Abstract
The oligomeric state in solution of four plant annexins, namely Anx23(Ca38), Anx24(Ca32), Anx(Gh1), and Anx(Gh2), was characterized by sedimentation equilibrium analysis and gel filtration. All proteins were expressed and purified as amino-terminal Hisn fusions. Sequencing of the Anx(Gh1) construct revealed distinct differences with the published sequence. Sedimentation equilibrium analysis of Anx23(Ca38), Anx24(Ca32), and Anx(Gh1) suggests monomer–trimer equilibria for each protein with association constants in the range of 0.9 × 1010−1.7 × 1011 M−2. All four proteins were subjected to analytical gel filtration under different buffer conditions. Observations from this experiment series agree quantitatively with the ultracentrifugation results, and strongly suggest calcium independence of the annexin oligomerization behavior; moreover, binding of calcium ions to the proteins seems to require disassembly of the oligomers. Anx(Gh2) showed a different elution profile than the other plant annexins; while having only a very small trimer content, this annexin seems to exist in a monomer–dimer equilibrium in solution.
Keywords: Annexin, Capsicum annuum, gel filtration, Gossypium hirsutum, sedimentation equilibrium analysis, ultracentrifugation
Despite the identification of annexin proteins in higher plants as early as 1989 (Boustead et al. 1989), it was only in the past 5 years that the field of plant annexins emerged to become a separate, intensely studied area. So far, annexins have been found in abundance in every plant where a search was initiated (for reviews, see Clark and Roux 1995; Delmer and Potikha 1997). Unlike in the animal kingdom, where a varying "bouquet" of up to 13 different annexins is expressed, the plants seem to possess a smaller number of annexin proteins, two of which are found most frequently and show very high sequence similarity throughout different plants. Among others (Blackbourn et al. 1991, 1992; Battey et al. 1996; Seals and Randall 1997; Proust et al. 1999), in bell pepper Anx23(Ca38) and Anx24(Ca32) (Proust et al. 1996) have been identified, as well as Anx(Le34) and Anx(Le35) in tomato (Smallwood et al. 1990) and Anx(Gh1) and Anx(Gh2) in cotton (Andrawis et al. 1993). All these proteins migrate within the range of 33 and 35 kD on SDS-PAGE; however, both proteins from one species clearly migrate differently from each other despite having similar molecular weights. However, in Arabidopsis seven annexin homologs have been identified so far (Clark et al. 2001), thereby giving rise to the speculation that annexins in other plants might also appear within a diverse multigene family. Additionally, annexin proteins have been reported that apparently are different from the ones mentioned above. The annexin-like proteins from celery (Seals et al. 1994) and tobacco (Seals and Randall 1997) associated with the vacuolar membrane show an apparent mass of 42 kD, and have been termed VCaB42. It seems very likely, though, that the latter protein is, in fact, the tobacco homolog of Anx23(Ca38) and Anx(Le35). Another type was reported with the fern annexin, having an apparent molecular weight of 70 kD, and therefore, could resemble the topology of annexin A6 with eight homologous repeats, although the authors could not rule out the possibility that this species is a dimer of 35 kD polypeptides (Clark et al. 1995). Due to their high sequence similarity, it was recently proposed that three annexins from tobacco, tomato, and bell pepper, namely Anx(Nt32), Anx(Le34), and Anx24(Ca32), might constitute a distinct type of Sp32 annexins (Proust et al. 1999).
Some mammalian annexins have been reported to self-associate in solution. Creutz et al. (1979) described this phenomenon for annexin A7 in solution, where the protein formed rods, bundles of rods, and paracrystalline arrays in a calcium-dependent fashion. A similar self-association event was seen with isolated annexin A4 from the ray Torpedo marmorata (Walker et al. 1983). Other members of the mammalian annexin subfamily, however, are claimed not to self-associate (Shadle et al. 1985).
The oligomerization states of mammalian annexins A1, A4, A5, A6, and the heterotetramer [AnxA2 p11]2 (Ahn et al. 1988), of annexin A7 (Creutz et al. 1979), as well as annexin C1-core from Dictyostelium discoideum (Liemann et al. 1997), have been investigated in solution using ultracentrifugation techniques. For all of these proteins a calcium-dependent monomer–dimer equilibrium has been observed with weak association constants in the range of 103 M−1; these studies were carried out using sedimentation equilibrium experiments in the presence of 10 mM CaCl2. The association constant for the heterotetramer constituted by annexin A2 and p11 shows a much higher affinity (ktetramer = 1.9 × 106 M−1) and indefinite isodesmic self-association of the tetramer was observed with an association constant of kiso = 2.8 × 105 M−1 (Ahn et al. 1988). The C-terminal core of annexin C1 was subjected to ultracentrifugation in the absence of calcium and found to be monomeric only. The half-maximal calcium concentrations for dimerization of mammalian annexins are in the range of 200 μM (annexin A7) (Creutz et al. 1979) and about 1 mM for other annexins (Südhof et al. 1982; Walker et al. 1983; Zaks and Creutz 1991). However, these oligomers of mammalian annexins are reported to be labile, and seem to gain stability only in the presence of membranes (Zaks and Creutz 1991).
