Abstract
The formation of fibrillar aggregates by β-lactoglobulin in the presence of urea has been monitored by using thioflavin T fluorescence and transmission electron microscopy (TEM). Large quantities of aggregated protein were formed by incubating β-lactoglobulin in 3–5 M urea at 37°C and pH 7.0 for 10–30 days. The TEM images of the aggregates in 3–5 M urea show the presence of fibrils with diameters of 8–10 nm, and increases in thioflavin T fluorescence are indicative of the formation of amyloid structures. The kinetics of spontaneous fibrillogenesis detected by thioflavin T fluorescence show sigmoidal behavior involving a clear lag phase. Moreover, addition of preformed fibrils into protein solutions containing urea shows that fibril formation can be accelerated by seeding processes that remove the lag phase. Both of these findings are indicative of nucleation-dependent fibril formation. The urea concentration where fibril formation is most rapid, both for seeded and unseeded solutions, is ∼5.0 M, close to the concentration of urea corresponding to the midpoint of unfolding (5.3 M). This result indicates that efficient fibril formation involves a balance between the requirement of a significant population of unfolded or partially unfolded molecules and the need to avoid conditions that strongly destabilize intermolecular interactions.
Keywords: Amyloid fibril, kinetics, β-lactoglobulin, thioflavin T, electron microscope, MALDI-TOF mass
It is now well established that under physiologic conditions proteins assume native folds that are unique to their amino acid sequences. But it is also clear that proteins are highly prone to form a variety of aggregated structures in vitro. Such aggregates can accumulate in biological environments giving rise to pathological conditions (Hurtley and Helenius 1989; Dobson 1999, 2001; Ellgaard et al. 1999). One particularly important example of these phenomena involves the formation of specific aggregates called amyloid fibrils. Such aggregates were first recognized in tissue taken from a patient suffering from Alzheimer's disease (Virchow 1853), but have now been observed in some 20 diseases including type II diabetes and the spongiform encephalopathies such as Creutzfeldt-Jakob disease (Tan and Pepys 1994; Kelly 2002). Moreover, it has been shown recently that many proteins and short peptides—whether or not they are known to be involved in disease—can be transformed in vitro into fibrillar aggregates identical to those associated with disease by searching for appropriate conditions (Dobson 1999, 2001). In general, experiments show that such transformations into fibrils occur under conditions that unfold the protein at least partially, but still favor the formation of noncovalent interactions within the ensemble of denatured conformations (Chiti et al. 1999, 2000; Bellotti et al. 2000; Liu et al. 2000; McParland et al. 2000; Fändrich et al. 2001).
Although there are differences in detail between the various morphologies observed for specific amyloid fibrils, analysis of their structures using a range of techniques including X-ray fiber diffraction and electron microscopy reveals common properties of the underlying structure. In particular, the fibrils usually have a diameter ranging from 5 to 20 nm (Vallat et al. 1979; Winer et al. 1979; Merz et al. 1983; Prusiner et al. 1983; Connors et al. 1985), a cross-β structure consisting of a core of β-strands (Kirshchner et al. 1987), along with an affinity to bind a range of dyes such as thioflavin T (thioT) (Naiki et al. 1989) and Congo red (Klunk et al. 1999; Westermark et al. 1999). Experimental data also indicate that the formation of amyloid fibrils is a nucleation-dependent process in which initial species produced by the association of specific regions of denatured proteins plays an important role in initiating the process (Jarrett and Lansbury 1993; Lomakin et al. 1996, 1997; Naiki and Nakakuki 1996; Perutz and Windle 2001). The experimental studies suggest that the ability to form amyloid fibrils is a common property of polypeptide chains, although the intrinsic propensities of sequences of amino acids to do so under given conditions will vary widely (Chiti et al. 2002). This observation is potentially of great importance to understand the evolved properties of native proteins, including their folding behavior, and the mechanism in which misfolding and aggregation are normally avoided in correctly functioning living systems (Dobson 2001; Bucciantini et al. 2002).
Bovine β-lactoglobulin (β-LG) is one of the major components of the whey of cow's milk. The protein assumes a dimeric structure at neutral pH, but dissociates into monomers at an acidic pH. The conformation of the protein at both neutral and acidic pH has been determined by X-ray crystallography (Brownlow et al. 1997; Qin et al. 1998, 1999) and NMR spectroscopy (Kuwata et al. 1999; Uhrinova et al. 2000). The structures at the different pH values possess the same basic topology, having nine antiparallel β-strands and one short and one long α-helix at the carboxyl terminus (Fig. 1 ▶). Although the biological function of β-LG is still unknown, the protein has the ability to bind to extended hydrophobic compounds such as retinol (Dodin et al. 1990; Dufour et al. 1991; Hambling et al. 1992). Interestingly, the amino acid sequence suggests a significant propensity for α-helical structure despite the highly β-rich fold (Nishikawa and Noguchi 1991). In accord with this observation, an early intermediate state of the protein during refolding has been found to form non-native α-helical structures (Hamada et al. 1996; Kuwajima et al. 1996; Hamada and Goto 1997) in the vicinity of the amino-terminal region of the sequence, a region corresponding to the A-strand in the native structure (Kuwata et al. 2001).
Fig. 1.
Schematic representation of the native structure of bovine β-LG drawn using WebLab Viewer Lite, Molecular Simulations Inc. (San Diego, CA). The β-strands and α-helices are colored in cyan and red, respectively.
The β-LG is known to form gels in solution under various conditions in which the protein is likely to be at least partially unfolded, for example, at high temperatures (Langton and Hermansson 1992; Bauer et al. 1998; Relkin et al. 1998), under high hydrostatic pressure (Zasypkin et al. 1996; Dumay et al. 1998), or in the presence of chemical denaturants (Katsuta et al. 1997; Dufour et al. 1998; Renard et al. 1999). Because the gels formed under certain conditions have different characters, there has been increasing interest in the utilization of β-LG gels to improve the quality of food products (Smithers et al. 1996). Importantly, similar gelation phenomena have been observed for several proteins under conditions where amyloid fibrils are formed (Charge et al. 1995; Guijarro et al. 1998; Chiti et al. 1999, 2000; Goda et al. 2000). Furthermore, electron micrographs of heat-induced gels of β-LG indicate the existence of network structures composed of long filaments with diameters of 4–10 nm (Hermansson 1986; Langton and Hermansson 1992; Kavanagh et al. 2000).
In the present work, we show that fibrillar structures can be readily formed by incubating bovine β-LG in the presence of urea at pH 7.0 and 37°C for 10–30 days. Transmission electron microscopy (TEM) of the solutions reveals the existence of well-defined fibrils with diameters of 8–10 nm. The solutions enhance the fluorescence of thioT, indicative of the presence of amyloid structures. Furthermore, the addition of aliquots of solutions containing preformed fibrils (i.e., seeding) dramatically reduces the lag time for the initiation of fibril formation and promotes fibril growth. The results indicate that bovine β-LG is converted into amyloid structures by incubating the protein under conditions where the protein is substantially unfolded but the formation of noncovalent interactions within the ensemble of denatured protein molecules is permitted.
