Abstract
A mutational analysis of three co-variant pairs of residues, located at the surface of a single-chain fragment, variable (scFv), remote from the antigen-binding site, was performed to investigate the tolerance of these positions to amino acid changes. The replacements consisted of the elimination or addition of charges, or in their replacement by a charge of opposite sign. As measured by Biacore, antigen-binding kinetics and specificity were essentially unaffected by the mutations. The purified scFvs remained mostly 100% active for 14 h, and their sensitivity to guanidinium-chloride denaturation was similar. These observations indicate that the mutations did not affect antigen-binding properties and that protein folding was conserved. However, the various scFvs differed greatly in half-life in periplasmic extracts (<4 h to >16 h at 25°C). The deleterious effect on half-life produced by single mutations could be reversed by introducing a second mutation that restores the natural combination of amino acids in the co-variant pair, indicating that the consequence of charge modifications at these locations depends on the sequence context. We propose that the differences in half-life result from differences in aggregation propensities with other periplasmic proteins, related to the presence of charged patches at the surface of the scFvs. The practical implication is that changes in surface charge may drastically affect the level of active molecules in complex protein mixtures, a potentially important consideration in engineering scFvs for biotechnological or medical purposes.
Keywords: Co-variance, antibody surface charges, Biacore, antigen-binding properties, half-life, recombinant protein yield
A mutational analysis of a recombinant antibody fragment was performed with two aims: (1) to gain a better understanding of the relationship between antibody sequence and function and (2) to identify candidate positions in engineering antibodies for biotechnological or medical applications.
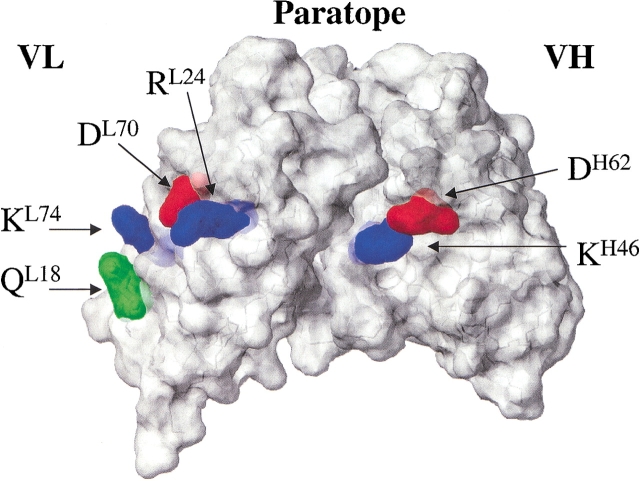
We are searching for protein engineering rules, applicable to at least a subgroup of antibodies, and have selected for mutagenesis residues located within the structurally conserved framework region, rather than in the highly variable complementarity determining regions (CDRs) that determine antigen-binding specificity. The residues in question are co-variant positions that were identified in an analysis of antibody germline sequence alignments (Choulier et al. 2000): Two positions are co-variant if the nature of amino acids at position A is not independent of the nature of amino acids at position B, indicating the existence of functional constraints linking these two residues (Altschuh et al. 1987). The study by Choulier et al. (2000) indicated the existence of a co-variance signal for framework positions located at the surface of the variable region. In particular, for two co-variant pairs identified in mouse antibody germline sequences (L18-L74, H46-H62, Kabat numbering), alternative amino acid types presented large differences in solvation free energy (Table 1), and the two positions of each pair were spatially close in the three-dimensional structure of antibodies (Fig. 1 ▶). A third pair (L24-L70) also showed co-variance of spatially close charged residues, but with two exceptions in an alignment of 42 sequences (Table 1; Fig. 1 ▶).
Table 1.
Alternative amino acid types observed in mouse germline sequences for three co-variant pairsa and mutants constructed
| Mutations introduced in scFv1F4 | |||||||
| Position 1 | Alternative amino acids | Position 2 | Alternative amino acids | No. of sequences (exceptions) | Single mutants | Double mutants | |
| L18 | T QPS/RKb | L74 | KER/TNSI | 42 (0) | QL18K | KL74I | QL18K-KL74I |
| L24 | S/RK | L70 | S/DQ | 42 (2) | RL24S | DL70T | RL24S-DL70T |
| H46 | K/EQ | H62 | DE/KRAST | 59 (0) | KH46E | DH62K | KH46E-DH62K |
a Based on the co-variance analysis by Choulier et al. (2000).
b Amino acid types in italics are found only once in the sequence alignment.
Fig. 1.
Location of co-variant pairs on a model of single-chain fragment, variable (scFv)1F4 constructed with program Modeler (Sali and Blundell 1993).
The patterns of amino acid changes at the three pairs of positions indicate that whether a charged residue is or is not tolerated depends on the nature of the side-chains at the corresponding co-variant position. For example, the L18-L74 pair appears to maintain one positive charge and one only: When position L18 contains a positively charged amino acid (RK; Table 1, right side in column "alternative amino acid types"), the spatially close position L74 is uncharged (TNSI). Conversely, when position L18 is uncharged (TQPS; Table 1, left side in column "alternative amino acid types"), position L74 almost always contains a positive charge (KR). For the pair H46-H62, a positive charge at position H46 (K) is always found together with a negative charge at the neighboring position H62 (DE), whereas H46 E (one Q) is found with either positively charged (KR) or small polar or neutral side-chains (AST) at position H62. Pair L24-L70 tends to contain either two oppositely charged residues or two serines.
The six positions were mutated to investigate their influence on several functional properties: The changes consisted in the removal or addition of a charge, or its replacement by a charge of opposite sign. The amino acid types introduced at the six positions were chosen among those occurring in natural sequences, and are therefore known to be compatible with function in their original atomic environment. Residues of each pair were replaced either individually or simultaneously (Table 1) to determine whether single replacements are more deleterious to function than double replacements, which would support the hypothesis of a functional significance of co-variance.
The single-chain variable fragment (scFv) 1F4 used for mutagenesis was elicited against protein E6 of human papillomavirus (HPV) 16 fused to glutathione S-transferase, and it recognizes a peptide corresponding to the N-terminal sequence of the protein (Giovane et al. 1999).