The first report on the oligomerization state of plant annexins described an annexin from Capsicum annuum, Anx(Ca35), purified from a natural source; only a partial amino acid sequence of this protein has been reported to date by Hoshino et al. (1995). When crosslinking the protein bound to phosphatidylinositol vesicles at calcium concentrations higher than 0.75 mM, these authors found a small fraction of annexin homodimers. This result allows only limited conclusions about the oligomerization state of the protein in solution, because the concentration of this annexin on the membrane surface and in its immediate vicinity may be very high under the calcium conditions used. Accidental crosslinking of two monomers can therefore occur without a real dimer being present.
In the course of our ongoing studies on structural investigation of plant annexins, in particular their calcium-bound forms, we encountered severe difficulties in obtaining protein crystals in the presence of calcium. To obtain a clearer picture about the effects of calcium on these proteins we elucidate in the current study the oligomerization state of four plant annexins: Anx23(Ca38), Anx24(Ca32), Anx(Gh1), and Anx(Gh2). Based on a strict amino acid sequence comparison and according to the respective homologies it is tempting to assume that Anx(Gh1) belongs to the class of Sp32 annexins (Proust et al. 1999) while Anx(Gh2) rather seems to be the homolog of Anx23(Ca38), and thus belongs to the class of Sp38 annexins (see Fig. 1 ▶). All four proteins were subcloned as N-terminal His-tag fusions, and the recombinant proteins were subjected to equilibrium sedimentation analysis as well as gel filtration to characterize their oligomerization behavior.
Fig. 1.
Alignment of amino acid sequences of Anx(Gh1) [acc. number u73746], Anx24(Ca32) [acc. number x93308], Anx(Gh2) [acc. number u73747], and Anx23(Ca38) [acc. number aj130956]. The alignment was generated with the programs PILEUP from the GCG suite (Genetics Computer Group 1996) and ALSCRIPT (Barton 1993).
We report for the first time the presence of plant annexin trimers in solution, as observed with annexins 23(Ca38), 24(Ca32), and Anx(Gh1). For Anx(Gh2), analytical gel filtration indicates the presence of a dimeric species in solution.
Results
Sequencing of Anx(Gh1)
Routine sequencing after subcloning showed some differences for the fusion construct of Anx(Gh1) in pRSET_6d compared to the GenBank sequence u73746. Resequencing of this annexin yielded the following deviations: positions 162 to 176 read VNMTLAKTEAKLLHE, and Val90 and Ile277 were determined to be Leu and Val, respectively. Pro241 showed a silent mutation in the third component of the codon (ccg instead of ccc). The GenBank entry (u73746) was updated with the new sequence.
Protein identification
The recombinant plant proteins were identified by LC-MS and N-terminal amino acid sequencing. The results from mass spectrometry are summarized in Table 1, and were in agreement with the theoretical values. In particular, the observed mass for Anx(Gh1) confirmed the validity of the newly determined DNA sequence. Amino acid sequencing revealed the correct sequences within the first 10 residues for each protein. With Anx(Gh1) and Anx23(Ca38), the first methionine residue was found to be processed according to mass spectrometry, which agreed with the results from amino acid sequencing; for Anx24(Ca32) and Anx(Gh2) Met1 was present in mass spectrometry but absent according to the sequencing results.
Table 1.
Results from mass spectrometry
| Experimental mass in g/mole | Theoretical mass in g/mole | Mass difference | |
| Anx(Gh1) | 36,448 | 36,457a,b, 36,454b,c | −6 g/mole |
| Anx(Gh2) | 36,873 | 36,873 | ±0 |
| Anx24(Ca32) | 36,901 | 36,907d | −6 g/mole |
| Anx23(Ca38) | 37,031 | 37,036b | −5 g/mole |
a According to GenBank sequence u73746 (plus N-terminal fusion).
b Met1 processed.
c Mass according to newly determined sequence (plus N-terminal fusion).
d Adduct with 1 Na+.