Results
Urea-induced unfolding of β-lactoglobulin
It has been established for a number of proteins that formation of amyloid fibrils occurs in the region of the protein unfolding transition (Stevens et al. 1995; Guijarro et al. 1998; Chiti et al. 1999, 2000; Bellotti et al. 2000; Liu et al. 2000; McParland et al. 2000; Fändrich et al. 2001). Therefore, we explored possible conditions for the conversion of β-LG into ordered aggregates by monitoring the urea unfolding transition of β-LG using intrinsic tryptophan fluorescence at pH 7.0 and 37°C.
For β-LG at a concentration of 0.05 mg/mL, a cooperative transition with a midpoint at a urea concentration of 3.1 ± 0.1 M was obtained by monitoring the fluorescence intensity at 343 nm (Fig. 2A ▶, circles). This value is significantly lower than the midpoint of the transition (5.3 ± 0.1 M) obtained from the plot of the peak position of these spectra as a function of urea concentration (Fig. 2B ▶, triangles). The difference in the transition midpoints obtained by fluorescence intensity and peak position suggests that at least one conformational species, other than the native dimer or the fully unfolded state, is present under at least some denaturing conditions.
Fig. 2.

Urea-induced unfolding of β-LG at pH 7.0 and 37°C. (A) Data obtained by monitoring the fluorescence intensity at 343 nm in the presence of 0.05 (○) or 1.0 (□) mg/mL of β-LG. The ordinate is the relative value to the fluorescence of unfolded protein in 8.0 M urea. (B) Data obtained by monitoring the frequency shift of the fluorescence maximum in the presence of 0.05 (▵) and 1.0 () mg/mL of β-LG, and the fluorescence intensity of ANS (⋄) in the presence of 0.05 mg/mL of β-LG. (C) Fractions of native dimeric, native monomeric, and unfolded states as a function of urea concentration in the presence of 1 mg/mL of β-LG. The curves were drawn using the parameters shown in the text.
As discussed, β-LG assumes a dimeric structure at a neutral pH. There is, therefore, a possibility that the dimer can be dissociated by an increase in urea concentration into either native or at least only partially unfolded monomers before the formation of fully unfolded structures. In comparison with the unfolding transition observed at a β-LG concentration of 0.05 mg/mL, the transition observed by the change in fluorescence intensity at 343 nm for the solution containing 1 mg/mL of β-LG is shifted to a much higher urea concentration. In contrast, no significant shift could be observed for the transition curve obtained from the change in the position of the fluorescence maximum. This difference suggests that the transition detected by the change in fluorescence intensity, which occurs at a lower urea concentration, is the result of the dissociation of the native dimer into native monomers, whereas the transition revealed by the shift of the peak position corresponds to unfolding of such monomers into partially or fully unfolded species (Fig. 2 ▶).
The hydrophobic dye ANS shows an intense fluorescence signal at ∼480 nm when it binds to exposed hydrophobic clusters on the surface of partially folded intermediate states of proteins (Semisotnov et al. 1987). In contrast, significant fluorescent changes are rarely observed for ANS molecules in the presence of native or fully unfolded proteins. Exceptionally, native β-LG stimulates an increase in ANS fluorescence due to its intrinsic property to bind to hydrophobic molecules (Collini et al. 2000). Nevertheless, ANS binding can detect the presence of a partially folded intermediate with non-native α-helical structure during the folding and unfolding of this protein at pH 2.0 (D. Hamada and Y. Goto, unpubl.).
In the present work, it has been found that the fluorescence intensity of ANS decreases with increasing concentration of urea (Fig. 2B ▶). The transition midpoint obtained by monitoring this fluorescence change is highly consistent with the unfolding curve obtained from the shift of the intrinsic tryptophan fluorescence peak. This observation suggests that the dissociation of ANS from β-LG occurs when the protein transforms into its fully unfolded state at high urea concentrations. Although ANS may affect the equilibrium among conformational states, it is rather likely that ANS stabilizes the partially folded states, if it exists, relative to the native state. It is, therefore, highly unlikely that a significant amount of a partially folded species is populated during the urea unfolding of β-LG at pH 7.0 and 37°C. The same conclusion has been drawn from the analysis of far- and near-UV circular dichroism spectra during unfolding of β-LG by urea at pH 7.0 and 25°C (M. Yagi, K. Sakurai, and Y. Goto, pers. comm.).
According to these observations, the unfolding transition of β-LG by urea are considered in the following scheme:
 |
Scheme 1. |
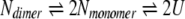 |
where Ndimer, Nmonomer, and U refer to the native dimeric, native monomeric, and unfolded states. On the basis of this scheme, the parameters for urea unfolding of β-LG are estimated and they are ΔGDM,0 = 50.3 ± 0.3 (kJ/mol); mDM = 4.9 ± 0.1 (kJ/mol/M); ΔGMU,0 = 20.1 ± 2.4 (kJ/mol); mMU = 3.8 ± 0.4 (kJ/mol/M). Importantly, the mMU value obtained here at neutral pH was relatively consistent with the value (5.0 ± 0.1 kJ/mol/M; Apenten 1998) obtained at pH 3.0, where the protein predominantly stabilizes the native monomer. This further supports the idea that the conformational state that accumulates during the transition of β-LG induced by urea is likely to be the native monomer.
Spontaneous fibril formation in urea solutions
The β-LG solutions containing a variety of concentrations of urea were incubated at 37°C and the existence of amyloid fibrils was probed by TEM and thioT fluorescence.
When solutions of the protein containing 3–5 M urea were incubated for periods of 10–30 days, opalescent precipitates became visible, and the presence of fibrillar aggregates was shown by TEM (Fig. 3 ▶). The electron micrographs reveal that these aggregates are unbranched, twisted fibrils with diameters of 8–10 nm (Table 1), comparable to the diameters of typical amyloid fibrils (4–20 nm) (Vallat et al. 1979; Winer et al. 1979; Merz et al. 1983; Prusiner et al. 1983; Connors et al. 1985; Chamberlain et al. 2000). Interestingly, micrographs of the fibrils formed in 3 M urea indicate the presence of some thicker fibrils, with diameters of 15 nm, relative to those formed at the higher urea concentrations.
Fig. 3.
TEM images of aggregates formed by incubating β-LG for 30 days at 37°C in (A) 3.0, (B) 4.0, (C) 5.0 M urea at pH 7.0. The scale bars represent 100 nm.
Table 1.
