Abstract
The partly folded states of protein members of the lysozyme (LYS)/α-lactalbumin (LA) superfamily have been analyzed by circular dichroism (CD) measurements and limited proteolysis experiments. Hen, horse, dog, and pigeon LYSs and bovine LA were used in the present study. These are related proteins of 123- to 129-amino-acid residues with similar three-dimensional structures but low similarity in amino acid sequences. Moreover, notable differences among them reside in their calcium-binding properties and capability to adopt partly folded states or molten globules in acid solution (A-state) or on depletion of calcium at neutral pH (apo-state). Far- and near-UV CD measurements revealed that although the structures of hen and dog LYS are rather stable in acid at pH 2.0 or at neutral pH in the absence of calcium, conformational transitions to various extents occur with all other LYS/LA proteins herewith investigated. The most significant perturbation of tertiary structure in acid was observed with bovine LA and LYS from horse milk and pigeon egg-white. Pepsin and proteinase K were used as proteolytic probes, because these proteases show broad substrate specificity, and therefore, their sites of proteolysis are dictated not by the specific amino acid sequence of the protein substrate but by its overall structure and dynamics. Although hen LYS at pH 2.0 was fully resistant to proteolysis by pepsin, the other members of the LYS/LA superfamily were cleaved at different rates at few sites of the polypeptide chain and thus producing rather large protein fragments. The apo-form of bovine LA, horse LYS, and pigeon LYS were attacked by proteinase K at pH 8.3, whereas dog and hen LYSs were resistant to proteolysis when reacted under identical experimental conditions. Briefly, it has been found that the proteolysis data correlate well with the extent of conformational transitions inferred from CD spectra and with existing structural informations regarding the proteins herewith investigated, mainly derived from NMR and hydrogen exchange measurements. The sites of initial proteolytic cleavages in the LYS variants occur at the level of the β-subdomain (approximately chain region 34–57), in analogy to those observed with bovine LA. Proteolysis data are in agreement with the current view that the molten globule of the LYS/LA proteins is characterized by a structured α-domain and a largely disrupted β-subdomain. Our results underscore the utility of the limited proteolysis approach for analyzing structure and dynamics of proteins, even if adopting an ensemble of dynamic states as in the molten globule.
Keywords: Lactalbumin, lysozyme, circular dichroism, limited proteolysis, molten globule, protein folding
The strong interest in partly folded states of proteins, or folding intermediates, resides in the fact that likely by studying the molecular features of these intermediates, it will be possible to elucidate the mechanism of how proteins fold into their specific biologically active conformations (Kim and Baldwin 1990; Matthews 1995; Privalov 1996; Dill and Chan 1997; Kuwajima and Arai 1999). In recent years, the difficulty in detecting and characterizing the transient intermediate species formed during the protein folding process prompted a variety of structural studies on the intermediates that can be generated at equilibrium by dissolving a protein in acid solution or in the presence of moderate concentrations of a protein denaturant, as well as by removing protein-bound metal ions or prosthetic groups (Dobson 1994; Fink 1995; Arai and Kuwajima 2000). These studies led to the development of the concept of the molten globule (MG) state, shown to be an equilibrium intermediate between the native and unfolded states of a number of globular proteins. Until recently, the MG state was considered to be a partly folded state having a stable native-like secondary structure but lacking a specific tertiary structure (Dolgikh et al. 1981; Ohgushi and Wada 1983). However, in several studies it has been shown that a great variety of MGs do exist, ranging from those having specific tertiary interactions or native-like topology to those resembling the unfolded state of a protein (Fink et al. 1998; Uversky et al. 1998). Evidence has been provided that the MGs of some proteins share conformational features with those transiently observed in kinetic experiments of protein folding (Ikeguchi et al. 1986a; Ptitsyn et al. 1990; Jennings and Wright 1993; Engelhard and Evans 1995; Raschke and Marqusee 1997; Roder and Colón 1997; Mizuguchi et al. 1998). Therefore, the detailed characterization of equilibrium intermediates in terms of structure and dynamics appears to be relevant for solving the protein folding problem (Fink 1995; Ptitsyn 1995; Chamberlain and Marqusee 2000).