Gel filtration
Analytical gel filtration was used in a qualitative manner to assess the nature of annexin oligomers (see Fig. 2 ▶). While a buffer containing high salt concentrations should increase the amount of an oligomer based on hydrophobic interactions and decrease the amount of oligomer in the case of polar interactions, the opposite is expected for a buffer containing a detergent. Additionally, the effect of 10 mM CaCl2 in a low-salt buffer was investigated. Annexin A5 was used as a control with each buffer condition. Monomer, dimer, and trimer species could be distinguished by their elution times, which are summarized in Table 2.
Fig. 2.
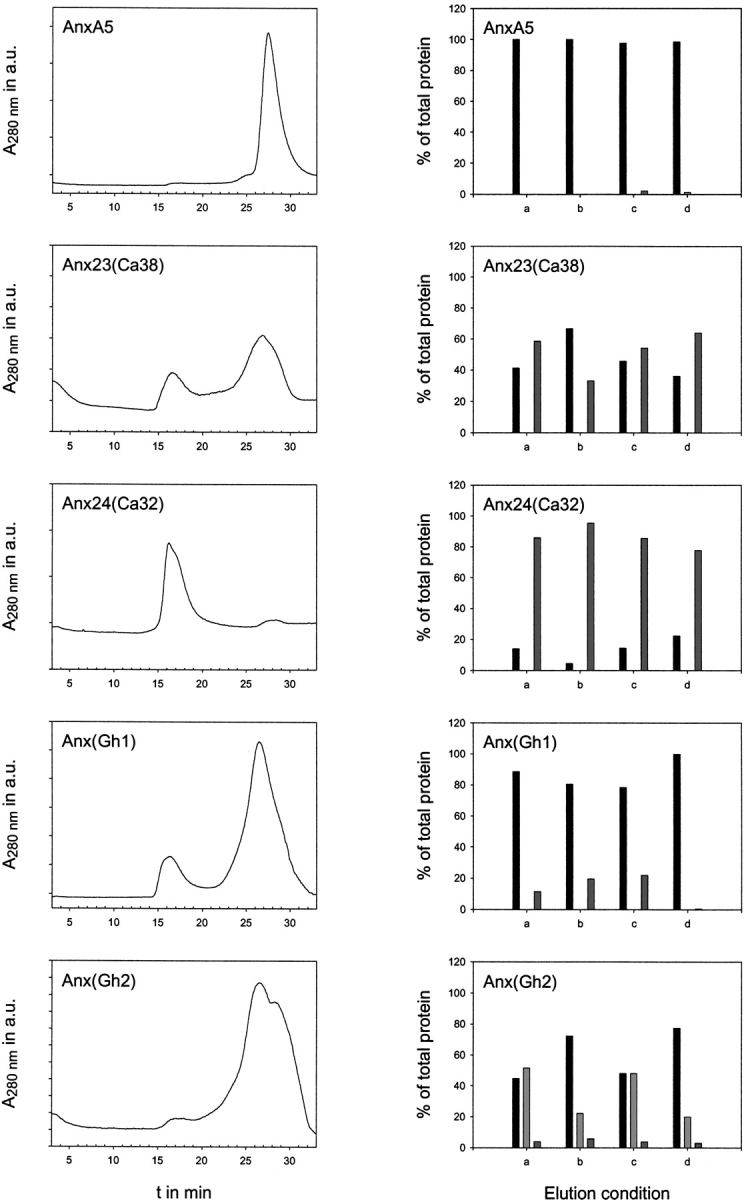
Gel filtration results. The left panel depicts representative gel filtration runs on a Superose-12 column with the five annexins under low salt conditions. Shown on the right panel are the oligomer distributions for the different proteins. The elution conditions are as follows: (a) low salt, (b) high salt, (c) low salt with 0.2% Tween20, and (d) low salt with 10 mM CaCl2. The monomer content is shown as black bar, the dimer as light gray, and the trimer contents as strong gray bars, respectively.
Table 2.
Elution times of the tested annexins on superose-12
| Monomer | Dimer | Trimer | |
| AnxA5 | 28.3 (0.8) | 25.9 (1.1) | 16.9 (0.4) |
| Anx23(Ca38) | 27.3 (0.5) | — | 16.3 (0.3) |
| Anx24(Ca32) | 28.3 (1.0) | — | 16.4 (0.4) |
| Anx(Gh1) | 27.0 (0.5) | — | 16.3 (0.4) |
| Anx(Gh2) | 28.6 (0.5) | 25.8 (1.3) | 16.8 (0.3) |
Shown are average elution times in minutes; the average error is given in parentheses.