Modifying charges at the surface of a protein may affect various properties. Charged amino acids at protein-protein interfaces can significantly affect binding free energies of complex formation, as well as the diffusion-controlled association rates (for review, see Gabdoulline and Wade 1999; Sheinerman et al. 2000). Although the positions mutated in this study are remote from the antigen-antibody interface, their influence on the electric field around the scFv that would affect the association rate cannot be excluded. Changes in the conformation of the paratope owing to long-range structural rearrangements could modify the dissociation rate parameter of antigen binding. A number of studies have indicated that surface charges may influence the thermodynamic stability of proteins (for review, see Pace et al. 2000; Martin et al. 2001), as well as their aggregation properties (Elcock and McCammon 1998; Yang et al. 1999). Functional parameters that were analyzed for scFv1F4-WT, and its mutants are antigen-binding kinetics and binding specificity, the half-time of antigen-binding activity at 25°C, and the influence of guanidinium-chloride (Gdn-HCl) denaturation on antigen-binding activity. These parameters were quantified using the Biacore technology.
Results
Kinetic measurements
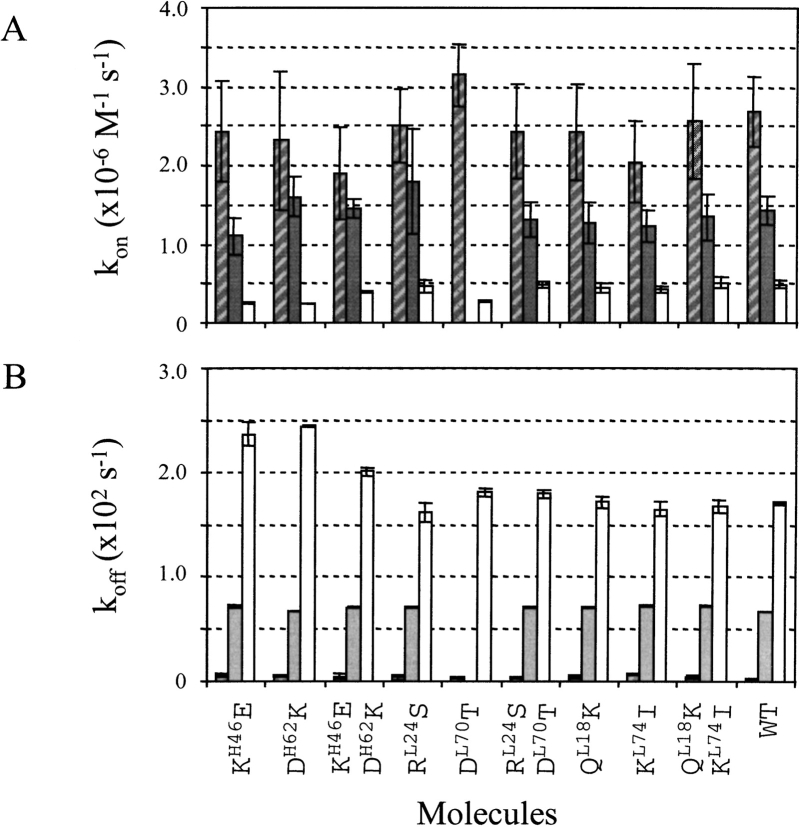
Kinetic parameters in HEPES buffered saline (HBS) were measured for the interaction of the different scFvs with the wild-type (WT) peptide antigen and two antigen variants. Absolute values of the kinetic rate parameters indicate whether the antigen-binding capacity of the various scFvs is conserved. The ranking of kinetic rates for binding to the three peptides reflects the ability of the scFvs to discriminate between related antigens, and is used to evaluate if their specificity is conserved. The peptides were WTE6-C, Q13AE6-C, and R15AE6-C. Kinetic data could be obtained for all interactions except for the scFv1F4-DL70T/Q13AE6-C interaction because of the low concentration of active scFv in periplasmic extracts. The range and ranking of kinetic rate parameters for binding to the antigen variants were similar for the WT and mutant antibody fragments (Fig. 2 ▶), indicating that binding affinity and specificity were not significantly affected by the mutations. The largest rate variations relative to WT were decreases in association rate parameter observed for the interaction between three mutants and peptide R15AE6-C (scFv1F4-KH46E, −48%; scFv1F4-EH62K, −43%, scFv1F4-DL70T, −43%).
Fig. 2.
Kinetic parameters for the interaction of single-chain fragment, variable (scFv)1F4-wild type (WT) and the nine mutants with peptides WTE6-C (hatched), Q13AE6-C (grey), and R15AE6-C (white). (A) Association rate parameters. (B) Dissociation rate parameters.
The effect on kinetic parameters of changing pH from 6.0 to 8.0 was investigated for five mutants. Both association (kon) and dissociation rate parameters (koff) were faster at pH 6 compared to pH 7.4 or 8 for all five mutants (Fig. 3 ▶). The effect on kinetics of temperature was investigated for three molecules (scFvs WT, KL74I, and KH46E-DH62K). The kon values measured for the interaction with peptide WTE6-C at 37°C were comparable to those observed at 25°C (ratio kon37°C/kon25°C between 0.4 and 0.9). The koff values were increased by a factor about 10 (ratio koff37°C/koff25°C between 7 and 13), leading to a weaker equilibrium affinity (KA = 5 × 108 M−1).
Fig. 3.
Effect of pH changes on association (A) and dissociation (B) rate parameters for the interaction of five single-chain fragments, variable (scFvs)1F4 with peptide wild type (WT)E6-C: scFv1F4-WT (*), mutants KH46E (empty squares), KH46E/DH62K (solid squares), RL24S (empty circles), and RL24S/DL70T (solid circles).
A Western blot analysis of periplasmic extracts indicated that the WT and mutant proteins were produced at similar levels (data not shown). Their active concentrations in periplasmic extracts varied, however, in particular with time of incubation at 25°C (see below). The validity of kon calculations, which require that the concentration of analyte is known, relies on the evaluation of the active scFv concentration in each preparation immediately before the kinetic run, a procedure that we apply routinely for kinetic determinations (Choulier et al. 1999). The observation that all mutants display similar antigen-binding parameters (Fig. 2 ▶) indicates that a proportion of antibody fragments is correctly folded in all scFv preparations, because any perturbation of the geometry of the paratope should affect binding affinity. Consequently a simple antigen-binding test can be applied to monitor changes in active concentration of the samples in different experimental conditions. We have followed the active concentrations as a function of time (half-life) and as a function of Gdn-HCl concentration.