Diameters of spontaneously formed β-LG fibrils
| Urea concentration (M) | Diameter of fibrilsa (nm) |
| 3.0 | 9.30 ± 1.41 |
| 15.90 ± 1.67b | |
| 4.0 | 9.50 ± 1.21 |
| 5.0 | 8.23 ± 2.06 |
a Determined from the diameters at more than 100 randomly selected sections of different fibrils.
b Average diameter of the minor population of the thicker fibrils shown in Fig. 3 ▶.
The solutions containing the fibrils exhibited an increase in thioT fluorescence indicative of the formation of amyloid structures (Fig. 4A ▶). No significant increase in thioT fluorescence was, however, observed for solutions containing 0–2 M urea even after 70 days of incubation. At the protein concentrations used in these experiments (1 mg/mL), β-LG assumes the native dimer structure at 0–2 M urea, whereas it dissociates into the native monomers at 2–4 M urea before the formation of fibrils (see Fig. 2C ▶). Therefore, it appears that the presence of the native dimer can efficiently prevent the formation and extension of fibril nuclei. This result is consistent with the observation that the stabilization of the native tetramer by substrate binding inhibits the formation of transthyretin fibrils (Miroy et al. 1996).
Fig. 4.

Increases in thioT fluorescence upon binding to aggregates of β-LG. (A) The fluorescence intensity of thioT versus urea concentration. (B) ThioT fluorescence at 0 (|aI|aU), 3 (○), 4 (□), 5 (▵), 6 () and 7 (⋄) M urea as a function of incubation time. Inset of B is the expansion to represent the data at 0, 6, and 7 M urea.
For the samples in 3–7 M urea, plots of thioT fluorescence as a function of incubation time produce sigmoidal curves showing a clear lag phase (Fig. 4B ▶). Similar kinetic behavior has been found to occur for many amyloidogenic proteins (Jarrett and Lansbury 1993; Lomakin et al. 1996; Naiki and Nakakuki 1996; Perutz and Windle 2001). Importantly, the urea concentrations showing the largest increase of thioT fluorescence (Fig. 4A ▶) are low (3–5 M) in comparison with the transition region for urea unfolding monitored by intrinsic tryptophan fluorescence (3–7 M) (see Fig. 2 ▶). Below the transition region (<3 M urea), the formation of aggregates is efficiently suppressed. Above the transition region where the protein begins to unfold (>3 M urea), the formation of fibrils is clearly observed. As the urea concentration increases, the rate and yield of fibril formation initially increase, reflecting the greater population of unfolded molecules able to aggregate. Above 5 M urea, however, both the rate and yield of fibril formation decrease substantially, a result that can be explained by the increased ability of urea to solvate the unfolded state. This result supports the idea that the fibrillogenesis of proteins is inhibited when noncovalent interactions are destabilized within the ensemble of unfolded structures (Chiti et al. 1999, 2000).
The sigmoidal curves such as these shown in Figure 4B ▶ were analyzed by nonlinear least squares curve-fitting to a stretched exponential function: 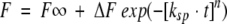 (Alvarez et al. 1991; Morozova-Roche et al. 1999; Jund et al. 2000). In this equation, F, F∞, and ΔF are the observed fluorescence intensity at time t, the final fluorescence intensity, and the fluorescence amplitude, respectively. ksp is the rate of spontaneous fibril formation. Although the interpretation of the parameters involved in this equation is not straightforward, these parameters are useful in empiric descriptions of complex reactions whose kinetics are not easily modeled (Morozova-Roche et al. 1999; Jund et al. 2000). For example, when the n value is close to 1, the curve can be expressed by a single exponential function with a rate constant of ksp. For 0 < n < 1, the kinetics can be approximated to multiple exponential functions indicative of multiple events. On the other hand, for n > 1, the curves represent a sigmoidal transition with an initial lag phase, suggestive of the involvement of intermediate species. The inverse of the rate constant ksp gives the relaxation time, that is, the time when the 1/e (∼36.8%) of the reaction is completed (where e is Euler's constant). For n > 1, a reaction becomes more cooperative as the value of n increases.
(Alvarez et al. 1991; Morozova-Roche et al. 1999; Jund et al. 2000). In this equation, F, F∞, and ΔF are the observed fluorescence intensity at time t, the final fluorescence intensity, and the fluorescence amplitude, respectively. ksp is the rate of spontaneous fibril formation. Although the interpretation of the parameters involved in this equation is not straightforward, these parameters are useful in empiric descriptions of complex reactions whose kinetics are not easily modeled (Morozova-Roche et al. 1999; Jund et al. 2000). For example, when the n value is close to 1, the curve can be expressed by a single exponential function with a rate constant of ksp. For 0 < n < 1, the kinetics can be approximated to multiple exponential functions indicative of multiple events. On the other hand, for n > 1, the curves represent a sigmoidal transition with an initial lag phase, suggestive of the involvement of intermediate species. The inverse of the rate constant ksp gives the relaxation time, that is, the time when the 1/e (∼36.8%) of the reaction is completed (where e is Euler's constant). For n > 1, a reaction becomes more cooperative as the value of n increases.
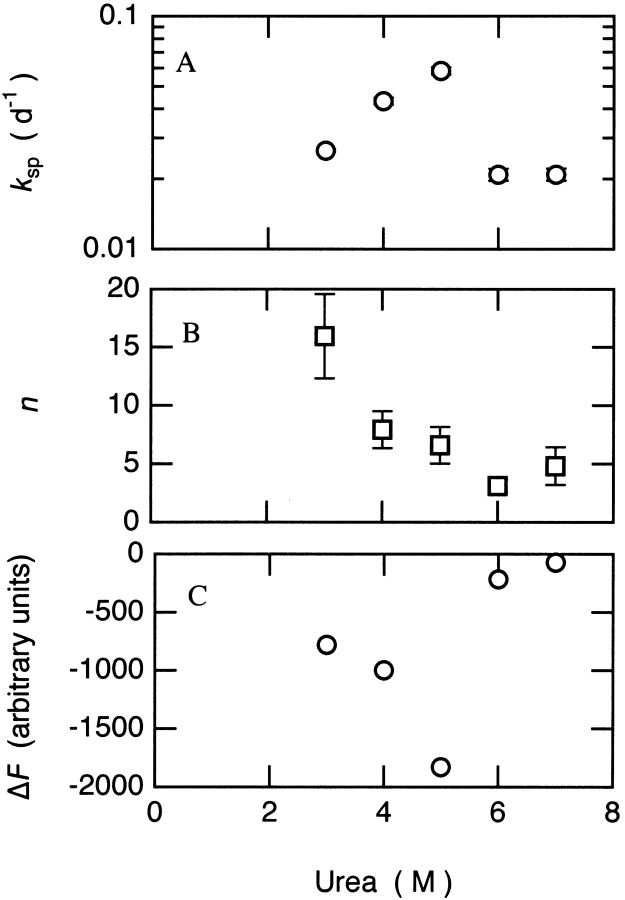
The ΔF, ksp, and n values determined in this way for the different urea concentrations are shown in Figure 5 ▶. Values of n more than 5 were obtained for all the samples showing a significant increase of thioT fluorescence; this result indicates the presence of a lag phase and the relatively high cooperativity of the growth phase that follows. The maximum ksp value (0.058 d−1) was found for the fibril growth in 5.0 M urea. This concentration of urea is close to the midpoint (5.3 M) of the unfolding transition of the monomeric species shown in Figure 2 ▶. Thus, at this concentration of urea, there is a substantial population of unfolded protein molecules in which the polypeptide chain is exposed so as to permit efficient aggregation. At higher urea concentrations, the urea molecules can solvate the denatured state and destabilize aggregated species. This effect is likely to reduce significantly the propensity for fibril formation.
