Abstract
The structure of the C-terminal region of the third cytoplasmic loop (IC3) of the cannabinoid receptor one (CB1) bound to Gαi1 has been determined using transferred nuclear Overhauser effects (NOEs). The wild-type IC3 sequence is helical when associated with Gαi1. In contrast, a peptide containing the amino-acid inversion, Ala341-Leu342 adopts a single turn. These findings correlate with the attenuated Gi association of CB1 with the Ala341-Leu342 mutation previously observed in vivo and the diminished stimulation of Gαi1 GTPase activity by the corresponding peptide demonstrated in vitro here. These results, the first to report the structure of a GPCR domain while associated with G protein, imply the C-terminus of CB1 IC3, a region with high-sequence conservation among G-protein coupled receptors, must be helical for efficient coupling and activation of the Gi protein.
Keywords: Cannabinoid receptor (CB1), G-protein coupled receptors, G-protein association, constitutively active receptors, third cytoplasmic loop (IC3), transferred NOEs
Two subtypes of G-protein coupled cannabinoid receptors, CB1 and CB2, have been identified and cloned. The CB1 receptors are found predominantly in the central nervous system (Gerard et al. 1991) while CB2 receptors are found mainly in association with immune cells, including the marginal zone of the spleen and in macrophages (Munro et al. 1993). The receptors have been implicated in mediation of cannabinoid-induced analgesia, antiinflammation, and immunosuppression. Recently, cannabinoid receptor agonism has been shown to control spasticity in a multiple sclerosis mouse model (Baker et al. 2000) and may regulate food intake as part of the neural circuitry regulated by leptin (Di Marzo et al. 2001).
CB1 and CB2 heterologously expressed in cultured cells have been shown to inhibit the accumulation of cAMP in a ligand-dependent manner (Felder et al. 1995; Slipetz et al. 1995), consistent with the notion that coupling to inhibitory G proteins (Gi) is a major signaling pathway for CB receptors (Howlett et al. 1986). CB1, but not CB2, can also couple to Gs but the functional significance of this association is not yet known (Glass and Felder 1997).
The identification of the regions of the receptors involved in the coupling to the G proteins has been an area of active research (Mukhopadhyay and Howlett 2001; Nie and Lewis 2001b). The C terminus of CB1 has been shown to play an important role in G protein coupling (Nie and Lewis 2001a), as well as in the desensitization and internalization of CB1 (Hsieh et al. 1999). Phosphorylation (Garcia et al. 1998) and peptide studies (Howlett et al. 1998) of the CB1 receptor, along with a number of studies in analogous regions of other GPCRs (Cotecchia et al. 1990; Ren et al. 1993; Samama et al. 1993), have shown that the C-terminal region of the third intracellular loop plays a significant role in G-protein activation. Previously, we have shown that the third cytoplasmic loop of CB1 is involved in coupling and activation of the G protein, and that exchange of the position of Leu341 and Ala342 resulted in altered specificity to constitutive Gs coupling (Abadji et al. 1999). Here, we examine the structure and function of this region of CB1. The structure of the wild-type sequence (WT-IC3: D338IRLAKTLV346) and mutant containing an inversion of residues 341 and 342 (AL-IC3: D338IRALKTLV346) while associated with the G protein is solved. This is the first example of a GPCR domain structure associated with G protein. Correlating these findings with the functional assays (stimulation of GTPase) illustrates important features for efficient receptor coupling and activation of the G protein.
