Abstract
The patient with word-finding difficulty presents a common and challenging clinical problem. The complaint of ‘word-finding difficulty’ covers a wide range of clinical phenomena and may signify any of a number of distinct pathophysiological processes. Although it occurs in a variety of clinical contexts, word-finding difficulty generally presents a diagnostic conundrum when it occurs as a leading or apparently isolated symptom, most often as the harbinger of degenerative disease: the progressive aphasias. Recent advances in the neurobiology of the focal, language-based dementias have transformed our understanding of these processes and the ways in which they breakdown in different diseases, but translation of this knowledge to the bedside is far from straightforward. Speech and language disturbances in the dementias present unique diagnostic and conceptual problems that are not fully captured by classical models derived from the study of vascular and other acute focal brain lesions. This has led to a reformulation of our understanding of how language is organized in the brain. In this review we seek to provide the clinical neurologist with a practical and theoretical bridge between the patient presenting with word-finding difficulty in the clinic and the evidence of the brain sciences. We delineate key illustrative speech and language syndromes in the degenerative dementias, compare these syndromes with the syndromes of acute brain damage, and indicate how the clinical syndromes relate to emerging neurolinguistic, neuroanatomical and neurobiological insights. We propose a conceptual framework for the analysis of word-finding difficulty, in order both better to define the patient's complaint and its differential diagnosis for the clinician and to identify unresolved issues as a stimulus to future work.
Keywords: aphasia, progressive aphasia, anomia, dementia, speech and language
Introduction
‘Word-finding difficulty’ is a common and challenging problem in neurological practice. In many cases, patients will complain of word-finding difficulty or, not uncommonly, the difficulty is identified by the neurologist in the course of the assessment. In both situations, the basis for the word-finding problem needs to be established but this is often not straightforward. Spoken communication depends on a sequence of cognitive processes, and disruption of any of these processes can affect word-finding (Fig. 1). Furthermore, processing occurs in a distributed network of brain areas that is vulnerable to a variety of acute and chronic pathological states (Levelt, 1989; Price et al., 1993; Levelt, 2001; Blank et al., 2002; Gorno-Tempini et al., 2004). The differential diagnosis of word-finding difficulty therefore encompasses a wide spectrum of acute and chronic disorders as diverse as delirium (Geschwind, 1964), aphasic stroke (Kertesz and McCabe, 1977), encephalitis (Okuda et al., 2001), major depression (Georgieff et al., 1998) and psychosis (Critchley, 1964), head injury (Levin et al., 1976), temporal lobectomy (Langfitt and Rausch, 1996) and metabolic and genetic disorders (Spinelli et al., 1995). In particular, however, it is a leading symptom of a number of degenerative conditions: the progressive aphasias (Mesulam, 1982, Hodges et al., 1992; Mesulam, 2003; Gorno-Tempini et al., 2004). In the degenerative diseases, in contrast to many of the other conditions associated with word-finding difficulty, the cause of the word-finding problem may not be obvious or it may be the presenting complaint: accurate diagnosis therefore depends on detailed characterization of the language deficit. It is accordingly in the context of degenerative disease that word-finding difficulty usually presents the greatest diagnostic challenge, yet the classical approach to the clinical assessment of language (which is based largely on the accumulated experience of aphasia in acute stroke: Hillis, 2007) may not be adequate. This reflects the often unique problems posed by speech and language breakdown in the degenerative dementias (Warrington, 1975; Mesulam, 2003).
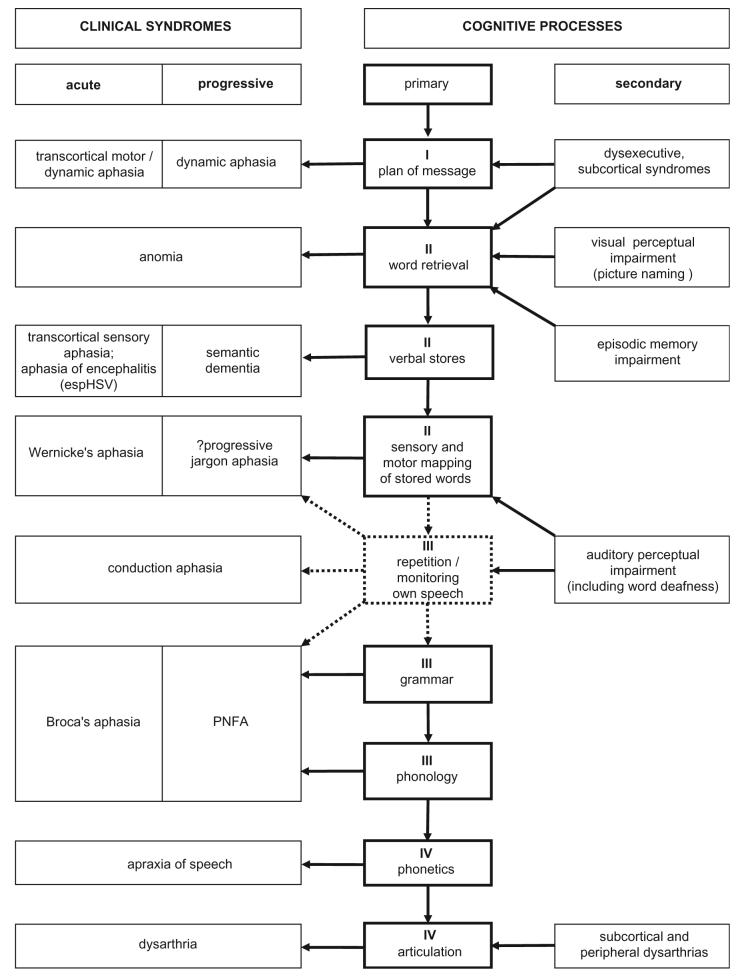
Fig. 1.
An outline of clinical syndromes and underlying functional deficits in patients with word-finding difficulty. Relations between acute and chronic syndromes and primary and secondary functional deficits are shown. Numerals refer to the operational stages in the language output pathway (dotted lines indicate processes that are related to but not essential for language output): I, generation of a verbal message; II, sense of the verbal message; III, structure of the verbal message; IV, motor programming of speech. Key: esp, especially; HSV, Herpes simplex encephalitis.
Here we use ‘word-finding difficulty’ as a shorthand for a class of symptoms which patients and carers commonly volunteer when describing impaired language output. Progressive cognitive syndromes with circumscribed deficits and preserved intellect have been recognized for many years (for example, Pick, 1892; Serieux, 1893, see also Luzzatti and Poeck, 1991; De Bleser, 2006 for other historical cases) and may preferentially affect a variety of cognitive domains, however the comparatively recent renaissance of interest in the language-based dementias (Mesulam, 1982) has transformed our picture both of disease biology in neurodegeneration and the organization of the human language system. The focal dementias pose considerable nosological and neurobiological difficulties. While circumscribed atrophy on structural brain imaging can support the impression of a focal dementia, diagnosis remains essentially clinical. Primary progressive aphasia (PPA) is a clinical syndrome of progressive language impairment with relative sparing of other aspects of cognitive function until late in the course (Mesulam, 1982, 2001, 2003). This broad definition subsumes substantial clinical, anatomical and pathological heterogeneity, and a spectrum of clinical subtypes of PPA has been described. While these subtypes have more or less distinctive profiles of speech and language disturbance, even where clinical characterization is robust (for example, in the distinction between ‘fluent’ and ‘non-fluent’ forms of PPA) understanding of the underlying pathophysiological mechanisms remains limited (Mesulam and Weintraub, 1992; Grossmann, 2002; Mesulam et al., 2003) Moreover, the overlap between clinical subtypes is substantial, incomplete syndromes are frequent (Grossmann, 2002; Mesulam et al., 2003), and none has been shown to have a unique correspondence with either anatomy or tissue pathology. This presents serious and unresolved nosological difficulties, and for the clinician, a substantial diagnostic dilemma. Furthermore, the stimulus of the focal language-based dementias has led to a wider appreciation of speech and language dysfunction in other neurodegenerative conditions, including Alzheimer's disease (AD) (Emery, 2000; Croot et al., 2000) and the problem of the differential diagnosis of ‘progressive aphasia’ in this broader sense. Accordingly, a conceptual framework is needed to allow the clinician to interpret the patient's complaint of word-finding difficulty in line with emerging evidence for language network dysfunction in neurodegenerative diseases.
Here we outline such a framework for the clinical analysis of ‘word-finding difficulty’. We propose a clinical scheme that can be used at the bedside to categorize the nature of the problem and to formulate a differential diagnosis, with reference in particular to the degenerative dementias, presented in Fig. 2. This scheme has speech as its focus because word-finding difficulty in spoken language is generally the dominant complaint in the progressive aphasias. Our scheme is informed by evidence emerging from the experimental brain sciences, and contemporary information-processing accounts of language processing (Levelt, 1989; Warren and Warrington, 2007; Hillis, 2007) (Fig. 1). Application of the scheme generates a taxonomy of clinical syndromes arising from different operational stages in the language output pathway and with distinct anatomical substrates. Our approach is based on a series of steps that probe the key stages in language output (Fig. 1). These steps are elaborated in the following sections and in Tables 1-4. The pattern of performance at each step identifies the cognitive processing stage that is principally affected and builds up a detailed profile of the speech syndrome. Both these levels of analysis are of clinical relevance: the broad cognitive operational level allows the deficit to be localized (Fig. 3), while the detailed syndromic description guides the differential diagnosis of the likely pathological process (Fig. 2). Our intention is to provide the neurologist with a bridge between the dilemmas of the bedside and the theoretical constructs of the brain sciences, rather than a comprehensive neurolinguistic treatise on the progressive aphasias. At the same time, however, we hope to show that understanding of the pathophysiology of these diseases can be advanced by the characterization of clinical phenomena that are difficult to reconcile with theoretical models of language function and dysfunction.
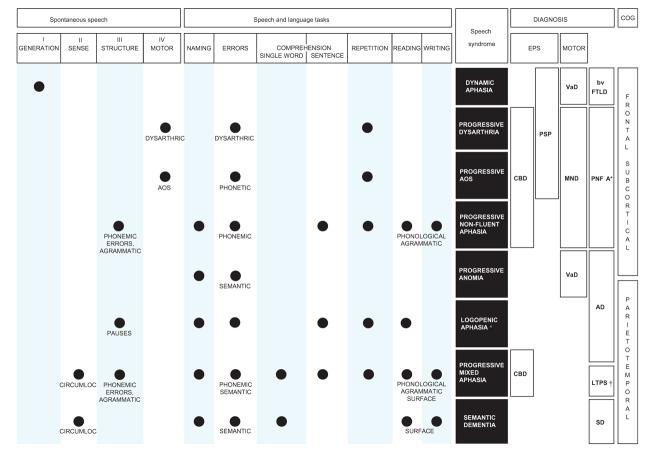
Fig. 2.
A clinical scheme for assessing the patient with word-finding difficulty, particularly in the context of degenerative disease. The scheme is organized as a ‘grid’ in which each column represents a key step in the clinical assessment, and each row represents a speech or language syndrome. Each entry in the grid represents an abnormality. Based on the initial assessment of features of the patient's spontaneous speech directed toward key language operations (left; see also Fig. 1), followed by key speech and language tasks (centre), the clinical speech or language syndrome is characterized. Identification of the clinical syndrome allows a differential diagnosis to be formulated, based on associated clinical features (right) including both cognitive and other neurological abnormalities. These associated features also allow primary and secondary effects on word-finding to be interpreted (Fig.1). See text for details. Key: filled circle: abnormal; AOS: apraxia of speech: *: as used in consensus criteria; †: nosological status not established; AD: Alzheimer's disease; bvFTLD: behavioural variant of frontotemporal lobar degeneration; CBD: corticobasal degeneration syndrome; CIRCUMLOC: empty, circumlocutory speech; COG: cognitive features; EPS: extrapyramidal syndrome; LTPS: lateral temporo-parietal syndrome; MND: motor neuron disease; PNFA: progressive nonfluent aphasia; PSP: progressive supranuclear palsy; SD: semantic dementia; SURFACE: surface (regularization) errors; VaD: vascular dementia.
Table 1.
History of the problem
|
Table 4.
Specific speech and language tasks and the functions they assess (see text for examples)
| Naming |
| Lack of content words and proper nouns in spontaneous speech (see Tables 2 and 3) |
| Naming of familiar items from pictures |
| Naming from verbal description |
| Effect of word frequency |
| Effect of category (e.g. animate/inanimate; special cases, e.g. colours) |
| Type of error (phonemic, speech sounds; semantic or neologistic, meaning) |
| Effect of cueing (initial letter/semantic association) |
| Functions: retrieval of words from verbal knowledge store, verbal output |
| Speech comprehension |
| Single words: vocabulary (point to items named by examiner, provide definitions, choose synonyms, categorise) |
| Functions: speech signal input, verbal knowledge storage |
| Sentences: grammar (perform a short series of actions to command, identify a picture from description) |
| Functions: manipulation of on-line verbal information and grammatical relations |
| Speech repetition |
| Monosyllabic words, polysyllabic words, phrases and sentences |
| Functions: speech signal input, verbal output, input:output transfer |
| Reading, writing and spelling |
| Read a short passage aloud (including both irregular words and ‘nonsense’ words such as proper nouns) |
| Write a sentence |
| Spelling of regular and irregular words |
| Functions: verbal processing in other language channels |
| Sentence generation and completion |
| Sentence generation around a target word |
| Sentence completion using terminal nouns (predictable versus open-ended) |
| Function: novel verbal thoughts and messages |
| Motor assessment |
| Repetition of single syllables |
| Function: articulation |
| Repetition of syllable combinations |
| Function: phonetic encoding |
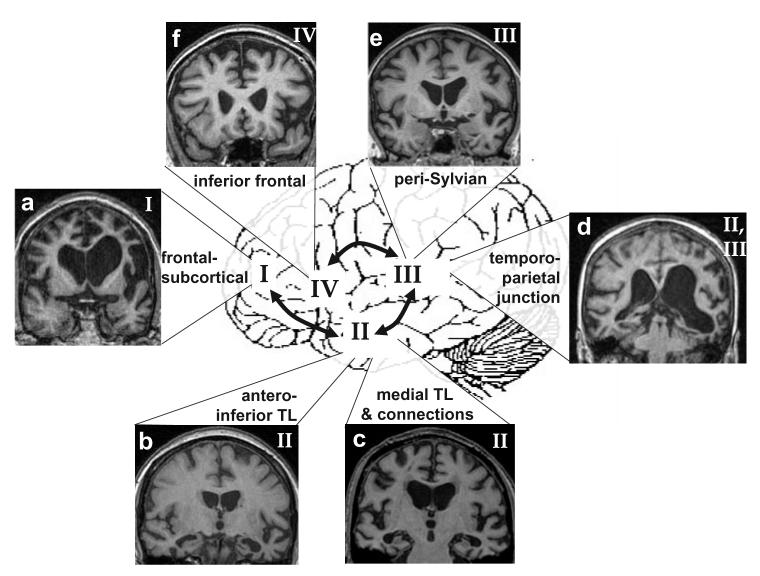
Fig. 3.
Structural anatomy of word-finding difficulty in degenerative disorders. Numerals and connecting arrows refer to the operational stages in the language output pathway (coded in Fig.1 and Table 2). Key anatomical areas are indicated. Arrows are bi-directional to indicate that flow of information between these areas is likely to be reciprocal. Brain magnetic resonance images illustrate some degenerative disorders with word-finding difficulty (the left hemisphere is on the right side in all coronal sections; TL, temporal lobe): (a) asymmetric (left greater than right) frontal lobe atrophy, dynamic aphasia; (b) focal left anterior/inferior temporal lobe atrophy, semantic dementia; (c) bilateral mesial temporal atrophy, Alzheimer's disease (anomia); (d) left posterior superior temporal/inferior parietal atrophy, progressive ‘mixed’, logopenic or jargon aphasia; (e) focal left superior temporal lobe/insular atrophy, progressive nonfluent aphasia; (f) focal left inferior frontal gyrus/frontal opercular atrophy, progressive apraxia of speech.
The clinical background
The complaint of word-finding difficulty should not be taken at face value. The first task is to determine what is meant: defective speech output of various kinds may be described as a problem ‘finding words’, ‘finding’ (or ‘remembering’) names’, ‘getting words out’, ‘using the wrong words’, ‘jumbled’ or ‘mixed up’ words. Patients may complain that their vocabulary is reduced (particularly for more specialized material), there may be an inability to convey precise shades of meaning or loss of facility with crossword puzzles. Carers may have become aware of speech sound or grammatical errors in the patient's spoken or written output, or the appearance (or reappearance) of a stutter or alteration in voice quality. However, specific descriptions of this kind (though valuable) often must be actively elicited.
Although word-finding is central to normal communication, word-finding difficulty should not be equated with aphasia. Problems with word-finding may develop in the setting of otherwise normal language as a result of a problem in another cognitive domain. A major goal of clinical assessment, therefore, is to decide whether word-finding difficulty reflects a primary language disorder, or whether the problem is secondary to other non-linguistic cognitive deficits. Primary word-finding difficulty may occur as an isolated language disturbance or may occur as part of a more extensive cognitive or behavioural syndrome. Secondary word-finding difficulty occurs when a deficit within another cognitive domain interferes with the function of a more or less intact language system. For example, a patient in whom failure to name household objects on bedside testing is accompanied by a failure to locate or correctly use the same items may have a primary visual perceptual problem, patients who participate less in conversations may be deaf, while difficulty remembering the names of acquaintances or in delivering messages may indicate a more general problem with episodic memory. Conversely, patients with a primary word-finding difficulty and their carers often describe their symptoms in terms of memory failure (they may say that they ‘forget’ the names of people or things) or a perceptual defect (impaired speech comprehension is not uncommonly ascribed to ‘deafness’ by the patient's family). It is also important to recognize the wide spectrum of normal variation in word-finding ability, and the potential effects of fatigue, anxiety or mood disorders. The evaluation of word-finding ability therefore requires both an objective assessment of performance, and an awareness of the wider context in which the problem has developed and its impact on the patient's daily life.
