Abstract
Purpose
In human subjects, only a small percentage of oral pentosanpolysulfate (PPS) is found in the urine. Commercially available PPS is a heterogeneous mixture with varying molecular weights. Our hypothesis was that only the low molecular weight fraction reaches the urine.
Materials and Methods
Urine was obtained from IC patients chronically taking PPS. The amount and molecular size of PPS in the urine were determined by ELISA and molecular sieve chromatography. PPS was purified from Elmiron capsules and fractionated into low molecular weight (LMW) and high molecular weight (HMW) fractions. Urine recovery of PPS was measured in rabbits after oral or intravenous administration of unfractionated, LMW or HMW PPS.
Results
Median urine PPS level for 34 IC patients was 1.2 ug/ml (range, 0.5 to 27.7 ug/ml). All of the PPS recovered from IC urine was low molecular weight. After intravenous administration in rabbits, the median recoveries in urine were 47.2 % (range 19.7 to 73.2 %) for unfractionated PPS, 74.6% (range 31.4 to 96.3 %) for LMW and 3.3% (range 2.5 to 5.0 %) for HMW. After oral administration in rabbits, the median recoveries in urine were 7.4% (range 2.1 to 46.0 %) for LMW and 0.10 % (range 0.0 to 0.3%) for HMW.
Conclusions
In IC patients taking oral PPS, the PPS recovered in the urine is all of low molecular weight. In rabbits, the high molecular weight fraction of PPS is recovered in very small amounts from the urine after intravenous administration, and not at all after oral administration.
Keywords: cystitis, interstitial, urine; cystitis, interstitial, drug therapy; pentosan sulfuric polyester, pharmacokinetics; pentosan sulfuric polyester, urine; pentosan sulfuric, polyester, therapeutic use
INTRODUCTION
Pentosanpolysulfate (PPS) is the only oral medication approved by the U.S. Food and Drug Administration for interstitial cystitis (IC). Its mechanism in IC is unknown, but several theories are proposed.1–3 Many patients fail to improve, possibly due to low urine and bladder concentrations of PPS. Its large size and high negative charge (similar to heparin) imply low gastrointestinal absorption and glomerular filtration.
Few previous studies addressed oral PPS absorption. Two groups compared oral vs. parenteral PPS using coagulation tests in humans, and concluded that zero4 to 10%5 of an oral dose reached the circulation. Another paper described that 1–2% of an oral dose and about 10% of an intravenous dose was recovered in human urine.6 In rats given radiolabeled PPS orally or intravenously, autoradiograms showed that oral PPS remained mostly in the large intestine.7
PPS administered to patients (Elmiron) is a heterogeneous mixture of various molecular sizes. Our hypothesis was that only the low-molecular weight (LMW) fraction would be absorbed from the gastrointestinal tract and excreted into the urine. First we analyzed the urine of IC patients who were taking PPS, and found only LMW PPS. Further investigation was carried out in rabbits by oral and intravenous administration of unfractionated, LMW and high molecular weight (HMW) PPS.
MATERIALS AND METHODS
PPS
PPS was prepared by extracting the contents of Elmiron capsules (Ortho-McNeil Pharmaceuticals, Raritan, NJ) with water at 4 °C, centrifuging and lyophilizing the clear supernatant. The yield (>95 mg per capsule) was close to the expected 100 mg per capsule.
Preparation of Anti-PPS antibodies
Antibodies against PPS were generated by immunizing rabbits (immunizations and bleedings done by Lampire Biological Laboratories, Pipersville, PA) with a complex of PPS and methylated bovine serum albumin (BSA) (Sigma, St. Louis, MO), as previously described.8 The titer was followed by using PPS conjugated to keyhole limpet hemocyanin (Sigma). The high titer antibody was purified by chromatography on a column of Avid AL according to the supplier (UNISYN Technologies, San Diego, CA).
Enzyme-Linked Immunosorbent Assay (ELISA) for PPS
Due to the low amounts of PPS in urine, specimens had to be concentrated before the assay. However, the high (glyco)protein content of concentrated urine caused interference in the assay. Therefore, before ELISA urine was treated as follows to eliminate the interfering material. Urine (10 mL) was lyophilized, redissolved in 1 mL of 50mM Tris HCl buffer, pH 8.0 containing 10 mM calcium chloride and incubated with Pronase (two additions of 50 ug at 0 and 24 h) at 37 °C for 48 h to degrade the (glyco)proteins. The Pronase was heat inactivated (5 min, 100 °C) and the degradation products removed by chromatography on desalting columns using water as the eluant. The fractions containing the PPS were lyophilized, redissolved in 0.5 mL of PBS and analyzed by competitive ELISA using the purified anti-PPS antibody.