Results
To reveal structural features of CB1 important for coupling with G protein, we exploited the differences in Gα subunit specificity exhibited in vivo by the wild type and the mutant Ala341-Leu342 receptor (Abadji et al. 1999). Peptides corresponding to the IC3 region were examined for GTPase stimulatory activity in vitro using Gαi1. The Gαi1 subunit, which coimmunoprecipitates with CB1 (Mukhopadhyay et al. 2000), exhibits endogenous GTPase activity (Fig. 1A ▶) (Kleuss et al. 1994). Previous studies have demonstrated that the toxin mastoparan (Higashijima et al. 1990) and peptides from the α2-adrenergic receptor (Wade et al. 1996) stimulate this activity by promoting GDP release in a manner that reflects the response to agonist-induced activation of an intact GPCR (Dalman and Neubig 1991; Taylor et al. 1994). We observed a small enhancement in the GTPase activity of G& alpha;i1 with mastoporan consistent with a previous report (Higashijima et al. 1990) and a comparable effect with the flAL-IC3 peptide (Fig. 1A ▶). In contrast, the stimulation with the flWT-IC3 peptide is marked, a more than fourfold enhancement compared to the latter peptides. The GTPase stimulation is dependent on peptide concentration (Fig. 1B ▶), GTP concentration (Fig. 1C ▶), and is saturable. Remarkably, the flAL-IC3 peptide differs from the flWT-IC3 peptide in sequence only by the conversion of Leu341-Ala342 to Ala341-Leu342 yet the impact on Gαi1 stimulation is dramatic, underscoring the critical nature of this region of the receptor.
Fig. 1.
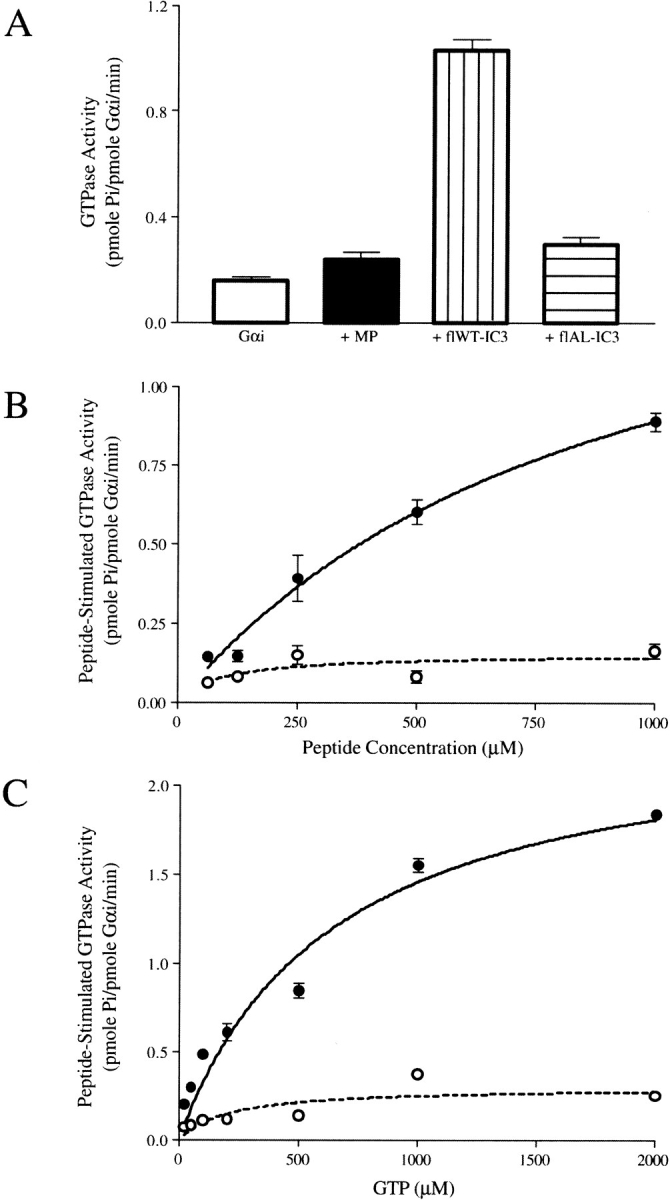
GTPase activity of Gαi1. The enzyme assays were conducted as described in Materials and Methods. Each data point represents an average of triplicate assays +/− SE. (A) Gαi1 GTPase activity in the absence and presence of 1 mM peptides and 200 μM GTP. (B) The dependence of Gαi1 GTPase activity on the concentration of the wild-type IC3 peptide (filled circles) and the Ala341-Leu342 variant (open circles). (C) The dependence of the peptide-stimulated Gαi1 GTPase activity on GTP concentration. The concentration of the wild-type IC3 peptide (filled circles) and the Ala341-Leu342 variant (open circles) was 1 mM.