Half-life of the antibody fragments
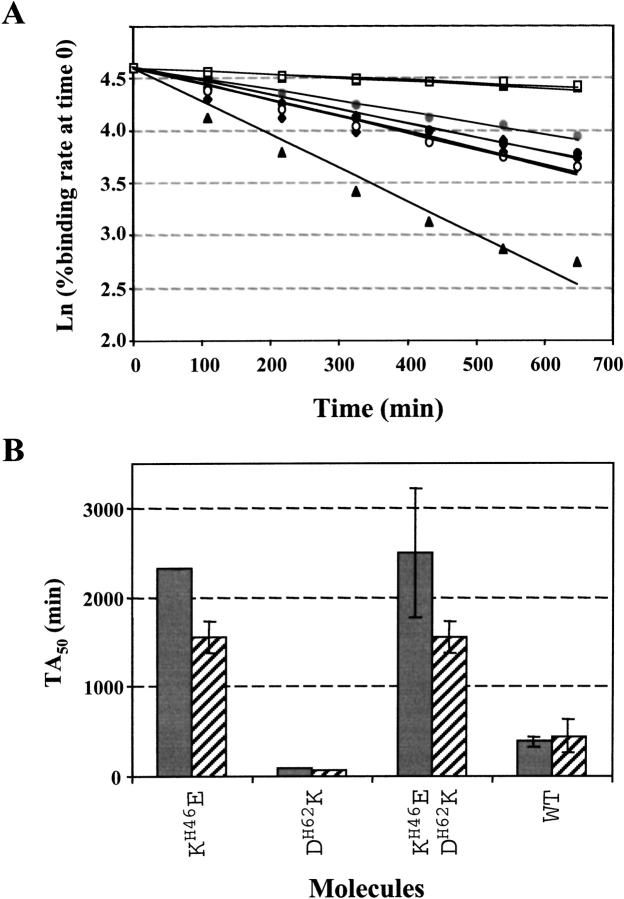
The binding rate of the fragments in HBS containing 200 mM urea was recorded as a function of time at 25°C and expressed as percentage of the binding rate at time 0, using sensor surfaces carrying large amounts of peptide. In these conditions, the binding rate immediately after analyte injection is directly proportional to the active protein concentration (Karlsson et al. 1993). Typical data are shown in Figure 4A ▶ for the WT and the three mutants of the L24-L70 pair. Results from two independent experiments are shown in Figure 4B ▶ for the WT and the three mutants of the H46-H62 pair. Absolute half-life (TA50) values showed some variation in independent experiments, with standard deviations up to 60%, in particular for the mutants with stable activities (see scFvs KH46E and KH46E-DH62K in Fig. 4B ▶), owing to the small slope of the ln(initial binding rate) versus time plots. Data are summarized in Table 2 as absolute TA50 values and as %TA50 relative to WT, which was included as reference in each experiment. The TA50 ranking was conserved in independent experiments as shown by the good reproducibility of results expressed in %TA50 relative to WT.
Fig. 4.
Variation of active single-chain fragment, variable (scFv) concentrations in periplasmic extracts with time at 25°C. (A) ln(% binding rate at time 0) as a function of time. The scFv1F4-WT (diamonds) and mutants RL24S (circles) and RL24S/DL70T (squares) were used at two initial concentrations of 57 and 101, 43 and 95, and 57 and 97 nM, respectively. Mutant DL70T (triangles) was used at an initial concentration of 30 nM. (B) Histogram representation of the TA50 values calculated for scFv1F4-WT and the three mutants of the H46-H62 pair, in two independent experiments.
Table 2.
Half-lives of scFv1F4-WT and of the nine mutants at 25°C in HEPES buffered saline containing 200 mM urea
| scFv1F4 | TA50 (min)a | SD (%) | TA50 relative to WT (%)b | SD (%) |
| QL18K | 730 ± 305 | 42 | 107 ± 6 | 6 |
| KL74I | 2455 ± 1429 | 58 | 343 ± 45 | 13 |
| QL18K-KL74I | 1738 ± 537 | 31 | 261 ± 45 | 17 |
| KH46E | 1935 ± 531 | 27 | 472 ± 176 | 37 |
| DH62K | 146 ± 2 | 2 | 35 ± 3 | 9 |
| KH46E-DH62K | 2031 ± 667 | 33 | 497 ± 211 | 42 |
| RL24S | 397 ± 108 | 27 | 91 ± 21 | 23 |
| DL70T | 170 ± 53 | 31 | 39 ± 9 | 23 |
| RL24S-DL70T | 1455 ± 780 | 54 | 328 ± 153 | 47 |
| WT | 556 ± 248 | 44 | 100 |
ScFv indicates single-chain fragment; variable; WT, wild type, and TA50, absolute half-life.
a Mean and SD from two to three independent experiments (four for scFv1F4-WT).
b Mean of %TA50 values relative to WT calculated from each independent experiment.
The molecules displayed large differences of their half-lives in periplasmic extracts (Table 2), and were divided into three groups. The active concentrations of scFv1F4-DL70T and scFv1F4-DH62K decreased rapidly with time, with a TA50 relative to WT <40%. Two single mutants behaved as the WT (QL18K, RL24S). Two single (KH46E and KL74I) and the three double mutants remained active longer than did scFv1F4-WT, with a TA50 relative to WT >200%. The absolute TA50 values for the three groups of molecules were <250 min, between 250 and 1000 min (WT-like), and mostly >1000 min, respectively. The TA50 values of the double mutants were always larger than those of one or both single mutants, indicating that the deleterious effect of single replacements can be reversed by introducing the second replacement of a co-variant pair.
Five fragments with different half-lives at 25°C were selected for measurements at 37°C to evaluate their behavior at physiological temperature: scFv1F4-WT, scFvs DH62K and DL70T (TA50 < 250 min), scFvs KL74I and KH46E-DH62K (TA50 > 1000 min). The half-lives of the scFvs were reduced about 10-fold at 37°C in the absence of urea, compared to those measured at 25°C in the presence of urea, but their ranking was conserved: The TA50 at 37°C of scFvs DH62K and DL70T was not measurable because of a rapid loss of binding activity. That of scFvs KL74I and KH46E-DH62K was between 100 and 200 min, compared to 35 min for scFv1F4-WT. Therefore, surface charge modifications may improve the behavior of the molecules in physiological conditions, despite the reduced half-life and antigen-binding affinity of the scFvs at 37°C compared to 25°C.