For the ELISA, the coating of different microtiter plates directly with PPS was examined using tritium-labeled PPS, but very little PPS adhered to the plastic. Therefore, Immulon 2 (Dynatech) microtiter plates were first coated with poly-L-lysine (Sigma) (100 uL of 100ug/mL in 0.1 M sodium bicarbonate, pH 9.6). After washing with phosphate-buffered saline (PBS)-0.5 % Tween 20, the wells were coated by incubating with PPS (100 uL of a 10ug /mL PBS solution) and the non-specific sites blocked by treatment with BSA (1.0% BSA in PBS - 0.1 % Tween 20). About 77 % of the applied 3H-PPS bound to the poly-lysine coated plates. Aliquots (200uL) of urine, pre-treated as above, and PPS standards (six to eight dilutions in the range 2 to 80 ug/mL in PBS) were incubated for 18 h at 4 °C with 200 uL of the purified anti-PPS antibody at a dilution of 1:2000 in the blocking buffer. Triplicate 100 uL aliquots of each pre-incubated sample or standard were added to the PPS coated wells in the microtiter plates. After incubation, the anti-PPS antibody bound to the plate was estimated using goat anti-rabbit IgG conjugated to alkaline phosphatase followed by color development (absorbance at 405 nm) with p-nitrophenol phosphate as substrate. The concentration of the PPS in the urine samples was calculated from standard curve generated for each plate. The antibody was specific against PPS and interference from sulfated glycosaminoglycans in concentrated urine was low. For example, 100 ug of chondroitin sulfate, heparan sulfate, and heparin reacted in the assay as 0.13, 0.23 and 0.66 ug PPS, respectively. The antibody was used as such since absorption with heparin did not eliminate the interference, in agreement with observations of Callahan et al.8 With this protocol, the recovery of PPS added to urine was almost identical to that of PPS added to PBS (Figure 1).
Figure 1.
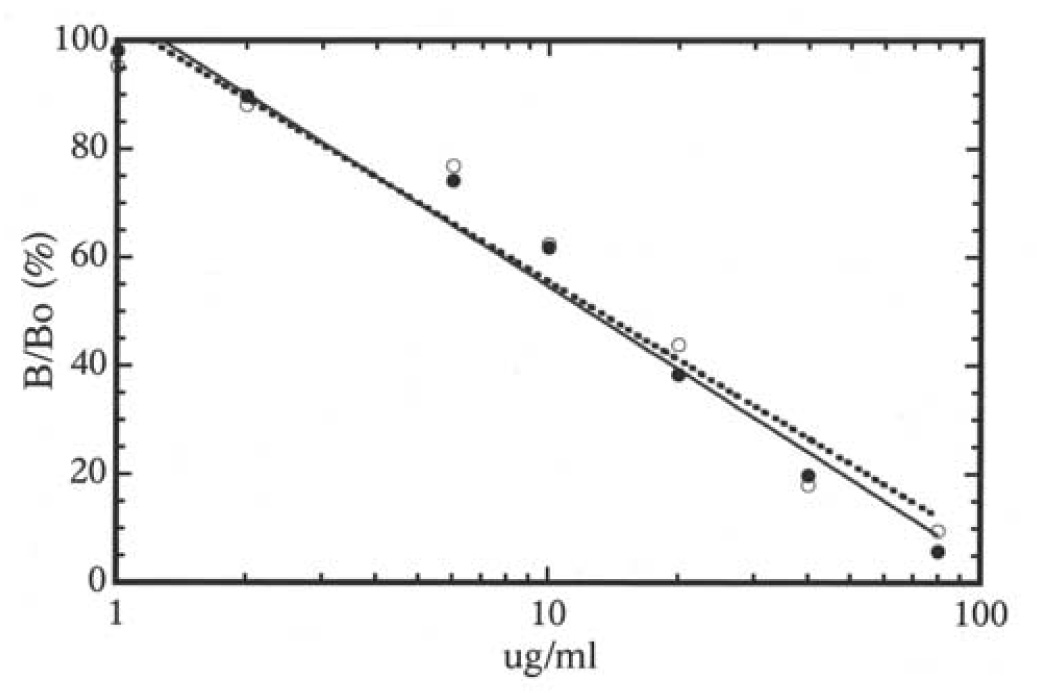
Estimation of PPS in phosphate buffer (closed circles, continuous line) or urine (open circles, broken line) by competitive ELISA using rabbit anti-PPS antibody. Data on the Y-axis is presented as B/Bo (%), where B and Bo are absorbance at 405 nm of PPS samples and zero blank, respectively.
