biochemistry 386918–6934. [DOI] [PubMed] [Google Scholar]
- Hoekema, L., Nicolaes, G.A., Hemker, H.C., Tans, G., and Rosing, J. 1997. Human factor Va1 and factor Va2: Properties in the procoagulant and anticoagulant pathways. Biochemistry 363331–3335. [DOI] [PubMed] [Google Scholar]
- Jenny, R.J., Pittman, D.D., Toole, J.J., Kriz, R.W., Aldape, R.A., Hewick, R.M., Kaufman, R.J., and Mann, K.G. 1987. Complete cDNA and derived amino acid sequence of human factor V. Proc. Natl. Acad. Sci. 844846–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalafatis, M., Rand, M.D., and Mann, K.G. 1994a. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J. Biol. Chem. 26931869–31880. [PubMed] [Google Scholar]
- Kalafatis, M., Rand, M.D., and Mann, K.G. 1994b. The mechanism of inactivation of human factor V and human factor Va by activated protein C. J. Biol. Chem. 26931869–31880. [PubMed] [Google Scholar]
- Kojima, Y., Heeb, M.J., Gale, A.J., Hackeng, T.M., and Griffin, J.H. 1998. Binding sites for coagulation factor Xa involving residues 311–335 in factor Va. J. Biol. Chem. 27314900–14905. [DOI] [PubMed] [Google Scholar]
- Kraulis, P.J. 1991. MOLSCRIPT: A program to produce both detailed and schematic plots of protein structures. J. Appl. Cryst. 24946–950. [Google Scholar]
- Krishnaswamy, S., Church, W.R., Nesheim, M.E., and Mann, K.G. 1987. Activation of human prothrombin by human prothrombinase: Influence of factor Va on the reaction mechanism. J. Biol. Chem. 2623291–3299. [PubMed] [Google Scholar]
- Macedo-Ribeiro, S., Bode, W., Huber, R., Quinn-Allen, M.A., Kim, S.W., Ortel, T.L., Bourenkov, G.P., Bartunik, H.D., Stubbs, M.T., Kane, W.H., and Fuentes-Prior, P. 1999. Crystal structures of the membrane-binding C2 domain of human coagulation factor V. Nature 402434–439. [DOI] [PubMed] [Google Scholar]
- Mann, K.G., Hockin, M.F., Begin, K.J., and Kalafatis, M. 1997. Activated protein C cleavage of factor Va leads to dissociation of the A2 domain. J. Biol. Chem. 27220678–20683. [DOI] [PubMed] [Google Scholar]
- McRee, D.E. 1992. XtalView: A visual protein crystallographic software system for X11/XView. J. Mol. Graph. 1044–47. [Google Scholar]
- Merritt, E.A., and Bacon, D.J. 1997. Raster3D: Photorealistic molecular graphics. Methods Enzymol. 277505–524. [DOI] [PubMed] [Google Scholar]
- Mesters, R.M., Houghten, R.A., and Griffin, J.H. 1991. Identification of a sequence of human activated protein C (residues 390–404) essential for its anticoagulant activity. J. Biol. Chem. 26624514–24519. [PubMed] [Google Scholar]
- Nesheim, M.E., Taswell, J.B., and Mann, K.G. 1979. The contribution of bovine factor V and factor Va to the activity of prothrombinase. J. Biol. Chem. 25410952–10962. [PubMed] [Google Scholar]
- Nicolaes, G.A., Villoutreix, B.O., and Dahlback, B. 1999. Partial glycosylation of Asn2181 in human factor V as a cause of molecular and functional heterogeneity. Modulation of glycosylation efficiency by mutagenesis of the consensus sequence for N-linked glycosylation. Biochemistry 3813584–13591. [DOI] [PubMed] [Google Scholar]
- Nicolaes, G.A.F., Tans, G., Thomassen, M.C.L.G.D., Hemker, H.C., Pabringer, I., Varadi, K., Schwarz, H.P., and Rosing, J. 1995. Peptide bond cleavages and loss of functional activity during inactivation of factor Va and factor VaR506Q by activated protein C. J. Biol. Chem. 27021158–21166. [DOI] [PubMed] [Google Scholar]
- Pabo, C.O., and Suchanek, E.G. 1986. Computer-aided model-building strategies for protein design. Biochemistry 255987–5991. [DOI] [PubMed] [Google Scholar]
- Pellequer, J.L., Gale, A.J., Getzoff, E.D., and Griffin, J.H. 2000. Three-dimensional model of coagulation factor Va bound to activated protein C. Thromb. Haemost. 84849–857. [PubMed] [Google Scholar]
- Perry, L.J., and Wetzel, R. 1984. Disulfide bond engineered into T4 lysozyme: Stabilization of the protein toward thermal inactivation. Science 226555–557. [DOI] [PubMed] [Google Scholar]
- Rosing, J., Bakker, H., Thomassen, M.C.L.G.D., Thomassen, L., Hemker, H., and Tans, G. 1993. Characterization of two forms of human factor Va with different cofactor activities. J. Biol. Chem. 26821130–21136. [PubMed] [Google Scholar]
- Rosing, J., Tans, G., Govers-Riemslag, J.W.P., Zwaal, R.F.A., and Hemker, H.C. 1980. The role of phospholipids and factor Va in the prothrombinase complex. J. Biol. Chem. 255274–283. [PubMed] [Google Scholar]
- Sauer, R.T., Hehir, K., Stearman, R.S., Weiss, M.A., Jeitler-Nilsson, A., Suchanek, E.G., and Pabo, C.O. 1986. An engineered intersubunit disulfide enhances the stability and DNA binding of the N-terminal domain of lambda repressor. Biochemistry 255992–5998. [DOI] [PubMed] [Google Scholar]
- Sowdhamini, R., Srinivasan, N., Shoichet, B., Santi, D.V., Ramakrishnan, C., and Balaram, P. 1989. Stereochemical modeling of disulfide bridges. Criteria for introduction into proteins by site-directed mutagenesis. Protein Eng. 395–103. [DOI] [PubMed] [Google Scholar]
- Villafranca, J.E., Howell, E.E., Voet, D.H., Strobel, M.S., Ogden, R.C., Abelson, J.N., and Kraut, J. 1983. Directed mutagenesis of dihydrofolate reductase. Science 222782–788. [DOI] [PubMed] [Google Scholar]
- Villoutreix, B.O., Bucher, P., Hofmann, K., Baumgartner, S., and Dahlbäck, B. 1998. Molecular models for the two discoidin domains of human blood coagulation factor V. J. Mol. Model. 4268–275. [Google Scholar]
- Villoutreix, B.O., and Dahlback, B. 1998. Structural investigation of the A domains of human blood coagulation factor V by molecular modeling. Protein Sci. 71317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, J.A., and Powers, D.B. 1986. In vivo formation and stability of engineered disulfide bonds in subtilisin. J. Biol. Chem. 2616564–6570. [PubMed] [Google Scholar]