Fig. 5.
Kinetic parameters for spontaneous fibril formation by β-LG. The data were fitted to stretched exponential functions F = F∞ + ΔF exp (−[ksp • t]n). (A) Rate constant (ksp) versus (urea). (B) Exponent (n) versus (urea). (C) Amplitude (ΔF) versus (urea).
The amplitude (ΔF) of the fluorescence can indicate the approximate quantity of fibrils formed in each solution, because fluorescence intensity is associated with fibril formation and is unlikely to vary substantially with urea concentration under these conditions. As in the case for the ksp value, the absolute value of ΔF is maximum in solutions containing 5.0 M urea. The differences in ΔF for the solutions containing varying urea concentrations suggests that the system is fairly closely balanced between polymerization and depolymerization. Interestingly, the plot of ksp versus ΔF is approximately linear, with a correlation coefficient of 0.94 (Fig. 6 ▶). Although a range of factors (e.g., the rates of fibril nucleation, extension, and depolymerization) will affect ksp, it appears to be primarily influenced by the length of the lag phase. As the latter reflects the rate of nucleation in the nucleation-dependent conversion process, the correlation found in Figure 6 ▶ implies that the total amount of protein molecules converted into the fibrils increases when the rate of nucleation becomes more rapid. Such a result may reflect the increased probability of the formation of organized fibrils rather than amorphous aggregates.
Fig. 6.
Rate constant of spontaneous fibril formation (ksp) versus the final quantity of protein incorporated into fibrils (ΔF). Experimental data are represented by circles. The line corresponds to the best fit of experimental data to a straight line.
The effect of seeding on the kinetics of fibril formation
Experimental data for other proteins suggest that the reaction associated with amyloid formation can be approximated by a nucleation-dependent model and that the reaction can be decomposed into at least two steps, namely the nucleation and the extension processes (Jarrett and Lansbury 1993; Lomakin et al. 1996, 1997; Naiki and Nakakuki 1996). This idea is supported by observations that the addition of preformed fibrils (seeds) promotes the fibril extension reaction. If the nucleation step has been achieved by the seeding process, provided that the lag phase has been completely abolished, the seeding experiments can provide information concerning the intrinsic rate of extension of fibrils under different conditions. To explore a further aspect of the process of fibril formation by β-LG, the effects of seeding on the kinetics were examined.
In agreement with the nucleation model, the initial lag time for β-LG fibrillogenesis was found to be significantly diminished when seeding was carried out with aliquots of the solutions incubated at the various concentrations of urea (Fig. 7A ▶). In contrast to the results for spontaneous fibrillogenesis, small but distinctive increases in thioT fluorescence could be monitored even for the solutions containing low concentrations of urea (0–2 M); this finding suggests that spontaneous formation of fibrils can occur under these conditions, but at a very slow rate. The experimental data, therefore, suggest that the conversion of soluble β-LG into amyloid fibrils in urea proceeds, as in other cases, according to a nucleation-dependent manner. Under ideal conditions, the rate of fibril extension can be obtained by fitting the data to a single exponential function (Naiki and Gejyo 2000). However, the kinetics shown here, even after seeding, are relatively slow and there could be a contribution to the extension kinetics from spontaneously formed nuclei, particularly at the later stage of fibrillogenesis. Therefore, to provide a more accurate description of the rate of intrinsic fibril extension, the initial slope for the reaction (dF/dt)t = 0 was considered in our analysis.
Fig. 7.

Seeding effects on fibril extension monitored by thioT fluorescence. (A) ThioT fluorescence intensity as a function of time. Data were obtained at 0 (▹◃), 1 (⊕), 2 (⊞), 3 (○), 4 (□), 5 (▵), 6 (), and 7 (⋄) M urea. (B) Initial rates of fibril extension (dF/dt)t = 0 in the seeding experiments, as described in the text, as a function of these urea concentrations.
Figure 7B ▶ shows a plot of (dF/dt)t = 0 determined from the rates of fibril formation measured by thioT fluorescence under the seeding conditions as a function of the urea concentration. In this situation, the value of (dF/dt)t = 0 correlates well with the rate of fibril extension. The intrinsic extension rate can be seen to become faster with increasing urea concentrations up to 5 M, but then to decrease as the urea concentration is increased. Interestingly, logarithms of (dF/dt)t = 0 in both the acceleratory and deceleratory regions are approximately linearly dependent on the urea concentration. The results of seeding experiments also indicate that the maximum rate of fibril extension is obtained under conditions where the rate of fibril nucleation is optimum (i.e., ∼5 M urea) (Figs. 4 and 7 ▶ ▶). In Figure 8 ▶, the values of (dF/dt)t = 0 for the seeding experiment are plotted against the ksp values obtained for spontaneous fibrillogenesis. The plot shows a high correlation between these parameters (the correlation coefficient is 0.96), indicating that the two processes are closely related.
Fig. 8.
Comparison of the rate of fibril formation for unseeded (ksp) and seeded (dF/dt)t = 0 solutions. Experimental data are represented by circles. The continuous line corresponds to the best fit of experimental data to a straight line.
Analysis of the protein in the fibrils
It has previously been shown that the formation of a misfolded dimer containing non-native intermolecular disulfide bonds plays a role in the thermally induced aggregation of β-LG (Shimada and Cheftel 1989).The protein contains five cysteine residues, one of which is free (C121) and the other four are involved in the formation of two disulfide bridges (C66–C160 and C102–C124) (see Fig. 1 ▶) (Brownlow et al. 1997). To examine whether such dimeric species are involved in the fibril formation by β-LG in the presence of urea, and more generally to probe for any degradation of the protein molecules during the time taken for fibril formation to occur, we used matrix associate laser disorption-time of flight (MALDI-TOF) mass spectrometry to characterize β-LG after its extraction from the fibrillar structures.
