Abstract
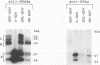
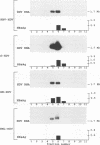
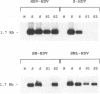
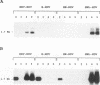
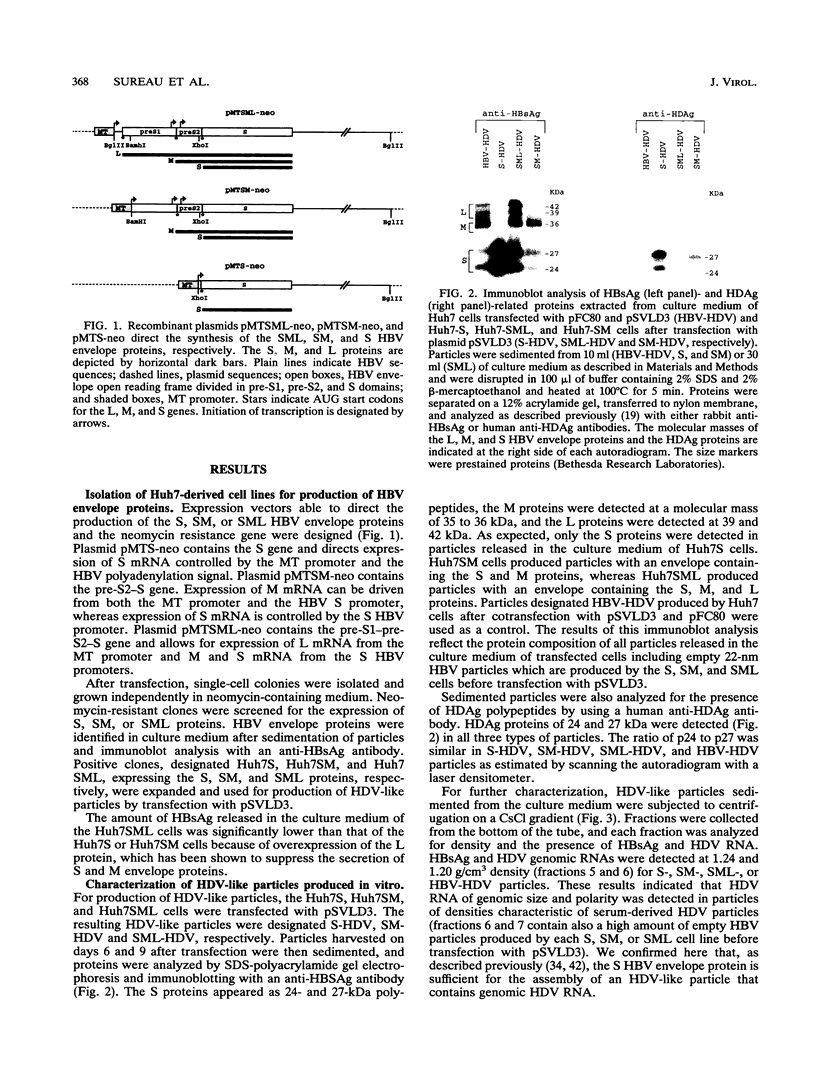
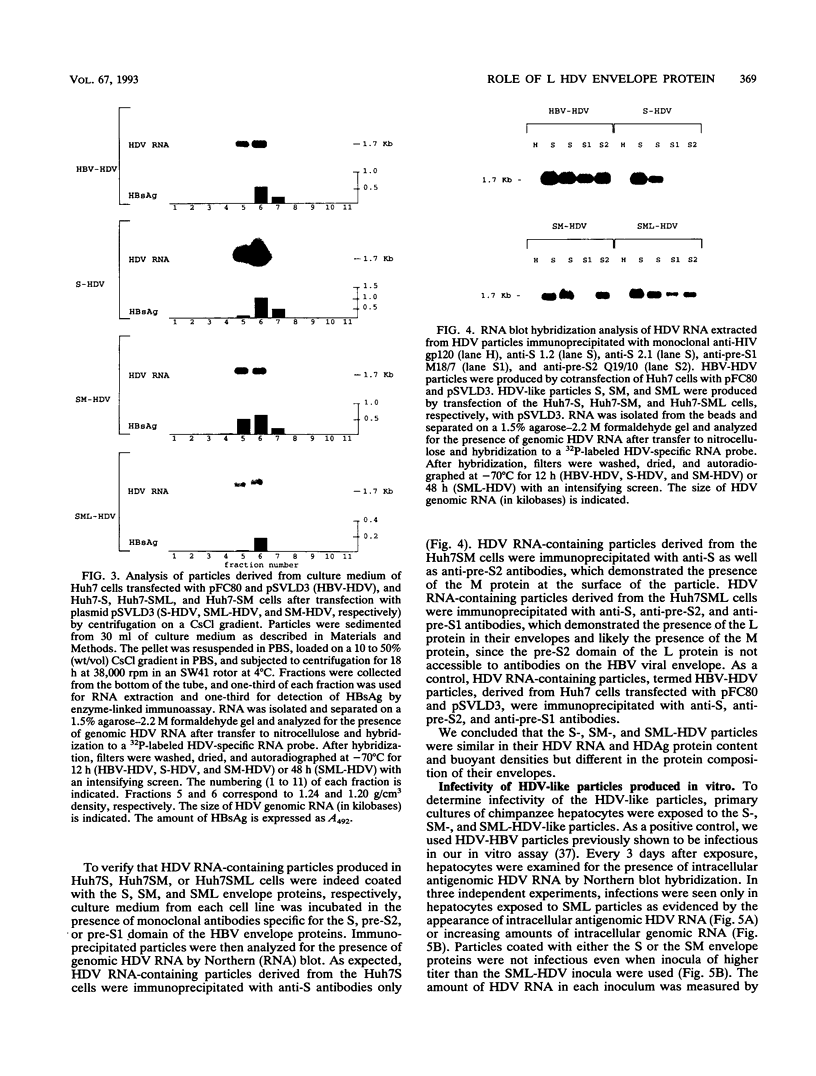
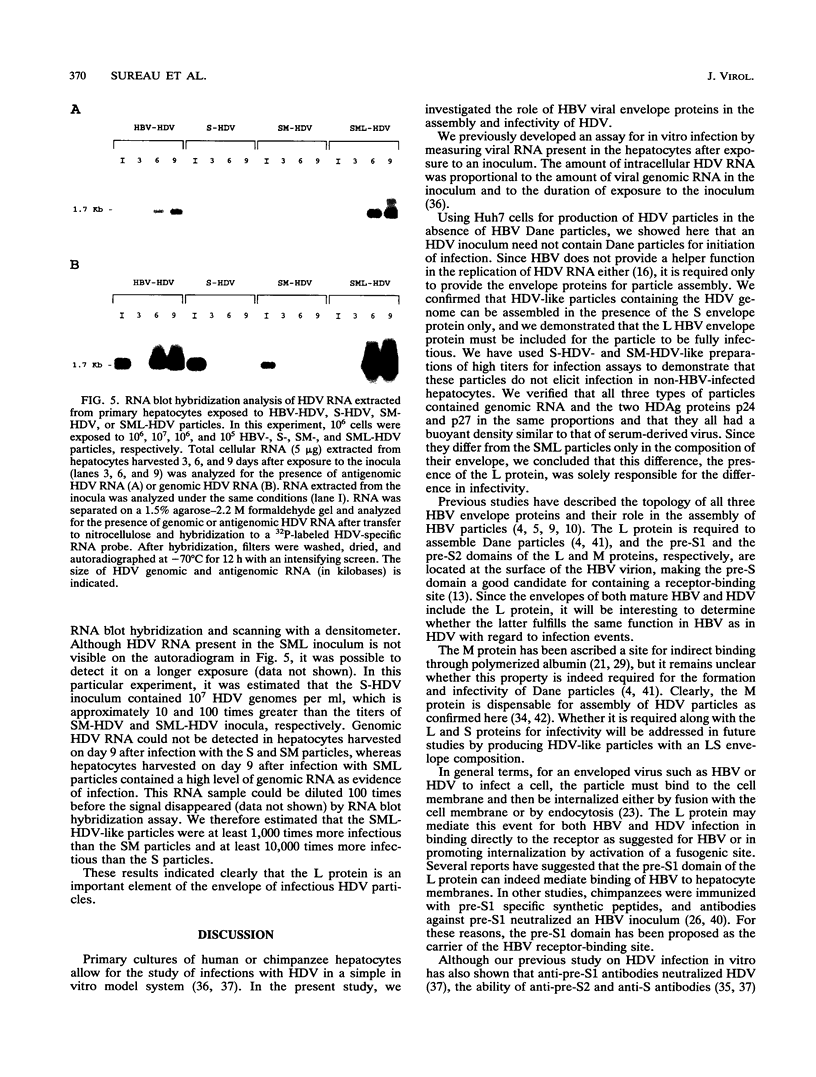
The hepatitis delta virus (HDV) is coated with large (L), middle (M), and small (S) envelope proteins encoded by coinfecting hepatitis B virus (HBV). To study the role of the HBV envelope proteins in the assembly and infectivity of HDV, we produced three types of recombinant particles in Huh7 cells by transfection with HBV DNA and HDV cDNA: (i) particles with an envelope containing the S HBV envelope protein only, (ii) particles with an envelope containing S and M proteins, and (iii) particles with an envelope containing S, M, and L proteins. Although the resulting S-, SM-, and SML-HDV particles contained both hepatitis delta antigen and HDV RNA, only particles coated with all three envelope proteins (SML) showed evidence of infectivity in an in vitro culture system susceptible to HDV infection. We concluded that the L HBV envelope protein, and more specifically the pre-S1 domain, is important for infectivity of HDV particles and that the M protein, which has been reported to bear a site for binding to polymerized albumin in the pre-S2 domain, is not sufficient for infectivity. Our data also show that the helper HBV is not required for initiation of HDV infection. The mechanism by which the L protein may affect HDV infectivity is discussed herein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann K. F., Gerin J. L. Antigens of hepatitis delta virus in the liver and serum of humans and animals. J Infect Dis. 1986 Oct;154(4):702–706. doi: 10.1093/infdis/154.4.702. [DOI] [PubMed] [Google Scholar]
- Bonino F., Heermann K. H., Rizzetto M., Gerlich W. H. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol. 1986 Jun;58(3):945–950. doi: 10.1128/jvi.58.3.945-950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonino F., Hoyer B., Shih J. W., Rizzetto M., Purcell R. H., Gerin J. L. Delta hepatitis agent: structural and antigenic properties of the delta-associated particle. Infect Immun. 