Human subjects
Subjects gave informed consent and the research was approved by the Institutional Review Board at the Pennsylvania State University College of Medicine. Subjects were 34 female IC patients (ages 22–86 years) who had been taking PPS 100 mg tid for at least three months and 18 healthy female volunteers (ages 26–87 years). All patients met the history and physical exam aspects of the National Institute of Diabetes, Digestive and Kidney Diseases criteria.9 Most patients also met the cystoscopic criteria,9 but some were treated empirically without cystoscopy. Voided urine was centrifuged to remove cells and the supernatants were either used immediately or frozen and stored at −70 C until assayed. The thawed samples were centrifuged again before analysis. Seventeen patients answered University of Wisconsin (UW) symptom scores the day of urine collection. Total UW score was calculated as the sum of the seven bladder items on the UW scale (each item scored from 0 to 6 for a total score of 0–42).
Molecular weight determinations of PPS in urine
Aliquots of urine (human or rabbit) containing about 20 ug of PPS were lyophilized, reconstituted in buffer and treated with Pronase as described above. After inactivation of the enzyme, the enzyme digest was microfuged and the supernatant applied on a calibrated column of Sephadex G-50. The column was eluted with 0.1 M pyridine acetate, one mL fractions collected, dried and analyzed for PPS as described above.
Rabbit studies
PPS was purified from Elmiron capsules as described above and fractionated into HMW and LMW species by column chromatography on a Sephadex G-50 column. PPS (unfractionated, HMW or LMW fractions) was administered intravenously (1–1.2 mg) or orally (25 mg) to rabbits in metabolic cages. Urine was collected every 24 hours for up to five days. Urine volumes were noted and PPS was analyzed by the above ELISA. The total PPS excreted in the urine was calculated as a percent of that administered.
RESULTS
Human urine PPS
Control specimens had apparent PPS levels between 0 and 0.8 ug/ml (median 0.55 ug/ml). These background values are attributed to endogenous urine glycosaminoglycans which, unlike glycoproteins, are not eliminated by Pronase treatment.
Most IC patients had low urine PPS levels (median 1.2 ug/ml, range 0.5 to 27.7) The six highest levels were 4.9, 5.9, 8.4, 11.6, 12.7 and 27.2 ug/ml, respectively. Five of these patients were elderly women with ulcer type IC.
For the patients who had UW scores, one was in remission (total score = 2) on amitriptyline and PPS. Her urine PPS level was 0.8 ug/ml. The other 16 patients were taking PPS in various combinations with other medications and had persistent symptoms (median total score 25.5, range 15 to 40).
For eight patients, urine PPS was evaluated by its elution profile on a column of Sephadex G-50 using 14C-glucose as an internal reference. In all cases, urine PPS was of much lower molecular size than the PPS in the Elmiron capsules. The elution profile of one sample is illustrated in Figure 2 a. The results of the other seven samples were very similar; i.e. the peak elution did not differ by more than two fractions when normalized to the elution position of glucose. The average molecular size of PPS excreted by one patient was estimated to be 2000 Daltons compared to 3100 Daltons for the starting material, using a heparin fragment and sialyloligosaccharides of known molecular sizes as calibration standards (Figure 2b).
Figure 2.