To find conditions where β-LG incorporated into the fibrils formed in 5 M urea could be solubilized, we tested a range of denaturants including 8 M urea, 8 M guanidium hydrochloride, and 95% (v/v) acetonitrile/H2O containing 0.05% trifluoroacetic acid; only the acetonitrile/H2O mixture was found to dissolve the fibrils and then only to a limited extent. The mass spectrum obtained for the protein solubilized using this procedure shows the presence of a single species with a molecular mass of 18,432.3 ± 9.2 daltons. This value is close to the molecular mass of monomeric β-LG (18,463.2 ± 9.2 daltons) determined from a reference spectrum of native β-LG in water. Thus, it is unlikely that any misfolded dimeric species play a significant role in fibril formation by β-LG in the presence of urea, or that any significant degradation of the protein takes place during this process.
Discussion
An increasing body of evidence suggests that the ability to form amyloid fibrils is a generic property of polypeptide chains, although the propensity of different proteins and of different regions of proteins to form such structures varies substantially (Dobson 1999, 2001). Therefore, if a given polypeptide is incubated under appropriate conditions, it should be possible to promote its conversion into amyloid fibrils. An important, but by no means sufficient, characterization of such conditions is likely to be that the native structure of a globular protein is substantially destabilized. The present results for β-LG support this idea and indicate that controlled variation of the concentrations of common chemical denaturants such as urea can be a valuable approach to find the appropriate conditions under which fibrils are formed. A range of urea concentrations could be identified such that large quantities of bovine β-LG fibrils were formed. These fibrils were shown to be amyloid in character from their appearance in TEM images, and from their ability to enhance the fluorescence intensity of thioT.
Fibril formation was found to be particularly rapid in the transition region for the unfolding of β-LG by urea, probably due to the increased population of unfolded protein molecules. However, the rate of formation decreased more than 5 M urea, although the population of unfolded protein molecules increased (Fig. 2C ▶). This observation can be attributed to the need for a high population of unfolded proteins for aggregation to occur and for these unfolded molecules to interact strongly with each other. The former property increases with the concentration of a denaturant such as urea, whereas the latter decreases (Chiti et al. 1999; Bellotti et al. 2000). It is, however, still unclear which conformational species is a direct precursor for fibril formation. There is a possibility for the involvement of partially folded species that accumulates only to a small amount. Such conformational species is more likely to be stabilized under the conditions where the unfolded state is populated and still promote the protein aggregation. As with other amyloid fibrils, the rate of formation was found to be promoted by the addition of preformed fibrils, a finding indicative of a nucleation-dependent process. The present results indicate further that the rate of nucleation correlates well with the rate of fibril extension, a finding suggesting that the interactions required for the nucleation event of fibrillogenesis are similar to those required for fibril extension.
Polymorphism of the structural morphology of amyloid fibrils has been demonstrated for several proteins (Ionescu-Zanetti et al. 1999, Chamberlain et al. 2000; Zurdo et al. 2001). Consistent with these studies, different morphologies were found for fibrils formed at the various urea solutions. In addition, the existence of a variety of network structures composed of filaments with diameters ranging from 4 to 10 nm has been reported for heat-induced β-LG gels (Hermansson 1986; Langton and Hermansson 1992; Kavanagh et al. 2000). Such polymorphism in the fibril structures formed from a single protein could be caused by differences in the number of protofilaments assembled into the mature fibrils. It could also, however, result from the incorporation in different regions of the sequence of the polypeptide chain with various types of fibrils. Although this latter possibility cannot be eliminated at this stage, the fact that the addition of fibrils formed at 5.0 M urea to the protein solutions at other concentrations of urea successfully promoted fibril extension suggests that the regions of the amino acid sequence that are incorporated into the core region of fibrils are essentially the same in the solutions at different urea concentrations.
Summary
The present results indicate that amyloid fibril formation by bovine β-LG is promoted under conditions where significant accumulation of unfolded proteins occurs, but is inhibited under conditions where higher denaturant concentrations destabilize intermolecular interactions. These experimental data support strongly the hypothesis that the ability to form amyloid fibrils is an intrinsic property of polypeptide chains, although the propensity to form such fibrils is strongly dependent on the amino acid sequence, and as demonstrated clearly here, on the solution conditions under which the proteins are incubated.
Materials and methods
Chemicals and EM grids
The A variant of bovine β-LG was purchased from Sigma (St. Louis, MO). Other chemicals of reagent grade were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). The polyvinylformal-carbon-coated 200-mesh copper grids (200-A) were obtained from Oken Shoji Co.,Ltd. (Tokyo, Japan).
Preparation of seeding solutions
Fibrils for seeding experiments were prepared by centrifuging protein solutions (1 mg/mL) in 5.0 M urea containing fibrils at 20,000 g for 10 min. The pellets were resuspended in water and centrifuged again, and the process repeated twice. The resulting precipitates were resuspended in distilled water, and used for seeding experiments. The amount of protein incorporated into fibrils was determined by measuring the weight of dried protein in the tube after lyophilizing aliquots of the solutions.
Urea unfolding by tryptophan and ANS fluorescence
All experiments were carried out in 10 mM sodium phosphate buffer at pH 7.0 and 37°C. The fluorescence intensity of either tryptophan or ANS was monitored using a F4500 fluorimeter (Hitachi, Tokyo, Japan). For intrinsic tryptophan fluorescence, protein solutions of 0.05 or 1 mg/mL with varying concentrations of urea were excited at 290 nm and the fluorescence at 300–400 nm was monitored. For ANS-binding analysis, solutions of 5 μM ANS in 0.05 mg/mL β-LG and various concentrations of urea were excited at 398 nm and the fluorescence intensity at 500–600 nm was monitored. For each experiment, the protein sample was incubated for 5 min at 37°C before taking the measurement.
Analysis of the unfolding transitions
The unfolding transition of β-LG by urea are considered to follow the scheme 1. Thus, the fractions of Ndimer, Nmonomer, and U (fD, fM, and fU, respectively) can be expressed as:
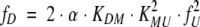 |
(1) |
 |
(2) |
 |
(3) |
where α is the total protein concentration (M) in the solution.
Provided that the free energy differences between the dimeric and monomeric native states (ΔGDM) and between the monomeric native and unfolded states (ΔGMU) are dependent on urea concentration, they can be expressed as:
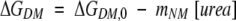 |
(4) |
 |
(5) |
where ΔGDM,0 and ΔGMU,0 are ΔGDM and ΔGMU in the absence of urea, mDM and mMU are the measures of cooperativity of transitions between the dimeric and monomeric native states and between the monomeric native and unfolded states, respectively. ΔGDM,0, ΔGMU,0, mDM, and mMU were calculated by full deconvolution of all the tryptophan fluorescence spectra obtained at different urea concentrations according to the functions shown in eqs. 1–5.
