Abstract
The recently described inhibitor of cysteine proteinases from Trypanosoma cruzi, chagasin, was found to have close homologs in several eukaryotes, bacteria and archaea, the first protein inhibitors of cysteine proteases in prokaryotes. These previously uncharacterized 110–130 residue-long proteins share a well-conserved sequence motif that corresponds to two adjacent β-strands and the short loop connecting them. Chagasin-like proteins also have other conserved, mostly aromatic, residues, and share the same predicted secondary structure. These proteins adopt an all-β fold with eight predicted β-strands of the immunoglobulin type. The phylogenetic distribution of the chagasins generally correlates with the presence of papain-like cysteine proteases. Previous studies have uncovered similar trends in cysteine proteinase binding by two unrelated inhibitors, stefin and p41, that belong to the cystatin and thyroglobulin families, respectively. A hypothetical model of chagasin–cruzipain interaction suggests that chagasin may dock to the cruzipain active site in a similar manner with the conserved NPTTG motif of chagasin forming a loop that is similar to the wedge structures formed at the active sites of papain and cathepsin L by stefin and p41.
Keywords: Protease inhibitor; thiol protease; conserved domain; binding mechanism, convergent evolution
Protein inhibitors of proteolytic enzymes, found in animal, plant, and microbial cells, have important roles in regulating the activity of endogenous proteinases and in preventing the deleterious effects of exogenous proteinases (Laskowski and Kato 1980; Barrett and Salvesen 1986; Valueva and Mosolov 1995). In humans, for example, disturbance of the normal protease–inhibitor balance has been shown to lead to such pathological conditions as rheumatoid arthritis, cancer, neurologic disorders, osteoporosis, and lysosomal storage diseases (Chapman et al. 1997; Turk et al. 2000, 2001). The mechanisms of proteinase–inhibitor binding have been extensively studied, mostly using various inhibitors of serine proteases. Despite significant differences in their structures, the majority of those inhibitors were found to follow a common mechanism, which is based on the formation of a stable enzyme–substrate-like complex (Laskowski and Kato 1980; Bode and Huber 1992, 2000; Mosolov 1994).
The importance of cysteine proteinases (Chapman et al. 1997; Barrett et al. 1998; McGrath, 1999; Turk et al. 2000, 2001) led to the proliferation of studies of their inhibitors also. Most characterized inhibitors of cysteine proteases, including cystatin, stefin, and kininogen groups, had similar structural features and were unified in the cystatin superfamily (Barrett and Salvesen 1986; Turk and Bode 1991). Detailed structural studies of the cystatin–papain complexes showed that these inhibitors form a wedge-like structure, blocking the active site of the enzyme (Bode et al. 1988; Stubbs et al. 1990). Recently, crystal structure of the enzyme–inhibitor complex of cathepsin L with p41 invariant chain fragment, which belongs to thyropins, a different family of proteinase inhibitors that contain thyroglobulin type I domain, was determined (Lenarcic and Bevec 1998; Guncar et al. 1999). A comparison of the mechanisms of protease inhibition by cystatins and thyropins showed that, despite a lack of general structural resemblance between those two groups, there were certain similarities in the mode of their interaction with the enzyme active site (Guncar et al. 1999). This was interpreted as a case of convergent structural evolution of these two evolutionarily unrelated groups of cysteine protease inhibitors (Guncar et al. 1999).
In addition, cysteine proteases could be inhibited by proteins that are related to the classical inhibitors of serine proteinases (Krizaj et al. 1993; Schick et al. 1998). Finally, several novel protein inhibitors of cysteine proteases have been recently reported, some of which are homologous to the proteinase propeptide regions and some that do not appear to be homologous to any of the previously known inhibitors (Mosolov 1998; Yamamoto et al. 1999; Brzin et al. 2000).