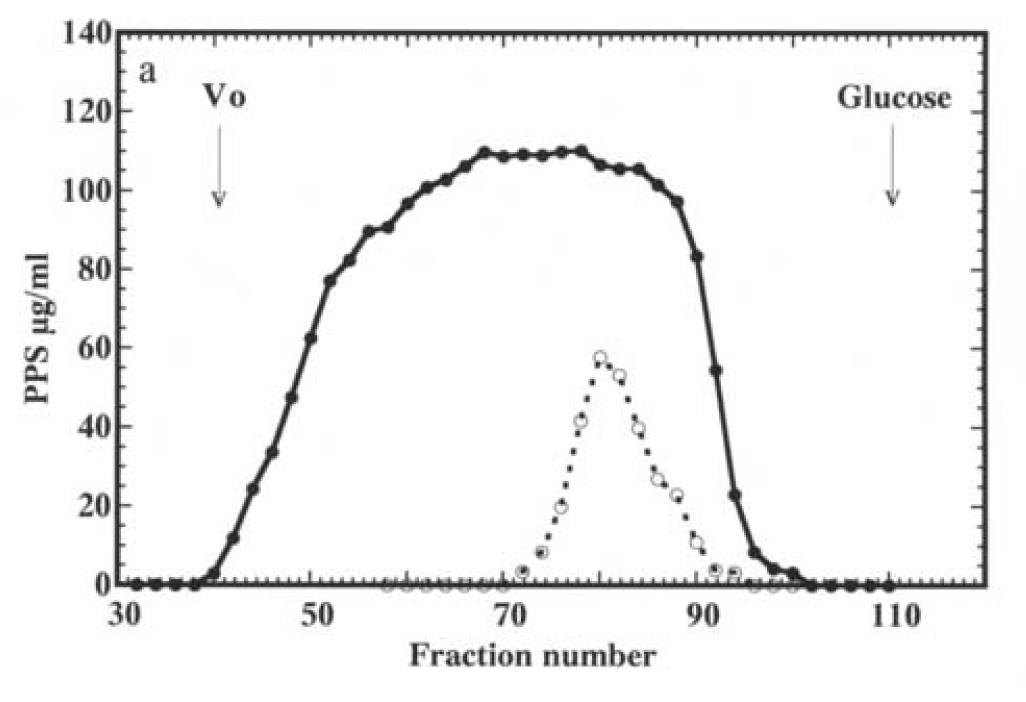
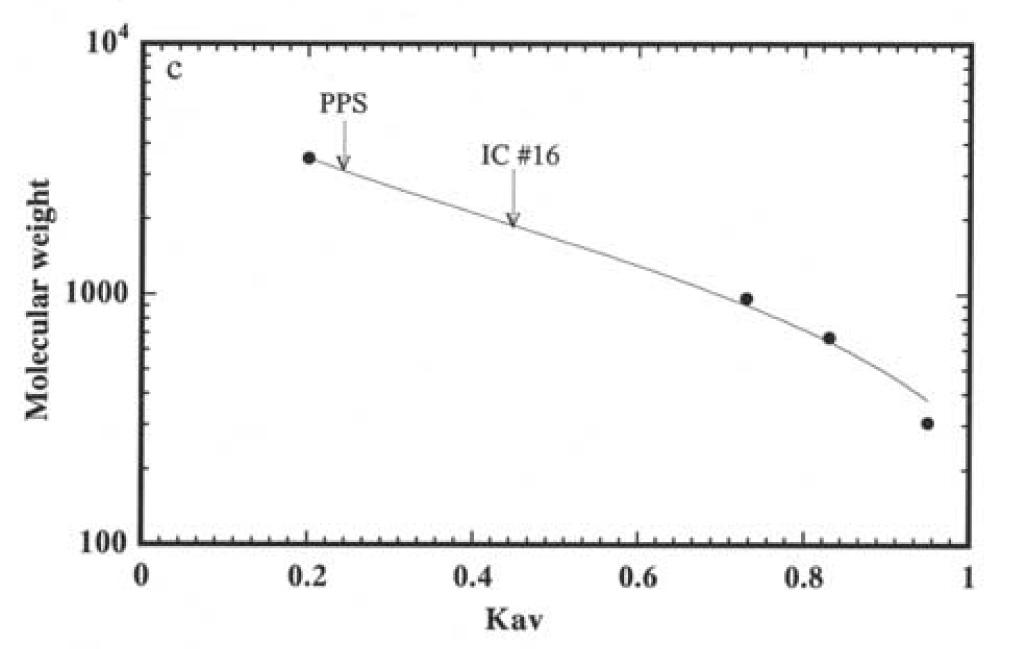
Chromatography of PPS isolated from Elmiron capsules (panel a, closed circles) and PPS recovered from urine of an IC patient taking PPS chronically (panel a, open circles) on a column of Sephadex G-50. The column was eluted with 0.1 N pyridine acetate, one mL fractions collected dried and analyzed for PPS by ELISA. The elution profiles from separate experiments are superimposed using peak elution of glucose as reference. Vo indicates the exclusion volume of the column. The average molecular sizes of the Elmiron PPS and PPS excreted by the IC patient were estimated based on calibration of the column with a heparin fragment (3500 Daltons) and sialyl saccharides of 966, 675 and 309 Daltons as calibration standards (panel b).