1984 Mar;43(3):1000–1005. doi: 10.1128/iai.43.3.1000-1005.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss V., Ganem D. Mutational analysis of hepatitis B surface antigen particle assembly and secretion. J Virol. 1991 Jul;65(7):3813–3820. doi: 10.1128/jvi.65.7.3813-3820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss V., Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane D. S., Cameron C. H., Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970 Apr 4;1(7649):695–698. doi: 10.1016/s0140-6736(70)90926-8. [DOI] [PubMed] [Google Scholar]
- Eble B. E., Lingappa V. R., Ganem D. Hepatitis B surface antigen: an unusual secreted protein initially synthesized as a transmembrane polypeptide. Mol Cell Biol. 1986 May;6(5):1454–1463. doi: 10.1128/mcb.6.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., MacRae D. R., Lingappa V. R., Ganem D. Multiple topogenic sequences determine the transmembrane orientation of the hepatitis B surface antigen. Mol Cell Biol. 1987 Oct;7(10):3591–3601. doi: 10.1128/mcb.7.10.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Heermann K. H., Ponzetto A., Crivelli O., Bonino F. Proteins of hepatitis delta virus. Prog Clin Biol Res. 1987;234:97–103. [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J. R., Eichberg J. W., Lanford R. E. In vitro replication and expression of hepatitis B virus from chronically infected primary chimpanzee hepatocytes. Hepatology. 1989 Dec;10(6):921–927. doi: 10.1002/hep.1840100605. [DOI] [PubMed] [Google Scholar]
- Kuo M. Y., Chao M., Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989 May;63(5):1945–1950. doi: 10.1128/jvi.63.5.1945-1950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. Y., Goldberg J., Coates L., Mason W., Gerin J., Taylor J. Molecular cloning of hepatitis delta virus RNA from an infected woodchuck liver: sequence, structure, and applications. J Virol. 1988 Jun;62(6):1855–1861. doi: 10.1128/jvi.62.6.1855-1861.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki K., Floreani M., Mimms L. T., Ganem D. Epitope mapping of the PreS1 domain of the hepatitis B virus large surface protein. Virology. 