One of these novel inhibitors of cysteine proteinases is chagasin, a 12-kD protein from Trypanosoma cruzi, the causative agent of Chagas' disease (Monteiro et al. 2001). A cathepsin L-like protease, cruzipain, is a well-known virulence factor for T. cruzi (Cazzulo et al. 1990; Eakin et al. 1992; McGrath et al. 1995a). Given the complexity of fighting this disease with traditional chemical drugs (Stoppani 1999), inhibiting cruzipain has been suggested as a viable method of controlling the Chagas' disease, which stimulated a vigorous search for its inhibitors (McGrath et al. 1995a; Caffrey et al. 2000; Cazzulo et al. 2001). Indeed, various cysteine proteinase inhibitors that were effective against cruzipain in vitro also affect the T. cruzi cells in vivo (Caffrey et al. 2000; Cazzulo et al. 1997, 2001). In contrast, chagasin has been discovered as an endogenous cruzipain inhibitor in T. cruzi cells, also effective against papain and related cysteine proteinases (Monteiro et al. 2001). Surprisingly, chagasin shared no significant sequence similarity with cystatins, thyropins, or any other previously described cysteine protease inhibitors. Secondary structure prediction methods identified chagasin as an all-beta protein, while threading suggested that it adopts an immunoglobulin-type fold, the first protease inhibitor to do so (Rigden et al. 2001). Here we report a detailed sequence analysis of this protein that found chagasin-like proteins encoded in the genomes of representatives of all three major domains of life and allowed us to identify its principal conserved residues. These, along with the conserved secondary structure prediction, suggest that three different classes of papain inhibitors, cystatins, thyropins, and chagasins, share a common trend in binding to their targets, namely a conserved loop flanked by β-strands, that directly binds in the active site cleft. A hypothetical, computational model of chagasin docked to the cruzipain active site is in accord with this idea, and should serve as a preliminary basis for further experiments.
Results and Discussion
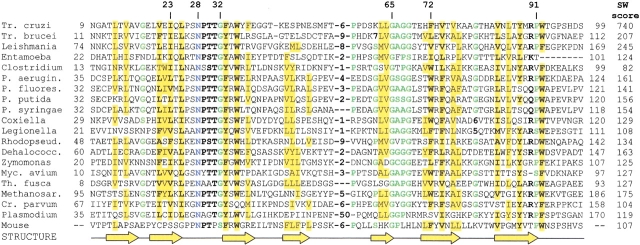
Previously, chagasin was considered to be a unique protein, specific for Trypanosoma cruzi (Monteiro et al. 2001; Rigden et al. 2001). However, a PSI-BLAST search of the nonredundant protein database at the NCBI revealed chagasin homologs in two other protozoans, Leishmania and Entamoeba, and two bacteria, Pseudomonas aeruginosa and Clostridium acetobutylicum (Fig. 1 ▶). Furthermore, searches of the EST data and unfinished microbial genome sequences revealed a family of highly conserved proteins in representatives of bacteria, archaea, and eukaryotes, including a closely related protein encoded in the mouse genome (Fig. 1 ▶). All these proteins have at least 25% identity to the T. cruzi chagasin in the 90-residue overlap and, with the single exception of the C. acetobutylicum protein CAC2467, give statistically significant scores of over 100 in Smith-Waterman searches with T. cruzi sequence (Fig. 1 ▶). Although bacterial inhibitors of serine proteinases have been known for quite some time (Chung et al. 1983; Strickler et al. 1992; Shiga et al. 1993; McGrath et al. 1995b), this appeared to be the first instance of a prokaryotic cysteine protease inhibitor.
Fig. 1.
Multiple alignment of the chagasin family proteins. The conserved residues are shown in bold. Conserved small residues (GASC) and Pro are colored green, conserved hydrophobic residues (ALICVMYFW) are shaded yellow. The positions of β-strands are as predicted by the PHD (Rost and Sander 1993) program. The following sequences were from the NCBI protein database: Trypanosoma cruzi (CAC39242), Leishmania major (CAC01987), Entamoeba histolytica (BAA22021), Pseudomonas aeruginosa (AAG04167), and Clostridium acetobutylicum (AAK80421); the Zymomonas mobilis sequence was translated from the GenBank entry AF102543. Trypanosoma brucei (gi2725738, corrected for a likely frameshift) and mouse (gi9068873, gi16980539) sequences were translated from the EST database. The remaining sequences were from unfinished genome projects at the Institute for Genomic Research (Coxiella burnetii, Dehalococcoides ethenogenes, Ps. putida, T. brucei), the DOE Joint Genome Institute (Methanosarcina barkeri, Ps. fluorescens, Rhodopseudomonas palustris, Thermobifida fusca), the Columbia University Genome Sequencing Center (Legionella pneumophila), and the University of Minnesota Microbial Genome project (Mycobacterium avium subsp. paratuberculosis, Cryptosporidium parvum). The residue numbering in T. cruzi chagasin reflects the mature protein; all other sequences are numbered starting from the first Met residue. The rightmost column shows Smith-Waterman scores for each protein, obtained in the search with T. cruzi chagasin sequence as probe.