The best-studied MG is that formed by α-lactalbumin (LA) in acid solution at pH 2.0 (A-state; Ptitsyn 1995; Kuwajima 1996). Over the years, numerous investigators used a variety of experimental approaches and techniques to analyze the A-state of LA, which can be considered the prototype MG. Actually, the original definition of the MG state was based on the structural and dynamic features of the A-state of bovine LA (Dolgikh et al. 1981; Ptitsyn 1995). Another partly folded state of LA can be generated at neutral pH and low salt concentration by removing the protein-bound calcium ion from this metalloprotein (Ikeguchi et al. 1986b; Kronman 1989; Kuwajima 1989, 1996; Kataoka et al. 1997; Permyakov and Berliner 2000). These studies were conducted on bovine, human, goat, and guinea-pig LA, but the conformational features of these homologous proteins are very similar (Pike et al. 1996). Thus, the results obtained with LA from different sources and their interpretations likely can be used interchangeably. Nevertheless, this assumption may not be generally valid, considering that, for example, the addition of a single Met residue at the N terminus in the Escherichia coli–expressed recombinant bovine LA (Chaudhuri et al. 1999; Veprintsev et al. 1999), as well as in human LA (Chaudhuri et al. 2000), significantly reduces protein stability to heat and denaturants, as well as calcium binding affinity.
Although hen egg-white lysozyme (LYS) and LA are homologous to each other (Fig. 1 ▶; Nitta and Sugai 1989), their equilibrium unfolding profiles are quite different, with that of LYS and LA obeying a two-state and three-state model, respectively (Kuwajima et al. 1976). In particular, although LA in acid at pH 2.0 attains a MG state, hen LYS remains native under similar acidic conditions. However, the homologous calcium-binding LYS from equine or canine milk or from pigeon egg-white can form the MG state when exposed to acid solution or on calcium depletion at neutral pH and (moderate) heating. More recently, the molecular features of the partly folded states of horse, pigeon, and dog LYS and their relative stabilities have been studied in great detail in several laboratories and compared with those of the MG of LA (Van Dael et al. 1993; Morozova et al. 1995; Morozova-Roche et al. 1997; Haezebrouck et al. 1998; Mizuguchi et al. 1998, 1999; Blanch et al. 2000; Kobashigawa et al. 2000; Koshiba et al. 2000, 2001).
Fig. 1.
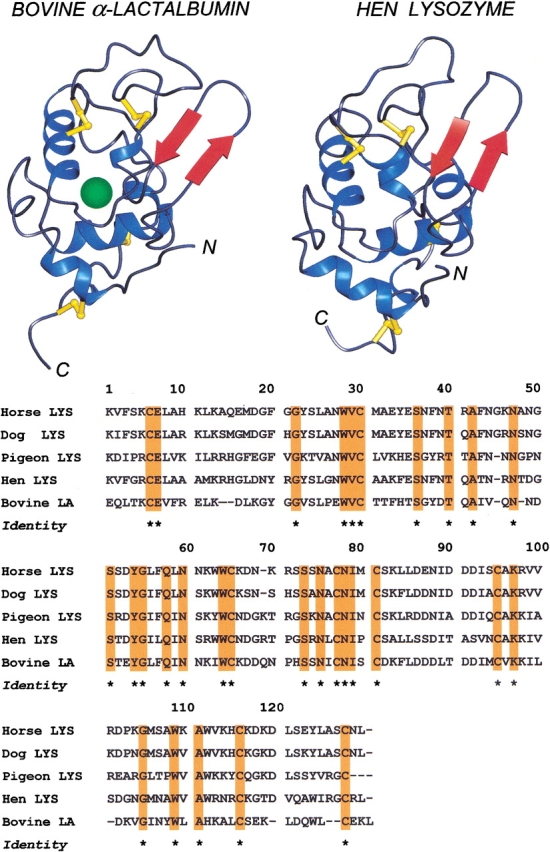
(Top) Schematic view of the native structures of bovine α-lactalbumin (LA) and hen egg-white lysozyme (LYS). The diagrams were drawn using the PDB files 1hfz and 1jpo for bovine LA and hen LYS, respectively, using the program PREPI (Molecular Simulations Inc.) on a Silicon Graphics Iris Indigo Workstation. The β-subdomain in both proteins is composed by the β-pleated sheets colored in red; the α-helices are colored in blue; and the disulfide bridges are represented by yellow sticks. In the case of bovine LA, the connectivities of the four disulfide bridges along the 123-residue chain of the protein are 6–120, 28–111, 61–77, and 73–91. In the LA structure, the calcium atom is shown by a solid sphere in green. (Bottom) Alignment of amino acid sequences of LYS from horse (McKenzie and Shaw 1985), dog (Grobler et al. 1994), pigeon (Menéndez-Arias et al. 1985), and hen egg-white (Canfield 1963) and bovine LA (Hurley and Schuler 1987). Dashes indicate deletions relative to the other sequences. Conserved residues are highlighted in orange and labeled by a asterisk.