Urine recovery of intravenous PPS in rabbits
Single intravenous doses of PPS (1–1.2 mg) were given. Six rabbits each received purified unfractionated PPS, HMW fraction or LMW fraction. The urine recoveries are summarized in Table 1, which shows only the first three days because the amounts of PPS recovered on days 4 and 5 were negligible. The median recoveries were 47.2% (range 19.7 to 73.2%) for unfractionated PPS, 74.6% (range 31.4 to 96.3%) for LMW-PPS and 3.3% (range 2.5 to 5.0%) for HMW-PPS. The difference between the recoveries of LMW-PPS and HMW-PPS was statistically significant (p < 0.0022).
TABLE 1.
PERCENT RECOVERY OF PPS IN URINE AFTER INTRAVENOUS ADMINISTRATION IN RABBITS
| Unfractionated PPS (median total recovery 47.2%) | ||||
|---|---|---|---|---|
| Rabbit | 1st 24 hours | 2nd 24 hours | 3rd 24 hours | Total recovery |
| 1 | 19.3 | 2.6 | 0 | 21.9 |
| 2 | 29.2 | 4.8 | 0 | 34.0 |
| 3 | 52.6 | 3.5 | 4.4 | 60.5 |
| 4 | 5.7 | 12.0 | 2.0 | 19.7 |
| 5 | 67.9 | 5.3 | 0 | 73.2 |
| 6 | 60.2 | 5.8 | 0 | 66.0 |
| Low molecular weight PPS (median total recovery 74.6%) | ||||
| Rabbit | 1st 24 hours | 2nd 24 hours | 3rd 24 hours | Total recovery |
| 1 | 76.7 | 8.4 | 2.3 | 87.4 |
| 2 | 89.6 | 2.9 | 3.8 | 96.3 |
| 3 | 85.9 | 3.2 | 0 | 89.1 |
| 4 | 61.7 | No urine | No urine | 61.7 |
| 5 | 48.0 | 12.3 | 0 | 60.3 |
| 6 | 22.0 | 9.4 | 0 | 31.4 |
| High molecular weight PPS (median total recovery 3.3%) | ||||
| Rabbit | 1st 24 hours | 2nd 24 hours | 3rd 24 hours | Total recovery |
| 1 | 1.5 | 1.4 | 0 | 2.9 |
| 2 | 2.2 | 1.4 | 0 | 3.2 |
| 3 | 2.6 | 2.4 | 0 | 5.0 |
| 4 | 2.6 | 0.8 | 0 | 3.4 |
| 5 | 3.0 | 1.9 | 0 | 4.9 |
| 6 | 1.0 | 1.5 | 0 | 2.5 |
The PPS recovered in the urine of rabbits given unfractionated PPS was analyzed by gel filtration and found to be also of low molecular weight, as in the case of IC patients (Figure 3).
Figure 3.
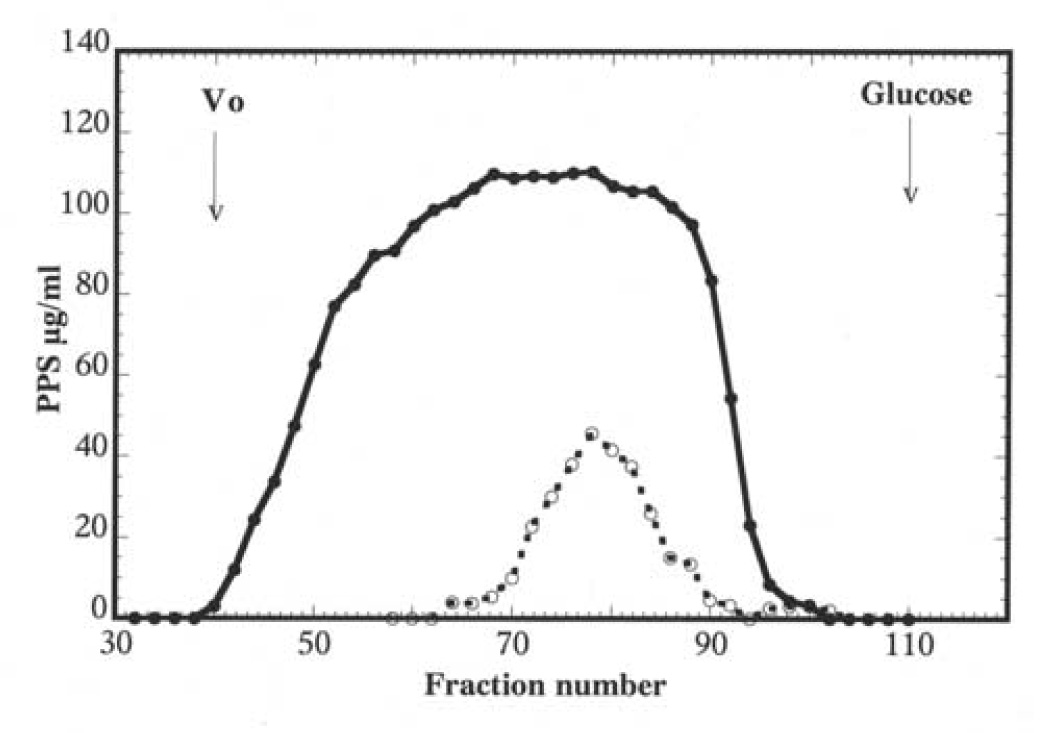
Chromatography of PPS isolated from Elmiron capsules (closed circles) and PPS recovered from urine of rabbit intravenously injected with PPS (open circles) on a column of Sephadex G-50. The column was eluted with 0.1 N pyridine acetate, one mL fractions collected dried and analyzed for PPS by ELISA. The elution profiles are superimposed using peak elution of glucose as reference. Vo indicates the exclusion volume of the column.
Urine recovery of oral PPS in rabbits
Rabbits were administered by gavage 25 mg of either LMW-PPS or HMW-PPS. Urine was collected every 24 h for three days and analyzed for PPS. The median recoveries were 7.4% (range 2.1 to 46.0 %) and 0.1% (range 0.0 to 0.3%) in 14 rabbits administered LMW-PPS and 7 rabbits administered HMW-PPS, respectively.
DISCUSSION
PPS is a heterogeneous mixture of molecules with varying size and charge. The rabbit experiments confirmed our expectations that the LMW fraction had greater gastrointestinal absorption and urine excretion. After intravenous administration, over 50% of LMW-PPS was recovered from the urine, but less than 5% of HMW-PPS was recovered. After oral administration, urine recovery was lower for both fractions, but it remained higher for LMW-PPS (averaging 7%) than HMW-PPS (essentially zero).