Obtaining an accurate history of word-finding difficulty (Table 1) depends on interviewing both the patient and an informant who knows the patient well. A complaint of word-finding difficulty must be interpreted in light of a patient's premorbid verbal skills. Information about bilingualism (was English the first language, and if not, what level of competence was achieved?), educational attainment and literacy, occupation and any premorbid disabilities (such as developmental dyslexia) is essential. The family history may be relevant not only to the diagnosis in general but also to the interpretation of the word-finding problem in particular: an example is the emerging association of mutations in the progranulin gene with familial forms of progressive non-fluent aphasia (PNFA) (Cruts et al., 2006; Mesulam et al., 2007). Establishing the mode of onset and time course of the word-finding difficulty will assist in distinguishing acute processes (for example, stroke, encephalitis, delirium), chronic processes with static or fluctuating deficits (for example, head-injury or seizures) and chronic processes with progressive deficits (for example, a degenerative dementia). This information is particularly critical where the process leading to language breakdown has developed insidiously and there may be few other clinical clues to aetiology [for example, the interictal ‘pseudodementia’ of temporal lobe epilepsy: (Mayeux et al., 1980)]. The context in which the problem developed may be crucial. Whereas in acute disease processes, associated disturbances of alertness, perceptual and motor functions are often prominent (or may dominate the clinical presentation); in chronic disease processes, associated features may be subtle. However, the distinction between acute and chronic processes is not always clear. Patients who have sustained an acute event may present later with ongoing word-finding difficulty: accurate diagnosis then depends on establishing the degree of initial recovery and whether the word-finding deficit has evolved over time. Conversely, neurodegenerative disease can occasionally appear to present acutely following a particular event e.g. surgery (Larner, 2005). This may be secondary to a superimposed acute confusional state or due to the fact that mild word-finding or cognitive difficulties had previously gone unnoticed: the key to diagnosis here is to establish a background of more insidious or progressive difficulty prior to the acute presentation. The history often provides clues to the nature of the word-finding difficulty and associated cognitive, behavioural or neurological features which can then be explored systematically during the examination.
Analysis of spontaneous speech
Systematic analysis of an extended sample of the patient's spontaneous (propositional) speech (Table 2) is the single most valuable aspect of the examination. Where little spontaneous conversation is offered, the patient can be asked to describe a scene in a photograph or drawing (an example is shown in Fig. 4A). This is preferable to asking the patient to recount an event in their daily routine, as it allows speech to be evaluated independently of episodic memory and provides a standard with which to compare speech characteristics in different clinical situations. Examples of scene descriptions produced by patients with canonical speech disorders are presented in Table 3. Valuable information is often obtained from observing the patient's general behaviour and approach to the clinical interview. The inert patient with a frontal dementia, who offers little speech at all and sits passively throughout the interview, makes a very different impression from the patient with PNFA, who is obviously frustrated by their difficulties and struggles to compensate with an excess of non-verbal gestures, and both contrast with the garrulous patient with semantic dementia (SD), who produces a steady stream of circumlocutory speech.
Table 2.
Analysis of spontaneous speech
|
Fig. 4.

Materials for assessing speech at the bedside. (Reproduced with permission of Professor EK Warrington.) (A) A beach scene, illustrating one means of eliciting conversational speech (see examples in Table 3). (B) A passage for reading aloud (see text).
Table 3.
Examples of spontaneous speech in progressive versus acute aphasias (each of these patients is describing a beach scene, shown in Fig. 4)
| Progressive aphasias |
| Semantic dementia |
| That's the father, playing with his son, that thing (points to ball) … hitting the thing in the air. (Pointing to boy falling out of boat) He's in the garden isn't he, playing that game again. I hope he doesn't fall down. Looks as if he's wobbling. (Pointing to sandcastle) I'm not quite sure. That's the water there, coming right up to there, and that stays there and he's working, he's pressing that down, isn't he? He's working it. He's moving it down there because that's the equivalent of that, and that goes there … both sides. I've seen something like that somewhere else. |
| Alzheimer's disease (‘logopenic aphasia’) |
| A beach scene … playing on the beach. A pier … (pause) and a building on the pier and a row of beach (pause) things. (long pause) In the middle ground, a father and child playing with a large ball on the … (pause). On the left..erm … a rower has overbalanced next to the beach really … and is falling out over the (pause) side of the erm.. (pause) rowing boat. In the foreground is a youngster building some (pause) sandcastles. |
| Progressive nonfluent aphasia/apraxia of speech |
| The sea … er … er … er … um … a man in a soup … no suit … with a panner (pointing at paddle) falling out of the boat. Er … nice stand … no sand next to the sea and the boy making a nice h.. h.. house … houses. Another (long pause) m.. m.. m.. man … abigmen … no man … and little g.. g.. g.. girl p.. p..p.. playing. The two skygurls (points to seagulls). Water round castle … |
| Acute aphasias |
| Broca's aphasia (left inferior frontal infarction) |
| It's picture of … er … ab … about a … a … er.. beach … er … holiday … er ….er … Father has gone down beach with his … er … (pause) three children … erm … He's playing with … er … a little … maybe a … er … chil … er … girl or boy. He's having a ball and the … the … choldren.. no … the child is reaching for it. |
| Wernicke's aphasia (left temporo-parietal infarction) |
| A little boy with spanks an sparras. These are the … It's got it on the high underground and a fly flow new boy, and the boy whose fallen in the water and the two children on the right there with one a bit two children. One childer and one in lyda and the child a boy in the flem of course. And that is the last one … is the last one in the bottom. |
The classification of aphasias as ‘expressive’ or ‘receptive’ (or ‘motor’ or ‘sensory’) is both overly simplistic and inaccurate (Geschwind, 1971): few patients present with either a pure speech production or comprehension deficit. This is true for acute lesions (Brust et al., 1976), but particularly relevant to the categorization of the progressive aphasias. Similarly, classifying speech disturbances as ‘fluent’ or ‘non-fluent’ also oversimplifies the clinical phenomenology and is open to misinterpretation. Fluency describes the flow of speech output, but it is multidimensional: ‘non-fluency’ may be due to a number of different factors, including decreased phrase length, agrammatism, poor articulation or slower speech rate (Hillis, 2007). As these impairments tend to occur together, an individual patient's speech can often be reliably categorized as fluent or non-fluent; moreover, certain dimensions (particular motor aspects such as rate and articulation) make a relatively greater contribution to the impression of dysfluency. However, the component processes are dissociable: thus, patients with milder forms of ‘non-fluent’ speech may still produce relatively long phrases or sentences, albeit containing many errors. Even in more advanced cases of ‘non-fluent’ speech, there may be stereotyped phrases comprising several words (e.g. ‘Hello, how are you?’): such phrases can be regarded as an expressive ‘unit’ serving a similar function to a single word. Conversely, patients with ‘fluent’ aphasias generally have empty speech due to an impaired ability to find appropriate content words but commonly also have conversational pauses during which they struggle to find the appropriate word: these gaps tend to reduce the overall number of words produced (‘logopenia’) and thus the fluency of the utterance as a whole. Although it remains clinically useful as a descriptive term, ‘fluency’ is therefore potentially misleading as a criterion for the categorization of speech and language syndromes, which is more usefully based on a combination of features (Fig. 2).
All propositional speech can be considered as an attempt to convey a thought or ‘message’ in verbal form, and the operational stages involved in this process (Fig. 1) suggest a broad classification of clinical deficits, according to whether the patient has difficulty initiating conversation, difficulty in conveying the sense of the message (a disturbance of speech content such that thought can no longer be conveyed coherently) or with message structure (a disturbance of word formation or word order). In practice, an individual patient's word-finding difficulty is rarely confined to a single one of these categories, although in many cases one category will predominate. Furthermore, deficits in these true word-finding categories may overlap with a difficulty in the motor programming of speech: production of intelligible words ultimately depends on an intact motor programme that enables correct articulation of a formulated utterance.
Generating a message: verbal thought
The ease of initiation of conversational (propositional) speech provides important information about the generation of verbal thought (the ability to express thoughts in words). This process involves the formulation of a plan for the verbal message (Fig. 1). Although patients with word-finding difficulty of all kinds may participate less in conversations as a non-specific result of reduced facility with language, a striking reduction in propositional speech is the hallmark of dynamic aphasia (Luria and Tsvetkova, 1967; Costello and Warrington, 1989; Robinson et al., 1998; Warren et al., 2003). The patient seems literally to have ‘nothing to say’. Such patients have a selective deficit at the level of the generation of verbal thought: although the amount of speech is reduced, the sense and structure of the message (provided it can be generated in the first place) usually remain intact. Sentence generation is dependent on context: a patient may be able to describe a simple picture but may not be able to talk to an everyday topic or may provide a sparse (but error-free) description of a complex scene (Fig. 4A). Compared to this decreased spontaneous output, speech can be produced relatively normally in specific contexts, such as naming tasks, repetition or reading. A similar decrease in speech output occurs in many patients with frontal and subcortical deficits who exhibit a generalized inertia and slowing of thought. However in pure dynamic aphasia there is retained ability to generate novel non-verbal material such as song, suggesting that dynamic aphasia is a true language disorder and not simply a consequence of abulia (Warren et al., 2003).
Some patients with impaired generation of verbal messages have defective (rather than simply absent) verbal output. The occurrence of spontaneous verbal stereotypies or echolalia (repetition of others' utterances) suggests a loss of capacity for self-generated verbal thought; such phenomena are often associated with other evidence of environmental dependency in patients with frontal lobe or fronto-subcortical damage (Denny-Brown, 1956; Bathgate et al., 2001).
The sense of the message: conceptual content and vocabulary
Once a plan for a verbal message is generated, the message must be elaborated with specific content and function words. The sense of a spoken thought or message depends on its conceptual content. It is possible to convey the constituent concepts of a message even where the structure is disorganized or degraded, and the converse is also true. To take the example of the message ‘the bird sat on the branch’: compare ‘bird sat branch or ‘the birt sit on the brench’ (content preserved, structure degraded) with ‘the thing pit on the tam’ (structure preserved, content degraded). The content of speech can be assessed at the level of individual words themselves, and the way they are combined to convey a more extended message in a sentence (Fig. 1).
Impaired content at the level of individual words is evident as a deficient vocabulary—the patient may use approximate or imprecise expressions (circumlocutions) that substitute for a single word (e.g. ‘the thing’, ‘the whatchamacallit’) and speech (though fluent) may seem vague and lacking in substance. Errors of meaning or ‘semantic paraphasias’ may be evident as context-inappropriate words (for example, ‘dog’ may be used when ‘pig’ is meant). Superordinate or generic terms (such as ‘animal’) are used rather than more specific ones (such as ‘squirrel’ or ‘lobster’) and often accompany the use of circumlocutory phrases in an attempt to compensate for the deficiency of vocabulary. There may also be increased reliance on stereotyped expressions, stock phrases and clichés. Such fluent but ultimately empty speech is characteristic of conditions in which there is damage to the verbal knowledge store, the paradigm for which is SD with focal degeneration of the left temporal lobe (Warrington, 1975, Snowden et al., 1989; Hodges et al., 1992; Chan et al., 2001). In this situation there is often evidence from the history and on further specific language tasks for impaired comprehension of single-word meaning. A more common scenario is difficulty retrieving words from storage despite evidence that comprehension of the meaning of words (at least initially) is well preserved: this situation prevails in a range of different disorders, including early AD, and indeed can be considered ‘word-finding difficulty’ in its purest operational sense. In this situation there may be prolonged word-finding pauses affecting both spontaneous discourse and naming. Linguistic deficits arise at an early stage in ∼10% of cases of typical amnestic AD: impaired verbal fluency is typically prominent (Emery, 2000), whereas speech production is characteristically relatively preserved in the earlier stages of the disease (Bayles and Kasniak, 1987). In some patients with progressive aphasias, idiosyncratic or novel expressions (neologisms) may dominate speech output, ‘jargon aphasia’ (Marshall, 2006): this is rare in degenerative disease (Ostberg et al., 2001; Rohrer et al., 2007).
Impairments of sentence-level content manifest as a lack of coherence in conveying the message—sentences may trail off unfinished, or tangential and context-inappropriate words or fragmentary phrases may be inserted, so that it is difficult to follow the patient's line of thought. Disordered speech of this kind is observed in acute brain syndromes, in which attentional and executive deficits may make organized or sustained verbal expression impossible (Chedru and Geschwind, 1972), and also in the intermediate and later stages of AD. Disorganized verbal output at the level of more complex narrative or discourse is a feature of the behavioural variant of frontotemporal lobar degeneration (bvFTLD), in which executive dysfunction is typically prominent (Ash et al., 2006). Though not conventionally considered with the canonical language syndromes, such higher-level difficulties with verbal output illustrate the wide range of phenomena that may impair patients' ability to communicate and the limitations of conventional models of ‘aphasia’.
The structure of the message: grammar and phonology
The structure of a verbal message can be considered at two levels: grammar, the ordering of words at the level of phrases and sentences, including the use of ‘function words’ (articles, prepositions and conjunctions); and phonology, the selection and ordering of individual sounds into syllables and words. Impaired grammatical structure (agrammatism) typically manifests as disjointed or ‘telegraphic’ speech composed of single words and short phrases, omitting function and connecting words (e.g. ‘bird sat branch’). Incorrect ordering of words may occur, grammatical elements such as plurals or tenses may be misused or binary grammatical alternatives (such as ‘yes – no’, ‘him – her’) may be confused (Frattali et al., 2003). Impaired phonological structure manifests as speech sound errors, or ‘phonemic (‘literal’) paraphasias' at the level of individual words and syllables, most commonly substitutions (‘crabon’ for ‘crayon’), transpositions (‘aminal’ for ‘animal’), omissions (‘elphant’ for ‘elephant’) or additions (‘hippopototamus’ for ‘hippopotamus’) (Duffy, 2005). Such errors often first appear and remain more evident with polysyllabic words. Agrammatism and phonemic errors are typical features of PNFA (Neary et al., 1998, Gorno-Tempini et al., 2004; Grossman and Ash, 2004) and help distinguish this syndrome from the language output difficulties observed in patients with AD (Mendez et al., 2003) (Table 3). Agrammatism and phonological breakdown commonly occur together but relatively pure dissociations have been described in degenerative disease (Caramazza et al., 2000). Agrammatism may be partly masked by other speech-production impairments, unless more detailed testing of the receptive aspects of sentence comprehension or written output is undertaken (Bak et al., 2001, 2006; Code et al., 2006).
Motor programming of speech: phonetics, articulation and prosody
Disorders of the motor programming of speech (Fig. 1) have a different clinical significance from true word-finding difficulty. Nevertheless such deficits frequently co-occur, and this assists in anatomical localization and diagnosis. Here we consider these deficits at some length, because they are difficult to characterize with precision and because they entail several concepts which continue to stimulate controversy in the literature on progressive aphasias. One example is apraxia of speech (AOS). This term has been used to describe a motor speech disorder which (by analogy with other ‘apraxias’) can be defined operationally as impairment of the motor gestures of speech that is not attributable to a primary motor deficit (Darley, 1969; Ogar et al., 2005). Although the cognitive basis of AOS remains controversial, it is likely to arise at the level of cortical programming of phonetics, the step in speech production where the phonological structure is converted into an ‘articulatory score’ that directs the relevant muscles of the vocal tract to produce the word or phrase. AOS is probably therefore synonymous with phonetic breakdown or disintegration. The characteristic features of AOS are slow speech rate with hesitancy (difficulty initiating utterances), effortfulness (with articulatory groping, i.e. multiple attempts at trying to get to the right word and self-correction, worse with longer words), phonetic errors (errors in the shaping, timing and ordering of individual syllables) and dysprosody (abnormal rhythm, stress and intonation, attributable to poor phonetic sequencing rather than a primary prosodic deficit) (Dabul, 2000; Duffy, 2005; Ogar et al., 2005; Duffy, 2006). Patients may describe the problem as a stutter or stammer and there may be re-emergence of a childhood stutter. In a recent review of AOS in degenerative disease, only 10% of cases occurred in an isolated fashion, independently of aphasia or dysarthria (Duffy, 2006). It is associated particularly with PNFA (Josephs et al., 2006a; Duffy, 2006).
In principle, phonetic errors (errors in the execution of a programmed speech sound) are distinct from phonemic errors (errors in the selection of speech sounds to be executed): speech sounds may be selected correctly during the programming of an utterance but then articulated incorrectly or conversely, speech sounds may be selected incorrectly but then articulated correctly. However, in practice this is a difficult distinction to make at the bedside, and the two types of error frequently coexist. Clues to phonetic errors include the presence of distortions (commonly either distorted substitutions e.g. ‘brop-er-ty’ for the target word ‘property’, or additions e.g. prop-er-ta-ty') and the co-occurrence of other features of AOS. This is in contrast to patients with pure phonological or phonemic breakdown: true phonemic errors are not distorted and speech is not effortful (Caramazza et al., 2000).
Speech features such as volume, rate, rhythm and intonation relate principally to the motor programming of speech output. These non-verbal aspects of speech output are most commonly affected in extrapyramidal disease [for example, the disorder of speech timing in Huntington's disease (Darvesh and Freedman, 1996)], and with cerebellar and subcortical (pseudobulbar or bulbar) pathologies. Such speech disturbances are often subsumed under the term ‘dysarthria’. Although dysarthria is most commonly secondary to a ‘peripheral’ disorder, it can occasionally be produced by cortical damage (progressive ‘cortical’ dysarthria or anarthria) (Broussolle et al., 1996; Silveri et al., 2003a; Soliveri et al., 2003). Dysarthric patients are likely to complain of slurred speech (or rarely, an altered or ‘foreign’ accent: e.g. Luzzi et al., 2007), reduced voice volume or other motor symptoms. Dysarthric and phonetic speech errors are generally difficult to distinguish. However, patients with phonetic impairment (AOS) make variable, inconsistent sound errors, and may articulate a word correctly on one occasion but not another, whereas the patient with dysarthria tends to make consistent errors. Like progressive AOS, isolated progressive dysarthria is rare and also overlaps with PNFA. Indeed, it is likely that all three disorders have frequently been conflated in the literature (Duffy, 2006), due both to the overlap and difficulty in distinguishing them and still unresolved problems of definition. This is underlined by the plethora of terms for motor speech disorders in the literature: ‘pure progressive aphemia’ (Cohen et al., 1993), ‘primary progressive anarthria’ (Silveri et al., 2003a), ‘slowly progressive anarthria’ or ‘anterior opercular syndrome’ (Foix–Chavany–Marie syndrome) (Broussolle et al., 1996).
Patients with progressive AOS or cortical dysarthria classically have well-preserved writing, indicating that these are disorders of speech output and that language processing per se is spared (Broussolle et al., 1996; Silveri et al., 2003a). In contrast, impairment at the level of phonological structure will manifest as phonemic errors in both speech and writing. Comparison of the patient's speech and writing is therefore generally a useful means of distinguishing primary phonological and phonetic disorders at the bedside. The severity of the speech deficit also provides a clue: patients with impaired motor programming of speech often have profoundly impaired speech production eventually leading to mutism. However, mutism is an end-stage of a number of disease processes (Kertesz and Orange, 2000) and can occur as an early feature in PNFA (Gorno-Tempini et al., 2006).