The analysis of the molecular features of folding intermediates or MG states is made difficult by their dynamic nature and by the fact that they are an ensemble of conformational states (Plaxco and Dobson 1996; Fink et al. 1998). A variety of biochemical and biophysical techniques has been used for these studies, ranging from circular dichroism (CD), fluorescence emission, binding of hydrophobic dyes, Raman spectroscopy, viscosimetry, gel filtration, and solution X-ray scattering to differential scanning calorimetry (Evans and Radford 1994). However, multidimensional NMR spectroscopy and hydrogen/deuterium (H/D) exchange experiments provided the most useful data on the structural features of MG (Dobson 1994; Redfield et al. 1994, 1999; Schulman et al. 1997; Eliezer et al. 1998; Forge et al. 1999). Nevertheless, it is clear that each technique has advantages and drawbacks, and no one is fully superior to all others. As a matter of fact, it has been clearly emphasized that a coherent view of the properties of folding intermediates will be reached only by studying them using a variety of complementary techniques (Evans and Radford 1994).
In recent years, in our laboratory we have conducted a series of studies aimed to show that the simple biochemical technique of limited proteolysis can provide useful data of protein structure and dynamics, complementing those reached by using other more classical spectroscopic techniques (Fontana et al. 1995, 1997a, 1997b,Fontana et al. 1999; Spolaore et al. 2001). This experimental approach relies on the fact that limited proteolysis of globular proteins occurs at sites characterized by enhanced flexibility or local unfolding, as anticipated by the fact that peptide bond fission requires the binding and adaptation of an extended chain segment of 8- to 10-amino-acid residues of the polypeptide substrate at the precise stereochemistry of the active site of the protease (Schechter and Berger 1967; Fontana et al. 1986, 1993, 1997a, 1997b, 1999; Hubbard 1998). In previous studies, we have used limited proteolysis experiments to analyze the conformational features of bovine LA in its MG state at pH 2.0 (A-state) or at neutral pH in its apo-form (Polverino de Laureto et al. 1995, 1999, 2001). Both pepsin and proteinase K initially cleave bovine LA in its MG state at the level of the β-subdomain (chain region approximately from residues 34–57), indicating that this region of LA in both the A-state and apo-form is highly dynamic or even unfolded. Our results were in agreement with the current view that the MG of LA has a bipartite structure, characterized by a structured α-domain and a disordered β-subdomain (Fig. 1 ▶; Peng and Kim 1994; Peng et al. 1995; Wu et al. 1995; Schulman et al. 1997; Forge et al. 1999; Redfield et al. 1999).