Blood levels of PPS are higher after multiple doses than after single doses.10 One possible explanation is that heparin-like molecules bind to vascular endothelium. After a single dose, some of the PPS may bind to the endothelium, causing lower initial blood levels. Higher levels could develop after multiple doses, when the endothelial binding sites were saturated. Therefore, our rabbit studies may have shown higher urine recoveries if they had been performed after chronic PPS dosing. On the other hand, we would not expect chronic dosing to change our finding that LMW-PPS had higher urine recovery than HMW-PPS. For example, the IC patients were on chronic PPS and they had only the LMW fraction in their urine.
Several controlled trials have tested oral PPS for IC.11–15 The rates of significant improvement on PPS ranged from 28% to 63%, and only three trials concluded that PPS was more effective than placebo. The reasons for this modest efficacy are unknown. Possible explanations include: (1) 300 mg/day is an insufficient dose, (2) the amount of oral PPS that actually reaches the bladder is too low to exert any benefit, (3) for some IC patients, PPS has no effect on the disease, regardless of its urine or tissue levels.
The first possibility was recently addressed by comparing three different doses (100, 200 or 300 mg tid).16 Response rates were not dose-related. Side effects, including diarrhea, rectal bleeding and abdominal pain, were dose-related. Thus, tripling the oral dose of PPS resulted in increased side effects, but did not improve efficacy for IC. This is not surprising, since the absorption of oral PPS is so low that even tripling the dose may not lead to significant increases in PPS reaching the urine. Also, since most of an oral dose of PPS remains in the intestine, it is not surprising that the GI side effects were dose-related.
With regard to the second possibility, our IC urine PPS levels were very low. The median IC level exceeded the median background level by less than 1 ug/ml. The six highest PPS levels were between 4.5 and 27 ug/ml, and these six patients all had persistent symptoms. Thus, PPS may have failed because its urine or tissue levels were too low. The levels needed for in vivo efficacy are unknown, but most in vitro actions of PPS need greater concentrations than what we found in IC urine. For example, inhibition of mast cell degranulation required at least 10−5 M (approximately 50 ug/ml)2 and inhibition of complement activation required at least 30 ug/ml.17 One exception is inhibition of lipopolysaccharide-induced nuclear factor-kB activation, which occurred at 0.5, 5 and 50 ug/ml.3 Since it is unknown exactly how PPS improves IC symptoms, we cannot answer this question definitively.