Chagasin homologs are typically 110–130 amino acids long and do not contain any additional domains. The conserved core structure includes seven predicted β-strands (Fig. 1 ▶) with one or two more potential β-strands on the flanks. The most conserved sequence elements included the aliphatic residues in the predicted second β-strand, aromatic residues in the predicted third β-strand and the extremely conserved NPTTG motif in the loop connecting those two β-strands (Fig. 1 ▶). Two more predicted β-strands contained similarly located conserved aromatic residues (Fig. 1 ▶). The secondary structure prediction and threading suggested that chagasin might have a β-sandwich structure, formed by two β-sheets, as has been found in the immunoglobulin-type (Ig-type) domains (Rigden et al. 2001). Previous fold recognition and modeling experiments convincingly favored an Ig-type domain over other β-sandwich structures. Furthermore, comparison of the chagasin sequence with an antibody light-chain variable domain consensus sequence revealed conservation of key residues, including an invariant tryptophan and several neighbors, suggestive of a distant evolutionary relationship.
With the benefit of homologous sequences this proposed structural correspondence could be reexamined. A PSI-BLAST search of the NCBI protein database using a profile constructed from the alignment shown in Figure 1 ▶ retrieved several CD8 sequences with highly significant scores (E < 2 × 10−5). Hence, although specialized fold-recognition programs were previously necessary to match chagasins to the Ig-type fold, this relationship has now been supported by sequence comparison methods. The structure of CD8 closely resembles those of light-chain variable domains which, indeed, were used for its structure solution by molecular replacement (Leahy et al. 1992).
The core residue Trp35 and the neighboring Ile23 and Gly65, whose match to the variable light-chain domain consensus was noted previously (Rigden et al. 2001), are all well-conserved in the chagasin family (Fig. 1 ▶). Further conserved residues, the predominantly aromatic positions 33 and 72, extend this core (Fig. 2 ▶). Phe72 contacts both Ser27 and Thr31, possibly aiding in the correct positioning of this highly conserved loop. Thr31 also contacts Pro91 of T. cruzi chagasin, well-conserved in the sequence alignment. The preference for glycine, or other small amino acids at positions 65 and 67, may be readily explained by the steric clashes with this conserved hydrophobic core that would result from the presence of larger side chains.
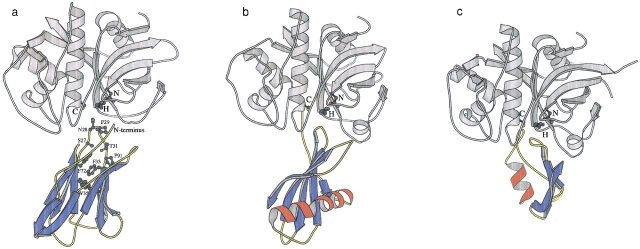
Fig. 2.
The final hypothetical model of the cruzipain–chagasin complex (a) compared to the complexes of papain with stefin (b) and cathepsin L with p41 fragment (c). The previously modeled structure of chagasin was used (Rigden et al. 2001) along with the X-ray structure of cruzipain (Gillmor et al. 1997). These were docked with the FTDOCK suite (Gabb et al. 1997; Moont et al. 1999) and the resultant complexes analyzed with the Protein–Protein Interaction Server (Jones and Thornton 1996) identifying the favored model shown (see Materials and Methods). The papain–stefin and cathepsin L–p41 complexes were drawn based on the 1stf (Stubbs et al. 1990) and 1icf (Guncar et al. 1999) structures, respectively. The catalytic triad is shown and labeled in each case along with key chagasin residues mentioned in the text. The figure was constructed using MOLSCRIPT (Kraulis 1991).
Given all the evidence for chagasin adopting the Ig-type fold, we attempted to predict the mode of interaction of chagasin with its target, cruzipain (Monteiro et al. 2001). As detailed in Materials and Methods, we assumed that chagasin would bind close to the enzyme's catalytic triad, leading to a direct blockage mechanism of inhibition (Bode and Huber 2000) and that the conserved NPTTG motif would be involved in binding. In recognition of the possible mistakes present in the chagasin model, relaxed criteria were adopted. Analysis of the 10 highest scoring putative complexes after application of standard docking and these additional specific filters showed the fourth-ranking complex to have characteristics much more typical of crystallographically determined enzyme–inhibitor complexes (Jones and Thornton 1996) than the other nine (Table 1). The area of the interface of the favored complex was greater than those of the other nine and its complementarity far superior, as shown by the lower gap volume index (Jones and Thornton 1996). The favored complex was also more planar than many of the alternatives, in line with typical enzyme–inhibitor complex values (Table 1). These scores improve further after refinement (Table 1), becoming comparable with those of known enzyme–inhibitor complexes so that this structure was taken as the final model. The complementarity values for alternative models also improved with similar refinement but in no case did the alternative modeled complex attain a typical (mean ± SD) value for complementarity or for interface surface area.
Table 1.