Other components of the motor programme that are functionally separate from phonetic encoding can also be disrupted by degenerative disease: a key example is prosody, the intonational pattern of pitch, stress and timing that constitutes the ‘melody’ of speech (Ross, 1981). Many patients with speech-production difficulties lose the normal rhythms of conversational speech and the ability to regulate fine pitch and accent shifts. If severe, dysprosody may disrupt the intelligibility of the utterance as a whole and could be misinterpreted as a primary verbal problem. Commonly, dysprosody is secondary to poor articulation but rare cases of primary progressive dysprosodia have been described (Confavreux et al., 1992; Ghacibeh and Heilman, 2003).
Specific speech and language tasks
The patient's word-finding difficulty can be further analysed using specific speech and language tasks (Table 4), which both corroborate the information obtained so far and may also expose additional deficits. As a result of these tasks, it should be possible to categorize the word-finding difficulty in terms of a core defect (summarized in Fig. 1), leading to a more detailed characterization of the speech or language syndrome (Fig. 2). Each of the suggested bedside tasks can be refined and amplified by more specialized and detailed neuropsychological tests. These allow the language disorder to be quantified or characterized in more detail than is usually possible at the bedside and may allow the identification of mild or ‘subclinical’ deficits that more fully define the cognitive phenotype. This is particularly useful in detecting and tracking disease progression. The information obtained at neuropsychometry, however, is most useful if the neuropsychologist is guided by information provided by the neurologist based on an initial bedside characterization of the problem and differential diagnosis.
Naming
Word-finding depends fundamentally on a capacity to retrieve words from the verbal knowledge store in the appropriate context. This is most conveniently assessed as the ability to name. However, this ability is not related simply to word retrieval: it is an active and multi-step process (Grossman et al., 2004) which calls upon many of the cognitive operations outlined in Fig. 1. Impaired naming, or anomia, is frequent in patients who complain of word-finding difficulties (indeed, patients and their carers frequently characterize the language deficit as a problem with names), and it is a feature of many different disorders. The diversity of clinical situations that lead to anomia underlines the need to evaluate other cognitive functions in order to arrive at a diagnosis. Although pure anomia is uncommon in degenerative settings, both primary verbal storage and word retrieval disorders typically present with anomia. Anomia is the most salient linguistic feature of early AD (Mendez et al., 2003; Blair et al., 2007): in this context, the diagnosis is usually based on impairments in other cognitive domains (notably, episodic memory; see next section). Early striking anomia is a characteristic feature of SD: in this situation, more sophisticated neuropsychological instruments may be required to expose the primary semantic defect (see for example, Howard and Patterson, 1992; Warrington et al., 1998). Because of its importance as a presenting symptom, the broad spectrum of clinical associations and the fundamental role of word retrieval in the language output pathway, we consider the problem of anomia and its practical evaluation in detail.
The evaluation of naming begins with the analysis of the patient's spontaneous speech (see previous section and Tables 2 and 3). Clues to anomia include a dearth of content words (especially low frequency or proper nouns), abundant circumlocutions or frequent word-finding pauses. The nature of the defect is established using a structured series of subtests designed to assess different aspects of naming. Poor performance on these naming tasks may lead to the characterization of a word-finding problem even in patients who do not present with a primary complaint of word-finding difficulty. Conversely, particular patterns of performance on naming tasks may help to establish that the basis for the word-finding impairment lies beyond (or is not confined to) the language system. Naming of objects in the environment depends on intact perceptual processing and activation of the appropriate semantic associations by the percept; only if these operations are successfully accomplished can verbal processing proceed.
Naming should be tested directly both in response to pictured items (confrontational naming) and from verbal description (e.g. ‘a large grey animal with a trunk’). Primary deficits of visual perception or visual knowledge manifest as a better performance in naming to verbal description than naming pictures. Having established a primary verbal deficit, naming performance should be assessed for words of both high and low frequency (e.g. ‘shoe’ versus ‘moat’) as subtle deficits may not emerge for confrontational naming of highly familiar items (Warrington, 1975). It should be established whether there is improvement with phonological (first letter) or semantic (associated item) cueing. Different categories of items should be presented (animals, inanimate objects, familiar faces, colours, nouns versus actions, etc.). Striking category effects are more commonly observed in acute brain damage (such as herpes simplex encephalitis) than in degenerative disease (Warrington and Shallice, 1984; Silveri et al., 1991; Laws et al., 2003), however selective deficits or sparing of noun categories have been described in SD (Robinson and Cipolotti, 2001; Incisa della Rochetta and Cipolotti, 2004; Zannino et al., 2006) and AD (Garrard et al., 1998). Naming deficits may be relatively specific for a particular grammatical class (for example, naming of verbs may be more impaired than naming of nouns in PNFA (Hillis et al., 2002), or selectively spared in AD (Robinson et al., 1999): it is debatable whether this is a primary verbal defect or part of a broader deficit involving knowledge of actions versus objects (Bak et al., 2006).
Naming errors
Any errors made on naming tasks should be recorded: the type of naming error provides important information about the primary defect. Deficits of visual perception manifest as ‘visual’ errors on confrontational naming tasks (for example, a line drawing of a teapot may be called a face). With primary involvement of the verbal knowledge store, there are typically highly consistent deficits that affect naming both to confrontation and from description, but which affect unusual (low frequency) items (e.g. hippopotamus) more than common (high frequency) items (e.g. cat). Naming errors take the form of semantic paraphasias: incorrect semantic categorisations (which may be from related categories: for example, a camel may be called a horse), or substitution of a generic category for a more specific one (for example, a hippopotamus and a lobster may both be called animals, or all animals may become ‘dog’). There may also be circumlocutory responses (e.g. a picture of a squirrel may elicit ‘they live in the garden, grey in colour’). Such errors are characteristically made by patients with SD, however similar errors are also observed not uncommonly in other dementias, including AD and vascular dementia (VaD) (Lukatela et al., 1998) and should be interpreted cautiously.
Deficits involving the process of word retrieval proper (common in early AD) lead to a relatively pure anomia: in this situation, knowledge about words and the phonological encoding of words are preserved, but the means for accessing these stores or linking stored word information with the appropriate phonological code is defective (Hillis, 2007). The selective nature of the anomia can be established from the overall pattern of performance on naming versus other speech and language tasks. On confrontational naming tasks, such patients may offer no response at all or they may produce circumlocutions or semantically (or phonologically) related alternatives to the target item, either due to aberrant activation of alternative stored word codes or in an attempt to compensate for their naming difficulty. Although the nature of circumlocutions and semantic paraphasias in nominal aphasia has been recognized for many years (Luria, 1970), these are frequently misinterpreted as evidence for a primary semantic (verbal knowledge store) defect. Clues to the true nature of the deficit are a tendency to hunt spontaneously through related items in the semantic field (‘it's not a fox … not a rat … it eats nuts … it's a squirrel’) or for naming performance to improve when such additional semantic associations are provided, and retained ability to recognize the correct name when alternatives are presented by the examiner. More conclusively, single word comprehension is intact (see later), whereas this is impaired from an early stage of the illness in diseases (particularly SD) with primary verbal semantic impairment.
Naming errors in patients with a primary breakdown in the phonological encoding of verbal concepts into speech sounds (as in PNFA) generally take the form of literal (phonemic) paraphasias (e.g. ‘hotapitamus’ for hippopotamus) that approximate the target item and which are usually also evident in other contexts (for example, speech repetition) (Mendez et al., 2003). Primary deficits of both word retrieval and phonological encoding (in contrast to primary verbal store defects) may benefit from cueing with the initial letter of the target word. Indeed, patients may complain that the words that elude them in conversation are ‘at the tip of the tongue’ (Delazer et al., 2003; Hillis, 2007). Personal names may present particular difficulties: this is likely to reflect the combined demands of accessing stored information about the subject's identity, retrieving that information from storage, and encoding it phonologically (since proper nouns are generally ‘non-words’ rather than part of the universal lexicon) (Delazer et al., 2003), though the rare occurrence of selectively spared proper names does raise the possibility of separable brain stores (De Bleser, 2006). Neologisms on confrontational naming tasks are comparatively rare in degenerative disease, however the presence of jargon should be noted as it may be of localising value (Fig. 3).
Speech comprehension
Speech-comprehension difficulties commonly coexist with word-finding and language output problems in both acute settings (such as left hemisphere stroke) and degenerative disease. Speech comprehension can be assessed at the level of single words, which depends both on intact perceptual mechanisms and the verbal knowledge store (vocabulary), and sentences, which depends on the ability to hold verbal information on line and to process grammatical relations between words.
Single-word comprehension
Impaired single-word perception manifesting as progressive word deafness has been described rarely in degenerative disease (Serieux, 1893; Mesulam, 1982; Ikeda et al., 1996; Otsuki et al., 1998): these patients have difficulty both in understanding and repeating spoken words but normal comprehension of written material, and speech output is often loud and dysprosodic and may contain phonemic substitutions. The perceptual deficit is likely to lie with auditory temporal acuity and discrimination of speech sounds (Otsuki et al., 1998) and often leads to associated impairments of environmental sound and/or music perception (Serieux, 1893; Otsuki et al., 1998): an apperceptive auditory agnosia. The auditory deficit can be demonstrated at the bedside by testing discrimination of phoneme pairs (e.g. ‘pat – tap, ‘gat - cat’).
Impaired comprehension of single words in the setting of intact acoustic analysis results from a breakdown in verbal knowledge systems. The most striking and selective deficits of single-word comprehension are associated with SD, however semantic impairment is also well documented in AD (Hodges et al., 1993; Garrard et al., 1998, 2005). Primary deficits of the verbal knowledge store lead to reduced vocabulary and also impair comprehension of both spoken and written material at single-word level. The comprehension of nouns can be assessed by asking the patient to point to items named or otherwise described by the examiner, to generate a definition or provide other information about a target word (e.g. ‘What is a squirrel?’), or to choose between alternative synonyms for a target word (e.g. does ‘trench’ mean ‘hedge’ or ‘ditch’?). This can be finessed according to the examiner's assessment of the patient's premorbid level of competence (for example, a highly competent patient with excellent premorbid verbal skills could be asked the difference between laziness and idleness). Defects of word knowledge can be further probed by asking the patient to classify items according to nominated criteria (for example, ‘Is a lion a mammal?’). Degradation of word knowledge typically progresses from more specific to superordinate categories (for example, loss of knowledge about dogs might evolve in the sequence: dachshund—dog—animal). Typically, meaning is retained for broad categories of nouns when more fine-grained classifications are impossible. It is important to take account of this when interpreting patients' responses, and to be prepared to probe for more detail than the patient may initially volunteer: asked ‘what is a hippo?’, the response ‘it's an animal’ indicates only a very general level of superordinate knowledge; one would expect to be able to elicit further information (‘big, lives in Africa, in the water’) if the verbal knowledge store is intact. Comprehension of verbs can also be assessed, for example by having the patient select an appropriate description of actions pantomimed by the examiner (‘pushing’ versus ‘pulling’, ‘catching’ versus ‘throwing’, etc.) or by asking them to produce actions nominated by the examiner. In patients with very impaired language output (for example, in the context of PNFA), gestures can also be used as a tool to assess comprehension of single words (nouns), provided readily manipulable items are chosen (for example, ‘shovel’ or ‘teapot’) and there is not an associated dyspraxia or significant motor deficit.
The organization of brain knowledge systems, in particular the extent to which different modalities and categories of knowledge are dissociable, remains a core theoretical issue in contemporary cognitive neuropsychology. Category-specific deficits of verbal knowledge have been documented in degenerative disease, but category effects are unusual and occur much less frequently than with acute pathologies. There may be selective impairment of the ability to comprehend the names of living things (McCarthy and Warrington, 1988; Lambon Ralph et al., 2003) or inanimate items (Silveri et al., 1997), or concrete versus abstract words (Warrington, 1975). Conversely, there may be relatively preserved comprehension of the names of body parts (Coslett et al., 2002), colours (Robinson and Cipolotti, 2001) or countries (Incisa della Rochetta et al., 1998). Though rare, category-specific deficits are of theoretical importance: the existence of such category effects, together with the consistency of deficits observed in SD and evidence for retention of partial knowledge in SD and AD (Murre et al., 2001; Garrard et al., 2005), argues for degradation of stored concepts (i.e. direct involvement of the knowledge store) rather than loss of access to the knowledge store. One well-established category effect in degenerative disease is the dissociation between noun and verb knowledge. Impairments of noun retrieval and comprehension are well documented (Silveri et al., 2003b) and usually are most salient in SD. Conversely, selective impairments of verb retrieval and comprehension have been demonstrated in patients with frontal dementia syndromes including frontotemporal dementia associated with motor neuron disease (FTD-MND) (Bak et al., 2001). Such patients have particular difficulty in processing verb phrases, and may rely more heavily on noun phrases (such as ‘laddering’ for ‘climbing’) and ‘superordinate’ verbs (such as ‘being’, ‘making’ or ‘having’).
Sentence comprehension
Under most circumstances in daily life, words must be processed not in isolation but combined into sentences. Difficulty with sentence comprehension may occur despite normal single-word comprehension. This pattern suggests that the processing of grammatical relations is deficient, and it may also be associated with particular difficulty understanding verbs rather than nouns (Price and Grossman, 2005). Having established that the comprehension of single words (nouns) is normal, the sentence level of comprehension can be assessed by asking the patient to perform a short sequence of actions according to different syntactic rules (e.g. ‘put the paper underneath the pen that is on the book’, ‘you pick up the watch and then give me the book’). Alternatively, the patient can be asked to identify a picture based on a syntactical sentence description (e.g. ‘point to the boy being chased by the dog’). The comprehension of grammar involves a number of different procedures (including determination of tense and number, interpretation of pronouns and prepositions, analysis of word order and subject-object relations and parsing of clauses). These procedures can be broadly classified as syntactical (relations between words) and morphological (word modifications according to grammatical context) and may have distinct neural bases. Some aspects of grammar processing may be dissociable from sentence comprehension (Cotelli et al., 2007) and can be assessed by asking the patient to detect grammatical errors within written sentences.
Patients with progressive aphasias may exhibit different types of deficits on sentence comprehension tasks, and these may assist in differential diagnosis. An early selective deficit in comprehending grammatical relations may be found in PNFA (Grossman, 2002; Grossman and Moore, 2005; Price and Grossman, 2005), whereas in SD, comprehension of syntactical constructions is typically intact within the limitations of reduced vocabulary. More subtle impairment of sentence comprehension has been documented in patients with AD: this is likely to be multifactorial in origin, including deficits in comprehension of pronouns (Almor et al., 1999) and in processing the structural and semantic coherence of sentences (Grossman and Rhee, 2001; Price and Grossman, 2005). However, other elements of grammar (such as gender, person and tense inflections) may be comprehended normally (Kavé and Levy, 2003). Sentence comprehension impairments have been documented in patients with bvFTLD not conventionally considered ‘aphasic’ (Cooke et al., 2003): in such patients, executive dysfunction and impaired working memory for complex syntactic constructions are likely to be responsible, emphasising the multidimensional nature of sentence comprehension and its susceptibility to a variety of different disease processes.
Speech repetition
Repetition of heard speech depends on intact input and output pathways and the ability to transfer information between these pathways. Accordingly, difficulties with speech repetition occur in patients with impaired processing of incoming speech signals (such as word deafness) and in those with impaired speech output. Like speech comprehension, repetition can be assessed at the level of words and sentences. Patients with word deafness or primary speech production problems may have difficulties even with single word repetition (especially for polysyllabic words) (Westbury and Bub, 1997). Repetition is hesitant and effortful and there are typically many phonemic errors. Patients with agrammatism may show a selective deficit in the repetition of phrases, particularly if these contain novel word combinations (clichés may be repeated more successfully, probably because they are processed as a single unit rather than a string of separate words). Single-word repetition is generally preserved in SD, though sentence repetition is influenced by the level of comprehension. Where comprehension of individual words is lost, there may be ‘migration’ of phonemes between words (e.g. ‘the flag was coloured bright red’ may become ‘the blag was fullered with a right breg’), suggesting that the utterance is encoded as an extended sequence of phonemes (and therefore susceptible to re-ordering) rather than a series of meaningful units (McCarthy and Warrington, 1987). Although overt speech repetition is seldom called upon outside the clinical setting, the cognitive operations that support speech repetition may be involved in processes such as monitoring of one's own spoken output, which is likely to improve the accuracy of communication. It is also likely that the editing of ‘inner speech’ and subvocal rehearsal play an important part in ensuring the coherence of spoken output (Head, 1926). Reduced phonological working memory (Nestor et al., 2003) and defective articulatory rehearsal (Silveri et al., 2003a) may contribute to errors in organizing and monitoring speech output in PNFA.
Reading, writing and spelling
Reading, writing and spelling deficits often accompany word-finding problems in speech, and the assessment of these other language channels is helpful in characterizing word-finding difficulty. Literacy skills are learned rather than innate capacities, and the neural mechanisms that sustain them are likely to have been at least partly adapted from brain systems that support more elementary functions. Deficits of literacy skills are often accompanied by, or secondary to, deficits of visual perceptual or knowledge systems, in addition to any speech disorder. Conversely, performance on literacy tests must take into account any specific longstanding limitation, such as developmental dyslexia. The classical neurological distinction between reading disorders without writing impairment (alexia without agraphia) and those accompanied by writing impairment (alexia with agraphia) corresponds loosely to an information-processing model of the acquired dyslexias (Warren and Warrington, 2007), in which disturbed visual analysis of written words produces a ‘peripheral’ dyslexia (often leaving written output unscathed) and disturbed analysis of written words for sound or meaning produces a ‘central’ dyslexia (often with associated deficits of written output). ‘Central’ dyslexia can be further sub-classified according to which of two functionally parallel routes to reading is predominantly affected: analysis for sound (the phonological encoding of written syllables) and analysis of meaning (sight vocabulary). An analogous information processing model can be used to classify dysgraphia into ‘central’ disorders affecting spelling processes and ‘peripheral’ (output) disorders affecting the motor programming and execution of writing. These classifications have both neuroanatomical and clinical implications. However, mixed forms of dyslexia and dysgraphia are common in degenerative disease, and the extent to which alternative sound- and meaning-based routes to reading and spelling are functionally separate has not been finally resolved.