1990 Jun;176(2):620–624. doi: 10.1016/0042-6822(90)90032-m. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Carey K. D., Estlack L. E., Smith G. C., Hay R. V. Analysis of plasma protein and lipoprotein synthesis in long-term primary cultures of baboon hepatocytes maintained in serum-free medium. In Vitro Cell Dev Biol. 1989 Feb;25(2):174–182. doi: 10.1007/BF02626175. [DOI] [PubMed] [Google Scholar]
- Leenders W. P., Glansbeek H. L., de Bruin W. C., Yap S. H. Binding of the major and large HBsAg to human hepatocytes and liver plasma membranes: putative external and internal receptors for infection and secretion of hepatitis B virus. Hepatology. 1990 Jul;12(1):141–147. doi: 10.1002/hep.1840120122. [DOI] [PubMed] [Google Scholar]
- Machida A., Kishimoto S., Ohnuma H., Miyamoto H., Baba K., Oda K., Nakamura T., Miyakawa Y., Mayumi M. A hepatitis B surface antigen polypeptide (P31) with the receptor for polymerized human as well as chimpanzee albumins. Gastroenterology. 1983 Aug;85(2):268–274. [PubMed] [Google Scholar]
- Macrae D. R., Bruss V., Ganem D. Myristylation of a duck hepatitis B virus envelope protein is essential for infectivity but not for virus assembly. Virology. 1991 Mar;181(1):359–363. doi: 10.1016/0042-6822(91)90503-4. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro F., Korba B. E., Forzani B., Baroudy B. M., Brown T. L., Gerin J. L., Ponzetto A. Hepatitis delta virus (HDV) and woodchuck hepatitis virus (WHV) nucleic acids in tissues of HDV-infected chronic WHV carrier woodchucks. J Virol. 1989 Apr;63(4):1612–1618. doi: 10.1128/jvi.63.4.1612-1618.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N., Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell. 1986 Aug 1;46(3):429–436. doi: 10.1016/0092-8674(86)90663-x. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Seto B., Strick N. Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine. 1989 Jun;7(3):234–236. doi: 10.1016/0264-410x(89)90235-1. [DOI] [PubMed] [Google Scholar]
- Peden K. W., Charles C., Sanders L., Tennekoon G. I. Isolation of rat Schwann cell lines: use of SV40 T antigen gene regulated by synthetic metallothionein promoters. Exp Cell Res. 1989 Nov;185(1):60–72. doi: 10.1016/0014-4827(89)90037-2. [DOI] [PubMed] [Google Scholar]