Comparison of interface characteristics for putative cruzipain–chagasin complexes
| Favored complex (fourth-ranking in FTDOCK output) | ||||||
| Nonfavored complexes | ||||||
| Characteristic | Minimum | Maximum | Mean | Before refinement | After refinement | Typical enzyme-inhibitor interface (mean ± SD)a |
| Interface accessible surface area (Å2)b | 487 | 665 | 582 | 731 | 738 | 785 ± 75 |
| Gap volume indexc | 4.9 | 7 | 6 | 3.8 | 2.3 | 2.2 ± 0.5 |
| Planarityd | 1.8 | 2.7 | 2.3 | 2.7 | 2.7 | 2.7 ± 0.4 |
a PDB codes 1ach, 1cho, 1cse, 1mct, 1mcc, 1stf, 1tab, 1tgs, 2ptc, 2sic (see Jones and Thornton 1996).
b Defined as described by Lee and Richards (1971).
c Defined as the gap volume (empty space between the two interacting molecules) divided by the interface surface area (Jones and Thornton 1996).
d Defined as the R.M.S. deviation of interface atoms from the best fit plane through the interface (Jones and Thornton 1996).
Although it must be remembered that the model is purely hypothetical, and that docking remains a challenging problem, as well as the favorable geometrical properties of the final model, other attractive features are apparent. In the final, refined model (Fig. 2 ▶), 17 residues contribute to the interface with cruzipain, namely His2-Lys6, Asn9, Pro26-Thr30, Thr69-His71, and Thr93-Pro95. Regions 26–34 and 90–98 correspond, respectively, to complementarity-determining regions (CDRs) 1 and 3 in the homologous variable light-chain domains. Thus, the prediction is that, in common with Ig-family members in general (Bork et al. 1994), chagasins bind cysteine proteases through regions corresponding to CDRs, with the additional involvement of the N-terminal region and another loop, around residue 70, sited on the same side of the structure. Also, consistent with the conservation of the NPTTG motif, the region from Pro26 to Thr30 contributes almost one-third of the interface area and lies at the heart of the enzyme's catalytic site.
The experimentally determined cysteine protease–inhibitor complexes, papain in complex with stefin B (1stf; Stubbs et al. 1990) and p41 fragment in complex with cathepsin L (1icf; Guncar et al. 1999), revealed very different modes of inhibition, lacking, for example, the canonical interactions of many serine protease inhibitors with their cognate enzymes (Bode and Huber 2000). However, they share two general characteristics with the proposed chagain–cruzipain interaction—an inhibitor structure composed almost entirely of β-strands, and a loop that penetrates deep into the cysteine protease catalytic site (Fig. 2 ▶). In addition, each inhibitor makes hydrophobic interactions with the conserved tryptophan at the center of the catalytic site (Trp177 in cruzipain). These involve the highly conserved Pro29 in chagasin (Fig. 1 ▶), Ala56 in stefin B, and Gly232 in the p41 fragment. The proline residues at position 29 of the chagasins may also be important for maintaining the loop conformation (MacArthur and Thornton 1991), as proposed for the corresponding proline in CD8, whose mutation almost abolishes CD8 binding to HLA I (Giblin et al. 1994).
The emergence of chagasin in the trypanosomal lineage has been previously proposed to occur through horizontal gene transfer (Rigden et al. 2001). This idea gets further support from the patchy phylogenetic distribution of chagasins in the three domains of life. Indeed, while chagasin is encoded in every sequenced representative of the genus Pseudomonas (Fig. 1 ▶), it is not encoded in E. coli, Vibrio cholerae, and many other gamma-proteobacteria. So far, Clostridium acetobutilicum has been the only member of the Bacillus/Clostridium clade that encodes chagasin: B. subtilis, B. halodurans, Staphylococcus aureus, Streptococcus pyogenes, or S. pneumoniae do not carry such a gene. Likewise, chagasin is encoded in the archaebacterium Methanosarcina barkeri, but is missing in the two other methanogens, Methanococcus jannashii and Methanobacterium thermoautotrophicum, or in any other archaeal genomes sequenced to this date. Finally, although genes encoding chagasin-like proteins were found in several mouse ESTs (Fig. 1 ▶), we found no such genes in the genomes or EST libraries of other metazoa.