The third possibility cannot be addressed by our data. We know that our IC patients had low PPS levels, but we do not know whether they would have achieved better symptom relief if their PPS levels had been higher. This question could be addressed in future studies if a way to improve PPS bioavailability were available. One possible method would be to use only the LMW fraction of PPS, which could deliver higher urine levels. Another method, placing PPS into different vehicles, was recently described.18
These questions have not been previously addressed in peer-reviewed IC literature, probably because it is difficult to measure urine PPS. The methods for the previous human study6 are in a foreign journal that is not listed in Medline or PubMed. In preparation for this study we investigated two chemical approaches to measure urine PPS, both without success. One approach was cellulose acetate electrophoresis followed by Alcian blue staining, but this had significant interference from endogenous urine glycoconjugates. In the other approach urine was subjected to chromatography on a diethylaminoethyl-Sephacel column. The polyanionic material, including PPS, was recovered free of glycoproteins by elution with 2M lithium chloride and dialysis. The PPS level was estimated by acid hydrolysis followed by analysis of the xylose content by high-performance anion-exchange chromatography.19 This method can identify nanogram levels of xylose but was complicated by a contaminant from the dialysis bag (made of cellulose derivatives) that eluted close to the xylose peak in the chromatography. Additional steps, such as gel filtration to remove the contaminant, made the assay excessively laborious. We also considered a previous assay for PPS in plasma PPS, based on competition with 125I-PPS for binding to polycation-conjugated Sephadex.20 However, this seemed unsuitable for urine because a polycation would also bind endogenous urine glycosaminoglycans nonspecifically, giving erroneous results.
The successful assay was an adaptation of the competitive ELISA previously reported.8 A potential problem with this approach is that small fragments or degradation products of PPS might be missed by the antibody. If this occurred, then the actual recoveries of PPS in the urine would be higher than what we found. However, it would not detract from our conclusion that large PPS molecules were not recovered in the urine.
CONCLUSIONS
After intravenous administration of different PPS fractions in rabbits, urine recovery of the LMW fraction was much higher than that of the HMW fraction. After oral administration in rabbits, essentially none of the HMW fraction was recovered. For IC patients taking PPS chronically, all PPS recovered in the urine was of low molecular weight. These findings indicate that the molecular size of PPS affects its urinary excretion.
ACKNOWLEDGMENT
This study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases, DK-57266 and DK-57281.
KEY OF DEFINITIONS FOR ABBREVIATIONS
- PPS
Pentosanpolysulfate
- IC
Interstitial cystitis
- LMW
Low molecular weight
- HMW
High molecular weight
- BSA
Bovine serum albumin
- ELISA
Enzyme-linked immunosorbent assay
- PBS
Phosphate-buffered saline
- UW
University of Wisconsin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Parsons CL. Epithelial coating techniques in the treatment of interstitial cystitis. Urology. 1997;49 suppl 5A:100. doi: 10.1016/s0090-4295(97)00180-5. [DOI] [PubMed] [Google Scholar]
- 2.Chiang G, Patra P, LeTourneau R, Jeudy S, Boucher W, Green M, et al. Pentosanpolysulfate inhibits mast cell histamine secretion and intracellular calcium ion levels: an alternative explanation of its beneficial effect in interstitial cystitis. J Urol. 2000;164:2119. [PubMed] [Google Scholar]
- 3.Sadhukhan PC, Tchetgen MB, Rackely RR, Vasavada SP, Liou L, Bandyopadhyay SK. Sodium pentosan polysulfate reduces urothelial responses to inflammatory stimuli via an indirect mechanism. J Urol. 2002;168:289. [PubMed] [Google Scholar]