Here, we present the results of comparative experiments of limited proteolysis conducted on four LYS species, derived from hen and pigeon egg-white and from equine and canine milk (Fig. 1 ▶), exposed to acid solution or at neutral pH in their calcium-free form. The extent and kinetics of proteolysis, as well as the location of initial proteolytic peptide bond fissions along the LYS polypeptide chains, have been correlated with the conformational features of the various proteins deduced from far- and near-UV CD spectroscopy, as well as interpreted in the light of the detailed studies conducted recently in other laboratories using NMR, hydrogen exchange, and differential scanning calorimetry (see above). Overall, our results do correlate well with the available information regarding structure, dynamics, and stability of protein members of the LYS/LA superfamily. For example, hen egg-white LYS was found to be fully resistant to proteolysis, in agreement with the fact that hen LYS does not form a MG. The results of this study greatly extend the scope and utility of our previous studies on the structural and dynamic characteristics of the MG state of bovine LA (Polverino de Laureto et al. 1995, 1999, 2001) and provide additional proof that the limited proteolysis approach can provide useful insights into the conformational features of proteins (Fontana et al. 1997a, 1997b, 1999; Spolaore et al. 2001).
Results
Circular dichroism
The conformational features of hen, horse, dog, and pigeon LYSs and bovine LA have been compared by far- and near-UV CD measurements. The spectra were recorded in acid solution at pH 2.0 or at neutral pH in the presence or absence of calcium ions. The aromatic chromophores (Trp, Tyr, Phe), responsible for dichroic signals in the near-UV region (Strickland 1974), are not completely conserved in the amino acid sequences of LYS from various species and LA (Fig. 1 ▶), and therefore, different near-UV spectra are expected with the LYS/LA proteins herewith investigated.
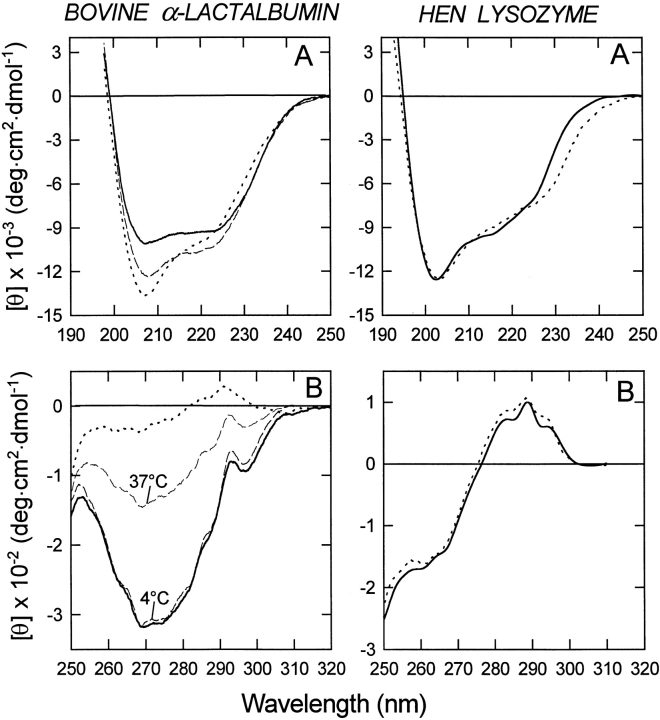
In Figure 2 ▶, the CD spectra of bovine LA and hen LYS in the far-UV (top) and near-UV (bottom) are reported. At neutral pH and room temperature, bovine LA displays a typical spectrum of helical polypeptides, with two minima of ellipticity near 208 and 220 nm (Chen et al. 1974). In acid solution or on removal of calcium by EDTA at neutral pH, LA maintains a native-like content of α-helical secondary structure. The near-UV CD spectrum of LA at neutral pH and in the presence of calcium is characterized by an intense negative signal near 270 nm, in agreement with previous CD measurements conducted under similar conditions (Ikeguchi et al. 1986a; Griko and Remeta 1999). At pH 7.5 and 4°C, the near-UV CD spectrum of apo-LA resembles that of the holo-protein, and only on moderate heating at 37°C, is the native tertiary structure of LA partially lost. These CD data are in agreement with the fact that apo-LA only in the absence of salts and on heating adopts the MG state (Griko and Remeta 1999). At pH 2.0, the tertiary structure of LA appears to be almost completely disrupted (Fig. 2 ▶, left, dotted line), because the intensity of the dichroic signals in the 250- to 300-nm region are much reduced.