Thioflavin T assay
Solutions (1.2 mL) of 1 mg/mL protein and various concentrations of urea were prepared in 1.5-mL plastic tubes with lids, and the tubes were then tightly sealed to prevent evaporation. The samples were placed in an air incubator at 37°C. For each incubation time, 40-μL aliquots of the solutions were taken and mixed with 360 μL of 5 μM thioT solution in 10 mM sodium phosphate buffer at pH 7.0. The fluorescence at 465–665 nm was monitored using 5-mm cuvettes. The excitation wavelength was 450 nm. A stretched exponential function, expressed as 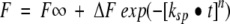 , was used in the curve-fitting analysis of the kinetics of spontaneous fibril formation determined from thioT fluorescence. The ksp and n values are the rate constant (day−1) and heterogeneity parameter, respectively (Morozova-Roche et al. 1999; Jund et al. 2000). The F∞ and ΔF values are the fluorescence intensity at the end of reaction and the amplitude change during the reaction. For the seeding experiments, 10 μg of aliquots of preformed fibrils were added to the protein solutions (1 mg/mL) containing various amounts of urea. The initial rates of fibril extension were determined to eliminate possible errors due to spontaneous nucleation.
, was used in the curve-fitting analysis of the kinetics of spontaneous fibril formation determined from thioT fluorescence. The ksp and n values are the rate constant (day−1) and heterogeneity parameter, respectively (Morozova-Roche et al. 1999; Jund et al. 2000). The F∞ and ΔF values are the fluorescence intensity at the end of reaction and the amplitude change during the reaction. For the seeding experiments, 10 μg of aliquots of preformed fibrils were added to the protein solutions (1 mg/mL) containing various amounts of urea. The initial rates of fibril extension were determined to eliminate possible errors due to spontaneous nucleation.
TEM imaging
TEM images of samples showing a typical fluorescence increase by thioT were acquired with a JEM 1010 or JEM-1200EX II transmission electron microscope (JEOL, Tokyo, Japan). The acceleration voltage was 80 or 85 kV. The samples were negatively stained by either uranium acetate or phosphotungstate.
Mass spectroscopy
Mass spectra of resolubilized fibrils formed by β-LG were obtained using a Voyager DE MALDI-TOF mass spectrometer from PerSeptive Biosystems (Framingham, Massachusetts, USA). Sinapinic acid was used to form the matrix complex. Fibrils prepared as described above were resolubilized using 95% acetonitrile/5% H2O, containing 0.05% trifluoroacetic acid.
Acknowledgments
We thank Dr. Shingo Takagi for his assistance in the TEM study and Prof. Saburo Aimoto and Dr. Takashi Higurashi for performing the MALDI-TOF mass spectrometry experiments. We also acknowledge Prof. Yuji Goto for his valuable comments on this work. DH was supported by a JSPS Postdoctoral Fellowships for Research Abroad at the beginning of this work in Oxford and is currently supported by JSPS Research Fellowships for Young Scientists (PD). This is a contribution from the Oxford Centre for Molecular Sciences, which is funded by BBSRC, EPSRC. and MRC. The research of CMD is supported in part by a Programme Grant from the Wellcome Trust.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
NMR, nuclear magnetic resonances
TEM, transmission electron microscopy
ANS, 1-anilino-8-naphthalene sulfonic acid
β-LG, β-lactoglobulin
thioT, thioflavin T
MALDI, matrix associate laser disorption
TOF, time of flight
d−1, day −1
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0217702.
References
- Alvarez, F., Alegría, A., and Colmenero, J. 1991. Relationship between the time-domain Kohlraush-Willams-Watts and frequency-domain Havriliak-Nagami relaxation functions. Phys. Rev. B 44 7306–7312. [DOI] [PubMed] [Google Scholar]
- Apenten, R.K.O. 1998. Protein stability function relations: β-Lactoglobulin-A sulphydryl group reactivity and its relationship to protein unfolding stability. Int. J. Biol. Macromol. 23, 19–25. [DOI] [PubMed] [Google Scholar]
- Bauer, R., Hansen, S., and Øgendal, L. 1998. Detection of intermediate oligomers, important for the formation of heat aggregates of β-lactoglobulin. Int. Dairy J. 8 105–112. [Google Scholar]
- Bellotti, V., Mangione, P., and Merlini, G. 2000. Review: Immunoglobulin light chain amyloidosis—the archetype of structural and pathogenic variability. J. Struct. Biol. 130 280–289. [DOI] [PubMed] [Google Scholar]
- Brownlow, S., Morais Cabral, J.H., Cooper, R., Flower, D.R., Yewdall, S. J., Polikarpov, I., North, A.C., and Sawyer, L. 1997. Bovine β-lactoglobulin at 1.8 Å resolution—still an enigmatic lipocalin. Structure 5 481–495. [DOI] [PubMed] [Google Scholar]
- Bucciantini, M., Giannoni, E., Chiti, F., Baroni, F., Formigli, L., Zurdo, J., Taddei, N., Ramponi, G., Dobson, C.M., and Stefani, M. 2002. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416 507–511. [DOI] [PubMed] [Google Scholar]
- Chamberlain, A.K., MacPhee, C.E., Zurdo, J., Morozova-Roche, L.A., Hill, H.A.O., Dobson, C.M., and Davis, J.J. 2000. Ultrastructural organization of amyloid fibrils by atomic force microscopy. Biophys. J. 79 3282–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charge, S.B., de Koning, E.J., and Clark, A. 1995. Effect of pH and insulin on fibrillogenesis of islet amyloid polypeptide in vitro. Biochemistry 34 14588–14593. [DOI] [PubMed] [Google Scholar]
- Chiti, F., Webster, P., Taddei, N., Clark, A., Stefani, M., Ramponi, G., and Dobson, C.M. 1999. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc. Natl. Acad. Sci. 96 3590–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti, F., Taddei, N., Bucciantini, M., White, P., Ramponi, G., and Dobson, C.M. 2000. Mutational analysis of the propensity for amyloid formation by a globular protein. EMBO J. 19 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti, F., Taddei, N., Baroni, F., Capanni, C., Stefani, M., Ramponi, G., and Dobson, C.M. 2002. Kinetic partitioning of protein folding and aggregation. Nature Struct. Biol. 9 137–143. [DOI] [PubMed] [Google Scholar]