- 4.Faaij RA, Srivastava N, van Griensven JM, Schoemaker RC, Kluft C, Burggraaf J, et al. The oral bioavailability of pentosan polysulphate sodium in healthy volunteers. Eur J Clin Pharmacol. 1999;54:929. doi: 10.1007/s002280050577. [DOI] [PubMed] [Google Scholar]
- 5.Marsh NA, Peyser PM, Creighton LJ, Mahmoud M, Gaffney PJ. The effect of pentosan polysulphate (SP54) on the fibrinolytic enzyme system – a human volunteer and experimental animal study. Thromb Haemost. 1985;54:833. [PubMed] [Google Scholar]
- 6.Fellstrom B, Backman U, Danielson B, Wikstrom B. Treatment of renal calcium stone disease with the synthetic glycosaminoglycan pentosan polysulphate. World J Urol. 1994;12:52. doi: 10.1007/BF00182052. [DOI] [PubMed] [Google Scholar]
- 7.Odlind B, Dencker L, Tengblad A. Preferential localization of 3H-pentosanpolysulphate to the urinary tract in rats. Pharmacol Toxicol. 1987;61:162. doi: 10.1111/j.1600-0773.1987.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 8.Callahan HJ, Shupp-Byrne D, Pizzo M, Parsons CL, Mulholland SG. The production of antibodies to pentosan polysulfate (Elmiron - SP54) J Immunol Methods. 1991;136:53. doi: 10.1016/0022-1759(91)90249-f. [DOI] [PubMed] [Google Scholar]
- 9.Striker GE. KUH notes. J Urol. 1989;142:139. doi: 10.1016/s0022-5347(17)38687-1. [DOI] [PubMed] [Google Scholar]
- 10.Marshall JL, Wellstein A, Rae J, DeLap RJ, Phipps K, Hanfelt J, et al. Phase I trial of orally administered pentosan polysulfate in patients with advanced cancer. Clin Cancer Res. 1997;3:2347. [PubMed] [Google Scholar]
- 11.Parsons CL, Mulholland SG. Successful therapy of interstitial cystitis with pentosanpolysulfate. J Urol. 1987;138:513. doi: 10.1016/s0022-5347(17)43243-5. [DOI] [PubMed] [Google Scholar]
- 12.Sant GR, Propert KJ, Hanno PM, Burks D, Culkin D, Diokno AC, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810. doi: 10.1097/01.ju.0000083020.06212.3d. [DOI] [PubMed] [Google Scholar]
- 13.Parsons CL, Benson G, Childs S, Hanno P, Sant GR, Webster G. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosan polysulfate. J Urol. 1993;150:845. doi: 10.1016/s0022-5347(17)35629-x. [DOI] [PubMed] [Google Scholar]
- 14.Mulholland SG, Hanno P, Parsons CL, Sant GR, Staskin DR. Pentosan polysulfate sodium for therapy of interstitial cystitis. Urology. 1990;35:552. doi: 10.1016/0090-4295(90)80116-5. [DOI] [PubMed] [Google Scholar]
- 15.Holm-Bentzen M, Jacobsen F, Nerstrom B, Lose G, Kristensen JK, Pedersen RH, et al. A prospective double-blind clinically controlled multicenter trial of sodium pentosanpolysulfate in the treatment of interstitial cystitis and related painful bladder disease. J Urol. 1987;138:503. doi: 10.1016/s0022-5347(17)43241-1. [DOI] [PubMed] [Google Scholar]
- 16.Nickel JC, Barkin J, Forrest J, Mosbaugh PG, Hernandez-Graulau J, Kaufman D, et al. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium (PPS) for interstitial cystitis (IC) Urology. 2005;65:654. doi: 10.1016/j.urology.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 17.Kilgore KS, Naylor KB, Tanhehco EJ, Park JL, Booth EA, Washington RA, et al. The semisynthetic polysaccharide pentosan polysulfate prevents complement-mediated myocardial injury in the rabbit perfused heart. J Pharm Exp Ther. 1998;285:987. [PubMed] [Google Scholar]
- 18.Dong L, Yum A, Nguyen J, Wong P. Enhanced ileal absorption of a hydrophilic macromolecule, pentosan polysulfate sodium (PPS) J Biomater Sci Polym. 2004;15:671. doi: 10.1163/156856204323046924. [DOI] [PubMed] [Google Scholar]
- 19.Lee YC. High-performance anion-exchange chromatography for carbohoydrate analysis. Analytic Biochem. 1990;189:151. doi: 10.1016/0003-2697(90)90099-u. [DOI] [PubMed] [Google Scholar]
- 20.MacGregor IR, Dawes J, Pepper DS, Prowse CV, Stocks J. Metabolism of sodium pentosan polysulphate in man measured by a new competitive binding assay for sulphated polysaccharides – comparison with effects upon anticoagulant activity, lipolysis and platelet alpha-granule proteins. Thromb Haemostas. 1985;53:411. [PubMed] [Google Scholar]