The WT and seven out of the nine mutants were purified by affinity-chromatography. scFv1F4-DL70T and scFv1F4-QL18K could not be obtained in sufficient amounts for further experiments. The half-life measurements were repeated with the purified molecules. Except for mutants DH62K and RL24S, which lost ∼15% and 35%, respectively, of their initial activity, all molecules remained 100% active for 864 mn (data not shown), indicating that the decrease in active concentration over time depends on the presence of other periplasmic proteins.
Four of the purified molecules (scFvs WT, KL74I, KH46E, and KH46E/DH62K) were diluted in the same periplasmic extract. Their half-lives were measured at 30°C, together with those of the purified molecules diluted in HBS (data not shown). The ranking of the TA50 values previously observed for the unpurified molecules at 25°C was reproduced, with a TA50 relative to WT > 190% for the three mutants. These results indicate that although the decrease of activity in periplasmic extracts depends on the presence of other proteins, the rapidity of the decrease is related to a property intrinsic to the scFvs.
The loss of active scFv fragments with time could originate from proteolysis, denaturation, and/or aggregation. The TA50 value for scFv1F4-WT was measured in the presence and absence of a protein inhibitor cocktail. Results are superimposable (data not shown), indicating that the decrease in active concentration is not caused by protease activity in the periplasmic extracts. Aggregation may occur through "sticky" patches present at the surface of the folded molecules or may result from thermodynamic instability leading to unfolding and exposure of hydrophobic residues. The observation that the purified mutants mostly remain 100% active for >14 h in HBS containing 200 mM urea indicates that the molecules remain folded in these experimental conditions and should display similar stabilities, which was investigated by comparing their sensitivity to Gdn-HCl denaturation.
Sensitivity to Gdn-HCl denaturation
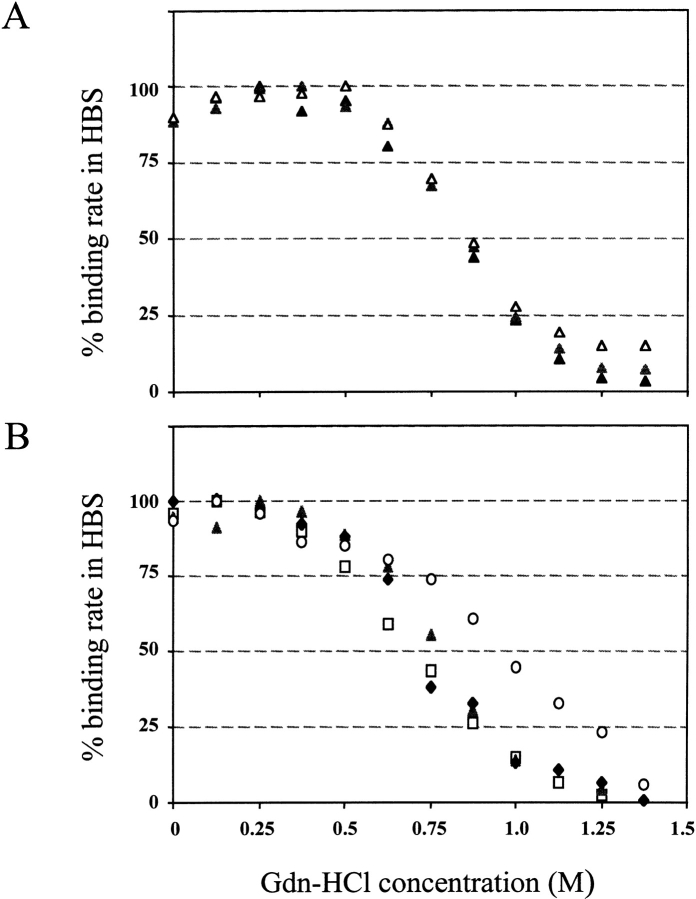
The presence of a denaturing agent is expected to amplify a putative instability that could cause denaturation and loss of binding activity. The purified scFv response on highly immobilized surfaces was recorded in the presence of Gdn-HCl at final concentrations ranging from 0 to 1.375 M. Typical results are shown in Figure 5 ▶. The concentration at which half of the activity remains was in the range 0.625 to 1.0 M for all molecules analyzed, with a range 0.625 to 0.75 M for scFv1F4-WT, indicating that all molecules have a similar sensitivity to Gdn-HCl denaturation. The concentration of half-activity was found to be consistently higher by 0.125 M for double mutant KH46E/DH62K compared with that for scFv1F4-WT (Fig. 5B ▶) in three independent experiments. Mutant DH62K was also more stable than was scFv1F4-WT, but could be analyzed only once because of the difficulty of purifying sufficient amount of the molecule. Mutant DL70T could not be analyzed in this study, but its thermal stability was shown to be slightly higher than that of scFv1F4-WT by Weidenhaupt et al. (2002).
Fig. 5.
Sensitivity of antigen binding to guanidinium chloride (Gdn-HCl) denaturation. The single-chain fragment, variable (scFv) response was recorded after the end of the injection phase for increasing Gdn-HCl concentrations and expressed in % of the response in HEPES buffered saline. (A) Triplicate experiment for scFv1F4-KH46E. (B) Data for scFv1F4-WT (squares) and double mutants QL18K-KL74I (triangles), RL24S-DL70T (diamonds), and KH46E- DH62K (circles).
The shapes of the denaturation curves were highly reproducible, as illustrated for mutants KH46E in Figure 5A ▶, and were not always parallel to that of the WT (mutant KH46E/DH62K; Fig. 5B ▶). No attempt was made to fit the data with known models. Denaturation is assessed indirectly through the loss of antigen-binding activity, and not through a physical signal for unfolding such as fluorescence. A decrease in active concentration should be observed as soon as the paratope geometry is modified, for example, by a change in domain pairing or in CDR conformation.
The Gdn-HCl denaturation experiments indicate that the mutations do not favor scFv denaturation. Therefore, the most likely explanation for the short half-lives in periplasmic extracts is aggregation of folded proteins through ‘sticky patches.’