- Collini, M., D'Alfonso, L., and Baldini, G. 2000. New insight on β-lactoglobulin binding sites by 1-anilinonaphthalene-8-sulfonate fluorescence decay. Protein Sci. 9 1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors, L.H., Shirahama, T., Skinner, M., Fenves, A., and Cohen, A.S. 1985. In vitro formation of amyloid fibrils from intact β2-microglobulin. Biochem. Biophys. Res. Commun. 131 1063–1068. [DOI] [PubMed] [Google Scholar]
- Dobson, C.M. 1999. Protein misfolding, evolution and disease. Trends Biochem. Sci. 24 329–332. [DOI] [PubMed] [Google Scholar]
- ———. 2001. The structural basis of protein folding and its links with human disease. Philos. Trans. Biol. Sci. 356 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodin, G., Andrieux, M., and Kabbani, A. 1990. Binding of ellipticine to β-lactoglobulin. A physico-chemical study of the specific interaction of an antitumor drug with a transport protein. Eur. J. Biochem. 193 697–700. [DOI] [PubMed] [Google Scholar]
- Dufour, E., Marden, M.C., and Haertle, T. 1991. β-Lactoglobulin binds retinol and protophophyrin IX at two different binding sites. FEBS Lett. 277 223–226. [DOI] [PubMed] [Google Scholar]
- Dufour E., Robert, P., Renard, D., and Llamas, G. 1998. Investigation of β-lactoglobulin gelation in water/ethanol solutions. Int. Dairy J. 8 87–93. [DOI] [PubMed] [Google Scholar]
- Dumay, E.M., Kalichevsky, M.T., and Cheftel, J.C. 1998. Characteristics of pressure-induced gels of β-lactoglobulin at various times after pressure release. Food Sci. Technol. 31 10–19. [Google Scholar]
- Ellgaard, L., Molinari, M., and Helenius, A. 1999. Setting the standards: Quality control in the secretary pathway. Science 286 1882–1888. [DOI] [PubMed] [Google Scholar]
- Fändrich, M., Fletcher, M.A., and Dobson, C.M. 2001. Amyloid fibrils from muscle myoglobin. Nature 410 165–166. [DOI] [PubMed] [Google Scholar]
- Goda, S., Takano, K., Yamagata,Y., Nagata, R., Akutsu, H., Maki, S., Namba, K., and Yutani K. 2000. Amyloid protofilament formation of hen egg lysozyme in highly concentrated ethanol solution. Protein Sci. 9 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijarro, J.I., Sunde, M., Jones, J.A., Campbell, I.D., and Dobson, C.M. 1998. Amyloid fibril formation by SH3 domain. Proc. Natl. Acad. Sci. 95 4224–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada, D. and Goto, Y. 1997. The equilibrium intermediate of β-lactoglobulin with non-native helical structure. J. Mol. Biol. 269 479–487. [DOI] [PubMed] [Google Scholar]
- Hamada, D., Segawa, S., and Goto, Y. 1996. Non-native α-helical intermediate in the refolding of β-lactoglobulin, a predominantly β-sheet protein. Nature Struct. Biol. 3 868–873. [DOI] [PubMed] [Google Scholar]
- Hambling, S.G., McAlpine, A.S., and Sawyer, L. 1992. β-Lactoglobulin. In Advanced dairy chemistry (ed. Fox, P.F.), vol. 1, pp. 140–190. Elsevier Applied Science, UK.
- Hermansson, A.-M. 1986. Water and fat-holding. In Functional properties of food macromolecules (eds. J.R. Michell, and D.A. Ledward), pp. 273–314. Elsevier Applied Science, UK.
- Hurtley, S.M. and Helenius, A. 1989. Protein oligomerization in the endoplasmic reticulum. Annu. Rev. Cell. Biol. 5 277–307. [DOI] [PubMed] [Google Scholar]
- Ionescu-Zanetti, C., Khurana, R., Gillespie, J.R., Petrick, J.S., Trabachino, L.C., Minert, L.J., Carter, S.A., and Fink, A.L. 1999. Monitoring the assembly of Ig light-chain amyloid fibrils by atomic force microscopy. Proc. Natl. Acad. Sci. 96 13175–13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett, J.T. and Lansbury P.T. Jr. 1993. Seeding "one-dimensional crystallization" of amyloid: A pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73 1055–1058. [DOI] [PubMed] [Google Scholar]
- Jund, P., Jullien, R., and Campbell, I. 2000. Random walks on fractals and stretched exponential relaxation. Phys. Rev. 63 036131. [DOI] [PubMed] [Google Scholar]
- Katsuta, K., Hatakeyama, M., and Hiraki, J. 1997. Isothermal gelation of proteins. 1. Urea-induced gelation of whey proteins and their gelling mechanism. Food Hydrocolloids 11 367–372. [Google Scholar]
- Kavanagh, G.M., Clark, A.H., and Ross-Murphy, S.B. 2000. Heat-induced gelation of globular proteins: part 3. Molecular studies on low pH β-lactoglobulin gels. Int. J. Biol. Macromol. 28 41–50. [DOI] [PubMed] [Google Scholar]
- Kelly, J.W. 2002. Towards an understanding of amyloidogenesis. Nature Struct. Biol. 9 323–325. [DOI] [PubMed] [Google Scholar]
- Kirschner, D.A., Inouye, H., Duffy, L.K., Sinclair, A., Lind, M., and Selkoe, D.J. 1987. Synthetic peptide homologous to β protein from Alzheimer disease forms amyloid-like fibrils in vitro. Proc. Natl. Acad. Sci. 84 6953–6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk, W.E., Jacob, R.F., and Mason, P. 1999. Quantifying amyloid by Congo red spectral shift assay. Method Enzymol. 309 285–305. [DOI] [PubMed] [Google Scholar]
- Kuwajima, K., Yamaya, H., and Sugai, S. 1996. The burst-phase intermediate in the refolding of β-lactoglobulin studied by stopped-flow circular dichroism and absorption spectroscopy. J. Mol. Biol. 264 806–822. [DOI] [PubMed] [Google Scholar]
- Kuwata, K., Hoshino, M., Forge, V., Era, S., Batt, C.A., and Goto, Y. 1999. Solution structure and dynamics of bovine β-lactoglobulin A. Protein Sci. 8 2541–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwata, K., Shastry, R., Cheng, H., Hoshino, M., Batt, C.A., Goto., Y., and Roder, H. 2001. Structural and kinetic characterization of early folding events in β-lactoglobulin. Nature Struct. Biol. 8 151–155. [DOI] [PubMed] [Google Scholar]
- Langton, M. and Hermansson, A.M. 1992. Fine-stranded and particulate gels of β-lactoglobulin and whey protein at varying pH. Food Hydrocolloids 6 523–539. [Google Scholar]
- Liu, K. Cho, H.S., Lashuel, H.A., Kelly, J.W., and Wemmer, D.E. 2000. A glimpse of a possible amyloidogenic intermediate of transthyretin. Nature Struct. Biol. 7 754–757. [DOI] [PubMed] [Google Scholar]