To establish a structural basis for the functional differences, transferred nuclear Overhauser effect (NOE) experiments were carried out with the Gαi1 subunit and peptides containing the C terminus of IC3, centered on Leu341-Ala342, WT-IC3 and AL-IC3. Both WT-IC3 and AL-IC3 displayed no ordered structure in aqueous solution. All of the nuclear magnetic resonance (NMR) parameters, including chemical shift, coupling constants, and NOEs (or ROEs) indicated the lack of secondary structural elements.
The peptide-to-protein ratio for the trNOE experiments was determined by titrating Gαi1 into solutions containing the peptides and monitoring line widths in one-dimensional proton spectra. The peptides proved to be very soluble at high concentrations (4 mM peptide), even upon addition of G protein. A series of NOESY spectra (mixing times of 50, 80, 100, and 120 ms) was collected at the optimal conditions of a G-protein:peptide ratio of 1:20 (200 μM Gαi1).
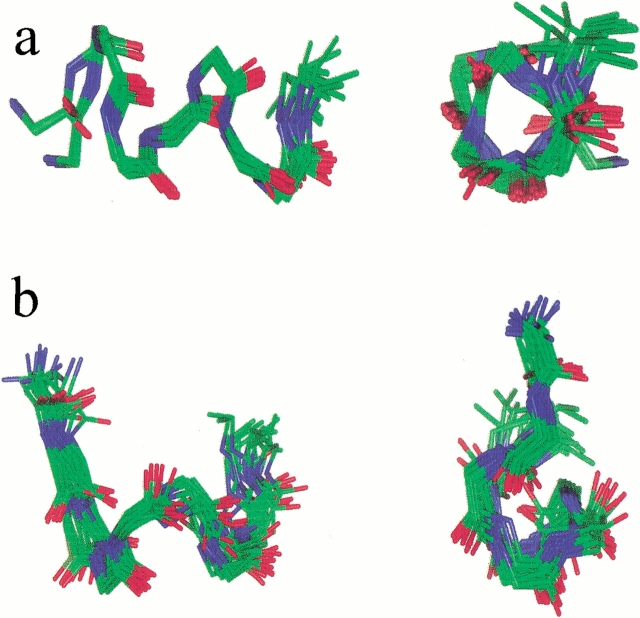
The initial ensemble of 20 peptide structures generated by metric matrix distance geometry (DG) calculations using NOE-derived distance restraints are shown superimposed in Fig. 2 ▶. For WT-IC3, a helical structure with some fraying at the terminal Asp1 and Val9 is observed. A number of the 93 trNOEs observed are consistent with a helix (Table 1) including αN(i)(i+3) i = 1,5,6, βN(i)(i+3) i = 1,2,3,4,6 and NN(i,i+1) i = 2,3,4. The average pairwise RMSD for the backbone atoms of residues 2–8 is 0.62 Å. The conformation of the side chains is well defined, with an average RMSD value of 1.4 Å for the heavy atoms of residues 2–8. The side chains of Leu4 and Ala5 are particularly well resolved with RMSD values of 0.79 and 0.71 Å, respectively (Table 1).
Fig. 2.
Superposition of the ensemble of 20 structures from the DG calculations: (a) WT-IC3 and (b) AL-IC3. The heavy backbone atoms were used in the superposition.
Table 1.