Anx24(Ca32) was predominantly observed as a trimer (approx. 80%) in this series. Although the detergent had almost no effect on its elution profile, high salt increased the amount of the trimer by 10%. Calcium, however, slightly lowered the trimer content. Anx23(Ca38) showed almost the same elution profile (monomer–trimer ratio approx. 40:60) for the low-salt conditions in the presence and absence of calcium and in the presence of detergent. High-salt concentrations reversed the monomer–trimer ratio to 60:40. With Anx(Gh1) the dominant species was a monomer that constituted about 90% of the protein content. High salt or the presence of detergent lowered this fraction to about 80% and increased the amount of trimer by 10%. The presence of calcium quantitatively suppressed oligomerization and yielded almost pure monomer.
Anx(Gh2) exhibits a high tendency to precipitate upon storage. Nevertheless, the supernantant after centrifugation was subjected to gel filtration, which revealed a monomer (40%) and a dimer (50%) as predominant species. The presence of detergent did not affect this elution profile significantly, but high-salt conditions as well as the presence of calcium decreased the amount of dimer substantially to 20% and resulted in an increase of monomeric Anx(Gh2). A trimeric species was found at almost constant levels (4%) in all conditions tested.
Sedimentation equilibrium analysis
Sedimentation equilibrium experiments were carried out with Anx23(Ca38), Anx24(Ca32), and Anx(Gh1). Anx(Gh2) was not subjected to analytical ultracentrifugation because of its strong tendency to precipitate at concentrations above 0.5 mg/mL. For the three tested plant annexins, monomer–trimer equilibria were found with the trimer constituting 55% to 65% of the total protein content (see Table 3). The association constants obtained from best fit of a reversible monomer–trimer association model under standard buffer conditions range from 0.9 × 1010−1.7 × 1011 M−2. A number of different association models were evaluated when fitting the equilibrium sedimentation data including models presuming a term for a dimer as well as terms for species higher than a trimer. However, all of the alternative models failed to provide reasonably good fits. Extensive self-association of Anx23(Ca38) was quite obvious, because precipitation occurred during storage and after dialysis; the data of this protein were therefore not obtained at thermodynamic equilibrium, and have to be treated with caution. Nevertheless, the association constant for the homo-trimer and the associated trimer content fit well into the context of the numbers obtained from Anx24(Ca32) and Anx(Gh1). Attempting to suppress the nonspecific aggregation of Anx23(Ca38), the experiment was repeated in the presence of 0.5 M urea. This led to an apparent increase of the trimer content but did not affect the nonspecific self-association.
Table 3.
Results from sedimentation equilibrium experiments
| Sample | Addition | Speed in 1000 rpm | Maveragea in kD | Ka(monomer–trimer)b in M−2 | Trimer contentc in % |
| Anx(Gh1) | None | 10 | 79.3 (1.3) | 1.1 (0.3) × 1011 | 60 (3) |
| 12 | 73.3 (1.6) | 0.9 (0.2) × 1010 | 49 (5) | ||
| Anx23(Ca38) | none | 10 | 95.0 (2.5) | 1.7 (0.5) × 1011 | 64 (9) |
| 12 | 77.6 (2.3) | 4.8 (0.8) × 1010 | 36 (5) | ||
| 5 mM EDTA, 0.5 M urea | 10 | 105.5 (5.0) | 2.8 (1.5) × 1012 | 85 (5) | |
| 12 | 84.8 (4.5) | 2.1 (1.0) × 1011 | 40 (5) | ||
| Anx24(Ca32) | none | 12 | 92.0 (3.0) | 1.1 (0.3) × 1011 | 55 (6) |
| 15 | 77.0 (3.4) | 1.0 (0.3) × 1011 | 49 (8) | ||
| 5 mM EDTA | 12 | 84.2 (3.5) | 8.1 (0.4) × 1010 | 53 (9) | |
| 15 | 78.1 (3.1) | 7.2 (0.6) × 1010 | 48 (7) | ||
| 5 mM CaCl2 | 12 | 89.7 (4.7) | 1.8 (0.5) × 1011 | 56 (5) | |
| 15 | 80.2 (2.0) | 8.8 (1.3) × 1010 | 54 (7) |
The buffer condition was 100 mM NaCl, 20 mM TRIS (pH = 8.0) plus additives as specified. Standard deviations are given in parentheses.
a Weight-average molecular weight for global fits of three protein concentrations.
b Association constant obtained from best fit using a monomer–trimer reversible association model.
c Average content of trimer species in the mixture for three protein concentrations.