Fig. 2.
Far-UV (A) and near-UV (B) circular dichroism (CD) spectra of bovine α-lactalbumin (LA) and hen lysozyme (LYS). Spectra were obtained at 20°–22°C in 0.01 M Tris/0.1 M KCl (pH 7.5), containing 5 mM CaCl2 (continuous lines) or 1 mM EDTA (dashed lines) and in 0.01 M HCl/0.1 M KCl (pH 2.0; dotted lines). In the case of bovine LA, near-UV CD spectra were recorded also in Tris/EDTA buffer(pH 7.5) at 4° and 37°C (dashed lines). The far- and near-UV CD spectra of hen LYS at pH 7.5 in the presence of 1 mM EDTA (data not shown) were identical to those recorded in Tris buffer only.
The CD spectra in the near- and far-UV of hen LYS (Fig. 2 ▶, right) at pH 2.0 are essentially identical to those at neutral pH. Indeed, hen LYS does not adopt a MG state in acid solution and is rather stable to extreme pH (Kuwajima et al. 1976). The CD spectra of hen LYS are not influenced by the presence of calcium in the protein solution, because LYS does not bind calcium ions.
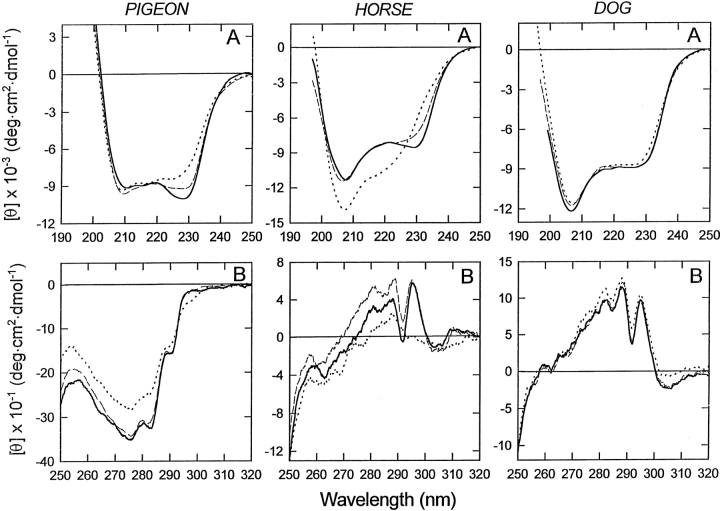
The spectrum of pigeon LYS in the near-UV region (Fig. 3B ▶, left) resembles that of LA at neutral pH and in the presence of calcium, showing a negative signal near 270 nm (continuous line). The apo-form of the protein does not show significant differences in comparison with the holo-protein (dashed line) at 20°C–22°C. At pH 2.0 (dotted line) the shape of the CD spectra is similar, but the intensity of the signal is lower, indicating a partial denaturation of the tertiary structure of the protein and/or enhanced flexibility. It is worthwhile observing that the minimum at 220 nm, typical of an α-helix, is shifted to 228 nm in the case of pigeon LYS, as well as equine and canine LYSs, at neutral pH and in the presence of calcium. This shift can be attributed to a contribution of aromatic chromophores in the 228-nm to 250-nm region (Woody 1978, 1994).
Fig. 3.