Distribution of trNOEs and structural statistics of WT-IC3 and AL-IC3
| Distribution of NOEs | WT-IC3 | AL-IC3 |
| NOEs | 93 | 93 |
| intraresidue | 66 | 64 |
| i, i + 1 | 16 | 19 |
| i, i + 2 | 1 | 2 |
| i, i + 3 | 10 | 8 |
| Atomic RMSD values (heavy atoms) of the ensemble of 20 DG structures | ||
| WT-IC3 | AL-IC3 | |
| Backbone, side chain (Å) | ||
| Asp1 | 1.05, 2.07 | 1.48, 2.62 |
| Ile2 | 0.64, 1.39 | 0.86, 1.58 |
| Arg3 | 0.54, 2.37 | 0.88, 2.37 |
| Leu/Ala4 | 0.36, 0.79 | 0.92, 1.28 |
| Ala/Leu5 | 0.43, 0.71 | 0.71, 1.71 |
| Lys6 | 0.67, 1.53 | 0.72, 2.19 |
| Thr7 | 0.81, 1.64 | 0.81, 1.29 |
| Leu8 | 0.68, 1.41 | 0.82, 1.76 |
| Val9 | 1.00, 1.85 | 1.18, 2.31 |
| NMR R-factor from IRMA Calculations (Gonzales et al., 1991) | ||
| Iteration | WT-IC3 | AL-IC3 |
| 1 | 0.84 | 0.86 |
| 2 | 0.75 | 0.88 |
| 3 | 0.74 | 0.81 |
| 4 | 0.74 | 0.79 |
| 5 | 0.74 | 0.79 |
In contrast to the helical structure observed for the wild-type IC3, the center of AL-IC3 adopts a well-defined turn involving Arg3-Lys6 (Fig. 2 ▶). Out of 93 total NOEs, eight are determinant in defining the structure of the turn, including Arg3 HN-Ala4 HN, Arg3 HN-Leu5 HN, Arg3 HN- Ala4 Hβ Arg3 Hβ-Ala4 HN, Ala4 Hβ-Leu5 HN, and Ile2 Hα–Leu5 HN. The average pairwise RMSD for the ensemble of structures is 0.81 Å for the backbone atoms of residues 2–8, and 1.7 Å for the side-chain atoms of residues 2–8.
The ensemble of DG structures was utilized as a starting point for the ensemble-IRMA calculations (Boelens et al. 1989; Bonvin et al. 1993). Extensive MD simulations with the ensemble-IRMA generated distance restraints based on the integrated NOE cross-peak volumes and the starting ensemble produced the next generation ensemble of structures. This procedure was iterated until the R-factor (Gonzales et al. 1991) did not further decrease. The resulting structures are illustrated in Figure 3 ▶.
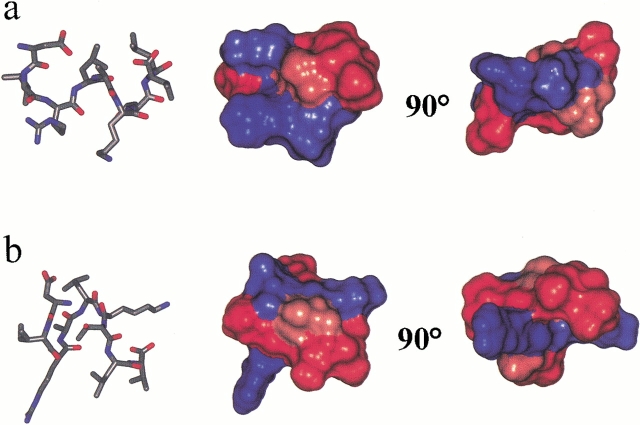
Fig. 3.
Structure of (a) WT-IC3 and (b) AL-IC3 after IRMA refinement. The Connolly surface representation of the molecules are shown color coded by hydrophobicity (red represents hydrophobic, blue represents hydrophilic).
Discussion
We have examined the structure and function of the interaction of the third intracellular loop of the CB1 receptor with Gαi1. In the peptides corresponding to the C-terminus of IC3, the conservative mutation, L341A/A342L, reduces the stimulation of Gαi1 GTPase compared to wild type. This correlates with the results from the corresponding mutation in vivo in which we observe a shift in preference toward Gs when both Gi and Gs α subunits are available in the cell (Abadji et al. 1999). It should be noted that when cells harboring the mutant receptor are treated with cholera toxin so that coupling via Gs is not possible, inhibition of cAMP accumulation via Gi coupling is observed. This is consistent with the NMR analysis, which demonstrates that the peptide with the mutant sequence does bind Gi, and the GTPase results that show stimulation to a level comparable to mastoparan. However, the strong parallelism observed between the diminished preference for Gi in vivo, the reduced extent of GTPase stimulation relative to the wild type, and the nonhelical structure of the Gi-associated form argue that the mutant sequence diminishes the efficiency of the interaction with Gi though it does not abrogate it altogether. The L341A/A342L mutation disrupts the LAKT motif important for receptor-G protein interaction. This motif is 100% conserved in the 11 cannabinoid receptors that have been characterized to date. Examining the database of 1026 GPCRs, a sequence of AAKM is observed ∼50% of the time, underscoring the critical nature of this segment of the receptor (Horn et al. 1998). Transferred NOE experiments have allowed us to directly observe the structure of this motif and the AL mutation when bound to the Gαi1 protein.