Ultracentrifugation experiments were performed with Anx24(Ca32) in the presence of calcium and EDTA. None of these additives changed the sedimentation behavior of this protein.
Discussion
Sedimentation equilibrium experiments with Anx23(Ca38), Anx24(Ca32), and Anx(Gh1) show that these annexins exist in a monomer–trimer equilibrium in solution. The association constants under standard buffer conditions are in the range of 1011 M−2, which indicates a considerable affinity; this translates to about 60% trimer content at concentrations about 1 mg/mL. Parvalbumin, for example, has been shown to form trimers with association constants of 106 to 108 M−2, depending on the buffer conditions (Henzl et al. 1995). With yeast arginase a monomer–trimer equilibrium has been reported as well and the association constant was determined to 2 × 1010 M−2 (Green et al. 1991). Compared to these proteins the plant annexin trimerization described here, exhibited association constants on the high end of the scale. This behavior certainly distinguishes the plant members of the annexin family from their mammalian counterparts. The latter proteins have been shown to form calcium-dependent dimers (see introduction section) with apparently weaker affinity. Results from gel filtration runs show that the presence of calcium does not affect the elution behavior of Anx23(Ca38) but lowers the amount of trimeric species for all other annexins. In case of Anx(Gh2), which exhibits a strong tendency to precipitate, the remaining supernatant shows only a low content of trimeric species, but instead contains a considerable amount of dimeric species. Even more, the presence of calcium negatively affects the dimer formation in accordance with the observation of the effect of calcium on the trimerization of the other annexins tested. We therefore conclude that the Anx(Gh2) dimer is not calcium-dependent either.
Both the ultracentrifugation and gel filtration results suggest that plant annexins investigated in this study exhibit calcium-independent self-association. These findings add further weight to the hypothesis that calcium-binding in the case of plant annexins works differently than for their mammalian relatives. As evident from the primary sequences, plant annexins do not show the high conservation of the endonexin sequence, which is responsible for the creation of type II calcium binding sites in mammalian annexins (Huber et al. 1992). Furthermore, Anx24(Ca32), the only plant annexin for which structural information is currently available, does not seem to provide the structural requirements for binding of calcium ions within the membrane-binding loops. The current study clearly shows the presence of a calcium-independent trimer of this plant annexin. Thus, the head-to-head dimer revealed by the crystal structure seems to be an assembly forced by the crystal packing (Hofmann et al. 2000; Hofmann and Huber, 2002).
Conclusions
This study provides for the first time a characterization of the solution state of different plant annexins. Generally, three members of the annexin family of proteins investigated in this study exhibit monomer–trimer equilibria in solution. For Anx(Gh2), only a small fraction of the protein was found to be trimeric; as observed by gel filtration experiments, this annexin exists mainly in a monomer–dimer equilibrium. Calcium is proven not to be required for the formation of these oligomers. In the case of the cotton annexins, it seems that binding of calcium requires dissolution of the oligomers since the amounts of the trimeric or dimeric species are reduced in the presence of the divalent cation. Thus, plant annexin behavior in solution, and especially calcium affinity, is clearly distinct from that of their mammalian relatives. The comparison within this study also shows that different plant annexins, although showing very high sequence similarity, behave differently regarding oligomerization and calcium affinity. The effect of ATP on the structure of plant annexins as well as characterization of membrane binding of these proteins are currently under investigation.
Materials and methods
Cloning, expression, and purification of recombinant proteins
All plant annexins in this study were subcloned into expression vectors yielding N-terminal His-tag fusion constructs. The tags were introduced together with appropriate restriction sites via polymerase chain reactions using Turbo Pfu polymerase and 10% Me2SO in the reaction mixtures. Annexins 23 and 24 from C. annuum were subcloned from clones p38 and E511 (Proust et al. 1999) into the pRSET_5d vector (Schoepfer 1993) via NcoI/EcoRI restriction sites. Annexin Gh1 from Gossypium hirsutum was subcloned from clone pDEL162 (Potikha and Delmer 1997) into pRSET_6d (Schoepfer 1993) using NdeI and BamHI restriction sites. The annexin Gh2 construct originated from clone pDEL138 (Potikha and Delmer 1997) and was cloned into pRSET_6d (Schoepfer 1993) via NcoI and XhoI restriction sites. The N-terminal fusion in all cases was the octapeptide MAHHHHHH, except for Anx(Gh1), which carried a hexapeptide fusion, MAHHHH.