Far-UV (A) and near-UV (B) circular dichroism (CD) spectra of pigeon (left), horse (middle), and dog (right) lysozyme (LYS). Spectra were obtained at 20°–22°C in 0.01 M Tris/0.1 M KCl (pH 7.5), containing 5 mM CaCl2 (continuous lines), in 0.01 M HCl/0.1 M KCl (pH 2.0; dotted lines), and in 10 mM Tris/0.1 M KCl (pH 7.5) containing 1 mM EDTA (dashed lines).
The CD spectra of horse LYS are reported in Figure 3 ▶ (middle). In the presence or absence of calcium, the CD spectra of the native form (continuous line) and of the apo-form (dashed line) are quite similar both in the far-UV (A) and near-UV (B) region. In particular, the near-UV CD spectrum shows fine structure, with maxima at 282, 287, and 294 nm, in agreement with previous CD measurements (Van Dael et al. 1993). Of interest is the fact that the near-UV CD spectrum of horse LYS is characterized by positive signals between 270 to 295 nm, much different from that of bovine LA and pigeon egg-white LYS and similar to that of hen LYS. In acid solution (pH 2.0), the far-UV CD spectrum (dotted line) indicates a high content of α-helical secondary structure with an increase of the signal at 208 nm, in analogy to bovine LA (Fig. 2A ▶, left). The near-UV CD spectrum recorded in acid solution is indicative of a partial unfolding of the protein, with a complete loss of the characteristic maximum of the CD signal at 294 nm.
At variance from bovine LA, LYS from pigeon egg-white, and equine LYS, the structure of canine LYS appears to be strongly resistant to environmental variations. In fact, there are no changes in shape and intensity of CD signals at low pH or after removal of the protein-bound calcium ion (Fig. 3 ▶, right panel). In the near-UV region, the CD spectrum has a specific fine structure, characterized by maxima at 294 and 298 nm, attributable to the optical activity of tryptophan residues (Strickland 1974; Kobashigawa et al. 2000).
Limited proteolysis
In this study, the limited proteolysis approach (Fontana et al. 1986, 1997a, 1997b, 1999) is used to analyze the partly folded or MG states of LYS from pigeon, horse, and dog obtained by exposing the protein at pH 2.0 or on removal of the protein-bound calcium ion by EDTA at neutral pH. The results obtained are compared with those previously obtained for bovine LA (Polverino de Laureto et al. 1995, 1999, 2001). The identification of proteolysis sites was established by N-terminal sequencing and matrix-assisted laser-desorption ionization (MALDI) mass spectrometry of the peptide material taken from reaction mixtures and separated by reverse-phase high-pressure liquid chromatography (RP-HPLC) and by comparison of these data with the known amino acid sequences of LYS from different sources. Details of these analyses are not given here. The reader is referred to our previous studies on the use of limited proteolysis for probing partly folded states of proteins for experimental details and methods used (Polverino de Laureto et al. 1999).
Pepsin
The proteolysis with pepsin has been carried out at pH 2.0 using an enzyme-to-substrate (E/S) ratio of 1:500 (by weight) at 20° to 22°C. Pepsin cleaves bovine LA in its A-state at pH 2.0 only at few sites of the polypeptide chain, and these sites are located at the chain region encompassing the β-sheet region (34–57) of the protein (Polverino de Laureto et al. 1995, 1999, 2001). In fact, the initial nicking of LA occurs at peptide bonds Ala40-Ile41 and Leu52-Phe53, followed by subsequent hydrolysis of Tyr103-Trp104 (Polverino de Laureto et al. 1995, 1999). Figure 4 ▶ (left, top) shows the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis (reducing conditions) of the proteolytic mixture of bovine LA with pepsin. The protein at pH 2.0 is digested by pepsin in few minutes, and in the Coomassie-stained gel, the electrophoretic bands of fragments 53–123, 53–103, and 1–40 (Fig. 4 ▶, top) are apparent. Initially a protein species given by fragments 1–40 and 53–123, covalently linked by the four disulfide bridges of the protein, are formed. Subsequently, fragment 53–103 is excised from this species.
Fig. 4.