Despite the fact that Trypanosoma brucei and Entamoeba histolytica represent very distant branches of the phylogenetic tree, the finding of chagasins in these organisms is hardly surprising, as both of them have been shown to encode cruzipain-like cysteine proteases (Eakin et al. 1990). In fact, the presence of different sequences in EST libraries for these organisms shows that each of these organisms carries at least two different chagasin genes. These probably arise from duplication of an ancestral gene, and may reflect the importance of chagasins in the regulation of protease activity. Similarly, other organisms that carry chagasin genes could be expected to encode cruzipain like cysteine proteases. Indeed, a search of unfinished genome sequences identified cysteine proteases in Methanosarcina barkeri, Pseudomonas syringae, and Porphyromonas gingivalis (data not shown). In Clostridium acetobutylicum, a cruzipain-like cysteine protease CAP0004 is encoded on its pSOL1 plasmid, whereas the chagasin gene is located on the chromosome. These observations suggest that regulation of cysteine proteases by chagasin-like proteins is a common trend in all three domains of life. On the other hand, P. aeruginosa and several other bacteria that encode chagasins do not seem to encode cruzipain-like cysteine proteases and might use chagasins for some other function. However, with the exception of C. acetobutylicum and P. aeruginosa, all other genomes that encode chagasins are incomplete, and may still encode papain-like proteases. It is easy to imagine that the corresponding genes could be toxic for the host(s) and would be most likely to be missed in the genome survey-type sequencing projects. Remarkably, in P. aeruginosa, the chagasin-encoding gene PA0778 is located next to and probably forms an operon with the gene PA0779 that encodes a Lon-type protease. The reason for such adjacency, if any, is still not clear, as the possibility of serine protease inhibition by chagasin-related proteins has not yet been studied. It is interesting to note that some molecular scaffolds are known to support different kinds of protease inhibitory activity, sometimes combined in bifunctional inhibitors, perhaps because of the overall similarities common to inhibitor segments that interact with proteases (Tyndall and Fairlie 1999). The Kunitz inhibitor family (Bode and Huber 2000) is an excellent example, containing inhibitors of serine, cysteine, and aspartic proteases (Ritonja et al. 1990; Krizaj et al. 1993; Ravichandran et al. 1999).
Finally, the finding of the chagasin homolog expressed in the intestinal mucosa of the mouse (Fig. 1 ▶) suggests that this protein might play a role in the protection of the mucosal cells from exogenous (e.g., plant) thiol proteinases. A putative human homolog of chagasin was found encoded on a single EST in GenBank; it would therefore be premature at this time to speculate on the presence or absence of chagasins in humans.
In conclusion, chagasins comprise a family of conserved proteins with a patchy phylogenetic distribution that are unrelated to either cystatins or thyropins, but which may share with them common trends in mode of interaction with cysteine proteases. This suggests a three-way convergence of structural features in those evolutionary distinct families of protease inhibitors. Although any conclusions regarding convergent evolution should be made with great care (Doolittle 1994), the evolution of proteases and their inhibitors offers certain cases where such convergence seems very likely (Russell 1998; Makarova and Grishin 1999). The hypothetical complex presented here should serve as a useful basis for further study of the structure–activity relationship in the chagasin family.
Materials and methods
Chagasin-like sequences were identified through BLAST (Altschul et al. 1997) searches of the T. cruzi chagasin sequence run against the following NCBI databases: the nonredundant protein database, the database of finished and unfinished microbial genomes, and the EST database. Sequence similarity of the putative chagasin homologs was evaluated using the Smith-Waterman algorithm, implemented in the SSEARCH program (Pearson 1991). The multiple alignment of the chagasin family (Fig. 1 ▶) was constructed based on the BLAST outputs, further improved by using the SAM-T99 (Karplus et al. 1998) program, and manually edited to reflect the secondary structure predictions for each of the sequences. The consensus secondary structure prediction shown in Figure 1 ▶ was made using PHD (Rost and Sander 1993).
The N-terminal Ser1, modeled ab initio, and therefore of lesser reliability, was removed from the published model structure of chagasin (Rigden et al. 2001). This structure was docked to the highest resolution available structure of cruzipain (1aim; Gillmor et al. 1997; modified to remove covalent modification) using the FTDOCK suite (Gabb et al. 1997; Moont et al. 1999) and pair potentials used to rerank the output (Moont et al. 1999). We used biochemical knowledge to further filter possible solutions and thereby improve the effectiveness of the docking procedure. Two assumptions were made. Direct catalytic site blockage is ubiquitous among cysteine protease inhibitors and is by far the most common protease inhibitor interaction mode in general (Bode and Huber 2000). We, therefore, required that an inhibitor atom lie within 6 Å of the catalytic triad of cruzipain, discarding all possible solutions not meeting this criterion. The location of the NPTTG motif in a surface loop makes it unlikely that its conservation arises purely from structural reasons, favoring the alternate hypothesis that it is important in chagasin function, that is, in the binding to cysteine proteases. We, therefore, also required that possible solutions place the completely conserved Asn28 within 6 Å of the enzyme in hypothetical complexes. Both these criteria are more relaxed than the default 4.5Å (Gabb et al. 1997) to allow for errors due to the chagasin structure being modeled rather than experimentally determined. Putative complexes were analyzed with the Protein–Protein Interaction Server (http://www.biochem.ucl.ac.uk/bsm/PP/server/; Jones and Thornton 1996) to enable the comparison of key parameters—surface complementarity, interface surface area, and planarity—with those generally observed for enzyme–inhibitor complexes. In this way we made use of knowledge of general characteristics of enzyme–inhibitor interfaces as well as specific characteristics of the present case to help guide the docking process. The final model complex was energy minimized for 100 cycles using CNS (Brunger et al. 1998).