The patient should be asked to read aloud a passage that includes both irregular words and non-words (e.g. proper nouns); an example is shown in Fig. 4B. The types of error made when reading a passage aloud provides information about the core reading defect. Patients who exhibit letter-by-letter reading have a defect in processing visual word forms: a syndrome of higher order visual perception (the input to the verbal lexicon) rather than a primary language deficit. Mild forms of peripheral dyslexia are not uncommon in AD (Glosser et al., 2002) and more dramatic examples may accompany posterior cortical atrophy (Mendez et al., 2007). Patients with deficits of the verbal knowledge store (in particular, SD) will often ‘regularize’ irregular words (e.g. reading ‘yacht’ as ‘yatched’): this is a ‘surface dyslexia’ (Marshall and Newcombe, 1973; Warrington, 1975), in which reading is based on superficial rules for translating written words to speech sounds, rather than a learned vocabulary that governs the pronunciation of the particular word. Regularization errors are more prominent for lower-frequency words. Analogous deficits occur in languages besides English: for example, a Japanese patient with SD developed selective dyslexia for kanji script (for which pronunciation is constrained by semantic context) but not phonetically regular kana (Fushimi et al., 2003). In contrast, patients with impairment at the level of phonological encoding may have particular difficulty reading non-words, either ‘nonsense’ words (e.g. ‘tegwop’) or proper nouns (e.g. ‘Gifford’): this is a ‘phonological dyslexia’ (Beauvois and Derouesne, 1979; Diesfeldt, 1991), in which learned vocabulary (for both regular and irregular words) is intact but the rules for translating written words to speech sounds are lost, so that novel words cannot be sounded correctly. Phonological dyslexia is frequently observed in PNFA (Mendez et al., 2003) and AD (Friedman et al., 1992). Patients with motor programming deficits tend to stumble reading polysyllabic words.
Analogous errors occur in written spelling of irregular and non-words, respectively. Impaired spelling from vocabulary (‘surface’ dysgraphia) manifests as phonologically plausible renderings of words with irregular or ambiguous spelling (e.g. ‘juice’ may be spelled ‘juse’) (Baxter and Warrington, 1987). Loss of spelling vocabulary is characteristic of the SD syndrome (Graham et al., 2000), but occurs in other settings and is probably the most common disorder of writing in AD (Graham, 2000). Impaired spelling by sound (‘phonological’ dysgraphia) leads to particular difficulty writing grammatical function words and non-words despite competent rendering of nouns, and occurs in PNFA (Graham, 2000) and AD (Luzzatti et al., 2003). Involvement of another language channel (writing) indicates a disturbance of language rather than speech production per se, and may be helpful in distinguishing a true word-finding difficulty from a motor speech disorder. It should be noted however that written expression is often relatively better preserved with fewer errors than speech in patients with primary disturbances of speech production (for, example, early in the course of PNFA). In patients with a disorder of written spelling, the capacity to spell aloud is in general comparably affected. However, relatively selective impairment of oral spelling has been described in patients with AD (Croisile et al., 1996) and the reverse dissociation in VaD (Lesser, 1990). Progressive dysgraphia has rarely been described as a presentation of degenerative disease (O'Dowd and de Zubicaray, 2003): where spelling is disproportionately affected as an early feature, a posterior cortical process is likely.
Sentence generation and completion
Although the generation of a verbal thought or message is the earliest operational stage in the verbal output pathway (Fig. 1), this stage is most reliably assessed once it has been established that other language functions are intact. If dynamic aphasia is suspected based on the constellation of very impoverished propositional speech despite normal (or nearly normal) comprehension, repetition and reading (Luria, 1970; Costello and Warrington, 1989; Warren et al., 2003), the defect can be probed by tasks that require the generation of a novel verbal thought, such as production of a sentence incorporating a target word (e.g. ‘boat’) or completion of an unfinished sentence. In the latter task, performance is typically better if the completion is predictably implied by context (‘the boat passed easily under the …’) than if the completion is open-ended (the girl went to the supermarket to buy a ….'), underlining the ‘dynamic’ nature of the defect and its dependence on the requirement for active verbal planning (Snowden et al., 1996; Warren et al., 2003).
Motor assessment
Although they are not strictly part of the assessment of word-finding difficulty, it is useful to characterize deficits of motor programming at the bedside, in order to disambiguate these from any language deficit and more broadly, to advance the clinical diagnosis. The patient can be asked rapidly to repeat a single syllable (e.g. ‘pa, pa, pa ….’) (Dabul, 2000; Duffy, 2005). Performance will be inaccurate in dysarthric patients with changes in either rate or rhythm, whereas performance is usually relatively normal in AOS. However, patients with AOS have great difficulty when they are asked rapidly to repeat a combination of syllables such as the phrase ‘pa-ta-ka’ (Dabul, 2000; Duffy, 2005, 2006): the phrase is poorly sequenced and there are often distortions and/or additions.
A taxonomy of the progressive aphasias
The analysis of spontaneous speech and specific speech and language tasks together allow the patient's speech syndrome to be defined (Fig. 2). While it is usually possible to align the individual case with one of these syndromes predominantly, syndromes commonly overlap and fragmentary syndromes are common. Moreover, each of the syndromes can occur in isolation (albeit with widely varying frequency) or as part of a more widespread disorder. PNFA and SD are the most common and the best defined syndromes: they are the canonical subtypes of the progressive aphasias and form part of most clinical classifications of FTLD (e.g. Neary et al., 1998; Grossman and Ash, 2004). Considered as a group, however, the taxonomy of the progressive aphasias remains among the most problematic confronting clinical neurology. Despite these caveats, an appreciation of the relations between the progressive aphasia syndromes and their disease associations helps guide the assessment of the individual patient and the formulation of a differential diagnosis. Here we consider each of the syndromes as they are schematized in Fig. 2.
Dynamic aphasia generally occurs in the context of an executive syndrome such as progressive supranuclear palsy (PSP) (Esmonde et al., 1996; Robinson et al., 2006) or a frontal degeneration (Snowden et al., 1996; Warren et al., 2003). In contrast, PNFA frequently occurs without cognitive features beyond the domains of speech and language output or other neurological features. It does, however, overlap with other degenerative syndromes, such as the corticobasal degeneration (CBD) syndrome (Graham et al., 2003), MND (Caselli et al., 1993) and PSP (Josephs et al., 2005). Pure progressive dysarthria is rare (Soliveri et al., 2003) and commonly either heralds another disorder (such as MND or CBD) or occurs as part of an overlap syndrome with PNFA or progressive AOS. Isolated progressive AOS is also probably rare (Duffy, 2006) but commonly overlaps with PNFA (Josephs et al., 2006a, Duffy, 2006). The true status of ‘isolated’ PNFA [defined as telegraphic speech, agrammatism, phonemic (rather than phonetic) errors and anomia], independent of an articulatory disorder consistent with AOS, has recently been called into question (Josephs et al., 2006a, Duffy, 2006). Discrepancies in the classification of cases between published series precludes a resolution at present, however more accurate differentiation will be important in refinement of clinico-pathological correlations (Josephs et al., 2006a).
Pure progressive anomia is probably rare and there are few cases on record (Graham et al., 1995; Papagno and Capitani, 2001; Ingles et al., 2007). All patients who have been followed for a substantial period of time have subsequently developed more typical features of SD, suggesting that such cases represent an atypical slowly progressive SD rather than a separate syndrome (Knibb and Hodges, 2005). Logopenic aphasia has been described as an isolated phenomenon in a small number of cases to date (Kertesz et al., 2003; Gorno-Tempini et al., 2004; Rosen et al., 2006). It is described as language output that is ‘slow in rate, grammatically simple but correct, and halted by frequent word-finding pauses’ (Gorno-Tempini et al., 2004). In the only detailed study (Gorno-Tempini et al., 2004), 10 patients who met ‘general PPA clinical criteria’ (i.e. those of Mesulam, 2001), but ‘did not show a pattern of speech and language deficit compatible with PNFA or SD’ were designated as having logopenic aphasia. Detailed neuropsychological evaluation in this group showed that as well as the speech output characteristics of slow rate with word-finding pauses, patients had sentence comprehension difficulties, impaired repetition, dyslexia (with errors on both irregular and nonwords) and anomia but with relatively preserved semantics and phonology. There was also evidence of impaired verbal memory. This clinical picture would be compatible with an atypical variant of AD, and indeed, prominent word-finding pauses are commonly observed in cases of AD with more typical amnestic presentations.
The classical SD syndrome rarely forms part of a more widespread disorder (Hodges et al., 1992; Rossor et al., 2000). However, a ‘mixed’ progressive aphasia with features of both PNFA and SD has been described (Grossman and Ash, 2004): these patients may be fluent initially but become non-fluent as the disease progresses. Unlike in typical PNFA/progressive AOS, phonetic and motor impairments are not a prominent feature, and unlike in classical SD, parietal lobe features frequently develop (Rohrer et al., in press). In contrast to logopenic aphasia, word-finding pauses are not salient, and evidence for an association with progranulin mutations (Rohrer et al., in press) suggests that the spectrum of pathological associations may also be distinct. The nosological status of this progressive mixed aphasia syndrome and its relationship to the other canonical progressive aphasia syndromes remain to be defined.
Associated clinical features
Speech and language syndromes in degenerative disease are rarely isolated, and in general it is necessary and often helpful to consider associated cognitive and neurological features in localizing the disease process and arriving at a differential diagnosis (Figs. 1 and 2). Clinical judgement is required, first, in deciding whether word-finding difficulty is in fact likely to be secondary to deficits in one of these other domains. In many cases the clue to this lies with the history, and examination can then be directed toward an initial evaluation of other deficits before embarking on a detailed and potentially misleading analysis of word-finding proper (for example, significant visual perceptual impairment may preclude any meaningful assessment of word retrieval based on picture naming tasks). A second key objective is to determine whether the patient has a focal language-based dementia, or whether word-finding difficulty is a leading feature of a more generalized process.
Episodic memory
Impairment of episodic memory, the record of events and episodes from the individual's daily life, is a hallmark of AD and is also seen in many other dementias. Pauses in conversation while the patient struggles to recall a name or other detail are commonly described as difficulty in finding words (or names). In particular, patients may lose the thread of a sentence and simply ‘forget’ how the sentence was intended to end: the problem here lies primarily with memory and attentional processes rather than with word-finding per se. The evaluation of memory is particularly important in deciding whether the patient's word-finding difficulty is a manifestation of a progressive aphasia (in which case episodic memory is typically well preserved) or an alternative diagnosis with more widespread cognitive impairment, in particular AD. An impression of this is usually formed from the history: patients with progressive aphasias generally are able to indicate detailed knowledge of current affairs and rarely have significant topographical difficulty, whereas deficits in these aspects of episodic memory typically occur early in the course of Alzheimer's disease. Consensus criteria for the clinical diagnosis of PNFA require the absence of ‘severe amnesia’ (Neary et al., 1998). Available evidence suggests that, while working memory may be deficient in the context of an associated dysexecutive syndrome, episodic memory is generally well preserved in PNFA (Libon et al., 2007). The situation in SD is more complex: amnesia for episodic material is typically not a major clinical issue in these patients, however the use of verbal material on more formal neuropsychological testing (as in tests of recognition memory for words) could in principle confound the assessment of episodic memory per se. Where appropriate indices are used, episodic memory can be shown to be comparable to that of healthy subjects in only a proportion of cases (Nestor et al., 2006, Scahill et al., 2005), and this effect is not wholly attributable to disease stage. The clinical message is that episodic memory impairment should not be equated uncritically with AD (just as semantic deficits do not equate to SD): the relative preponderance of deficits in the episodic and semantic domains of memory, and the more qualitative aspects of the clinical history, are likely to be more reliable for differential diagnosis, pending a more sophisticated understanding of the detailed interaction of these different memory systems in different degenerative diseases.
Semantic memory
In addition to verbal knowledge, which is probed by tests of single-word comprehension, the non-verbal domains of semantic memory collectively comprise the individual's stored fund of conceptual knowledge about the world. While deficits of semantic memory are well described in AD (Rogers et al., 2006), it is particularly relevant to assess these non-verbal domains when SD (or the ‘temporal lobe variant of FTLD’) is suspected, and visual knowledge is the most convenient domain to test at the bedside. Recognition of familiar faces (a privileged category of visual knowledge) can be assessed by having the patient provide information about public figures from their pictures and comparing this with recognition from verbal description and ability to match faces based on perceptual (rather than semantic) criteria. More general aspects of visual object knowledge can be assessed by having the patient draw or colour objects from memory, classify pictures based on semantic criteria (e.g. farm animals versus wild animals) or match pictures of objects according to semantic relatedness (e.g. Egyptian pyramid with a palm rather than a fir tree).
There remains controversy over the relationship between so-called fluent PPA (Mesulam et al., 2003) and SD (Knibb and Hodges, 2005; Adlam et al., 2006). The most prominent features in patients with early SD are anomia, single-word comprehension difficulties and fluent, empty, circumlocutory speech. As verbal deficits generally predominate without obvious non-verbal deficits, it has been argued that these patients should be considered to have a fluent form of PPA (Mesulam, 2001, 2003; Mesulam et al., 2003), reserving the term ‘semantic dementia’ for patients who also have an early associative face- or object- recognition deficit (Mesulam et al., 2003). In information processing terms, these alternatives would represent (in ‘fluent PPA’) a selective defect in linking stored semantic representations for words with otherwise intact aspects of stored semantic knowledge, versus (in ‘SD’) a defect of semantic knowledge more generally (Hillis, 2007). While this distinction has theoretical support, in practice patients with progressive fluent aphasia and seemingly isolated verbal deficits later develop prominent non-verbal deficits (e.g. associative agnosia in the visual and auditory domains) (Hodges et al., 1992; Bozeat et al., 2000). Furthermore, recent studies have suggested that when tested on a series of more demanding tasks, patients who would fit proposed diagnostic criteria for fluent PPA do have associated deficits in non-verbal domains (Adlam et al., 2006), suggesting that ‘fluent PPA’ is equivalent to early SD. ‘Gogi aphasia’, a progressive loss of word meaning described in Japanese patients, is based on a primary amodal semantic deficit, suggesting that this entity, too, is a manifestation of SD (Lambon Ralph and Howard, 2000).
Executive functions, verbal fluency and behaviour
Deficits of executive functions such as abstraction (interpretation of proverbs, cognitive estimates, explaining similarities and differences), response inhibition (as in the ‘go-no go’ task) or motor sequencing (e.g. alternating hand movements) are frequently associated with impaired verbal fluency and more rarely with dynamic aphasia (Warren et al., 2003). Patients with frontal lobe and fronto-subcortical disease may have prominent behavioural disturbances (disinhibition, environmental dependency or apathy), however these are not invariable; conversely, they may occur despite well preserved language skills. Disruption of fronto-subcortical circuitry (for example, in disorders with basal ganglia involvement such as PSP and dementia with Lewy bodies, DLB) commonly leads both to impaired executive function and reduced cognitive processing speed (bradyphrenia) (Cummings and Benson, 1988), a hallmark of the ‘subcortical dementia’ syndrome.
Verbal fluency depends on an efficient mechanism for searching the verbal knowledge store and is properly considered a frontal-executive rather than a primary language function. It requires generation of a strategy for producing verbal output de novo according to some rule or criterion nominated by the examiner. Impaired verbal fluency is often accompanied by other evidence of executive dysfunction, notably in patients with frontal lobe damage (Perret, 1974; Alvarez and Emory, 2006). However, it is worth noting that patients with deficits of the verbal knowledge store itself (e.g. in SD) will also have decreased verbal fluency. Verbal fluency can be assessed as the ability to produce a list of common animals (‘category fluency’) or words beginning with a nominated letter (‘phonological’ or ‘phonemic fluency’). Reductions in fluency may be useful in distinguishing progressive aphasias from other degenerative conditions (Marczinski and Kertesz, 2006), and in particular, reduced letter fluency is a pointer to PNFA (Clark et al., 2005). Performance on such tasks can be scored as the number of words produced in one minute; a useful bedside rule of thumb is that patients should be able to produce words as quickly as the examiner can write them down. Subtle or variable reductions in fluency should be attributed with caution; fluency tasks are more difficult to interpret in patients with deficits of speech production (in which the output pathway is itself affected), and ‘blocking’ due to anxiety is common in healthy people. Poor performance on these tasks should be explored with further tests to identify the nature of the difficulty more precisely.
PNFA is rarely associated with a behavioural syndrome early in the illness (Rosen et al., 2006) although patients are often frustrated and can become depressed at their inability to communicate. In contrast, SD is associated with behavioural features similar to bvFTLD (Snowden et al., 2001; Rosen et al., 2006), which may be related to increased right temporal lobe involvement as the disease progresses. Symptoms include irritability, apathy, disinhibition and altered eating behaviour. Behavioural features may be qualitatively different in SD compared to bvFTLD: for example, food fads are common in SD versus overeating in bvFTLD, and compulsions are more common in SD (Snowden et al., 2001).
Orofacial praxis
Orofacial apraxia refers to an impairment of volitional coughing, yawning or other complex orofacial actions despite intact reflex movements. It frequently (though not invariably) accompanies disorders with impaired speech production and AOS, such as PNFA, CBD or FTD-MND (Tyrrell et al., 1991; Lang, 1992; Chapman et al., 1997; Ozsancak et al., 2004; Duffy et al., 2007). Orofacial apraxia is also described in atypical PSP syndromes (Josephs et al., 2005) including a recent case study of a patient with ‘progressive oculo-orofacial-speech apraxia (POOSA)’ (Roth et al., 2006) associated with a supranuclear gaze palsy and a number of behavioural symptoms (including altered eating behaviour).
Posterior cerebral functions
Posterior cerebral functions including visual perceptual and spatial processing, calculation and limb praxis should be assessed both to ensure that apparent word-finding difficulties are interpreted correctly and to provide a complete picture of the cognitive syndrome, which may in turn suggest a particular diagnosis [such as corticobasal degeneration, CBD (Graham et al., 2003), the posterior variant of AD (Benson et al., 1988; McMonagle et al., 2006) or DLB (Gibb et al., 1987; McKeith et al., 2004)]. Significant early posterior cortical dysfunction is unusual in PNFA, SD and the FTLD spectrum in general, though emerging evidence suggests that apraxia and other posterior hemispheric deficits may be relatively more common in patients with mutations in the progranulin gene (Rohrer et al., in press).
General neurological examination
The general neurological examination is frequently normal in many of the degenerative speech and language disorders. However, associated neurological features, if present, can be diagnostically helpful in certain situations. Orofacial apraxia is a special instance, due to the intimate relation between the control of speech and other orofacial movements, however certain other features should also be sought specifically. Particularly relevant to the complaint of word-finding difficulty are associated behavioural abnormalities (bvFTLD or PSP), dysphagia (fronto-subcortical processes), primitive reflexes (frontal lobe disorders), upper motor neuron signs (VaD), fasciculations and amyotrophy (MND) or extrapyramidal features (parkinsonian syndromes). Some conditions have signature neurological abnormalities (for example, gaze palsy and postural instability in PSP, an asymmetric akinetic-rigid syndrome and alien limb in the CBD syndrome). Asymmetric (predominantly right-sided) extrapyramidal signs are not uncommon in patients with PNFA (Mesulam and Weintraub, 1992; Mesulam et al., 2003; Gorno-Tempini et al., 2004). It remains unclear what proportion of PNFA cases with hemiparkinsonism should be classified within the spectrum of the CBD syndrome.