Discussion
We have analyzed the functional consequences of modifying charges located at a distance from the paratope at the surface of scFv1F4. The six target positions were selected because of their nonrandom patterns of amino-acid changes in sequence alignments. The changes had only minor effects on the antigen-binding properties of the molecules and on their sensitivity to Gdn-HCl denaturation, indicating that all molecules are properly folded and of similar stabilities. However, the half-lives of the various scFvs in periplasmic extracts differed drastically, although the purified molecules mostly remained 100% active for >l4 h in HBS containing 200 mM urea. We propose that the decrease of active scFv concentration in periplasmic extracts is owing to aggregation of the antibody fragments with other proteins, and that the aggregation propensity depends on the surface charge composition of the molecules. In this view, engineering of surface charges may prove a relatively straightforward method to influence levels of active scFv fragments in complex environments, at least for molecules presenting a high positive charge. Such substitutions are unlikely to change the conformation and activity of the molecules.
Surface charges and antigen binding kinetics
Kinetic measurements were performed with three different peptide antigens, of which one contained one less positive charge, and in buffers of different pH values, allowing comparison of the effects on kinetic rates of several factors: charge modifications of the peptide epitope presumably in or near the binding interface; the scFv remote from the binding interface; pH; and temperature. In HBS at 25°C, the measured koff ranged from 2.4 × 10−4 to 2.4 × 10−2 s−1, corresponding to a 100-fold variation, whereas kon values ranged from 2.5 × 105 to 5.7 × 106 M−1•s−1, corresponding to a 23-fold variation. The major effects on both kon and koff were caused by modifications in the peptide epitope (compare kon and koff for the three peptide variants in Fig. 2A ▶). pH variations in the range of 6 to 7.4 were found to influence kon by a factor of two to three and koff by a factor of three to six for all interactions studied (Fig. 3 ▶), whereas temperature affected koff by a factor 10. In contrast, modifications at the surface of the scFv remote from the binding interface had little if any effects on kinetic rates or specificity as also observed by Weidenhaupt et al. (2002).
The engineering of non-specific electrostatic charges in or near the binding interface has been efficiently used for modifying the kon of molecular interactions (Schreiber and Fersht 1996). However, charges play multiple and complex roles in binding (Gibas et al. 1997; Sheinerman et al. 2000). In the present study, significant effects on kon (peptide and pH changes) were always concomitant with effects on koff, indicating that association rates should prove difficult to modulate in a significant and controlled manner independently of dissociation rates. Limited rate parameter variations may more effectively and easily be achieved by optimizing the buffer (Andersson et al. 1999a,b), in particular pH or temperature, than by engineering new molecules, which may be useful for in vitro applications.
Surface charges and aggregation
Several observations are consistent with the hypothesis that the shortened half-life of the scFvs in periplasmic extracts is owing to aggregation through charged patches. (1) The amino acid replacements in the scFv only involved polar and charged side-chains except for the change KL74I (Table 1), and have therefore not significantly increased hydrophobic surfaces. (2) The theoretical pI values of VL and VH domains were calculated using the "Compute pI/Mw" tool of the ExPASy Molecular Biology Server (Swiss Institute of Bioinformatics, http://www.expasy.ch/; Appel et al. 1994). Average pI values for VL and VH were calculated using the sequences of the unique set of antibody structures made available by Dr. A. Martin (http://www.bioinf.org.uk/abs/). The theoretical pI of the VL domain of scFv1F4-WT is above average with a value of 9.1 compared to an average of 7.6±1.5, which may promote aggregation with negatively charged surfaces. The theoretical pI for VH is 7.9, compared to an average of 7.7±1.4. (3) The two mutants with the shortest half-lives (scFv1F4-DL70T and scFv1F4-DH62K) have one negative charge removed compared with the WT. The two single mutants with longest half-lives have one less positive charge (scFv1F4-KL74I) or a replacement of a positive by a negative charge (scFv1F4-KH46E). These examples indicate some correlation between the net charge of the molecules and their half-life. The trend was not true for other single or double mutants. The charge distribution at the surface of the molecule, as well as total charge, may be an important factor for the occurrence of patches critical for aggregation.
Engineering surface charges to ‘improve’ scFvs
The influence of surface charges on stability (Pace et al. 2000; Spector et al. 2000; Koide et al. 2001; Martin et al. 2001) and/or aggregation (Elcock and McCammon, 1998) has been largely documented in particular for thermophilic and halophilic proteins. In the case of antibodies, isolated examples have been reported in which a charge modification influences the stability and/or aggregation of scFv fragments, for example, at position H6 (Kipriyanov et al. 1997; Jung et al. 2001) and H66 (Wörn and Plückthun 1998), but no systematic analysis of the role of surface charges is available.
In their review on stabilization of antibody fragments, (Wörn and Plückthun (2001) have pointed out that among mutations that "improve" the properties of scFvs, some increase thermodynamic stability of scFv fragments, whereas others decrease their propensity to aggregate. Published mutations belonging to the first group act mainly by stabilizing the VL/VH interface (interdomain disulfide bonds, mutations that increase interface packing), or by increasing intrinsic domain stability through core mutations. Published mutations belonging to the second group involve the replacement of hydrophobic by hydrophilic residues at the surface of the fragment, mainly at the V/C (variable/constant regions) interface.
We highlight here a novel approach for improving the yield of active scFvs, namely, by presumably decreasing their propensity to aggregate through engineering surface charges. The concept should be general, although its applicability depends on the surface charge composition and distribution of each particular scFv. In addition, charge modifications could be used in attempts to stabilize the molecules (as suggested for mutants KH46E/DH62K, DL70T, and possibly DH62K), but stability and solubility may vary in opposite directions (mutants DH62K and DL70T), a phenomenon discussed for halophilic proteins (Elcock and McCammon, 1998).
Significance of co-variance
Consensus analyses of sequence alignments are often performed to predict the tolerance of a particular position to amino acid changes and have also been successfully used for antibody stability engineering (Kipriyanov et al. 1997; Chowdhury et al. 1998; Saldanha et al. 1999). However, the effect of most amino acid changes remains unpredictable because the tolerance to a given amino acid change depends on the sequence context. A relationship between the amino acids found at a limited set of positions (H9, H18, H67, H82) and scFv conformation had first been suggested by Saul and Poljak (1993) on the basis of sequence and structure analysis. A relationship between the conformation of the N-terminal segment of VH and the local sequence (H6, H7, H10) has been established by crystallographic analysis (Jung et al. 2001).