Acknowledgments
We acknowledge the availability of unfinished genome sequences from the Institute for Genomic Research (Coxiella burnetii, Dehalococcoides ethenogenes, Porphyromonas gingivalis, Pseudomonas putida), the DOE Joint Genome Institute (Methanosarcina barkeri, Pseudomonas fluorescens, Rhodopseudomonas palustris, Thermobifida fusca), the Columbia University Genome Sequencing Center (Legionella pneumophila), and the University of Minnesota Microbial Genome project (Mycobacterium avium subsp. paratuberculosis, Cryptosporidium parvum).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0207202.
References
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zheng, Z., Miller, W., and Lipman, D.J. 1997. Gapped BLAST and PSI-BLAST—A new generation of protein database search programs. Nucleic Acids Res. 25 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, A.J. and Salvesen, G.S. 1986. Proteinase inhibitors. Elsevier, Amsterdam.
- Barrett, A.J., Rawlings, N.D., and Woessner, J.F.J. 1998. Handbook of proteolytic enzymes. Academic Press, London.
- Bode, W. and Huber, R. 1992. Natural protein proteinase inhibitors and their interaction with proteinases. Eur. J. Biochem. 204 433–451. [DOI] [PubMed] [Google Scholar]
- Bode, W. and Huber, R. 2000. Structural basis of the endoproteinase–protein inhibitor interaction. Biochim. Biophys. Acta 1477 241–252. [DOI] [PubMed] [Google Scholar]
- Bode, W., Engh, R., Musil, D., Thiele, U., Huber, R., Karshikov, A., Brzin, J., Kos, J., and Turk, V. 1988. The 2.0 Å X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J. 7 2593–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork, P., Holm, L., and Sander, C. 1994. The immunoglobulin fold. Structural classification, sequence patterns and common core. J. Mol. Biol. 242 309–320. [DOI] [PubMed] [Google Scholar]
- Brunger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., Read, R.J., Rice, L.M., Simonson, T., and Warren, G.L. 1998. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54 905–921. [DOI] [PubMed] [Google Scholar]
- Brzin, J., Rogelj, B., Popovic, T., Strukelj, B., and Ritonja, A. 2000. Clitocypin, a new type of cysteine proteinase inhibitor from fruit bodies of mushroom Clitocybe nebularis. J. Biol. Chem. 275 20104–20109. [DOI] [PubMed] [Google Scholar]
- Caffrey, C.R., Scory, S., and Steverding, D. 2000. Cysteine proteinases of trypanosome parasites: Novel targets for chemotherapy. Curr. Drug Targets 1 155–162. [DOI] [PubMed] [Google Scholar]
- Cazzulo, J.J., Cazzulo Franke, M.C., Martinez, J., and Franke de Cazzulo, B.M. 1990. Some kinetic properties of a cysteine proteinase (cruzipain) from Trypanosoma cruzi. Biochim. Biophys. Acta 1037 186–191. [DOI] [PubMed] [Google Scholar]
- Cazzulo, J.J., Stoka, V., and Turk, V. 1997. Cruzipain, the major cysteine proteinase from the protozoan parasite Trypanosoma cruzi. Biol. Chem. 378 1–10. [DOI] [PubMed] [Google Scholar]
- Cazzulo, J.J., Stoka, V., and Turk, V. 2001. The major cysteine proteinase of Trypanosoma cruzi: A valid target for chemotherapy of Chagas disease. Curr. Pharm. Des. 7 1143–1156. [DOI] [PubMed] [Google Scholar]
- Chapman, H.A., Riese, R.J., and Shi, G.P. 1997. Emerging roles for cysteine proteases in human biology. Annu. Rev. Physiol. 59 63–88. [DOI] [PubMed] [Google Scholar]
- Chung, C.H., Ives, H.E., Almeda, S., and Goldberg, A.L. 1983. Purification from Escherichia coli of a periplasmic protein that is a potent inhibitor of pancreatic proteases. J. Biol. Chem. 258 11032–11038. [PubMed] [Google Scholar]
- Doolittle, R.F. 1994. Convergent evolution: The need to be explicit. Trends Biochem. Sci. 19 15–18. [DOI] [PubMed] [Google Scholar]
- Eakin, A.E., Bouvier, J., Sakanari, J.A., Craik, C.S., and McKerrow, J.H. 1990. Amplification and sequencing of genomic DNA fragments encoding cysteine proteases from protozoan parasites. Mol. Biochem. Parasitol. 39 1–8. [DOI] [PubMed] [Google Scholar]