When bound, WT-IC3 adopts a distorted helical conformation, which orients the side chains of Leu4 and Ala5 in the LAKT motif to form a hydrophobic ridge along one face of the helix (Fig. 3 ▶). In contrast, AL-IC3 is not helical but instead forms a turn at Ala4-Leu5. This may result from distortion of the side chains of leucine and alanine to fit into a binding pocket that is sterically optimized for the wild-type sequence. The structural difference has a major consequence of the relative topological display of the two positively charged residues, Arg3 and Lys6. As illustrated in Figure 3 ▶, the helical structure of WT-IC3 produces an amphipathic arrangement, with the Asp1, Arg3, and Lys6 all on one face. The turn structure observed for AL-IC3 disrupts this arrangement (Fig. 3b ▶). The dramatic alteration of the bound peptide structure consequent to a conservative mutation (two hydrophobic residues with only a slight difference in size) indicates that this part of the receptor makes very close contacts with the G protein. It is unlikely that such a small change would be able to induce a major shift in a distant binding site.
Based on homology with rhodopsin, the peptide sequence examined here is at the interface of IC3 and transmembrane helix (TM) 6 of the CB1 receptor (WT-IC3 corresponds to 247EKEVTRMVI255 of bovine rhodopsin); this region is α-helical in the X-ray structure of rhodopsin (Palczewski et al. 2000). Based on studies incorporating spin and fluorescent labels into this region of rhodopsin and the β2-adrenergic receptor (Farrens et al. 1996; Ghanouni et al. 2001), activation leads to a rearrangement of the TM helices, particularly TM6, possibly leading to accessibility of the LAKT domain. Our NMR data substantiate that, indeed, this critical region changes conformation upon interaction with G protein. The characterization of these structural changes with different G proteins will provide greater insight into the mechanism of specific G protein activation.
Materials and methods
Sample preparation
Using a pGEX fusion system (Amersham Biosciences), Gαi1 and IC3 peptides were expressed in BL21(DE3) (Invitrogen) from plasmids pGEX-6P1.Gαi1 (a gift from K. Wakanatsu and T. Kohno of Mitsubishi Kasei Institute of Life Science, Tokyo), pGEX-2T.CB1IC3 and pGEX-2T.CB1IC3AL (encoding full-length CB1 IC3, designated flWT-IC3, and that with the L341A/A342L mutation, designated flAL-IC3, respectively). The GST fusions were induced with IPTG (30°C for Gαi1 and 37°C for IC3 peptides) and purified using glutathione Sepharose 4B resin according to the manufacturer's protocol with minor modification. After PreScission protease cleavage, Gαi1 was isolated by Q-Sepharose chromatography followed by a second round of glutathione Sepharose 4B chromatography to >98% purity confirmed by SDS-PAGE. The IC3 peptides were separated from GST after thrombin cleavage, which results in a Gly and Ser amino terminal to the CB1 sequences, using a SP-Sepharose fast flow column and a linear gradient of ammonium acetate (0 mM to 500 mM). Pure peptide-containing fractions, monitored via SDS-PAGE, were pooled, lyophilized, dissolved in 5% acetic acid, and lyophilized again three times.
Peptides containing the C terminus of the IC3 of CB1, WT-IC3: DIRLAKTLV-NH2 and AL-IC3: DIRALKTLV-NH2, were synthesized and purified by reverse-phase HPLC (Tufts Analytical Core Facility). Purity was verified by mass spectrometry and NMR.
GTPase assays
GTPase activity was determined essentially as described previously (Higashijima et al. 1987, 1990; Wade et al. 1996). Reactions contained 50 mM Na HEPES (pH 8.0), 1 mM EDTA, 1.1 mM MgCl2, 1 mM DTT, 120 nM Gαi1, peptides, and GTP (Sigma) concentrations as indicated in the figure legends, and 20% glycerol. Nonenzymatic hydrolysis of [γ-32P]GTP, which was generally <5% of the total added, was subtracted from all responses.