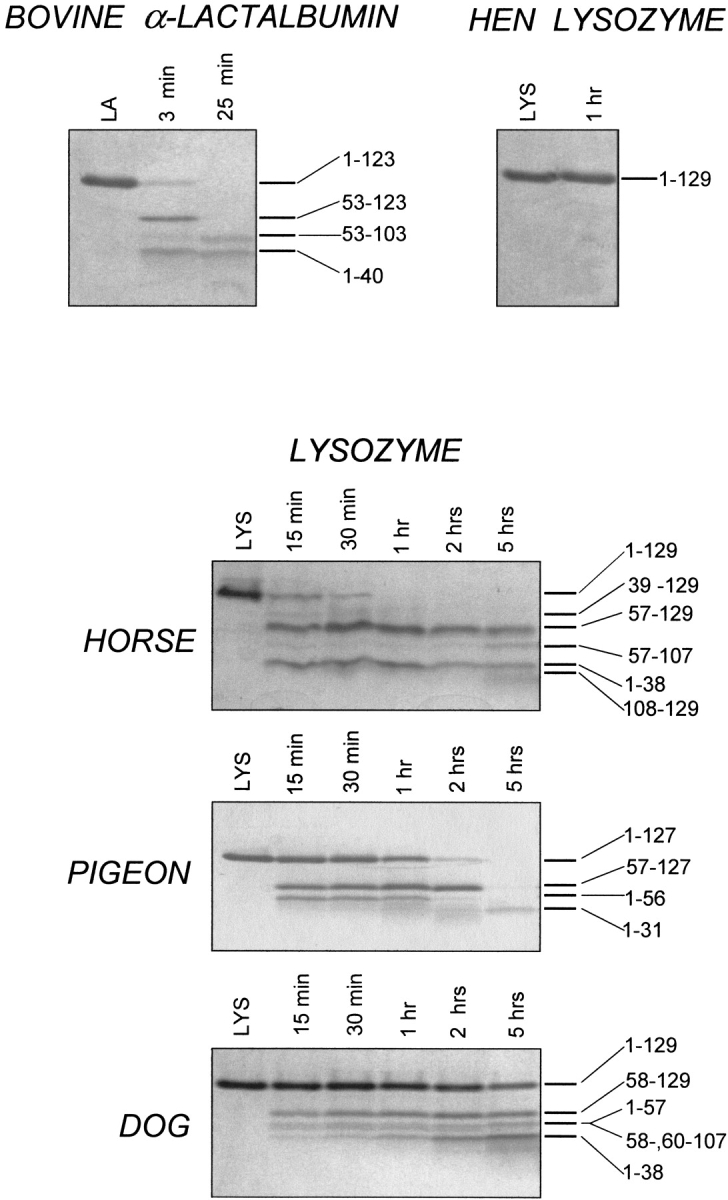
SDS-PAGE of the proteolytic digestion of hen, horse, dog, and pigeon lysozyme (LYS) and bovine α-lactalbumin (LA) by pepsin at pH 2.0. The proteolysis of bovine LA and hen LYS (top) and of horse, pigeon, and dog LYS (bottom) was conducted at 20°–22°C (enzyme-to-substrate ration [E/S], 1:500 by weight). Samples were taken from the reaction mixtures at the indicated times and stopped by alkalinization. LA and LYS refer to protein samples dissolved in acid solution without pepsin. The time-course digestion of LA and LYS polypeptide chain by pepsin was followed by SDS-PAGE under reducing conditions using the Tricine buffer system (Schägger and von Jagow 1987); under these conditions inter- and intramolecular disulfide bridges are broken, and protein fragments migrate in the gel as individual polypeptide chains. Numbers in the right part of the Coomassie-stained gel refer to the identity of the protein fragments produced during proteolysis.
At variance from bovine LA, hen egg-white LYS (Fig. 4 ▶, right, top) appears to be fully resistant to proteolysis with pepsin, as expected from the highly stable and native-like structure of the protein at pH 2.0 (Fig. 2 ▶, right). An aliquot of the proteolytic mixture after 1-h digestion of hen LYS with pepsin was loaded on a SDS-PAGE gel, and a strong band of the intact protein is seen in the stained gel, without bands at lower molecular mass.