- Petit M. A., Dubanchet S., Capel F., Voet P., Dauguet C., Hauser P. HepG2 cell binding activities of different hepatitis B virus isolates: inhibitory effect of anti-HBs and anti-preS1(21-47). Virology. 1991 Feb;180(2):483–491. doi: 10.1016/0042-6822(91)90062-g. [DOI] [PubMed] [Google Scholar]
- Pontisso P., Petit M. A., Bankowski M. J., Peeples M. E. Human liver plasma membranes contain receptors for the hepatitis B virus pre-S1 region and, via polymerized human serum albumin, for the pre-S2 region. J Virol. 1989 May;63(5):1981–1988. doi: 10.1128/jvi.63.5.1981-1988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontisso P., Ruvoletto M. G., Gerlich W. H., Heermann K. H., Bardini R., Alberti A. Identification of an attachment site for human liver plasma membranes on hepatitis B virus particles. Virology. 1989 Dec;173(2):522–530. doi: 10.1016/0042-6822(89)90564-3. [DOI] [PubMed] [Google Scholar]
- Ponzetto A., Cote P. J., Popper H., Hoyer B. H., London W. T., Ford E. C., Bonino F., Purcell R. H., Gerin J. L. Transmission of the hepatitis B virus-associated delta agent to the eastern woodchuck. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2208–2212. doi: 10.1073/pnas.81.7.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M., Canese M. G., Aricò S., Crivelli O., Trepo C., Bonino F., Verme G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977 Dec;18(12):997–1003. doi: 10.1136/gut.18.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M., Hoyer B., Canese M. G., Shih J. W., Purcell R. H., Gerin J. L. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6124–6128. doi: 10.1073/pnas.77.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu W. S., Bayer M., Taylor J. Assembly of hepatitis delta virus particles. J Virol. 1992 Apr;66(4):2310–2315. doi: 10.1128/jvi.66.4.2310-2315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau C., Jacob J. R., Eichberg J. W., Lanford R. E. Tissue culture system for infection with human hepatitis delta virus. J Virol. 1991 Jul;65(7):3443–3450. doi: 10.1128/jvi.65.7.3443-3450.