Neuroanatomy of the progressive aphasias
Traditionally in clinical neurology, the history of the mode of onset and development of the complaint suggests the type of disease process responsible, while the findings on examination allow anatomical localization. Applied to word-finding difficulty in degenerative disease, the bedside assessment (Fig. 2) often allows the patient's word-finding difficulty to be characterized according to the cognitive process primarily affected (Fig. 1), and in turn, to be localized generally within the brain network mediating different components of the word-finding process (Fig. 3). However, detailed anatomical correlation is problematic even in ‘focal’ dementias dominated by selective neurolinguistic defects. This reflects both the distributed nature of the language system (Hillis, 2007) and the nature of the underlying disease processes. While for some syndromes (notably, SD) clinico-anatomical correlation is relatively precise, in other syndromes brain atrophy is often subtle or equivocal in the early stages, many patients have mixed phenotypes that cannot be simply correlated with structural damage seen on the scan, and a number of degenerative diseases in which word-finding difficulty may be prominent (for example, CBD) lack diagnostic atrophy profiles. From the perspective of anatomical localization in progressive aphasia, group and longitudinal cohort studies therefore have a particularly important role to play, and are in general more informative than information derived from individual patients or detailed single case studies. Unbiased techniques for the analysis of group data such as voxel-based morphometry (VBM) can establish consistent neuroanatomical correlations at a population level that would be difficult to detect from visual inspection alone. A corollary of this is that clinical interpretation is essential to avoid misinterpreting potentially spurious correlations. In principle, the problem of anatomical correlation can be considered at the level of deficits in particular cognitive operations and at the level of syndromes, though these levels are frequently difficult to distinguish in practice. Here we consider available information concerning each of these levels of anatomical correlation, based on structural and functional imaging and pathological studies in patients with degenerative disease.
Message generation
Propositional speech production in normal subjects involves the left superior frontal gyrus, left frontal operculum and rostral left temporal cortex (Blank et al., 2002). In patients with dynamic aphasia and focal lesions, brain imaging has implicated the anterior left frontal lobe (Luria, 1970; Costello and Warrington, 1989; Snowden et al., 1996; Robinson et al., 1998). While it is not possible to draw firm conclusions regarding the macro-anatomical correlates of propositional speech failure in dynamic aphasia, it is likely that the syndrome results from damage involving a distributed left fronto-subcortical network (Warren et al., 2003).
Message sense
Word retrieval
Word retrieval has been studied using VBM in PNFA, SD, bvFTLD, CBD and AD (Grossman et al., 2004): the findings are consistent with multifocal interruption of a distributed, asymmetric (predominantly left-sided) brain network. Left lateral temporal cortex was involved in all disease groups and the volume of this region correlated with naming accuracy. Additional correlations were observed specifically in left inferior and lateral frontal areas in PNFA, anterior cingulate in AD and right inferior frontal and temporal areas in CBD. This evidence is consistent with partially distinct substrates for naming deficits in different diseases, arising from the disruption of component processes such as semantic memory and visual perceptual functions. Further evidence suggests distinct anatomical substrates for naming specific categories of objects (Brambati et al., 2006): in a mixed group of patients with different degenerative diseases, naming performance for drawings of animate items correlated with grey matter volume at the right temporal pole, while for inanimate items of equivalent familiarity, performance correlated with grey matter in the left posterior middle temporal gyrus. Functional imaging evidence in healthy subjects has demonstrated that the mesial temporal lobe is engaged during word retrieval (verbal fluency tasks) (Pihlajamaki et al., 2000), suggesting a potential substrate for the anomia observed in early AD.
Verbal knowledge
The consistent and relatively focal involvement of the left temporal pole, anterolateral and inferior left temporal lobe in SD (Galton et al., 2001; Chan et al., 2001) suggests that neocortical regions in the anterolateral and inferior temporal lobe are critical for verbal knowledge. The degree of atrophy of anterolateral left temporal neocortical areas correlates with VBM measures of semantic impairment (Mummery et al., 2000). However anterolateral temporal neocortical regions are not affected in isolation: there is frequently atrophy of the hippocampal formation (albeit asymmetrically and predominantly anteriorly), amygdala and entorhinal cortex (Galton et al., 2001; Chan et al., 2001), with variable extension into the posterior temporal lobe and inferior frontal lobe (Mummery et al., 2000). Disconnection between temporal lobe areas (Harasty et al., 2001) and from posterior and inferior regions that are distant from the site of maximal structural damage may also contribute to the pathogenesis of semantic deficits (Mummery et al., 1999). Although it is difficult to establish precise anatomical correlates for particular categories of word knowledge in degenerative diseases, knowledge of verbs has been specifically associated with pathological involvement of inferior frontal areas, perhaps implicating dorsal motor pathways concerned with action processing (Bak et al., 2001).
Message structure
Deficits in both the comprehension and production of grammar are associated with atrophy involving the inferior frontal gyrus and insula (Harasty et al., 2001). Impaired syntactic comprehension has been correlated with involvement of the left posterior temporal-inferior parietal lobe (Gorno-Tempini et al., 2004) and reduced activation of a distributed frontal network mediating grammatical encoding and working memory for syntactic structures (Cooke et al., 2003). Little evidence is available concerning the substrate of phonological encoding per se, however this is likely to involve a distributed left peri-Sylvian network involving the inferior frontal lobe, anterior and posterior superior temporal areas overlapping that implicated in grammatical processing (Harasty et al., 2001; Nestor et al., 2003; Gorno-Tempini et al., 2004).
Motor programming
Partially overlapping regions including the left frontal operculum and anterior insula have been identified in group and single-case studies of speech production breakdown in PNFA and cortical anarthria/AOS (Nestor et al., 2003; Gorno-Tempini et al., 2004), implicating these dominant anterior regions in the motor programming of speech. The region of metabolic abnormality extends widely beyond the relatively circumscribed tissue destruction detected on structural imaging (Tyrrell et al., 1991; Nestor et al., 2003). The insula may play a crucial role in linking grammatical, phonological and articulatory networks (Harasty et al., 2001). Early mutism has been associated with atrophy involving the pars opercularis and its subcortical connections (Gorno-Tempini et al., 2006). The anatomical basis of progressive dysprosody is poorly defined, but predominantly right-sided peri-Sylvian and frontal atrophy has been demonstrated in individual cases (Confavreux et al., 1992; Ghacibeh and Heilman, 2003). The anatomical and pathophysiological substrates of the component operations of the speech output pathway are peculiarly difficult to isolate, and there is a pressing need for detailed neuroanatomical and neurophysiological correlation of specific functions and deficits (for example, to help resolve the difficult distinction between phonetic and phonemic deficits).
Syndromes
Whereas correlations between brain anatomy and particular cognitive deficits can be established by applying appropriate neuropsychological measures across syndromes and diseases, to establish the anatomical basis of a syndrome depends on how that syndrome is defined. The different syndromes within the progressive aphasia spectrum lack detailed, universally accepted consensus criteria, and interpretation of anatomical data derived from brain imaging and pathological studies remains difficult. Despite this caveat, a recent meta-analysis of 267 subjects with FTLD based on both VBM and metabolic imaging data concluded that alterations in specific brain networks could be identified in each of the canonical FTLD clinical subtypes, as defined using available consensus criteria (Schroeter et al., 2007): a medial and orbito-frontal network for bvFTLD, a predominantly left-sided anterior and inferior temporal network for SD, and a left superior temporal and frontal opercular network for PNFA. In general, dementias that produce selective impairments of speech and language processing are associated with asymmetric atrophy predominantly involving the left peri-Sylvian cortices and anterior temporal lobe, and certain broad patterns consistently emerge from both single-case and group studies in patients with focal dementia syndromes. However, involvement of the left peri-Sylvian cortex typically occurs in the context of more widespread involvement of other cortical and subcortical regions in both cerebral hemispheres (Ikeda et al., 1996; Broussolle et al., 1996; Snowden et al., 2007). Conversely, a particular anatomical region may be implicated in diverse language phenotypes (for example, the posterior superior temporal lobe—inferior parietal lobe region in logopenic and ‘mixed’ aphasias, and rare cases of progressive jargon aphasia: Gorno-Tempini et al., 2004; Mesulam et al., 2007; Rohrer et al., 2007).
Metabolic brain-imaging techniques (single photon emission computed tomography, SPECT; positron emission tomography, PET; and functional MRI, fMRI) suggest that dysfunction of left hemisphere language networks (Westbury and Bub, 1997; Mesulam, 2001; Diehl et al., 2004) predates and predicts the development of brain atrophy in the progressive aphasias. The functional derangement extends beyond the zone of tissue loss, and there may be abnormal (possibly compensatory) activation beyond the classical language areas (Mesulam, 2001; Sonty et al., 2003). Functional changes may be confined to the left hemisphere or bihemispheric (Westbury and Bub, 1997; Soriani-Lefèvre et al., 2003). ‘Non-fluent’ phenotypes are associated with hypometabolism and decreased perfusion of frontal peri-Sylvian language areas, while ‘fluent’ phenotypes are associated predominantly with temporal or temporo-parietal dysfunction (Tyrrell et al., 1991; Mesulam, 2001; Soriani-Lefèvre et al., 2003). Speech-production impairment associated with PNFA is likely to be attributable to involvement of the left anterior insula (Nestor et al., 2003). These patterns correlate with neuropsychological profiles and clinical evolution (Tyrell et al., 1991; Nestor et al., 2003). Bilateral involvement of posterior temporo-parietal association cortex has predictive value for AD rather than non-AD pathologies in patients with PNFA (Nestor et al., 2007). Partial cerebral reorganization has been documented both in PPA (Vandenbulcke et al., 2005) and probable AD (Nelissen et al., 2007), manifested as a relative shift of language processing to the right hemisphere, though the functional effects of such ‘laterality shifts’ remain difficult to predict. Proton magnetic resonance spectroscopy has documented asymmetric axonal injury within the arcuate fasciculus in PPA (Catani et al., 2003) consistent with the focal involvement of white matter tracts linking cortical language areas. This supports recent evidence for reduced connectivity between anterior and posterior language areas during language tasks in PPA (Sonty et al., 2007). Such evidence underlines the need for studies that move beyond anatomical profiling to assess alterations of anatomical connections and functional relationships within distributed language networks in the progressive aphasias.
Neurobiology of the progressive aphasias
This clinical analysis of the progressive aphasias raises a number of issues relevant to the neurobiology of these disorders. In this section we consider these issues under the rubric of three broad neurobiological problems: the basis for phenomenological differences between the progressive and acute aphasias; the relations between clinical phenotypes and tissue pathology; and the molecular genetics of inherited speech and language syndromes.
A comparison of acute and progressive disorders with word-finding difficulty
Although there is considerable overlap between the disorders of word-finding in acute disease states (in particular, the vascular aphasic syndromes) and in the progressive aphasias, certain features are more typically seen in one setting rather than the other. These divergences are both relevant to the clinical analysis of language dysfunction in these different disease states and of considerable interest for the pathophysiological insights they provide into language neurobiology. Key clinical features of the language disturbance in selected acute and progressive disorders with prominent word-finding difficulty are summarized in the appendix (Tables A1 and A2).
Table A1.
Comparison of some clinical syndromes with word-finding difficulty: acute
| Clinical features | Broca's | Wernicke's | Temporal lobe encephalitis (e.g. HSV) |
Delirium |
|---|---|---|---|---|
| General | Hesitant, effortful, ‘telegraphic’ (initially often global aphasia) |
Fluent, empty, circumlocutions and neologisms, jargon |
Fluent, empty, circumlocutions | Fluctuating impairment, perseveration |
| Message initiation | Sparse | Normal or increased | Sparse | Variable |
| Semantic errors/circumlocutions | Present | Often prominent | Present | Present: context-inappropriate words |
| Phonemic errors | Prominent | Present | Rare | Rare |
| Grammar | Agrammatic | Usually normal | Usually normal | Normal—may be fragmented |
| Articulation | Effortful | Normal | Normal | Normal |
| Prosody | Aprosodic | Normal or exaggerated | Normal | Normal |
| Naming | Anomia: mainly phonemic errors |
Anomia: semantic or mixed errors, neologisms |
Anomia: mainly semantic errors, may be category specific |
Anomia: perseveration, variable errors (depending on attention) |
| Comprehension | Single words may be intact; sentences impaired (agrammatism) |
Poor sentence comprehension, variable single word comprehension |
Mildly impaired | Intact though influenced by attention |
| Repetition | Difficulty with polysyllabic words |
Affected by task comprehension | Usually intact | Influenced by attention |
| Reading | Effortful with phonological errors |
Impaired, mixed errors | May have surface dyslexia | Influenced by attention |
| Writing | Sparse, agrammatic, phonological errors |
Impaired, mixed errors | May have surface dysgraphia | Influenced by attention |
| Sentence completion | Not disproportionately impaired |
Affected by task comprehension | Not disproportionately impaired |
Influenced by attention |
| Verbal fluency tasks | Reduced | Reduced | Reduced | Reduced |
| Other cognitive features | May have orofacial apraxia, often none |
Usually none | May have amnestic state, Kluver Bucy syndrome |
Disorientation Disturbed attention and alertness |
| General neurological examinationa |
Right hemiparesis | Right hemiparesis, right homonymous upper quadrantanopia |
Motor restlessness, carphology | |
| Primary deficit | Structure of the message/motor programming |
Sense of the message | Sense of the message | Variable, mixed |
HSV = Herpes simplex encephalitis.
Helpful if present.
Table A2.
Comparison of some clinical syndromes with word-finding difficulty: progressive
| Clinical features | AD | SD | PNFA/progressive AOS | bvFTLD | VaD/subcortical |
|---|---|---|---|---|---|
| General | ‘Logopenic’ with word- finding pauses, losing train of sentence |
Empty, circumlocutory, semantic errors |
Hesitant, effortful, ‘telegraphic’, phonemic errors |
Economy of speech with short, terse phrases |
Word-finding pauses, slow |
| Message initiation | Normal | Normal | Normal | May be difficult | Normal |
| Semantic errors | Present | Frequent | Rare | Usually none | Usually none |
| Phonemic errors | Rare | Rare | Frequent | Usually none | Rare |
| Grammar | Usually normal | Usually normal | Agrammatic | Usually normal | Usually normal |
| Articulation | Normal | Normal | Effortful, stuttering | Normal | May be impaired |
| Prosody | Normal | Normal | Aprosodic | Normal | Normal |
| Naming | Anomia: visual and semantic errors |
Anomia (severe): circumlocutions, superordinate terms, semantic errors |
Anomia: phonemic errors | Often normal | Anomia (often mild): mixed errors |
| Comprehension | Single words often intact; syntax may be impaired |
Poor single words | Single words often intact; sentences impaired (agrammatism) |
Often normal | Often normal |
| Repetition | May have difficulty with sentences |
Intact where comprehended | Difficulty with polysyllabic words |
Usually normal or spontaneously increased (echolalia) |
Usually normal |
| Reading | May have phonological dyslexia |
Surface dyslexia | Effortful phonological dyslexia |
Usually normal | Slow but few errors |
| Writing | May have phonological or mixed dysgraphia |
Surface dysgraphia | Phonological dysgraphia | Usually normal or increased (hypergraphia) |
Slow but few errors |
| Sentence completion | Not disproportionately impaired |
Not disproportionately impaired |
Not disproportionately impaired |
May be disproportionately impaired (dynamic aphasia) |
May be disproportionately impaired (dynamic aphasia) |
| Verbal fluency tasks | Reduced | Reduced (esp category) | Reduced (esp phonological) | Reduced | Reduced |
| Other cognitive features |
Episodic and topographical memory impairment early |
May have visual agnosia | May have orofacial apraxia, mild dysexecutive, often none |
Often dysexecutive | Dysexecutive, impaired attention, bradyphrenia |
| General neurological examinationa |
Generally normal. May have myoclonus |
Generally normal | May have parkinsonism, features of parietal lobe dysfunction in CBD, UMN/LMN signs in MND |
May have primitive reflexes | ‘Apraxic’ gait, brisk reflexes. May have features of specific diseases, e.g. supranuclear gaze palsy, postural instability in PSP |
| Primary deficitb | Sense of the message | Sense of the message | Structure of the message/ motor programming |
Initiation of speech, sense of the message |
Variable |
AD = Alzheimer's disease; AOS = apraxia of speech; bvFTLD = behavioural variant of frontotemporal lobar degeneration; CBD = corticobasal degeneration syndrome; esp = especially; LMN = lower motor neuron; MND = motor neuron disease; PNFA = progressive nonfluent aphasia; PSP = progressive supranuclear palsy; SD = semantic dementia; UMN = upper motor neuron; VaD = vascular dementia.
Helpful if present.
See text and Fig. 1.
Anomia occurs in all disorders that affect word-finding and is often accompanied by deficits in other language areas. In aphasic stroke it commonly remains as an isolated deficit as recovery occurs (Kertesz and McCabe, 1977), and it may be the only obvious disturbance of language in patients with chronic temporal lobe epilepsy and following temporal lobectomy (Mayeux et al., 1980; Langfitt and Rausch, 1996), whereas pure anomia is rare (or rarely persists as an isolated feature) in degenerative disease, reflecting the diffuse and progressive nature of the disease process.
Deficits of single-word comprehension are characteristic of the paradigmatic disorder of verbal knowledge, SD (Warrington, 1975; Snowden et al., 1989; Hodges et al., 1992) and are also common in acute lesions involving the anterior temporal lobe (notably herpes simplex encephalitis) (Warrington and Shallice, 1984; Noppeney et al., 2007) and the posterior superior temporal lobe (Hillis, 2007). Category effects are more common in the acute setting (Lambon Ralph et al., 2003; Noppeney et al., 2007), perhaps because they require complete destruction of a discrete functional region, rather than the more diffuse and partial damage that attends degenerative pathologies. Fluent aphasia arising from acute damage involving the posterior superior temporal lobe (so-called ‘Wernicke's area’) (Wernicke, 1874) tends to be associated with less-severe impairment of single-word comprehension and more prominent phonological errors and neologisms (‘jargon aphasia’) than are observed in the fluent aphasias of degenerative disease. It is likely that involvement of the posterior superior temporal—parietal lobe junction is necessary for jargon aphasia to occur in degenerative disease: indeed, neologisms are well described in AD (Nicholas et al., 1985), and progressive jargon aphasia and agraphia has been described as a presentation of FTLD with extension to the dominant parietal lobe (Ostberg et al., 2001; Graham et al., 2001; Rohrer et al., 2007) Emerging neurolinguistic models and experimental data suggest distinct core deficits that could plausibly give rise to these different forms of ‘fluent’ aphasia (Gotts and Plaut, 2002; Warren et al., 2005; Jefferies and Lambon Ralph, 2006; Hillis, 2007). Damage involving the posterior temporal lobe and its connections (principally, in acute stroke) is likely to affect the selection or mapping of stored word representations onto incoming speech signals and stored motor patterns, or neuromodulatory systems that govern semantic processing, while diseases predominantly involving the anterior temporal lobes (principally, focal neurodegenerations) affect the verbal store itself (Figs. 1 and 3).