- Lomakin, A., Chung, D.S., Benedek, G.B., Kirschner, D.A., and Teplow, D.B. 1996. On the nucleation and growth of amyloid β-protein fibrils: detection of nuclei and quantitation of rate constants. Proc. Natl. Acad. Sci. 93 1125–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin, A., Teplow, D.B., Kirschner, D.A., and Benedek, G.B. 1997. Kinetic theory of fibrillogenesis of amyloid β-protein. Proc. Natl. Acad. Sci. 94 7942–7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz, P.A., Wisniewski, H.M., Somerville, R.A., Bobin, S.A., Masters, C.L., and Iqbal, K. 1983. Ultrastructural morphology of amyloid fibrils from neuritic and amyloid plaques. Acta Neuropathol. (Berl) 60 113–124. [DOI] [PubMed] [Google Scholar]
- McParland, V.J., Kad, N.M., Kalverda, A.P., Brown, A., Kirwin-Jones, P., Hunter, M.G., Sunde, M., and Radford, S.E. 2000. Partially unfolded states of β2-microglobulin and amyloid formation in vitro. Biochemistry 39 8735–8746. [DOI] [PubMed] [Google Scholar]
- Miroy, G.J., Lai, Z., Lashuel, H.A., Peterson, S.A., Strang, C., and Kelly, J. 1996. Inhibiting transthyretin amyloid fibril formation via protein stabilization. Proc. Natl. Acad. Sci. 93 15051–15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova-Roche, L.A., Jones, J.A., Noppe, W., and Dobson, C.M. 1999. Independent nucleation and heterogeneous assembly of structure during folding of equine lysozyme. J. Mol. Biol. 289 1055–1073. [DOI] [PubMed] [Google Scholar]
- Naiki, H. and Gejyo, F. 2000. Kinetic analysis of amyloid fibril formation. Methods Enzymol. 309 305–318. [DOI] [PubMed] [Google Scholar]
- Naiki, H. and Nakakuki, K. 1996. First-order kinetic model of Alzheimer's β-amyloid fibril extension in vitro. Lab. Invest. 74 374–383. [PubMed] [Google Scholar]
- Naiki, H., Higuchi, K., Hosokawa, M., and Takeda, T. 1989. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal. Biochem. 177 244–249. [DOI] [PubMed] [Google Scholar]
- Nishikawa, K. and Noguchi, T. 1991. Predicting protein secondary structure based on amino acid sequence. Methods Enzymol. 202 21–24. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F. and Windle, A.H. 2001. Cause of neural death in neurodegenerative diseases attributable to expansion of glutamine repeats. Nature 412 143–144. [DOI] [PubMed] [Google Scholar]
- Prusiner, S.B., McKinley, M.P., Bowman, K.A., Bolton, D.C., Bendheim, P.E., Groth, D.F., and Glenner, G.G. 1983. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35 349–358. [DOI] [PubMed] [Google Scholar]
- Qin, B.Y., Bewley, M.C., Creamer, L.K., Baker, H.M., Baker, E.N., and Jameson, G.B. 1998. Structural basis of the Tanford transition of bovine β-lactoglobulin. Biochemistry 37 14014–14023. [DOI] [PubMed] [Google Scholar]
- Qin, B.Y., Bewley, M.C., Creamer, L.K., Baker, E.N., and Jameson, G.B. 1999. Functional implications of structural differences between variants A and B of bovine β-lactoglobulin. Protein Sci. 8 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relkin, P., Launay, B., and Liu, T.-X. 1998. Heat- and cold-setting gels of β-lactoglobulin solutions. A DSC and TEM study. Thermochemica Acta 308 69–74. [Google Scholar]
- Renard, D., Lefebvre, J., Robert, P., Llamas, G., Dufour, E., and Dufour, E. 1999. Structural investigation of β-lactoglobulin gelation in ethanol/water solutions .Int. J. Biol. Macromol. 26 35–44. [DOI] [PubMed] [Google Scholar]
- Semisotnov, G.V., Rodionova, N.A., Kutyshenko, V.P., Ebert, B., Blank, J., and Ptitsyn, O.B. 1987. Sequential mechanism of refolding of carbonic anhydrase B. FEBS Lett. 224 9–13. [DOI] [PubMed] [Google Scholar]
- Shimada, K. and Cheftel, J.C. 1989. Sulfhydryl group/disulfide bond inter-change reactions during heat-induced gelation of whey protein isolate. J. Agricul. Food Chem. 37 161–168. [Google Scholar]
- Smithers, G.W., Ballard, F.J., Copeland, A.D., De Silva, K.J., Dionysius, D.A., Francis, G.L., Goddard, C., Grieve, P.A., McIntosh, G.H., Mitchell, I.R., Pearce, R.J., and Regester, G.O. 1996. New opportunities from the isolation and utilization of whey proteins. J. Dairy Sci. 79 1454–1459. [DOI] [PubMed] [Google Scholar]
- Stevens, P.W., Raffen, R., Hanson, D.K., Deng, Y.L., Berrios-Hammond, M., Westholm, F.A., Murphy, C., Eulitz, M., Wetzel, R., and Solomon, A. 1995. Recombinant immunoglobulin variable domains generated from synthetic genes provide a system for in vitro characterization of light-chain amyloid proteins. Protein Sci. 4 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, S.Y. and Pepys, M.B. 1994. Amyloidosis. Histopathology 25 403–414. [DOI] [PubMed] [Google Scholar]
- Uhrinova, S., Smith, M.H., Jameson, G.B., Uhrin, D., Sawyer, L., and Barlow, P.N. 2000. Structural changes accompanying pH-induced dissociation of the β-lactoglobulin dimer. Biochemistry 39 3565–3574. [DOI] [PubMed] [Google Scholar]
- Vallat, M., Vallat, J.M., Loubet, R., Leboutel, M.J., and Loubet, A. 1979. Conjunctival biopsies in diffuse amyloid. J. Fr. Ophtalmol. 2 275–278. [PubMed] [Google Scholar]
- Virchow, R. 1853. Über eine im Gehirn und Rückenmark des Menschen aufgefundene Substanz mit der chemischen Reaktion der Cellulose. Virchows Arch. 6 135–138. [Google Scholar]
- Westermark, G.T., Johnson, K.H., and Westermark, P. 1999. Staining methods for identification of amyloid in tissue. Method Enzymol. 309 3–25. [DOI] [PubMed] [Google Scholar]
- Winer, R.L., Wuerker, R.B., Erickson, J.O., and Cooper, W.L. 1979. Ultrastructural examination of urinary sediment. Value in renal amyloidosis. Am. J. Clin. Pathol. 71 36–39. [DOI] [PubMed] [Google Scholar]
- Zasypkin, D.V., Dumay, E., and Cheftel, J.C. 1996. Pressure- and heat-induced gelation of mixed β-lactoglobulin/xanthan solutions. Food Hydrocolloids 19 203–211. [Google Scholar]
- Zurdo, J., Guijarro, J.I., Jimenez, J.L., Saibil, H.R., and Dobson, C.M. 2001. Dependence on solution conditions of aggregation and amyloid formation by an SH3 domain. J. Mol. Biol. 311 325–340. [DOI] [PubMed] [Google Scholar]