The mutational study performed here is based on a co-variance analysis of antibody sequence alignments. We show that the deleterious effect on half-life of single changes (DH62K and DL70T) may be reverted by replacing the second residue of the co-variant pair, restoring both amino acids found in natural sequences. The tolerance to charge modifications at the surface of the scFv therefore depends on the atomic environment, which has important implications for scFv engineering. A relationship between half-life and in vivo functional properties of the whole antibody is difficult to assess for several reasons. First, natural antibodies are large multidomain proteins in which the constant regions may predominantly determine solubility and stability. Second, the periplasmic extracts differ in protein content from the various environments (e.g., interstitial fluids, blood) in which antibodies are naturally found.
Perspective for biomedical applications of scFv1F4
The scFv1F4 is able to bind specifically the E6 oncoprotein of HPV16 and inhibits in vitro the degradation of p53 that is mediated by E6 (Giovane et al. 1999). It is therefore a possible candidate for targeting E6 in HPV16-infected cells if the molecule is folded and active intracellularly (Giovane et al. 1999). The general lack of scFv activity in cells has been attributed to the requirement for an intradomain disulfide bridge. Considerable effort is being devoted to the identification of scFv sequences that are stable in the absence of intradomain disulfide bond formation, which should allow them to fold in a reducing cytoplasmic environment (Chowdhury et al. 1998; Wörn and Plückthun 1998; Martineau and Betton 1999; Ohage and Steipe 1999; Wirtz and Steipe 1999). We show here that the aggregation behavior of scFvs in complex protein mixtures may be another factor limiting their functional efficiency in biological fluids in general and in cells in particular.
The necessity to control the behavior of therapeutic proteins in the cytoplasm, characterized by macromolecular crowding has been recently emphasized (Minton 2000; Ellis 2001). The Biacore approaches outlined in this work could be extended to investigate the behavior of proteins in various fluids and experimental conditions, to better evaluate the biological half-life of therapeutic proteins.
Materials and methods
Synthetic peptides
Peptides were obtained from Synthem (Montpellier, France). They were synthesized with an additional C-terminal cysteine to allow oriented chemical coupling on sensor chips for Biacore analysis. The WT peptide antigen corresponds to residues 7–15 of the E6 protein sequence, and is denoted WTE6-C. Two substituted peptides derived from this WT sequence were used: Q13AE6-C and R15AE6-C.
Site-directed mutagenesis
Site-directed mutagenesis was performed using the PCR-based Quick Change Site-Directed Mutagenesis Kit as described by the manufacturer (Stratagene). Mutagenized plasmids were systematically verified by sequencing the coding region of the antibody fragments on both strands.
Periplasmic expression
The scFv fragments were expressed in the periplasm of Escherichia coli using a protocol adapted from Ben Khalifa et al. (2000). BMH71–18 cells carrying derivative pGE20 plasmids encoding for antibody fragments (Orfanoudakis et al. 1993) were grown in Luria Bertrani supplemented with 100 μg/mL ampicillin at 37°C. In exponentially growing cultures (OD600 ∼0.5), IPTG-induced expression was performed for 14 h at 30° C. Cells were then harvested by centrifugation (4,000g for 20 min), resuspended in 1/10 culture volume of ice-cold spheroblast buffer (30mM Tris-HCl at pH 8.0; 1 mM EDTA, and 20% w/v sucrose), and incubated on ice with gentle shaking for 30 min. Periplasmic protein extracts were obtained by osmotic shock after diluting twice the cells with 0.5× spheroblast buffer supplemented with lysozyme (0.1 mg/mL, final concentration). Cells supernatants were extensively dialyzed against 1000 volumes of HBS (10 mM Hepes at pH 7.4, 150 mM NaCl, and 3.4 mM EDTA) extemporally supplemented with 1 mM phenylmethylsulfonyl fluorhyde. Dialyzed periplasmic extracts were concentrated by ultrafiltration through a 10-kD molecular-weight cut-off filter (Spinprep, Amicon) to give a final volume around 1/100 of initial culture volume. Aliquots were stored at −20°C.
Biacore measurements
All experiments were performed at 25°C on a Biacore 2000 instrument. All solutions and buffers were filtered through a 0.22-μm filter (Millipore), and running buffers were extensively degassed under vacuum; 10 mM HEPES and 10 mM MES (2-[N-morpholino] ethanesulfonic acid) were used for buffering at pH 7.4 or 8.0, and at pH 6.0, respectively. Running buffers were supplemented with 3.4 mM EDTA, 150 mM NaCl, and 0.05% surfactant P20 (Biacore AB). All surfaces were regenerated by injecting 10 μL of a 50 mM HCl solution.
Preparation of sensor surfaces for Biacore measurements
B1 sensor chips, with a low degree of carboxylation, were used for analyzing the scFv1F4-peptide interactions to prevent nonspecific adsorption of scFv fragments on the dextran matrix. Two types of surfaces were prepared: Low amounts of peptides were immobilized for measuring the kinetics of binding between peptides and antibody fragments, and high amounts were immobilized for measuring the active concentrations of antibody fragments. The standard immobilization procedures (BIA-Applications Handbook, Biacore) were used as described previously (Choulier et al. 1999; Ben Khalifa et al. 2000). The desired peptide level was achieved by varying the peptide injection time. Both the activation and peptide injection times were between 5 and 10 min for preparing highly immobilized surfaces. The immobilized peptide levels were between 400 and 700 resonance units (RUs). For surfaces with low concentrations of peptide, the activation time was 10 min, whereas peptide injection times were between 6 and 60 sec (10 μL of peptide solution at a flow of 100 and 10 μL/min, respectively). Between six and 20 RUs of peptides were immobilized, to reach a maximum analyte response (RUmax) of 150 RUs of scFv1F4 at saturating analyte concentration. Blank surfaces were obtained in the same manner as kinetic surfaces, except that no peptide was injected.
Determination of the concentrations of active antibody fragments
Active concentrations of the WT and mutant scFvs were determined as previously described (Choulier et al. 1999; Ben Khalifa et al. 2000) by using a calibration curve. Dilutions of the periplasmic extracts containing the antibody fragments were injected at 10 μL/min on a surface where a high concentration of peptides had been immobilized (mass transport limitation), and the reaction rate was recorded 15 sec after injection. In these conditions, the reaction rate is directly proportional to the concentration of analyte (Karlsson et al. 1993).