A further key empirical distinction between acute vascular damage and degenerative disease lies in the phenomenon of refractory access dysphasia, in which single-word comprehension is variable and modulated by context. In this condition there is ‘refractoriness’ or temporary unavailability of stored words. Patients are better at finding the correct word (for example, in a word–picture-matching task) if there is a delay between presentations of the target and have more difficulty if distractor items are closely related semantically to the target. In contrast, performance is equivalent for high- and low-frequency words. This is the reverse of the pattern observed in (for example) SD, and indeed refractory access dysphasia appears to be peculiar to non-degenerative conditions (especially cerebrovascular disease) (Warrington and Cipolotti, 1996). It is likely that additional but related cognitive processes are required for activating the sensory and motor representations associated with stored word knowledge, before those representations can be associated with meaning or translated into spoken output. Rather than equating refractory dysphasia simply with interrupted ‘access’ to the verbal store, it may be more appropriate to regard refractory and storage disorders as arising from different kinds of damage involving stored semantic representations for words (Warrington and Cipolotti, 1996).
Phonemic errors are seen both in acute (‘Broca's aphasia’) and chronic progressive (PNFA) settings, and are classically associated with damage involving the left inferior frontal cortex (Broca, 1861) and especially with non-fluent aphasia. Phonological breakdown often co-exists with agrammatism, so that patients with PNFA or with Broca's aphasia typically have telegraphic or ‘agrammatic’ speech and concurrent deficits at the level of sentence comprehension (Grossman and Moore, 2005). Furthermore, just as PNFA is commonly associated with progressive AOS, so patients with a Broca's aphasia often have an accompanying AOS (Dronkers, 1996; Hillis, 2007). Sentence comprehension deficits and phonological and grammatical errors also occur in association with other acute and progressive disease processes affecting the peri-Sylvian language areas (for example in Wernicke's aphasia and in AD) (Grossman and White-Devine, 1998). The occurrence on a degenerative basis of ‘mixed aphasia’ with combined features of phonological breakdown, agrammatism and partial degradation of verbal semantic knowledge, but without jargon or motor programming deficits, suggests that the joint involvement of anterior and posterior language areas as a result of selective dominant lateral temporo-parietal damage may constitute a distinct aphasic syndrome of degenerative disease.
Classically, ‘transcortical’ and ‘conduction’ aphasias are considered to arise from acute damage respectively involving the cortical ‘centres’ for speech comprehension and production or the anatomical pathways connecting these centres (Lichtheim, 1885). ‘Transcortical’ sensory and motor aphasias are associated with relative sparing of speech repetition despite defective comprehension and production, respectively (Goldstein, 1912). Conversely, the hallmark of ‘conduction aphasia’ (Lichtheim, 1885; Bartha and Benke, 2003) is a relatively selective deficit of speech repetition at the level of phrases, with relatively well-preserved spontaneous speech, suggesting a disruption of the transfer of information between input and output speech pathways. There is typically an associated deficit of short-term memory (used in the neuropsychological sense of immediate memory). These different patterns are generally observed as acute vascular syndromes, but can be approximated by progressive aphasias in the FTLD spectrum. Transcortical motor aphasia has features similar to dynamic aphasia which may herald bvFTLD, PSP or other degenerative conditions, while transcortical sensory aphasia closely resembles the fluent aphasia of the SD syndrome, and conduction aphasia has been reported rarely as a presenting feature of FTLD (Hachisuka et al., 1999). By analogy with the explanation proposed for the greater preponderance of semantic category effects in the acute setting, it is likely that the transcortical and conduction syndromes require relatively discrete damage that removes a nodal region or disconnects it from other regions in a functional network. These conditions are most likely to be met in acute vascular damage, rather than degenerative disease, in which there is greater potential for incomplete damage involving a number of cortical regions and their functional connections. In terms of the cognitive operations and brain regions they affect (Fig. 1), the dynamic aphasia observed with head trauma (Luria, 1970) or cerebral tumours (Costello and Warrington, 1989) and the loss of single-word comprehension observed in temporal lobe encephalitis (Okuda et al., 2001) may be closer analogues of the degenerative aphasias than the classical transcortical aphasias of vascular disease.
These observations raise the fundamental issue of the basis for the observed dissimilarities between acute vascular and degenerative aphasic syndromes. To the extent that the acute and progressive aphasic syndromes both illustrate the effects of interruption of distributed functional networks, the acute and progressive aphasias are predicted to share certain phenomenological similarities. The many divergences between the progressive and acute syndromes of language breakdown illustrate the effects of chronic, evolving damage distributed amongst functionally connected brain areas, versus the acute failure of a single network component. The vascular anatomy of the human language cortices means that certain syndromes are intrinsically more likely (for example, jargon aphasia due to focal posterior superior temporal lobe damage) or less likely (for example, semantic disintegration due to anterior temporal lobe damage) to occur in the acute setting. Moreover, the degenerative aphasias result from subtotal damage simultaneously involving a number of cortical regions and their connections, and therefore in principle might have no precise acute analogue. In contrast to acute infarction, degenerative pathologies have the potential for continuing ‘noisy’ information processing within and between affected brain regions. Furthermore, it is likely that the microstructure of language networks is differentially affected by chronic diseases with abnormal protein deposition in surviving cellular components, and by acute necrosis affecting all components in a region uniformly.
Clinico-pathological correlations
Though rarely confirmed during life, prediction of the underlying pathological process is the ultimate goal of clinical diagnosis. However, clinico-pathological correlation in the progressive aphasias remains problematic. Recent years have seen a number of post-mortem case series of patients with progressive speech and language disorders (Rossor et al., 2000; Hodges et al., 2004; Shi et al., 2005; Josephs et al., 2006a,b; Forman et al., 2006; Davidson et al., 2007). It is clear from these studies that the majority of cases fall into one of the two main pathological groups in the FTLD spectrum, with abnormal tau-positive cellular inclusions (including Pick's disease, PSP and CBD), or with ubiquitin-positive (TDP-43-positive) tau-negative pathology (of which three subtypes have been described) (McKhann et al., 2001; Cairns et al., 2007). Patients described as having PNFA who have come to post-mortem have had either tau-positive (Pick's disease, CBD or PSP) or ubiquitin-positive (TDP-43-positive) pathology. In some case series the majority of cases have had tau pathology (Hodges et al., 2004), whilst in others ubiquitin-positive cases have predominated (Shi et al., 2005; Davidson et al., 2007). Two issues are pertinent to this discrepancy: firstly, whether the cases are sporadic [which appears to be most commonly associated with tau pathology (Knibb et al., 2006a)] or familial [commonly associated with type 3 (Sampathu/Neumann classification) ubiquitin-positive (TDP-43-positive) pathology and mutations in the progranulin gene; see below (Hodges et al., 2004; Snowden et al., 2007)]. A second issue is the phenomenology of the language syndrome, and how this is defined: for example, in a recent study specifically comparing progressive AOS and PNFA cases (Josephs et al., 2006a), all seven cases with isolated progressive AOS and all three with mixed PNFA/AOS had tau pathology (five PSP, four CBD and one Pick's disease). SD is associated mainly with ubiquitin-positive (TDP-43 positive) pathology (Rossor et al., 2000; Davies et al., 2005) and early reports suggest the most common subtype is type 1 pathology (Snowden et al., 2007). Despite the emphasis on pathological findings within the FTLD spectrum, a proportion of patients with a primary speech and language syndrome in life will have AD pathology at post-mortem, and indeed this was up to around 30% of PNFA and SD cases in one series (Knibb et al., 2006b). Progressive AOS has also been reported as being caused by AD pathology (Gerstner et al., 2007). The pathology of logopenic aphasia remains open to question. Circumstantial evidence (including an increased frequency of the ApoE4 allele) suggests that AD may account for a proportion of cases (Gorno-Tempini et al., 2004), and if this is a topographical syndrome based on involvement of the dominant inferior parietal lobe, additional disease associations (including CBD) might be predicted.
Clinical genetics
The recent discovery of a developmental speech and language disorder with agrammatism, phonological breakdown and oral apraxia caused by mutations in the FOXP2 gene has stimulated interest in the molecular genetic basis for language and other complex cognitive functions (Vargha-Khadem et al., 2005). While genetically mediated FTLD with autosomal dominant inheritance accounts for a substantial proportion of cases in most series (Goldman et al., 2005), true familial progressive aphasia has been considered rare (Krefft et al., 2003). Recent progress in the genetics of FTLD has refined this picture. Mutations in four genes are known to cause familial FTLD (microtubule-associated protein tau, MAPT, progranulin, GRN, valosin-containing protein, VCP and charged multivesicular body protein 2B, CHMP2B) (Cairns et al., 2007). There are now a number of reports of progressive language syndromes in relation to mutations in GRN. Although only limited details are available concerning the phenotypic spectrum, most of the cases on record have had PNFA (Cruts et al., 2006; Gass et al., 2006), or mixed features of PNFA and SD (Mesulam et al., 2007; Rohrer et al., in press). No cases of a pure SD syndrome have been shown so far to be related to a GRN mutation; indeed, SD is only very rarely familial (Goldman et al., 2005). Articulatory impairment (either AOS or dysarthria) appears to be uncommon. Furthermore, a number of patients also have asymmetrical extrapyramidal symptoms consistent with a CBD syndrome (Mesulam et al., 2007; Rohrer et al., in press). GRN mutations appear to be a candidate molecular substrate for the ‘left lateral temporo-parietal syndrome’ of mixed aphasia (Rohrer et al., in press; see Fig. 3). Primary speech and language syndromes in association with MAPT mutations appear to be uncommon (e.g. Janssen et al., 2002; van Swieten and Spillantini, 2007), though dynamic aphasia may develop in the context of frontal lobe dysfunction.
The role of other genetic factors in progressive aphasia remains poorly defined. Mutations in the amyloid precursor protein (APP), presenilin 1, (PS1) and presenilin 2 (PS2) genes are known to cause familial AD, which is much less common than familial FTLD. In light of emerging evidence that certain PS1 polymorphisms can be associated with GRN mutations, further evidence is required to establish whether mutations in any of these genes can cause a primary language syndrome. A single report has suggested that there is an association between prion protein codon 129 heterozygosity and PPA (Li et al., 2005), however this was not replicated in another study of specific progressive aphasia subtypes (Rohrer et al., 2006).
Conclusions and future directions
The rich phenomenology of the progressive aphasias presents a substantial clinical challenge and a unique window on the neurobiology of language. Accurate clinical diagnosis of the patient who presents with word-finding difficulty requires an appreciation of the taxonomy of the progressive speech and language syndromes and a systematic approach based on the principles of structured history-taking and examination, analogous to those that guide other areas of neurological practice. Here we have presented an approach to the clinical analysis of word-finding difficulty, both to assist diagnosis and to set clinical symptoms in the context of experimental evidence concerning the organization of the language system. However, any such analysis exposes problems that will only be resolved by a more detailed understanding of the pathophysiology of the progressive aphasias.
The progressive aphasias are more than the sum of their neurolinguistic parts: these are diseases of neural networks, distributed both in space (functionally connected brain regions) and time (evolution of deficits). Although broad correlations can be established to guide clinical localisation (Fig. 3), few if any clinical deficits are specific to dysfunction in a single brain region, while a particular brain region often participates in the development of several different syndromes. The search for correspondences between clinical syndromes and regional brain atrophy in the progressive aphasias is analogous to classical attempts to correlate acute aphasic syndromes with focal lesions. The language models of classical neurology that emphasized discrete cortical centres in the mediation of specific language functions (Lichtheim, 1885) have given way to neurolinguistic accounts that emphasise distributed functional networks (Levelt, 1989, 2001; Hillis, 2007). Despite longstanding interest in the so-called disconnection syndromes, the science of distributed neural networks has yet to be widely translated to clinical practice, yet this may hold the key to understanding the phenomenology of the progressive aphasias and the ways in which they depart from the acute syndromes of vascular disease. At present, the functional consequences of neural network disintegration remain difficult to predict a priori, and the mechanisms by which they give rise to clinical syndromes have not been elucidated in any detail. The progressive aphasias have thrown up fundamental issues that are often difficult to reconcile with classical models of language localization: the SD syndrome, for example, clearly illustrates the fundamental importance of the anterior temporal lobe in language, yet the relations of this region to the ‘classical’ language cortex in Broca's and Wernicke's areas within the wider language network remain problematic. In line with network accounts of the acute aphasias (Hillis, 2007), the overarching challenge of future work in the degenerative language syndromes will be to characterize particular syndromes as ‘pathway-opathies’ or dynamic profiles of correlated atrophy across brain regions. This perspective will be constrained by models of healthy brain function (see for example, Binder et al., 2005), and may help to resolve the many apparent discrepancies of structure: function correlation in degenerative disease (see for example, Nestor et al., 2006).
Despite the diversity and limitations of clinico-pathological correlation in the progressive aphasias, there are no grounds for nihilism: speech and language deficits may act as signatures of tissue pathology (Hodges et al., 2004; Snowden et al., 2007). If this clinico-pathological correspondence is to be refined, an improved understanding of the molecular pathogenesis of regional neuronal dysfunction and the pathophysiology of distributed neural networks will be required. The progressive aphasias illustrate the sometimes striking selective vulnerability of particular neuronal populations to degenerative disease (for example, the left anterior temporal lobe in SD). ‘Molecular neurolinguistics’ is a science in embryo, yet there are tantalizing indications that specific molecular defects may map onto specific clinical aphasic syndromes. Acquired alterations in critical gene products is a plausible mechanism for both regional neuronal destruction and specific neurolinguistic effects in neurodegenerative disease. To demonstrate these pathophysiological signatures, a multi-modal approach will be required. In addition to detailed correlation of tissue damage with specific language functions (Harasty et al., 2001; Knibb et al., 2006b), there is a need for complementary techniques such as metabolic and functional imaging (Nestor et al., 2003; Sonty et al., 2003, 2007), longitudinal imaging to map the evolution of deficits (Janssen et al., 2005) and diffusion tensor imaging and magnetic resonance spectroscopy to assess the integrity of axonal pathways linking cortical language areas (Catani et al., 2003). The diffuse nature of the pathological process and wide individual variation in the distribution of tissue damage favour the use of unbiased techniques such as VBM to establish macro-anatomical correlates of speech processing deficits at the group or population level (Grossman et al., 2004; Schroeter et al., 2007). Modulation of network function by pharmacological agents (Tivarus et al., 2007) and other interventions (for example, transcranial magnetic stimulation: see Hillis, 2007) is a further dimension.
A key theme emerging in any survey of the progressive aphasias is the need for improved syndrome definition that could form the basis for a rational taxonomy of these disorders and a uniform system of classification. The exuberant terminology of the progressive aphasias has probably hindered this: the conflation of clinical, anatomical and pathological levels of description has led to considerable confusion in the literature of these disorders. Neurolinguistics, the structural and functional brain-imaging modalities and molecular biology all potentially have an important role to play here, if collectively they can provide a coherent information processing model for the core deficits that underpin clinical syndromes. One pressing issue concerns the most appropriate way to classify the fluent and non-fluent phenotypes of the PPA spectrum, which in turn reflects the difficulties inherent in the concept of ‘fluency’ (Hillis, 2007). Our account favours the classification of progressive fluent aphasia with SD, and the separation of disorders of motor programming from primary language disorders; however, this remains challenging to implement at the bedside. A truly comprehensive description of the progressive aphasias will move beyond word-finding into the realms of prosody and other non-verbal phenomena that impact on vocal communication but are not well captured by traditional models and instruments.
For the neurologist, early and accurate diagnosis of patients with word-finding difficulty will become an increasingly urgent issue as specific therapies with the potential to salvage cognitive function become available. Advances in brain imaging and other techniques to aid diagnosis have only underlined the central importance of clinical evaluation: this reflects both the wide heterogeneity of the underlying disease processes, and the relative insensitivity of existing diagnostic modalities. Clinical neurology and neuropsychology will remain crucial in identifying problems and discrepancies; alertness to these may lead to fundamental conceptual advances (Warrington, 1975). For the neurobiologist, the clinical phenomenology of the progressive aphasias will continue to inform the experimental study of the human language system both in health and disease.