Nuclear magnetic resonance spectroscopy
NMR samples for the transferred NOE experiments were prepared by exchanging Gαi1 into 10 mM acetate buffer, pH 6.0, containing 1 mM deuterated DTT and TSP as an internal chemical shift reference and adding the protein in solution to the lyophilized peptide. All spectra were acquired on a 600 MHz Bruker AVANCE spectrometer at 25°C. The transferred NOE experiments of 4 mM WT-IC3 or AL-IC3 and 200 μM Gαi1 were carried out using mixing times of 50, 80, 120, 150, and 200 ms. Sequential assignments of the NOESY spectra were made using TOCSY spectra (mixing times 30 and 60 ms; 10 kHz spin locking field obtained with MLEV17). Both NOESY and TOCSY experiments used the WATERGATE 3–9–19 pulse sequence for water suppression (Piotto et al. 1992; Sklenar et al. 1993). Spectra were processed using Bruker XWIN-NMR software or NMRPipe (Delaglio et al. 1995), and Sparky (Goddard and Kneller 2001) was used to view and assign spectra, and to measure peak volumes and intensities.
Distance geometry calculations
Peak volumes from the assigned NOESY spectra were divided into strong, medium, and weak peaks to generate distance restraints (2.0–3.0 Å, 2.0–4.0 Å, and 2.0–5.0 Å, respectively). Pseudoatoms were used for methylene protons that could not be stereospecifically assigned. Distance geometry calculations were carried out employing the random metrization method of Havel (1991) to generate an ensemble of structures to be refined using IRMA (Boelens et al. 1989; Bonvin et al. 1993) following published procedures (Mierke et al. 1994; Pellegrini et al. 1996).
IRMA calculations
The ensemble IRMA (Bonvin et al. 1993) calculations were run from the InsightII software suite (Molecular Simulations, Inc.); the MD simulations were run using Discover (Molecular Simulations, Inc.). In the first iteration of IRMA, the 20 DG structures with the lowest penalty function were used as a starting ensemble. The result of the IRMA calculations is a set of distance restraints based on the integrated volumes of the NOEs and the starting ensemble. The distance restraints were then applied during NOE-restrained MD simulations and energy minimization to a randomly chosen set of ten structures from the original ensemble. These resulting structures then formed the starting ensemble for a second IRMA iteration. This procedure of IRMA followed by MD and EM was repeated for a total of five iterations, until the NOE R-factor (Gonzales et al. 1991) did not decrease further. The resulting structures and the distance restraints have been submitted to the PDB databank (access code 1LVQ and 1LVR).
Acknowledgments
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
AL-IC3, peptide sequence of IC3 with AL swap, DIRALKTLV
CB1, cannabinoid receptor one
CHAPS, 3-[(3-Cholamidopropyl)dimethylammonio]-1-propane-sulfonate
DG, distance geometry
DTT, dithiothreitol
EM, energy minimization
GPCR, G-protein coupled receptor
IC3, intracellular loop 3
IPTG, isopropyl-β-D-thio-galactopyranoside
IRMA, iterative relaxation matrix approach
MD, molecular dynamics
NOE, nuclear overhauser effect
PMSF, phenylmethanesulfonyl fluoride
RMSD, root mean squared deviation
ROE, rotating frame Overhauser effect
TM, transmembrane segment
TSP, 3-(trimethylsilyl) tetradeutero sodium propionate
WT-IC3, wild-type sequence of IC3, DIRLAKTLV
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0218402.