- Eakin, A.E., Mills, A.A., Harth, G., McKerrow, J.H., and Craik, C.S. 1992. The sequence, organization, and expression of the major cysteine protease (cruzain) from Trypanosoma cruzi. J. Biol. Chem. 267 7411–7420. [PubMed] [Google Scholar]
- Gabb, H.A., Jackson, R.M., and Sternberg, M.J. 1997. Modelling protein docking using shape complementarity, electrostatics and biochemical information. J. Mol. Biol. 272 106–120. [DOI] [PubMed] [Google Scholar]
- Giblin, P.A., Leahy, D.J., Mennone, J., and Kavathas, P.B. 1994. The role of charge and multiple faces of the CD8 alpha/alpha homodimer in binding to major histocompatibility complex class I molecules: Support for a bivalent model. Proc. Natl. Acad. Sci. 91 1716–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillmor, S.A., Craik, C.S., and Fletterick, R.J. 1997. Structural determinants of specificity in the cysteine protease cruzain. Protein Sci. 6 1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guncar, G., Pungercic, G., Klemencic, I., Turk, V., and Turk, D. 1999. Crystal structure of MHC class II-associated p41 Ii fragment bound to cathepsin L reveals the structural basis for differentiation between cathepsins L and S. EMBO J. 18 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S. and Thornton, J.M. 1996. Principles of protein–protein interactions. Proc. Natl. Acad. Sci. 93 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus, K., Barrett, C., and Hughey, R. 1998. Hidden Markov models for detecting remote protein homologies. Bioinformatics 14 846–856. [DOI] [PubMed] [Google Scholar]
- Kraulis, P.J. 1991. MOLSCRIPT: A program to produce both detailed and schematic plots of protein structures. J. Appl. Cryst. 24 946–950. [Google Scholar]
- Krizaj, I., Drobnic-Kosorok, M., Brzin, J., Jerala, R., and Turk, V. 1993. The primary structure of inhibitor of cysteine proteinases from potato. FEBS Lett. 333 15–20. [DOI] [PubMed] [Google Scholar]
- Laskowski, M., Jr. and Kato, I. 1980. Protein inhibitors of proteinases. Annu. Rev. Biochem. 49 593–626. [DOI] [PubMed] [Google Scholar]
- Leahy, D.J., Axel, R., and Hendrickson, W.A. 1992. Crystal structure of a soluble form of the human T cell coreceptor CD8 at 2.6 Å resolution. Cell 68 1145–1162. [DOI] [PubMed] [Google Scholar]
- Lee, B. and Richards, F. M. 1971. The interpretation of protein structures: Estimation of static accessibility. J. Mol. Biol. 55 379–400. [DOI] [PubMed] [Google Scholar]
- Lenarcic, B. and Bevec, T. 1998. Thyropins—New structurally related proteinase inhibitors. Biol. Chem. 379 105–111. [PubMed] [Google Scholar]
- MacArthur, M.W. and Thornton, J.M. 1991. Influence of proline residues on protein conformation. J. Mol. Biol. 218 397–412. [DOI] [PubMed] [Google Scholar]
- Makarova, K.S. and Grishin, N.V. 1999. Thermolysin and mitochondrial processing peptidase: How far structure–functional convergence goes. Protein Sci. 8 2537–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, M.E. 1999. The lysosomal cysteine proteases. Annu. Rev. Biophys. Biomol. Struct. 28 181–204. [DOI] [PubMed] [Google Scholar]
- McGrath, M.E., Eakin, A.E., Engel, J.C., McKerrow, J.H., Craik, C.S., and Fletterick, R.J. 1995a. The crystal structure of cruzain: A therapeutic target for Chagas' disease. J. Mol. Biol. 247 251–259. [DOI] [PubMed] [Google Scholar]
- McGrath, M.E., Gillmor, S.A., and Fletterick, R.J. 1995b. Ecotin: Lessons on survival in a protease-filled world. Protein Sci. 4 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro, A.C., Abrahamson, M., Lima, A.P., Vannier-Santos, M.A., and Scharfstein, J. 2001. Identification, characterization and localization of chagasin, a tight-binding cysteine protease inhibitor in Trypanosoma cruzi. J. Cell. Sci. 114 3933–3942. [DOI] [PubMed] [Google Scholar]
- Moont, G., Gabb, H.A., and Sternberg, M.J. 1999. Use of pair potentials across protein interfaces in screening predicted docked complexes. Proteins 35 364–373. [PubMed] [Google Scholar]