Acknowledgements
This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. The Dementia Research Centre is an Alzheimer's Research Trust Co-ordinating Centre. This work was also supported by the Alzheimer Research Trust, the Medical Research Council UK and the Wellcome Trust. J.D.R. is supported by a Wellcome Trust Research Training Fellowship. J.E.W. is supported by the Wellcome Trust UK. J.D.W. is supported by a Wellcome Trust Intermediate Clinical Fellowship. We thank Professor Elizabeth Warrington and Dr G.D. Schott for helpful discussion. Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Abbreviations
- PPA
primary progressive aphasia
- PNFA
progressive non-fluent aphasia
- PSP
progressive supranuclear palsy
- CBD
corticobasal degeneration
- AD
Alzheimer's disease
- AOS
apraxia of speech
- DLB
dementia with Lewy bodies
- FTD-MND
frontotemporal dementia associated with motor neuron disease
- SD
semantic dementia
- VaD
vascular dementia
Appendix
References
- Adlam AL, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, Hodges JR. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129:3066–80. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- Almor A, Kempler D, MacDonald MC, Andersen ES, Tyler LK. Why do Alzheimer patients have difficulty with pronouns? Working memory, semantics, and reference in comprehension and production in Alzheimer's disease. Brain Lang. 1999;67:202–27. doi: 10.1006/brln.1999.2055. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66(9):1405–13. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Bak TH, O'Donnan DG, Xuereb JH, et al. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain. 2001;124:103–20. doi: 10.1093/brain/124.1.103. [DOI] [PubMed] [Google Scholar]
- Bak TH, Yancopoulou D, Netsor PJ, Xuereb JH, Spilantini MG, Pulvermuller F, et al. Clinical, imaging and pathological correlates of a hereditary deficit in verb and action processing. Brain. 2006;129:321–32. doi: 10.1093/brain/awh701. [DOI] [PubMed] [Google Scholar]
- Bartha L, Benke T. Acute conduction aphasia: an analysis of 20 cases. Brain Lang. 2003;85:93–108. doi: 10.1016/s0093-934x(02)00502-3. [DOI] [PubMed] [Google Scholar]
- Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D. Behaviour in frontotemporal dementia, Alzheimer's disease and vascular dementia. Acta Neurol Scand. 2001;103:367–78. doi: 10.1034/j.1600-0404.2001.2000236.x. [DOI] [PubMed] [Google Scholar]
- Baxter DM, Warrington EK. Transcoding sound to spelling: single or multiple sound unit correspondence? Cortex. 1987;23:11–28. doi: 10.1016/s0010-9452(87)80016-3. [DOI] [PubMed] [Google Scholar]
- Bayles K, Kazniak A. Communication and cognition in normal aging and dementia. Boston: College Hill Press; 1987. [Google Scholar]
- Beauvois MF, Derouesne J. Phonological alexia: three dissociations. J Neurol Neurosurg Psychiatry. 1979;42:1115–24. doi: 10.1136/jnnp.42.12.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45:789–93. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–93. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Blair M, Marczinski CA, Davis-Faroque N, Kertesz A. A longitudinal study of language decline in Alzheimer's disease and frontotemporal dementia. J Int Neuropsychol Soc. 2007;13:237–45. doi: 10.1017/S1355617707070269. [DOI] [PubMed] [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton E, Wise RJ. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–38. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–15. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Myers D, Wilson A, Rankin KP, Allison SC, Rosen HJ, et al. The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci. 2006;18:1644–53. doi: 10.1162/jocn.2006.18.10.1644. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarques sur la siège de la faculté du language articulé; suivies d'une observation d'aphémie. (2nd ser).Bull soc Anar (Paris) 1861;6:330–357. [Google Scholar]
- Broussolle E, Bakchine S, Tommasi M, Laurent B, Bazin B, Cinotti L, et al. Slowly progressive anarthria with late anterior opercular syndrome: a variant form of frontal cortical atrophy syndromes. J Neurol Sci. 1996;144:44–58. doi: 10.1016/s0022-510x(96)00096-2. [DOI] [PubMed] [Google Scholar]
- Brust JC, Shafer SQ, Richter RW, Bruun B. Aphasia in acute stroke. Stroke. 1976;7(2):167–74. doi: 10.1161/01.str.7.2.167. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol (Berl) 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, Papagno C, Ruml W. The selective impairment of phonological processing in speech production. Brain Lang. 2000;75(3):428–50. doi: 10.1006/brln.2000.2379. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Windebank AJ, Petersen RC, Komori T, Parisi JE, Okazaki H, et al. Rapidly progressive aphasic dementia and motor neuron disease. Ann Neurol. 1993;33:200–7. doi: 10.1002/ana.410330210. [DOI] [PubMed] [Google Scholar]
- Catani M, Piccirilli M, Cherubini A, Tarducci R, Sciarma T, Gobbi G, et al. Axonal injury within language network in primary progressive aphasia. Ann Neurol. 2003;53:242–7. doi: 10.1002/ana.10445. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann Neurol. 2001;49:433–42. [PubMed] [Google Scholar]
- Chapman SB, Rosenberg RN, Weiner MF, Shobe A. Autosomal dominant progressive syndrome of motor-speech loss without dementia. Neurology. 1997;49:1298–306. doi: 10.1212/wnl.49.5.1298. [DOI] [PubMed] [Google Scholar]
- Chedru F, Geschwind N. Disorders of higher cortical functions in acute confusional states. Cortex. 1972;8:395–411. doi: 10.1016/s0010-9452(72)80004-2. [DOI] [PubMed] [Google Scholar]
- Clark DG, Charuvastra A, Miller BL, Shapira JS, Mendez MF. Fluent versus nonfluent primary progressive aphasia: a comparison of clinical and functional neuroimaging features. Brain Lang. 2005;94:54–60. doi: 10.1016/j.bandl.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Code C, Müller N, Tree J, Ball M. Syntactic impairments can emerge later: progressive agrammatic agraphia and syntactic comprehension impairment. Aphasiology. 2006;20:1035–58. [Google Scholar]
- Cohen L, Benoit N, Van Eeckhout P, Ducarne B, Brunet P. Pure progressive aphemia. J Neurol Neurosurg Psychiatry. 1993;56:923–4. doi: 10.1136/jnnp.56.8.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confavreux C, Croisile B, Garassus P, Aimard G, Trillet M. Progressive amusia and aprosody. Arch Neurol. 1992;49:971–6. doi: 10.1001/archneur.1992.00530330095023. [DOI] [PubMed] [Google Scholar]
- Cooke A, DeVita C, Gee J, Alsop D, Detre J, Chen W, et al. Neural basis for sentence comprehension deficits in frontotemporal dementia. Brain Lang. 2003;85:211–21. doi: 10.1016/s0093-934x(02)00562-x. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Saffran EM, Schwoebel J. Knowledge of the human body: a distinct semantic domain. Neurology. 2002;59:357–63. doi: 10.1212/wnl.59.3.357. [DOI] [PubMed] [Google Scholar]
- Costello AL, Warrington EK. Dynamic aphasia: the selective impairment of verbal planning. Cortex. 1989;25:103–14. doi: 10.1016/s0010-9452(89)80010-3. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Borroni B, Manenti R, Ginex V, Calabria M, Moro A, et al. Universal grammar in the frontotemporal dementia spectrum: evidence of a selective disorder in the corticobasal degeneration syndrome. Neuropsychologia. 2007 Jun 8; doi: 10.1016/j.neuropsychologia.2007.05.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Critchley M. The neurology of psychotic speech. Br J Psychiatry. 1964;110:353–64. doi: 10.1192/bjp.110.466.353. [DOI] [PubMed] [Google Scholar]
- Croisile B, Brabant MJ, Carmoi T, et al. Comparison between oral and written spelling in Alzheimer's disease. Brain Lang. 1996;54:361–87. doi: 10.1006/brln.1996.0081. [DOI] [PubMed] [Google Scholar]
- Croot K, Hodges JR, Xuereb J, Patterson K. Phonological and articulatory impairment in Alzheimer's disease: a case series. Brain Lang. 2000;75:277–309. doi: 10.1006/brln.2000.2357. [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–4. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Benson DF. Psychological dysfunction accompanying subcortical dementias. Annu Rev Med. 1988;39:53–61. doi: 10.1146/annurev.me.39.020188.000413. [DOI] [PubMed] [Google Scholar]
- Dabul B. Apraxia battery for adults. 2nd edn. Austin, TX: Pro-Ed; 2000. [Google Scholar]
- Darley FL. The classification of output disturbances in neurogenic communication disorders; American Speech and Hearing Association Annual Conference; Chicago: IL. 1969. [Google Scholar]
- Darvesh S, Freedman M. Subcortical dementia: a neurobehavioral approach. Brain Cogn. 1996;31:230–49. doi: 10.1006/brcg.1996.0043. [DOI] [PubMed] [Google Scholar]
- Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D, et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol (Berl) 2007;113:521–33. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain. 2005;128:1984–95. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- De Bleser R. A linguist's view on progressive anomia: evidence for Delbrück (1886) in modern neurolinguistic research. Cortex. 2006;42:805–10. doi: 10.1016/s0010-9452(08)70421-0. [DOI] [PubMed] [Google Scholar]
- Delazer M, Semenza C, Reiner M, Hofer R, Benke T. Anomia for people names in DAT- evidence for semantic and post-semantic impairments. Neuropsychologia. 2003;41:1593–8. doi: 10.1016/s0028-3932(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D. Positive and negative aspects of cerebral cortical function. N C Med J. 1956;17:295–303. [PubMed] [Google Scholar]
- Diehl J, Grimmer T, Drzezga A, Riemenschneider M, Forstl H, Kurz A. Cerebral metabolic patterns at early stages of frontotemporal dementia and semantic dementia. A PET study. Neurobiol Aging. 2004;25:1051–6. doi: 10.1016/j.neurobiolaging.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Diesfeldt HF. Impaired phonological reading in primary degenerative dementia. Brain. 1991;114:1631–46. doi: 10.1093/brain/114.4.1631. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–61. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Duffy J. Motor speech disorders: substrates, differential diagnosis, and management. 2nd edn. St Louis, MO: Elsevier Mosby; 2005. [Google Scholar]
- Duffy J. Apraxia of speech in degenerative neurologic disease. Aphasiology. 2006;20:511–27. [Google Scholar]
- Duffy JR, Peach RK, Strand EA. Progressive apraxia of speech as a sign of motor neuron disease. Am J Speech Lang Pathol. 2007;16:198–208. doi: 10.1044/1058-0360(2007/025). [DOI] [PubMed] [Google Scholar]
- Emery VO. Language impairment in dementia of the Alzheimer type: a hierarchical decline? Int J Psychiatry Med. 2000;30:145–64. doi: 10.2190/X09P-N7AU-UCHA-VW08. [DOI] [PubMed] [Google Scholar]
- Esmonde T, Giles E, Xuereb J, Hodges J. Progressive supranuclear palsy presenting with dynamic aphasia. J Neurol Neurosurg Psychiatry. 1996;60:403–10. doi: 10.1136/jnnp.60.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–62. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattali C, Duffy JR, Litvan I, Patsalides AD, Grafman J. Yes/no reversals as neurobehavioral sequela: a disorder of language, praxis, or inhibitory control? Eur J Neurol. 2003;10:103–6. doi: 10.1046/j.1468-1331.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- Friedman RB, Ferguson S, Robinson S, Sunderland T. Dissociation of mechanisms of reading in Alzheimer's disease. Brain Lang. 1992;43:400–13. doi: 10.1016/0093-934x(92)90109-r. [DOI] [PubMed] [Google Scholar]
- Fushimi T, Komori K, Ikeda M, Patterson K, Ijuin M, Tanabe H. Surface dyslexia in a Japanese patient with semantic dementia: evidence for similarity-based orthography-to-phonology translation. Neuropsychologia. 2003;41:1644–58. doi: 10.1016/s0028-3932(03)00108-8. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology. 2001;57:216–25. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Garrard P, Patterson K, Watson PC, Hodges JR. Category specific semantic loss in dementia of Alzheimer's type. Functional-anatomical correlations from cross-sectional analyses. Brain. 1998;121:633–46. doi: 10.1093/brain/121.4.633. [DOI] [PubMed] [Google Scholar]
- Garrard P, Lambon Ralph MA, Patterson K, Pratt KH, Hodges JR. Semantic feature knowledge and picture naming in dementia of Alzheimer's type: a new approach. Brain Lang. 2005;93:79–94. doi: 10.1016/j.bandl.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- Georgieff N, Dominey PF, Michel F, Cardine MM, Dalery J. Anomia in major depressive state. Psychiatry Res. 1998;77:197–208. doi: 10.1016/s0165-1781(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Gerstner E, Lazar RM, Keller C, Honig LS, Lazar GS, Marshall RS. A case of progressive apraxia of speech in pathologically verified Alzheimer disease. Cogn Behav Neurol. 2007;20:15–20. doi: 10.1097/WNN.0b013e31802b6c45. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Non-aphasic disorders of speech. Int J Neurol. 1964;4(3):207–14. [PubMed] [Google Scholar]
- Geschwind N. Current concepts: aphasia. N Engl J Med. 1971;284(12):654–6. doi: 10.1056/NEJM197103252841206. [DOI] [PubMed] [Google Scholar]
- Ghacibeh GA, Heilman KM. Progressive affective aprosodia and prosoplegia. Neurology. 2003;60(7):1192–4. doi: 10.1212/01.wnl.0000055870.48864.87. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Esiri MM, Lees AJ. Clinical and pathological features of diffuse cortical Lewy body disease (Lewy body dementia) Brain. 1987;110:1131–53. doi: 10.1093/brain/110.5.1131. [DOI] [PubMed] [Google Scholar]
- Glosser G, Baker KM, de Vries JJ, Alavi A, Grossman M, Clark CM. Disturbed visual processing contributes to impaired reading in Alzheimer's disease. Neuropsychologia. 2002;40:902–9. doi: 10.1016/s0028-3932(01)00165-8. [DOI] [PubMed] [Google Scholar]
- Goldman JS, Farmer JM, Wood EM, Johnson JK, Boxer A, Neuhaus J, et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology. 2005;65:1817–9. doi: 10.1212/01.wnl.0000187068.92184.63. [DOI] [PubMed] [Google Scholar]
- Goldstein K. Die transkortikalen Aphasien. Jena: Gustav Fischer; 1912. [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Ogar JM, Brambati SM, Wang P, Jeong JH, Rankin KP, et al. Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology. 2006;67:1849–51. doi: 10.1212/01.wnl.0000237038.55627.5b. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Plaut DC. The impact of synaptic depression following brain damage: a connectionist account of “access/refractory” and “degraded-store” semantic impairments. Cogn Affect Behav Neurosci. 2002;2:187–213. doi: 10.3758/cabn.2.3.187. [DOI] [PubMed] [Google Scholar]
- Graham NL. Dysgraphia in dementia. Neurocase. 2000;6:365–76. [Google Scholar]
- Graham NL, Bak T, Patterson K, Hodges JR. Language function and dysfunction in corticobasal degeneration. Neurology. 2003;61:493–9. doi: 10.1212/01.wnl.0000081230.09863.ed. [DOI] [PubMed] [Google Scholar]
- Graham KS, Patterson K, Hodges JR. Progressive pure anomia: Insufficient activation of phonology by meaning. Neurocase. 1995;1:25–38. [Google Scholar]
- Graham NL, Patterson K, Hodges JR. The impact of semantic memory impairment on spelling: evidence from semantic dementia. Neuropsychologia. 2000;38:143–63. doi: 10.1016/s0028-3932(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Graham N, Patterson K, Hodges J. The emergence of jargon in progressive fluent dysgraphia: The widening gap between target and response. Cogn Neuropsychol. 2001;18:343–61. doi: 10.1080/02643290125983. [DOI] [PubMed] [Google Scholar]
- Grossman M. Progressive aphasic syndromes: clinical and theoretical advances. Curr Opin Neurol. 2002;15:409–13. doi: 10.1097/00019052-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Grossman M, Ash S. Primary progressive aphasia: a review. Neurocase. 2004;10:3–18. doi: 10.1080/13554790490960440. [DOI] [PubMed] [Google Scholar]
- Grossman M, Moore P. A longitudinal study of sentence comprehension difficulty in primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2005;76:644–9. doi: 10.1136/jnnp.2004.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Rhee J. Cognitive resources during sentence processing in Alzheimer's disease. Neuropsychologia. 2001;39:1419–31. doi: 10.1016/s0028-3932(01)00059-8. [DOI] [PubMed] [Google Scholar]
- Grossman M, White-Devine T. Sentence comprehension in Alzheimer's disease. Brain Lang. 1998;62:186–201. doi: 10.1006/brln.1997.1898. [DOI] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–49. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Hachisuka K, Uchida M, Nozaki Y, Hashiguchi S, Sasaki M. Primary progressive aphasia presenting as conduction aphasia. J Neurol Sci. 1999;167:137–41. doi: 10.1016/s0022-510x(99)00147-1. [DOI] [PubMed] [Google Scholar]
- Harasty JA, Halliday GM, Xuereb J, et al. Cortical degeneration associated with phonologic and semantic language impairments in AD. Neurology. 2001;56:944–50. doi: 10.1212/wnl.56.7.944. [DOI] [PubMed] [Google Scholar]
- Head H. Aphasia and kindred disorders of speech. London: Cambridge University Press; 1926. [DOI] [PubMed] [Google Scholar]
- Hillis AE. Aphasia: progress in the last quarter of a century. Neurology. 2007;69:200–13. doi: 10.1212/01.wnl.0000265600.69385.6f. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J Cogn Neurosci. 2002;14:1099–108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Recognition and naming of famous faces in Alzheimer's disease: a cognitive analysis. Neuropsychologia. 1993;31:775–788. doi: 10.1016/0028-3932(93)90128-m. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and palm trees: a test of semantic access from pictures and words. Bury St Edmunds, UK: Thames Valley Test Company; 1992. [Google Scholar]
- Ikeda K, Akiyama H, Iritani S, Kase K, Arai T, Niizato K, et al. Corticobasal degeneration with primary progressive aphasia and accentuated cortical lesion in superior temporal gyrus: case report and review. Acta Neuropathol (Berl) 1996;92:534–9. doi: 10.1007/s004010050558. [DOI] [PubMed] [Google Scholar]
- Incisa della Rocchetta A, Cipolotti L. Preserved knowledge of maps of countries: implications for the organization of semantic memory. Neurocase. 2004;10:249–64. doi: 10.1080/13554790490495186. [DOI] [PubMed] [Google Scholar]
- Incisa della Rocchetta A, Cipolotti L, Warrington EK. Countries: their selective impairment and selective preservation. Neurocase. 1998;4:99–109. [Google Scholar]
- Ingles JL, Fisk JD, Passmore M, Darvesh S. Progressive anomia without semantic or phonological impairment. Cortex. 2007;43:558–64. doi: 10.1016/s0010-9452(08)70250-8. [DOI] [PubMed] [Google Scholar]
- Janssen JC, Warrington EK, Morris HR, Lantos P, Brown J, Revesz T, et al. Clinical features of frontotemporal dementia due to the intronic tau 10(+16) mutation. Neurology. 2002;58:1161–8. doi: 10.1212/wnl.58.8.1161. [DOI] [PubMed] [Google Scholar]
- Janssen JC, Schott JM, Cipolotti L, et al. Mapping the onset and progression of atrophy in familial frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2005;76:162–8. doi: 10.1136/jnnp.2003.032201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–47. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Boeve BF, Duffy JR, Smith GE, Knopman DS, Parisi JE, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11:283–96. doi: 10.1080/13554790590963004. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006a;129:1385–98. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006b;66:41–8. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- Kavé G, Levy Y. Sensitivity to gender, person, and tense inflection by persons with Alzheimer's disease. Brain Lang. 2003;87:267–77. doi: 10.1016/s0093-934x(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Kertesz A, McCabe P. Recovery patterns and prognosis in aphasia. Brain. 1977;100:1–18. doi: 10.1093/brain/100.1.1. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Orange JB. Primary progressive aphasia. The future of neurolinguistic and biologic characterization. Brain Lang. 2000;71:116–9. doi: 10.1006/brln.1999.2228. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, McCabe P, Takagi K, Munoz D. Primary progressive aphasia: diagnosis, varieties, evolution. J Int Neuropsychol Soc. 2003;9:710–19. doi: 10.1017/S1355617703950041. [DOI] [PubMed] [Google Scholar]