Expression was carried out in Escherichia coli BL21(DE3). A 1-liter culture of transformed cells was grown overnight at 37°C in LB medium containing 50 mg/L ampicillin. The overnight culture was used to inoculate 8 L of LB medium (50 mg/L ampicillin), which were incubated at 37°C until the absorbance at 600 nm exceeded 1.0. Isopropyl-1-thio-β-d-galactopyranoside was then added to a final concentration of 0.5 mM and the concentration of ampicillin was increased twofold. Cell growth was continued for 4–6 h.
The Capsicum annexins were purified by affinity chromatography using a Ni2+-NTA column. The Gossypium annexins were purified using affinity (Ni2+-NTA column) and anion exchange chromatography (Q-sepharose column). The buffer for affinity chromatography contained 100 mM NaCl, 20 mM TRIS (pH = 8.0) and 20 mM, 50 mM, 100 mM, or 200 mM imidazole. Elution was performed step-wise with constant imidazole concentration. For anion exchange chromatography a gradient 0–1 M NaCl in 20 mM TRIS (pH = 8.0) was generated with a concentric gradient mixer. Annexins eluted at 230–350 mM chloride. Human annexin A5 was purified as described earlier (Burger et al. 1993).
DNA sequencing
All expression constructs were sequenced using a T7 primer (5′-d[TAATACGACTCACTATAGGGAGA]-3′) to verify the correct DNA sequence. We found a different sequence for Anx(Gh1) than the one reported in the GenBank entry u73746. Sequencing of this annexin was therefore repeated with the following primers:
GH9 (coding) 5′-d(GGTGGACTTCAAGCAATCAAGTCC)-3′,
GH10 (coding) 5′-d(GGCACAGATCAATGCAACTCTG)-3′,
GH11 (non-coding) 5′-d(GGACTTGATTGCTTGAAGTCCACC)-3′,
GH12 (non-coding) 5′-d(CAGAGTTGCATTGATCTGTG CC)-3′.
Protein identification
N-terminal amino acid sequencing as well as liquid chromatography-mass spectrometry (LC-MS) was performed for all four recombinant plant proteins. SDS-PAGE was employed routinely for purity analysis.
The LC-MS system consisted of a Hewlett Packard binary pump, degasser, autosampler, and an HP1100 LC-Mass Selective Detector (MSD). Data were acquired on the HP ChemStation data system. The mass spectrometer was scanned from m/z 600 to 1700 every 4 sec. Nitrogen was used to assist nebulization and desolvation. Chromatographic separation was done after an initial wash of 25 min using a gradient from 5 to 100% acetonitrile within 55 min at 40°C on a Zorbax SB-C3 reversed phase column (150 × 2.1 mm i.d.) and a C3 guard column at a flow rate of 0.2 mL/min. Solvent A of the mobile phase consisted of 5% (v/v) acetic acid, solvent B consisted of 100% acetonitrile. The injection volume was 5 μL (50 pmole).
Gel filtration
Gel filtration was carried out with an ÄKTA FPLC system using a Superose-12 column (30 × 1 cm) from Amersham Pharmacia Biotech. The column was equilibrated with six column volumes (CV) of the appropriate buffer. Two hundred microliters of protein were applied to the column and eluted with 1.5 CV at 0.5 mL/min flow rate with each one of the following buffers: low salt (100 mM NaCl, 20 mM TRIS [pH = 8.0]), high salt (1 M NaCl, 20 mM TRIS [pH = 8.0]), low salt with Tween20 (100 mM NaCl, 0.2% Tween20, 20 mM TRIS [pH = 8.0]) and low salt with calcium (100 mM NaCl, 10 mM CaCl2, 20 mM TRIS [pH = 8.0]). Elution was monitored by UV absorption at λ = 280 nm. Sample concentrations ranged from 0.8 mg/mL to 1.4 mg/mL. The chromatograms were analyzed on-line with the provided software (Unicorn 3.21).