Kinetic measurements
The antibody fragments in crude periplasmic extracts diluted in the running buffer were injected, at five concentrations ranging between 5 and 100 nM, at a constant flow rate of 30 μL/min during 3 min, on the blank surface (cell 1) and two peptide surfaces (cells 2 and 3). Just before the kinetic run, each analyte solution was injected on a highly immobilized surface (cell 4) to measure the active concentration of antibody fragments (Choulier et al. 1999). A concentration measurement immediately before the kinetic run proved particularly valuable in the present study for a precise evaluation of kon, because a number of molecules had short half-lives at room temperature. At least two independent measurements on different surfaces were performed for each interaction.
Kinetic data were interpreted with the BIAevaluation 3.0 software (Biacore, Uppsala, Sweden) using a Langmuir model of interaction as previously described (Ben Khalifa et al. 2000). After data evaluation, values were rejected if (1) the Rmax value for one antibody fragment showed >20% difference compared with the RUmax value calculated for other fragments on that same surface, (2) the χ2 value was >10% of the Rmax value, (3) all antibody concentrations were <10 nM.
Half-life of active scFv in periplasmic extracts
Periplasmic extracts were adjusted to an initial active concentration between 20 and 100 nM in HBS buffer containing 200mM urea. The active concentration of antibody fragment in these solutions was measured at constant time intervals (108 min) during 14.4 h, using the same method as for active concentration measurements: 10 μL of the solutions were injected on a highly immobilized surface, and the initial binding rate was recorded. The Biacore racks were maintained at a constant temperature (25°, 30°, or 37°C) using a thermobath (Lauda RE104). Because the initial binding rate is directly proportional to the active analyte concentration, these values were not transformed into active concentrations, but directly expressed as percentages of the binding rate measured at time 0. The Ln of the %(binding rate at time 0) was then plotted as a function of time of incubation and fitted using a linear extrapolation. The rate of inactivation of each molecule was represented by the time of incubation at which half of the activity remains (denoted TA50). TA50 = Ln 0.5/a, were a is the slope of the straight line.
Some experiments were performed in the presence of a protease inhibitor cocktail (Complete, Roche). One tablet was dissolved in 1 mL HBS, and this solution was used at a final dilution of 1:50.
Sensitivity of purified scFvs to Gdn-HCl denaturation
The scFv fragments were purified by affinity chromatography on a chromium bromide–activated Sepharose 4B column (Pharmacia) immobilized with the antigenic fusion protein MBP-E6 as previously described (Giovane et al. 1999). 25 μL aliquots of the purified scFv at a constant concentration adjusted between 30 and 50 nM in HBS were mixed with 25 μL Gdn-HCl solutions of increasing concentrations, to reach final concentrations ranging between 0 and 1.37 M. Next, 10 μL aliquots of the solutions were injected on a highly immobilized surface, and the response was recorded immediately after the end of the injection. The Biacore racks were maintained at a constant temperature of 30°C using a thermobath. The responses at the various Gdn-HCl concentrations were expressed as a percentage of the highest response in HBS.
Acknowledgments
This work was supported by a grant from the Biotechnology program of the European Community (contract BIO4-CT98-0502) and partly by grants from the “Association pour la Recherche sur le Cancer” (contract 5173), and from the Ministry of Defense (contract 99 34 043/DSP/STTC). We thank Marianne Weidenhaupt for the gift of purified scFv1F4-WT.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0209302.
References
- Altschuh, D., Lesk, A.M., Bloomer, A.C., and Klug, A. 1987. Correlation of co-ordinated amino acid substitutions with function in viruses related to tobacco mosaic virus. J. Mol. Biol. 193 693–707. [DOI] [PubMed] [Google Scholar]
- Andersson, K., Areskoug, D., and Hardenborg, E. 1999a. Exploring buffer space for molecular interactions. J. Mol. Recognit. 12 310–315. [DOI] [PubMed] [Google Scholar]
- Andersson, K., Gulich, S., Hamalainen, M., Nygren, P. A., Hober, S., and Malmqvist, M. 1999b. Kinetic characterization of the interaction of the Z-Fragment of protein A with mouse-Igg3 in a volume in chemical space. Proteins 37 494–498. [DOI] [PubMed] [Google Scholar]
- Appel, R.D., Bairoch, A., and Hochstrasser, D.F. 1994. A New generation of information retrieval tools for biologists: The example of the Expasy Www Server. Trends Biochem. Sci. 19 258–260. [DOI] [PubMed] [Google Scholar]
- Ben Khalifa, M.B., Weidenhaupt, M., Choulier, L., Chatellier, J., Rauffer-Bruyere, N., Altschuh, D., and Vernet, T. 2000. Effects on interaction kinetics of mutations at the Vh-Vl interface of Fabs depend on the structural context. J. Mol. Recognit. 13 127–139. [DOI] [PubMed] [Google Scholar]
- Choulier, L., Rauffer-Bruyere, N., Ben Khalifa, M., Martin, F., Vernet, T., and Altschuh, D. 1999. Kinetic analysis of the effect on Fab binding of identical substitutions in a peptide and its parent protein. Biochemistry 38 3530–3537. [DOI] [PubMed] [Google Scholar]
- Choulier, L., Lafont, V., Hugo, N., and Altschuh, D. 2000. Covariance analysis of protein families: The case of the variable domains of antibodies. Proteins 41 475–484. [DOI] [PubMed] [Google Scholar]
- Chowdhury, P.S., Vasmatzis, G., Beers, R., Lee, B., and Pastan, I. 1998. Improved stability and yield of a Fv-Toxin fusion protein by computer design and protein engineering of the Fv. J. Mol. Biol. 281 917–928. [DOI] [PubMed] [Google Scholar]