References
- Abadji, V., Lucas-Lenard, J.M., Chin, C., and Kendall, D.A. 1999. Involvement of the carboxyl terminus of the third intracellular loop of the cannabinoid CB1 receptor in constitutive activation of Gs. J. Neurochem. 72 2032–2038. [DOI] [PubMed] [Google Scholar]
- Baker, D., Pryce, G., Croxford, J.L., Brown, P., Pertwee, R.G., Huffman, J.W., and Layward, L. 2000. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 404 84–87. [DOI] [PubMed] [Google Scholar]
- Boelens, R., Koning, T.M.G., van der Marel, G.A., Van Boom, H., and Kaptein, R. 1989. Iterative procedure for structure determination from proton-proton NOEs using a relaxation matrix approach, application to a DNA octamer. J. Magn. Reson. 82 290. [Google Scholar]
- Bonvin, A.M., Rullmann, J.A., Lamerichs, R.M., Boelens, R., and Kaptein, R. 1993. "Ensemble" iterative relaxation matrix approach: A new NMR refinement protocol applied to the solution structure of crambin. Proteins 15 385–400. [DOI] [PubMed] [Google Scholar]
- Cotecchia, S., Exum, S., Caron, M.G., and Lefkowitz, R.J. 1990. Regions of the α 1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function. Proc. Natl. Acad. Sci. 87 2896–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman, H.M. and Neubig, R.R. 1991. Two peptides from the α 2A-adrenergic receptor alter receptor G protein coupling by distinct mechanisms. J. Biol. Chem. 266 11025–11029. [PubMed] [Google Scholar]
- Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J., and Bax, A. 1995. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6 277–293. [DOI] [PubMed] [Google Scholar]
- Di Marzo, V., Goparaju, S.K., Wang, L., Liu, J., Batkai, S., Jarai, Z., Fezza, F., Miura, G.I., Palmiter, R.D., Sugiura, T., and Kunos, G. 2001. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410 822–825. [DOI] [PubMed] [Google Scholar]
- Farrens, D.L., Altenbach, C., Yang, K., Hubbell, W.L., and Khorana, H.G. 1996. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 274 768–770. [DOI] [PubMed] [Google Scholar]
- Felder, C.C., Joyce, K.E., Briley, E.M., Mansouri, J., Mackie, K., Blond, O., Lai, Y., Ma, A.L., and Mitchell, R.L. 1995. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 48 443–450. [PubMed] [Google Scholar]
- Garcia, D.E., Brown, S., Hille, B., and Mackie, K. 1998. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J. Neurosci. 18 2834–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard, C.M., Mollereau, C., Vassart, G., and Parmentier, M. 1991. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem. J. 279 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanouni, P., Steenhuis, J.J., Farrens, D.L., and Kobilka, B.K. 2001. Agonist-induced conformational changes in the G-protein-coupling domain of the β 2 adrenergic receptor. Proc. Natl. Acad. Sci. USA 98 5997–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, M. and Felder, C.C. 1997. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 17 5327–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard, T.D. and Kneller, D.G. 2001. Sparky 3.0. University of California, San Francisco.
- Gonzales, C., Rullmann, J.A.C., Bonvin, A.M.J.J., Boelens, R., and Kaptein, R. 1991. Toward an NMR R-factor. J. Magn. Reson. 91 659–666. [Google Scholar]
- Havel, T.F. 1991. An evaluation of computational strategies for use in the determination of protein structure from distance geometry constraints obtained by nuclear magnetic resonance. Prog. Biophys. Molec. Biol. 56 43–78. [DOI] [PubMed] [Google Scholar]
- Higashijima, T., Ferguson, K.M., Sternweis, P.C., Smigel, M.D., and Gilman, A.G. 1987. Effects of Mg2+ and the β γ-subunit complex on the interactions of guanine nucleotides with G proteins. J. Biol. Chem. 262 762–766. [PubMed] [Google Scholar]
- Higashijima, T., Burnier, J., and Ross, E.M. 1990. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J. Biol. Chem. 265 14176–14186. [PubMed] [Google Scholar]