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau C., Moriarty A. M., Thornton G. B., Lanford R. E. Production of infectious hepatitis delta virus in vitro and neutralization with antibodies directed against hepatitis B virus pre-S antigens. J Virol. 1992 Feb;66(2):1241–1245. doi: 10.1128/jvi.66.2.1241-1245.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau C., Romet-Lemonne J. L., Mullins J. I., Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986 Oct 10;47(1):37–47. doi: 10.1016/0092-8674(86)90364-8. [DOI] [PubMed] [Google Scholar]
- Sureau C., Taylor J., Chao M., Eichberg J. W., Lanford R. E. Cloned hepatitis delta virus cDNA is infectious in the chimpanzee. J Virol. 1989 Oct;63(10):4292–4297. doi: 10.1128/jvi.63.10.4292-4297.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Tsurimoto T., Matsubara K. Three envelope proteins of hepatitis B virus: large S, middle S, and major S proteins needed for the formation of Dane particles. J Virol. 1991 Jul;65(7):3521–3529. doi: 10.1128/jvi.65.7.3521-3529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. J., Chen P. J., Wu J. C., Patel D., Chen D. S. Small-form hepatitis B surface antigen is sufficient to help in the assembly of hepatitis delta virus-like particles. J Virol. 1991 Dec;65(12):6630–6636. doi: 10.1128/jvi.65.12.6630-6636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. S., Choo Q. L., Weiner A. J., Ou J. H., Najarian R. C., Thayer R. M., Mullenbach G. T., Denniston K. J., Gerin J. L., Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986 Oct 9;323(6088):508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Choo Q. L., Wang K. S., Govindarajan S., Redeker A. G., Gerin J. L., Houghton M. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J Virol. 1988 Feb;62(2):594–599. doi: 10.1128/jvi.62.2.594-599.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. C., Chen P. J., Kuo M. Y., Lee S. D., Chen D. S., Ting L. P. Production of hepatitis delta virus and suppression of helper hepatitis B virus in a human hepatoma cell line. J Virol. 1991 Mar;65(3):1099–1104. doi: 10.1128/jvi.65.3.1099-1104.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]