Analytical ultracentrifugation
A Beckman Optima Model XL-A analytical ultracentrifuge equipped with a four-place An-Ti rotor was used for sedimentation equilibrium experiments. Three 12-mm cells equipped with carbon-filled, double channel centerpieces and plane quartz windows were used. Protein solutions with absorbance at 280 nm ranging from 0.15 to 0.45 were loaded on the right hand side (200 μL/channel), with the corresponding reference buffer on the left-hand side (220 μL/channel). The reference buffer was the dialysate which contained 20 mM TRIS-HCl and 100 mM NaCl at pH 8.0 (unless otherwise stated); ρ* = 1.003 g/mL at 20°C, as determined with an Anton Paar Model DMA 58 densitometer. After equilibration at 3000 rpm and 20°C at which reference wavelength and radial scans were performed, the rotor was accelerated to the selected experimental speed (10,000, 12,000, or 15,000 rpm). Typically, the proteins were run at two of the three speeds. The scans of protein concentration profiles were collected at 4-h intervals for 56 h. Radial scans were recorded at 280 nm in a step mode with 0.001 cm steps and five averages. Equilibrium was attained typically after 40–44 h, when two consecutive scans taken 4 h apart became indistinguishable. After the data collection was complete, the rotor was accelerated to 40,000 rpm for 4–5 h and the protein sedimented to the bottom of the cell. The experimental centrifuge speed was restored and the baseline absorption values were immediately obtained from a single scan. Analysis of ultracentrifugation data was performed with the software package from Beckman, Inc., and A. P. Minton (NIDDK, NIH). Partial specific volumes of 0.721 mL/g for Anx24(Ca32), 0.717 mL/g for Anx23(Ca38), and 0.717 mL/g for Anx(Gh1), respectively, were calculated from amino acid sequences and the values reported by Zamyatnin (1984).
Acknowledgments
We thank Young Kim (Protein Chemistry Laboratory, SAIC, Frederick) for amino acid sequencing, and Lewis Pannell (Structural Mass Spectrometry Facility, Laboratory of Bioorganic Chemistry, NIDDK) for his support.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.4770102.
References
- Ahn, N.G., Teller, D.C., Bienkowski, M.J., McMullen, B.A., Lipkin, E.W., and de Haen, C. 1988. Sedimentation equilibrium analysis of five lipocortin-related phospholipase A2 inhibitors from human placenta. J. Biol. Chem. 263 18657–18663. [PubMed] [Google Scholar]
- Andrawis, A., Solomon, M., and Delmer, D.P. 1993. Cotton fibre annexins: A potential role in the regulation of callose synthase. Plant J. 3 763–772. [DOI] [PubMed] [Google Scholar]
- Barton, G.J. 1993. ALSCRIPT: A tool to format multiple sequence alignments. Protein Eng. 6 37–40. [DOI] [PubMed] [Google Scholar]
- Battey, N.H., James, N.C., and Greenland, A.J. 1996. cDNA isolation and gene expression of the maize annexins p33 and p35. Plant Physiol. 112 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackbourn, H.D., Barker, P.J., Huskisson, N.S., and Battey, N.H. 1992. Properties and partial protein sequence of plant annexins. Plant Physiol. 99 864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackbourn, H.D., Walker, J.H., and Battey, N.H. 1991. Calcium-dependent phospholipid-binding proteins in plants. Planta 184 67–73. [DOI] [PubMed] [Google Scholar]
- Boustead, C.M., Smallwood, M., Small, H., Bowles, D.J., and Walker, J.H. 1989. Identification of Ca2+-dependent phospholipid-binding proteins in higher plant cells. FEBS Lett. 244 456–460. [Google Scholar]
- Burger, A., Berendes, R., Voges, D., Huber, R., and Demange, P. 1993. A rapid and efficient purification method for recombinant annexin V for biophysical studies. FEBS Lett. 297 25–28. [DOI] [PubMed] [Google Scholar]
- Clark G.B. and Roux S.J. 1995. Annexins of plant cells. Plant Physiol. 109 1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, G.B., Sessions, A., Eastburn, D.J., and Roux, S.J. 2001. Differential expression of members of the annexin multigen family in Arabidopsis. Plant Physiol. 126 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, G.B., Turnwald, S., Tirlapur, U.K., von der Mark, K., Roux, S., and Scheuerlein, R. 1995. Induction and polar distribution of annexin-like proteins during phytochrome mediated rhizoid initiation and growth in spores of the ferns Dryopteris and Anemia. Planta 197 376–384. [DOI] [PubMed] [Google Scholar]
- Creutz, C.E., Pazoles, C.J., and Pollard, H.B. 1979. Self-association of synexin in the presence of calcium. J. Biol. Chem. 254 553–558. [PubMed] [Google Scholar]
- Delmer, D.P. and Potikha, T.S. 1997. Structures and functions of annexins in plants. Cell. Mol. Life Sci. 53 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetics Computer Group. 1996. Wisconsin package, version 9.0. Genetics Computer Group Inc., Madison, WI.