- Elcock, A.H. and McCammon, J.A. 1998. Electrostatic contributions to the stability of halophilic proteins. J. Mol. Biol. 280 731–748. [DOI] [PubMed] [Google Scholar]
- Ellis, R.J. 2001. Macromolecular crowding: An important but neglected aspect of the intracellular environment. Curr. Opin. Struct. Biol. 11 114–119. [DOI] [PubMed] [Google Scholar]
- Gabdoulline, R.R. and Wade, R.C. 1999. On the protein-protein diffusional encounter complex. J. Mol. Recognit. 12 226–234. [DOI] [PubMed] [Google Scholar]
- Gibas, C.J., Subramaniam, S., McCammon, J.A., Braden, B.C., and Poljak, R.J. 1997. Ph dependence of antibody/lysozyme complexation. Biochemistry 36 15599–15614. [DOI] [PubMed] [Google Scholar]
- Giovane, C., Trav, G., Briones, A., Lutz, Y., Wasylyk, B., and Weiss, E. 1999. Targetting of the N-terminal domain of the human papillomavirus type 16 E6 oncoprotein with monomeric Scfvs blocks the E6-mediated degradation of cellular P53. J. Mol. Recognit. 12 141–152. [DOI] [PubMed] [Google Scholar]
- Jung, S., Spinelli, S., Schimmele, B., Honegger, A., Pugliese, L., Cambillau, C., and Pluckthun, A. 2001. The importance of framework residues H6, H7 and H10 in antibody heavy chains: Experimental evidence for a new structural subclassification of antibody V(H) domains. J. Mol. Biol. 309 701–716. [DOI] [PubMed] [Google Scholar]
- Karlsson, R., Fagerstam, L., Nilshans, H., and Persson, B. 1993. Analysis of active antibody concentration: Separation of affinity and concentration parameters. J. Immunol. Methods 166 75–84. [DOI] [PubMed] [Google Scholar]
- Kipriyanov, S.M., Moldenhauer, G., Martin, A.C., Kupriyanova, O.A., and Little, M. 1997. Two amino acid mutations in an anti-Human Cd3 single chain Fv antibody fragment that affect the yield on bacterial secretion but not the affinity. Protein Eng. 10 445–453. [DOI] [PubMed] [Google Scholar]
- Koide, A., Jordan, M.R., Horner, S.R., Batori, V., and Koide, S. 2001. Stabilization of a fibronectin type Iii domain by the removal of unfavorable electrostatic interactions on the protein surface. Biochemistry 40 10326–10333. [DOI] [PubMed] [Google Scholar]
- Martin, A., Sieber, V., and Schmid, F.X. 2001. In-vitro selection of highly stabilized protein variants with optimized surface. J. Mol. Biol. 309 717–726. [DOI] [PubMed] [Google Scholar]
- Martineau, P. and Betton, J. M. 1999. In vitro folding and thermodynamic stability of an antibody fragment selected in vivo for high expression levels in Escherichia coli cytoplasm. J. Mol. Biol. 292 921–929. [DOI] [PubMed] [Google Scholar]
- Minton, A. P. 2000. Implications of macromolecular crowding for protein assembly. Curr. Opin. Struct. Biol. 10 34–39. [DOI] [PubMed] [Google Scholar]
- Ohage, E., and Steipe, B. 1999. Intrabody construction and expression, I: The critical role of Vl domain stability. J. Mol. Biol. 291 1119–1128. [DOI] [PubMed] [Google Scholar]
- Orfanoudakis, G., Karim, B., Bourel, D., and Weiss, E. 1993. Bacterially expressed Fabs of monoclonal antibodies neutralizing tumour necrosis factor α in vitro retain full binding and biological activity. Mol. Immunol. 30 1519–1528. [DOI] [PubMed] [Google Scholar]
- Pace, C.N., Alston, R.W., and Shaw, K. L. 2000. Charge-charge interactions influence the denatured state ensemble and contribute to protein stability. Protein Sci. 9 1395–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha, J.W., Martin, A.C., and Leger, O.J. 1999. A single backmutation in the human Kiv framework of a previously unsuccessfully humanized antibody restores the binding activity and increases the secretion in Cos cells. Mol. Immunol. 36 709–719. [DOI] [PubMed] [Google Scholar]
- Sali, A. and Blundell, T. L. 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234 779–815. [DOI] [PubMed] [Google Scholar]
- Saul, F.A. and Poljak, R.J. 1993. Structural patterns at residue positions 9, 18, 67 and 82 in the Vh framework regions of human and murine immunoglobulins. J. Mol. Biol. 230 15–20. [DOI] [PubMed] [Google Scholar]
- Schreiber, G. and Fersht, A.R. 1996. Rapid, electrostatically assisted association of proteins. Nat. Struct. Biol. 3 427–431. [DOI] [PubMed] [Google Scholar]
- Sheinerman, F.B., Norel, R., and Honig, B. 2000. Electrostatic aspects of protein-protein interactions. Curr. Opin. Struct. Biol. 10 153–159. [DOI] [PubMed] [Google Scholar]
- Spector, S., Wang, M., Carp, S.A., Robblee, J., Hendsch, Z.S., Fairman, R., Tidor, B., and Raleigh, D.P. 2000. Rational modification of protein stability by the mutation of charged surface residues. Biochemistry 39 872–879. [DOI] [PubMed] [Google Scholar]
- Weidenhaupt, M., Ben Khalifa, M., Hugo, N., Choulier, L., Altschuh, D., Vernet, T. 2002. Functional mapping of conserved, surface-exposed charges of antibody variable domains. J. Mol. Recognit. 15 94–103. [DOI] [PubMed] [Google Scholar]
- Wirtz, P. and Steipe, B. 1999. Intrabody construction and expression iii: Engineering hyperstable V(H) domains. Protein Sci. 8 2245–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wörn, A. and Plückthun, A. 1998. Mutual stabilization of Vl and Vh in single-chain antibody fragments: Investigated with mutants engineered for stability. Biochemistry 37 13120–13127. [DOI] [PubMed] [Google Scholar]
- ———. 2001. Stability engineering of antibody single-chain Fv fragments. J. Mol. Biol. 305 989–1010. [DOI] [PubMed] [Google Scholar]
- Yang, J., Jia, Z., and Xiang, J. 1999. Substitution of surface-exposed framework residues alters secretion of recombinant fusion protein Fv/tumor necrosis factor in Escherichia coli. IUBMB Life. 48 327–332. [DOI] [PubMed] [Google Scholar]
Web site references
- http://www.expasy.ch; “Compute pI/Mw” tool of the ExPASy Molecular Biology Server, Swiss Institute of Bioinformatics.
- http://www.bioinf.org.uk/abs; unique set of antibody structures made available by Dr. A. Martin.