- Mosolov, V.V. 1994. Protein inhibitors of proteolytic enzymes. Russian J. Bioorg. Chem. 20 153–161. [PubMed] [Google Scholar]
- Mosolov, V.V. 1998. Advances in studies of natural inhibitors of proteolytic enzymes. Russian J. Bioorg. Chem. 24 293–300. [PubMed] [Google Scholar]
- Pearson, W.R. 1991. Searching protein sequence libraries: Comparison of the sensitivity and selectivity of the Smith-Waterman and FASTA algorithms. Genomics 11 635–650. [DOI] [PubMed] [Google Scholar]
- Ravichandran, S., Sen, U., Chakrabarti, C., and Dattagupta, J.K. 1999. Cryocrystallography of a Kunitz-type serine protease inhibitor: The 90 K structure of winged bean chymotrypsin inhibitor (WCI) at 2.13 Å resolution. Acta Crystallogr. D Biol. Crystallogr. 55 1814–1821. [DOI] [PubMed] [Google Scholar]
- Rigden, D.J., Monteiro, A.C., and Grossi de Sa, M.F. 2001. The protease inhibitor chagasin of Trypanosoma cruzi adopts an immunoglobulin-type fold and may have arisen by horizontal gene transfer. FEBS Lett. 504 41–44. [DOI] [PubMed] [Google Scholar]
- Ritonja, A., Krizaj, I., Mesko, P., Kopitar, M., Lucovnik, P., Strukelj, B., Pungercar, J., Buttle, D.J., Barrett, A.J., and Turk, V. 1990. The amino acid sequence of a novel inhibitor of cathepsin D from potato. FEBS Lett. 267 13–15. [DOI] [PubMed] [Google Scholar]
- Rost, B. and Sander, C. 1993. Prediction of protein secondary structure at better than 70% accuracy. J. Mol. Biol. 232 584–599. [DOI] [PubMed] [Google Scholar]
- Russell, R.B. 1998. Detection of protein three-dimensional side-chain patterns: New examples of convergent evolution. J. Mol. Biol. 279 1211–1227. [DOI] [PubMed] [Google Scholar]
- Schick, C., Bromme, D., Bartuski, A.J., Uemura, Y., Schechter, N.M. and Silverman, G.A. 1998. The reactive site loop of the serpin SCCA1 is essential for cysteine proteinase inhibition. Proc. Natl. Acad. Sci. 95 13465–13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga, Y., Yamagata, H., and Udaka, S. 1993. Characterization of the gene encoding an intracellular proteinase inhibitor of Bacillus subtilis and its role in regulation of the major intracellular proteinase. J. Bacteriol. 175 7130–7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppani, A.O. 1999. The chemotherapy of Chagas disease. Medicina (Buenos Aires) 59 147–165. [PubMed] [Google Scholar]
- Strickler, J.E., Berka, T.R., Gorniak, J., Fornwald, J., Keys, R., Rowland, J.J., Rosenberg, M., and Taylor, D.P. 1992. Two novel Streptomyces protein protease inhibitors. Purification, activity, cloning, and expression. J. Biol. Chem. 267 3236–3241. [PubMed] [Google Scholar]
- Stubbs, M.T., Laber, B., Bode, W., Huber, R., Jerala, R., Lenarcic, B., and Turk, V. 1990. The refined 2.4 Å X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: A novel type of proteinase inhibitor interaction. EMBO J. 9 1939–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk, B., Turk, D., and Turk, V. 2000. Lysosomal cysteine proteases: more than scavengers. Biochim. Biophys. Acta 1477 98–111. [DOI] [PubMed] [Google Scholar]
- Turk, V., Turk, B., and Turk, D. 2001. Lysosomal cysteine proteases: Facts and opportunities. EMBO J. 20 4629–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk, V. and Bode, W. 1991. The cystatins: Protein inhibitors of cysteine proteinases. FEBS Lett. 285 213–219. [DOI] [PubMed] [Google Scholar]
- Tyndall, J.D. and Fairlie, D.P. 1999. Conformational homogeneity in molecular recognition by proteolytic enzymes. J. Mol. Recognit. 12 363–370. [DOI] [PubMed] [Google Scholar]
- Valueva, T.A. and Mosolov, V.V. 1995. Involvement of proteolytic enzymes and their inhibitors in plant protection. Appl. Biochem. Microbiol. 31 493–501. [Google Scholar]
- Yamamoto, Y., Watabe, S., Kageyama, T. and Takahashi, S.Y. 1999. A novel inhibitor protein for Bombyx cysteine proteinase is homologous to propeptide regions of cysteine proteinases. FEBS Lett. 448 257–260. [DOI] [PubMed] [Google Scholar]