- Green, S.M., Ginsburg, A., Lewis, M.S., and Hensley, P. 1991. Roles of metal ions in the maintenance of the tertiary and quarternary structure of arginase from Saccharomyces cerevisiae. J. Biol. Chem. 266 21474–21481. [PubMed] [Google Scholar]
- Henzl, M.T., Zhao, H., and Saez, C.T. 1995. Self-association of CPV3, an avian thymic Parvalbumin. FEBS Lett. 375 137–142. [DOI] [PubMed] [Google Scholar]
- Hofmann, A. and Huber, R. 2002. Structural conservation and functional versatility: Allostery as a common annexin feature. In Annexins: Biological importance and annexin-related pathologies (ed. J. Bandorowicz-Pikula), Landes Bioscience, in press.
- Hofmann, A., Proust, J., Dorowski, A., Schantz, R., and Huber, R. 2000. Annexin 24 from Capsicum annuum—X-ray structure and biochemical characterization. J. Biol. Chem. 275 8072–8082. [DOI] [PubMed] [Google Scholar]
- Hoshino, T., Mizutani, A., Chida, M., Hidaka, H., and Mizutani, J. 1995. Plant annexin form homodimer during Ca2+-dependent liposome aggregation. Biochem. Mol. Biol. Int. 35 749–755. [PubMed] [Google Scholar]
- Huber, R., Berendes, R., Burger, A., Schneider, M., Karshikov, A., Lücke, H., Römisch, J., and Paques, E.P. 1992. Crystal and molecular structure of human annexin V after refinement. Implications for structure, membrane binding and ion channel formation of the annexin family of proteins. J. Mol. Biol. 223 683–704. [DOI] [PubMed] [Google Scholar]
- Liemann, S., Bringemeier, I., Benz, J., Göttig, P., Hofmann, A., Huber, R., Noegel, A.A., and Jacob, U. 1997. Crystal structure of the C-terminal repeat from synexin (annexin VII) of Dictyostelium discoideum. J. Mol. Biol. 270 79–88. [DOI] [PubMed] [Google Scholar]
- Potikha, T.S. and Delmer, D.P. 1997. cDNA clones for annexin AnnGh1 (Accession No. U73746) and AnnGh2 (Accession No. U73747) from Gossypium hirsutum (cotton) (PGR97–003). Plant Physiol. 113 305.9008398 [Google Scholar]
- Proust, J., Houlne, G., Schantz, M.L., and Schantz, R. 1996. Characterisation and gene expression of an annexin during fruit development in Capsicum annuum. FEBS Lett. 383 208–212. [DOI] [PubMed] [Google Scholar]
- Proust, J., Houlne, G., Schantz, M.L., Shen, W.H., and Schantz, R. 1999. Regulation of biosynthesis and cellular localization of Sp32 annexins in tobacco BY2 cells. Plant Mol. Biol. 39 361–372. [DOI] [PubMed] [Google Scholar]
- Schoepfer, R. 1993. The pRSET family of T7 promoter expression vectors for Escherichia coli. Gene 124 83–85. [DOI] [PubMed] [Google Scholar]
- Seals, D.F. and Randall, S.K. 1997. A vacuole-associated annexin protein, VCaB42, correlates with the expansion of tobacco cells. Plant Physiol. 115 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals, D.F., Parrish, M.L., and Randall, S.K. 1994. A 42 kDa annexin-like protein is associated with plant vacuoles. Plant Physiol. 106 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadle, P.J., Gerke, V., and Weber, K. 1985. Three Ca2+-binding proteins from porcine liver and intestine differ immunologically and physicochemically and are distinct in Ca2+ affinities. J. Biol. Chem. 260 16354–16360. [PubMed] [Google Scholar]
- Smallwood, M.F., Gurr, S.J., McPherson, M.J., Roberts, K., and Bowles, D. 1990. Purification and partial sequence analysis of plant annexins. Biochem. J. 281 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof, T., Walker, J.H., and Obrocki, J. 1982. Calelectrin self-aggregates and promotes membrane aggregation in the presence of calcium. EMBO J. 1 1167–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, J.H., Obrocki, J., and Südhof, T.C. 1983. Calelectrin, a calcium-dependent membrane binding protein associated with secretory granules in Torpedo cholinergic electromotor nerve endings and rat adrenal medulla. J. Neurochem. 41 139–145. [DOI] [PubMed] [Google Scholar]
- Zaks, W.J. and Creutz, C.E. 1991. Ca2+-dependent annexin self-association on membrane surfaces. Biochemistry 30 9607–9615. [DOI] [PubMed] [Google Scholar]
- Zamyatnin, A. 1984. Amino acid, peptide, and protein volume in solution. Annu. Rev. Biophys. Bioeng. 13 145–165. [DOI] [PubMed] [Google Scholar]