- Knibb JA, Hodges JR. Semantic dementia and primary progressive aphasia: a problem of categorization? Alzheimer Dis Assoc Disord. 2005;19(Suppl 1):S7–14. doi: 10.1097/01.wad.0000183085.22562.13. [DOI] [PubMed] [Google Scholar]
- Knibb JA, Kipps CM, Hodges JR. Frontotemporal dementia. Curr Opin Neurol. 2006a;19:565–71. doi: 10.1097/01.wco.0000247606.57567.41. [DOI] [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006b;59:156–65. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Krefft TA, Graff-Radford NR, Dickson DW, Baker M, Castellani RJ. Familial primary progressive aphasia. Alzheimer Dis Assoc Disord. 2003;17:106–12. doi: 10.1097/00002093-200304000-00009. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Howard D. Gogi aphasia or semantic dementia? Simulating and assessing poor verbal comprehension in a case of progressive fluent aphasia. Cogn Neuropsychol. 2000;17:437–65. doi: 10.1080/026432900410784. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K, Garrard P, et al. Semantic dementia with category specificity: a comparative case series study. Cogn Neuropsychol. 2003;20:307–26. doi: 10.1080/02643290244000301. [DOI] [PubMed] [Google Scholar]
- Lang AE. Cortical basal ganglionic degeneration presenting with “progressive loss of speech output and orofacial dyspraxia”. J Neurol Neurosurg Psychiatry. 1992;55:1101. doi: 10.1136/jnnp.55.11.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfitt JT, Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Arch Neurol. 1996;53:72–6. doi: 10.1001/archneur.1996.00550010090021. [DOI] [PubMed] [Google Scholar]
- Larner AJ. “Dementia unmasked”: atypical, acute aphasic, presentations of neurodegenerative dementing disease. Clin Neurol Neurosurg. 2005;108:8–10. doi: 10.1016/j.clineuro.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Laws KR, Leeson VC, Gale TM. Inflated and contradictory category naming deficits in Alzheimer's disease? Brain Cogn. 2003;53:416–8. doi: 10.1016/s0278-2626(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Lesser R. Superior oral to written spelling. Evidence for separate buffers? Cogn Neuropsychol. 1990;7:347–66. [Google Scholar]
- Levelt W. Speaking: from intention to articulation. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- Levelt WJ. Spoken word production: a theory of lexical access. Proc Natl Acad Sci USA. 2001;98(23):13464–71. doi: 10.1073/pnas.231459498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Grossman RG, Kelly PJ. Aphasic disorder in patients with closed head injury. J Neurol Neurosurg Psychiatry. 1976;39:1062–70. doi: 10.1136/jnnp.39.11.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rowland LP, Mitsumoto H, Przedborski S, Bird TD, Schellenberg GD, et al. Prion protein codon 129 genotype prevalence is altered in primary progressive aphasia. Ann Neurol. 2005;58:858–64. doi: 10.1002/ana.20646. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–75. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Lichtheim L. On aphasia. Brain. 1885;7:433–84. [Google Scholar]
- Lukatela K, Malloy P, Jenkins M, Cohen R. The naming deficit in early Alzheimer's and vascular dementia. Neuropsychology. 1998;12:565–72. doi: 10.1037//0894-4105.12.4.565. [DOI] [PubMed] [Google Scholar]
- Luria AR. Traumatic aphasia: its syndromes, psychology and treatment. The Hague and Paris: Mouton; 1970. [Google Scholar]
- Luria AR, Tsvetkova LS. Towards the mechanisms of “dynamic aphasia”. Acta Neurol Psychiatr Belg. 1967;67(11):1045–57. [PubMed] [Google Scholar]
- Luzzatti C, Laiacona M, Agazzi D. Multiple patterns of writing disorders in dementia of the Alzheimer type and their evolution. Neuropsychologia. 2003;41:759–72. doi: 10.1016/s0028-3932(02)00328-7. [DOI] [PubMed] [Google Scholar]
- Luzzatti C, Poeck K. An early description of slowly progressive aphasia. Arch Neurol. 1991;48:228–9. doi: 10.1001/archneur.1991.00530140126029. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Viticchi G, Piccirilli M, Fabi K, Pesallaccia M, Bartolini M, et al. Foreign accent syndrome as the initial sign of primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2007 Jul 17; doi: 10.1136/jnnp.2006.113365. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Kertesz A. Category and letter fluency in semantic dementia, primary progressive aphasia, and Alzheimer's disease. Brain Lang. 2006;97:258–65. doi: 10.1016/j.bandl.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Marshall J. Jargon aphasia: what have we learned? Aphasiology. 2006;20(5):387–410. [Google Scholar]
- Marshall JC, Newcombe F. Patterns of paralexia: a psycholinguistic approach. J Psycholinguistic Res. 1973;2:175–99. doi: 10.1007/BF01067101. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Brandt J, Rosen J, Benson DF. Interictal memory and language impairment in temporal lobe epilepsy. Neurology. 1980;30:120–5. doi: 10.1212/wnl.30.2.120. [DOI] [PubMed] [Google Scholar]
- McCarthy R, Warrington EK. The double dissociation of short-term memory for lists and sentences. Evidence from aphasia. Brain. 1987;110:1545–63. doi: 10.1093/brain/110.6.1545. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Warrington EK. Evidence for modality-specific meaning systems in the brain. Nature. 1988;334:428–30. doi: 10.1038/334428a0. [DOI] [PubMed] [Google Scholar]
- McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, et al. Dementia with Lewy bodies. Lancet Neurol. 2004;3:19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Work Group on Frontotemporal Dementia and Pick's Disease. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- McMonagle P, Deering F, Berliner Y, Kertesz A. The cognitive profile of posterior cortical atrophy. Neurology. 2006;66:331–8. doi: 10.1212/01.wnl.0000196477.78548.db. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Clark DG, Shapira JS, Cummings JL. Speech and language in progressive nonfluent aphasia compared with early Alzheimer's disease. Neurology. 2003;61:1108–13. doi: 10.1212/01.wnl.0000090563.97453.90. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS, Clark DG. “Apperceptive” alexia in posterior cortical atrophy. Cortex. 2007;43:264–70. doi: 10.1016/s0010-9452(08)70481-7. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–8. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–32. [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia: a language-based dementia. N Engl J Med. 2003;349:1535–42. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S. Spectrum of primary progressive aphasia. Baillieres Clin Neurol. 1992;1:583–609. [PubMed] [Google Scholar]
- Mesulam MM, Grossman M, Hillis A, Kertesz A, Weintraub S. The core and halo of primary progressive aphasia and semantic dementia. Ann Neurol. 2003;54(Suppl 5):S11–4. doi: 10.1002/ana.10569. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Johnson N, Krefft TA, Gass JM, Cannon AD, Adamson JL, et al. Progranulin mutations in primary progressive aphasia: the PPA1 and PPA3 families. Arch Neurol. 2007;64:43–7. doi: 10.1001/archneur.64.1.43. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJS, et al. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122:61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, et al. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Murre JMJ, Graham KS, Hodges JR. Semantic dementia: relevance to connectionist models of long-term memory. Brain. 2001;124:647–75. doi: 10.1093/brain/124.4.647. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nelissen N, Vandenbulcke M, Fannes K, Verbruggen A, Peeters R, Dupont P, et al. Abeta amyloid deposition in the language system and how the brain responds. Brain. 2007;130(Pt 8):2055–69. doi: 10.1093/brain/awm133. Epub 2007 Jun 24. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Hodges JR. Declarative memory impairments in Alzheimer's disease and semantic dementia. NeuroImage. 2006;30:1010–20. doi: 10.1016/j.neuroimage.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive nonfluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–18. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Balan K, Cheow HK, Fryer TD, Knibb JA, Xuereb JH, et al. Nuclear imaging can predict pathologic diagnosis in progressive nonfluent aphasia. Neurology. 2007;68:238–9. doi: 10.1212/01.wnl.0000251309.54320.9f. [DOI] [PubMed] [Google Scholar]
- Nicholas M, Obler LK, Albert ML, Helm-Estabrooks N. Empty speech in Alzheimer's disease and fluent aphasia. J Speech Hear Res. 1985;28:405–10. doi: 10.1044/jshr.2803.405. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Patterson K, Tyler LK, Moss H, Stamatakis EA, Bright P, et al. Temporal lobe lesions and semantic impairment: a comparison of herpes simplex virus encephalitis and semantic dementia. Brain. 2007 Jan 24; doi: 10.1093/brain/awl344. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- O'Dowd BS, de Zubicaray GI. Progressive dysgraphia in a case of posterior cortical atrophy. Neurocase. 2003;9:251–60. doi: 10.1076/neur.9.3.251.15561. [DOI] [PubMed] [Google Scholar]
- Ogar J, Slama H, Dronkers N, Amici S, Gorno-Tempini ML. Apraxia of speech: an overview. Neurocase. 2005;11(6):427–32. doi: 10.1080/13554790500263529. [DOI] [PubMed] [Google Scholar]
- Okuda B, Kawabata K, Tachibana H, Sugita M, Tanaka H. Postencephalitic pure anomic aphasia: 2 year follow-up. J Neurol Sci. 2001;187:99–102. doi: 10.1016/s0022-510x(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Ostberg P, Bogdanovic N, Fernaeus SE, Wahlund LO. Jargonagraphia in a case of frontotemporal dementia. Brain Lang. 2001;79:333–9. doi: 10.1006/brln.2001.2491. [DOI] [PubMed] [Google Scholar]
- Otsuki M, Soma Y, Sato M, Homma A, Tsuji S. Slowly progressive pure word deafness. Eur Neurol. 1998;39(3):135–40. doi: 10.1159/000007923. [DOI] [PubMed] [Google Scholar]
- Ozsancak C, Auzou P, Dujardin K, Quinn N, Destee A. Orofacial apraxia in corticobasal degeneration, progressive supranuclear palsy, multiple system atrophy and Parkinson's disease. J Neurol. 2004;251:1317–23. doi: 10.1007/s00415-004-0530-0. [DOI] [PubMed] [Google Scholar]
- Papagno C, Capitani E. Slowly progressive aphasia: a four-year follow-up study. Neuropsychologia. 2001;39:678–86. doi: 10.1016/s0028-3932(01)00007-0. [DOI] [PubMed] [Google Scholar]
- Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974;12:323–30. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- Pick A. Über die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Med Wochenschrift. 1892;17:165–7. [Google Scholar]
- Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Laakso M, Partanen K, et al. Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol. 2000;47:470–6. [PubMed] [Google Scholar]
- Price BH, Gurvit H, Weintraub S, Geula C, Leimkuhler E, Mesulam M. Neuropsychological patterns and language deficits in 20 consecutive cases of autopsy-confirmed Alzheimer's disease. Arch Neurol. 1993;50:931–7. doi: 10.1001/archneur.1993.00540090038008. [DOI] [PubMed] [Google Scholar]
- Price CC, Grossman M. Verb agreements during on-line sentence processing in Alzheimer's disease and frontotemporal dementia. Brain Lang. 2005;94:217–32. doi: 10.1016/j.bandl.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Robinson G, Cipolotti L. The selective preservation of colour naming in semantic dementia. Neurocase. 2001;7:65–75. doi: 10.1093/neucas/7.1.65. [DOI] [PubMed] [Google Scholar]
- Robinson G, Blair J, Cipolotti L. Dynamic aphasia: an inability to select between competing verbal responses? Brain. 1998;121:77–89. doi: 10.1093/brain/121.1.77. [DOI] [PubMed] [Google Scholar]
- Robinson G, Rossor M, Cipolotti L. Selective sparing of verb naming in a case of severe Alzheimer's disease. Cortex. 1999;35:443–50. doi: 10.1016/s0010-9452(08)70812-8. [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Cipolotti L. Dynamic aphasia in progressive supranuclear palsy: a deficit in generating a fluent sequence of novel thought. Neuropsychologia. 2006;44:1344–60. doi: 10.1016/j.neuropsychologia.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Ivanoiu A, Patterson K, Hodges JR. Semantic memory in Alzheimer's disease and the frontotemporal dementias: a longitudinal study of 236 patients. Neuropsychology. 2006;20:319–35. doi: 10.1037/0894-4105.20.3.319. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Ogar JM, Amici S, Rose K, Dronkers N, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67:1752–6. doi: 10.1212/01.wnl.0000247630.29222.34. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Mead S, Omar R, Poulter M, Warren JD, Collinge J, et al. Prion protein (PRNP) genotypes in frontotemporal lobar degeneration syndromes. Ann Neurol. 2006;60:616. doi: 10.1002/ana.20931. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Omar R, Mead S, Beck J, Revesz T, et al. Parietal lobe deficits are a feature of frontotemporal lobar degeneration caused by a mutation in the progranulin gene. Arch Neurol. doi: 10.1001/archneur.65.4.506. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Fox NC, Rossor MN, Warrington EK, Warren JD. Jargon language in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2007;78:1014–38. (78) [Google Scholar]
- Ross ED. The aprosodias. Functional-anatomic organization of the affective components of language in the right hemisphere. Arch Neurol. 1981;38(9):561–9. doi: 10.1001/archneur.1981.00510090055006. [DOI] [PubMed] [Google Scholar]
- Rossor MN, Revesz T, Lantos PL, Warrington EK. Semantic dementia with ubiquitin-positive tau-negative inclusion bodies. Brain. 2000;123:267–76. doi: 10.1093/brain/123.2.267. [DOI] [PubMed] [Google Scholar]
- Roth HL, Eskin TA, Kendall DL, Heilman KM. Progressive oculoorofacial-speech apraxia (POOSA) Neurocase. 2006;12:221–7. doi: 10.1080/13554790600837347. [DOI] [PubMed] [Google Scholar]
- Scahill VL, Hodges JR, Graham KS. Can episodic memory tasks differentiate semantic dementia from Alzheimer's Disease? Neurocase. 2005;11:441–51. doi: 10.1080/13554790500287734. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Raczka K, Neumann J, Yves von Cramon D. Towards a nosology for frontotemporal lobar degenerations-a meta-analysis involving 267 subjects. Neuroimage. 2007;36:497–510. doi: 10.1016/j.neuroimage.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Serieux P. Sur un cas de surdite verbale pure. Rev Med. 1893;13:733–50. [Google Scholar]
- Shi J, Shaw CL, Du Plessis D, Richardson AM, Bailey KL, Julien C, et al. Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol (Berl) 2005;110:501–12. doi: 10.1007/s00401-005-1079-4. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Cappa A, Salvigni BL. Speech and language in primary progressive anarthria. Neurocase. 2003a;9:213–20. doi: 10.1076/neur.9.3.213.15555. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Perri R, Cappa A. Grammatical class effects in brain damaged patients: functional locus of noun and verb deficit. Brain Lang. 2003b;85:49–66. doi: 10.1016/s0093-934x(02)00504-7. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Daniele A, Giustolisi L, Gainotti G. Dissociation between knowledge of living and nonliving things in dementia of the Alzheimer type. Neurology. 1991;41:545–6. doi: 10.1212/wnl.41.4.545. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Gainotti G, Perani D, Cappelletti JY, Carbone G, Fazio F. Naming deficit for non-living items: neuropsychological and PET study. Neuropsychologia. 1997;35:359–67. doi: 10.1016/s0028-3932(96)00084-x. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol. 1989;2:167–82. [Google Scholar]
- Snowden JS, Griffiths HL, Neary D. Progressive language disorder associated with frontal lobe degeneration. Neurocase. 1996;2:429–40. [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–32. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol (Berl) 2007;114:31–8. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- Soliveri P, Piacentini S, Carella F, Testa D, Ciano C, Girotti F. Progressive dysarthria: definition and clinical follow-up. Neurol Sci. 2003;24:211–2. doi: 10.1007/s10072-003-0135-X. [DOI] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Thompson CK, Johnson NA, Weintraub S, Parrish TB, et al. Primary progressive aphasia: PPA and the language network. Ann Neurol. 2003;53:35–49. doi: 10.1002/ana.10390. [DOI] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Weintraub S, Johnson NA, Parrish TB, Gitelman DR. Altered effective connectivity within the language network in primary progressive aphasia. J Neurosci. 2007;27:1334–45. doi: 10.1523/JNEUROSCI.4127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriani-Lefevre MH, Hannequin D, Bakchine S, Menard JF, Manrique A, Hitzel A, et al. Evidence of bilateral temporal lobe involvement in primary progressive aphasia: a SPECT study. J Nucl Med. 2003;44:1013–22. [PubMed] [Google Scholar]
- Spinelli M, De Oliveira Rocha AC, Giacheti CM, Richieri-Costa A. Word-finding difficulties, verbal paraphasias, and verbal dyspraxia in ten individuals with fragile X syndrome. Am J Med Genet. 1995;60:39–43. doi: 10.1002/ajmg.1320600108. [DOI] [PubMed] [Google Scholar]
- Tivarus ME, Hillier A, Schmalbrock P, Beversdorf DQ. Functional connectivity in an fMRI study of semantic and phonological processes and the effect of l-Dopa. Brain Lang. 2007 Apr 5; doi: 10.1016/j.bandl.2007.02.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell PJ, Kartsounis LD, Frackowiak RS, Findley LJ, Rossor MN. Progressive loss of speech output and orofacial dyspraxia associated with frontal lobe hypometabolism. J Neurol Neurosurg Psychiatry. 1991;54:351–7. doi: 10.1136/jnnp.54.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbulcke M, Peeters R, Van Hecke P, Vandenberghe R. Anterior temporal laterality in primary progressive aphasia shifts to the right. Ann Neurol. 2005;58:362–70. doi: 10.1002/ana.20588. [DOI] [PubMed] [Google Scholar]
- van Swieten J, Spillantini MG. Hereditary frontotemporal dementia caused by Tau gene mutations. Brain Pathol. 2007;17:63–73. doi: 10.1111/j.1750-3639.2007.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci. 2005;6:131–8. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- Warren JD, Warrington EK. Cognitive neuropsychology of dementia syndromes. In: Growdon JH, Rossor MN, editors. Blue books of neurology: the dementias II. Philadelphia: Butterworth Heinemann; 2007. pp. 329–80. [Google Scholar]
- Warren JD, Warren JE, Fox NC, Warrington EK. Nothing to say, something to sing: primary progressive dynamic aphasia. Neurocase. 2003;9(2):140–55. doi: 10.1076/neur.9.2.140.15068. [DOI] [PubMed] [Google Scholar]
- Warren JE, Wise RJ, Warren JD. Sounds do-able: auditory-motor transformations and the posterior temporal plane. Trends Neurosci. 2005;28:636–43. doi: 10.1016/j.tins.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol. 1975;27:635–57. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Cipolotti L. Word comprehension. The distinction between refractory and storage impairments. Brain. 1996;119:611–25. doi: 10.1093/brain/119.2.611. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107:829–54. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McKenna P, Orpwood L. Single word comprehension: a concrete and abstract word synonym test. Neuropsychol Rehabil. 1998;8:143–54. [Google Scholar]
- Wernicke C. Der aphasische Symptomenkomplex: Eine psychologische Studie auf anatomischer Basis. Breslau: Cohn and Weigert; 1874. [Google Scholar]
- Westbury C, Bub D. Primary progressive aphasia: a review of 112 cases. Brain Lang. 1997;60:381–406. doi: 10.1006/brln.1997.1840. [DOI] [PubMed] [Google Scholar]
- Zannino GD, Perri R, Pasqualetti P, Di Paola M, Caltagirone C, Carlesimo GA. The role of semantic distance in category-specific impairments for living things: evidence from a case of semantic dementia. Neuropsychologia. 2006;44:1017–28. doi: 10.1016/j.neuropsychologia.2005.11.006. [DOI] [PubMed] [Google Scholar]