- Horn, F., Weare, J., Beukers, M.W., Horsch, S., Bairoch, A., Chen, W., Edvardsen, O., Campagne, F., and Vriend, G. 1998. GPCRDB: An information system for G protein-coupled receptors. Nucleic Acids Res. 26 275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett, A.C., Qualy, J.M., and Khachatrian, L.L. 1986. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol. Pharmacol. 29 307–313. [PubMed] [Google Scholar]
- Howlett, A.C., Song, C., Berglund, B.A., Wilken, G.H., and Pigg, J.J. 1998. Characterization of CB1 cannabinoid receptors using receptor peptide fragments and site-directed antibodies. Mol. Pharmacol. 53 504–510. [DOI] [PubMed] [Google Scholar]
- Hsieh, C., Brown, S., Derleth, C., and Mackie, K. 1999. Internalization and recycling of the CB1 cannabinoid receptor. J. Neurochem. 73 493–501. [DOI] [PubMed] [Google Scholar]
- Kleuss, C., Raw, A.S., Lee, E., Sprang, S.R., and Gilman, A.G. 1994. Mechanism of GTP hydrolysis by G-protein α subunits. Proc. Natl. Acad. Sci. USA 91 9828–9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierke, D.F., Scheek, R.M., and Kessler, H. 1994. Coupling constant restraints in ensemble calculations. Biopolymers 34 559–563. [Google Scholar]
- Mukhopadhyay, S. and Howlett, A.C. 2001. CB1 receptor-G protein association. Subtype selectivity is determined by distinct intracellular domains. Eur. J. Biochem. 268 499–505. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, S., McIntosh, H.H., Houston, D.B., and Howlett, A.C. 2000. The CB(1) cannabinoid receptor juxtamembrane C-terminal peptide confers activation to specific G proteins in brain. Mol. Pharmacol. 57 162–170. [PubMed] [Google Scholar]
- Munro, S., Thomas, K.L., and Abu-Shaar, M. 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365 61–65. [DOI] [PubMed] [Google Scholar]
- Nie, J. and Lewis, D.L. 2001a. The proximal and distal C-terminal tail domains of the CB1 cannabinoid receptor mediate G protein coupling. Neuroscience 107 161–167. [DOI] [PubMed] [Google Scholar]
- Nie, J. and Lewis, D.L. 2001b. Structural domains of the CB1 cannabinoid receptor that contribute to constitutive activity and G-protein sequestration. J. Neurosci. 21 8758–8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski, K., Kumasaka, T., Hori, T., Behnke, C.A., Motoshima, H., Fox, B.A., Le Trong, I., Teller, D.C., Okada, T., Stenkamp, R.E., Yamamoto, M., and Miyano, M. 2000. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289 739–745. [DOI] [PubMed] [Google Scholar]
- Pellegrini, M., Gobbo, M., Rocchi, R., Peggion, E., Mammi, S., and Mierke, D.F. 1996. Threonine(6)-bradykinin: Conformational study of a flexible peptide in dimethyl sulfoxide by NMR and ensemble calculations. Biopolymers 40 561–569. [DOI] [PubMed] [Google Scholar]
- Piotto, M., Saudek, V., and Sklenar, V. 1992. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR 2 661–665. [DOI] [PubMed] [Google Scholar]
- Ren, Q., Kurose, H., Lefkowitz, R.J., and Cotecchia, S. 1993. Constitutively active mutants of the α 2-adrenergic receptor. J. Biol. Chem. 268 16483–16487. [PubMed] [Google Scholar]
- Samama, P., Cotecchia, S., Costa, T., and Lefkowitz, R.J. 1993. A mutation-induced activated state of the β 2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 268 4625–4636. [PubMed] [Google Scholar]
- Sklenar, V., Piotto, M., Leppik, R., and Saudek, V. 1993. Gradient-tailored water suppression for 1H-15N HSQC experiments optimized to retain full sensitivity. J. Magn. Reson. A 102 241–245. [Google Scholar]
- Slipetz, D.M., O'Neill, G.P., Favreau, L., Dufresne, C., Gallant, M., Gareau, Y., Guay, D., Labelle, M., and Metters, K.M. 1995. Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Mol. Pharmacol. 48 352–361. [PubMed] [Google Scholar]
- Taylor, J.M., Jacob-Mosier, G.G., Lawton, R.G., Remmers, A.E., and Neubig, R.R. 1994. Binding of an α 2 adrenergic receptor third intracellular loop peptide to G β and the amino terminus of G α. J. Biol. Chem. 269 27618–27624. [PubMed] [Google Scholar]
- Wade, S.M., Scribner, M.K., Dalman, H.M., Taylor, J.M., and Neubig, R.R. 1996. Structural requirements for G(o) activation by receptor-derived peptides: Activation and modulation domains of the α 2-adrenergic receptor i3c region. Mol. Pharmacol. 50 351–358. [PubMed] [Google Scholar]