Figure 4 ▶ (bottom) shows the time-course analysis of the peptic digestion of horse, pigeon, and dog LYSs at pH 2.0, as followed by SDS-PAGE. After a few minutes of incubation of the protein substrate with the protease, the polypeptide chains of LYS from different sources were cleaved at very few peptide bonds, as given by the presence of few electrophoretic bands of rather large protein fragments, besides the band of the intact protein (lane 2, 15-min reaction). Moreover, some fragments appear to be quite resistant to further proteolysis, because their electrophoretic bands remain in the stained gel after several hours of reaction.
It is worthwhile to underline the differences to hydrolytic attack by pepsin within the various LYS species (Fig. 4 ▶, bottom). In the case of horse LYS, the electrophoretic band corresponding to the intact protein disappears from the stained gel after a 30-min reaction, and the protein is completely converted to fragments of lower molecular mass. Pigeon LYS was found to be somewhat more resistant than is horse LYS to peptic digestion, because the band of the intact protein is still visible after a 2-h reaction. Dog LYS instead is almost completely resistant to proteolysis, as evidenced by the fact that even after a 5-h reaction, a strong band of intact protein is still seen in the stained gel (Fig. 4 ▶, bottom).
In the case of horse LYS reacted with pepsin at pH 2.0, the various protein fragments indicated in the stained gel (Fig. 4 ▶, bottom) were identified. A kinetic analysis of the peptic digestion conducted using both SDS-PAGE and RP-HPLC (data not shown) allowed us to conclude that the initial nicking of the polypeptide chain of horse LYS occurs at peptide bonds Phe38-Asn39 and Leu56-Phe57, followed by a slower cleavage at peptide bond Ala107-Trp108. The species 1–38/57–129 and 1–38/108–129 were isolated after RP-HPLC and shown to be constituted by two fragments crosslinked by the four disulfide bonds of the intact protein.
The main product of the proteolysis of pigeon LYS by pepsin is the fragment species 1–56/57–127, given by fragments 1–56 and 57–127 covalently linked by disulfides. Although the electrophoretic band of fragment 57–127 is present in the stained gel even after 2 h of proteolysis, the band relative to the fragment 1–56 during proteolysis fades from the gel, and a new band of lower molecular mass is detectable. Analysis by RP-HPLC of the proteolytic mixture, combined with N-terminal sequencing and mass spectrometry of isolated fragments, revealed that the major product of degradation of the N-terminal fragment 1–56 is fragment 1–31. Therefore, fast initial nicking of pigeon LYS occurs at Phe56-Gln57 peptide bond, followed by a much slower cleavage at Leu31-Val32.
At variance from LYS from horse milk and egg-white pigeon, canine LYS is rather resistant to proteolysis by pepsin at pH 2.0 (Fig. 4 ▶, bottom). The fragment species indicated in the Coomassie-stained SDS-PAGE gel have been identified by isolation of protein fragments by RP-HPLC and subsequent analyses for their identity (above). Briefly, it was concluded that major cleavages occur at the level of the Phe57-Gln58 and Phe38-Asn39 peptide bonds, and a minor cleavage at Leu59-Asn60. As an additional proof of the sites of cleavage in dog LYS, we may mention that we have isolated after RP-HPLC of an aliquot of the reaction mixture the peptides corresponding to the chain segments 39–53 and 39–57 (data not shown).
Proteinase K
Although the calcium-loaded LA is fully resistant to proteolysis by proteinase K, the calcium-free form of the protein is amenable to proteolytic digestion (Fig. 5 ▶, top; Polverino de Laureto et al. 1999, 2001). However, if the reaction is conducted at 4°C, the protein appears to be resistant to proteolysis, because at low temperature, apo-LA maintains a native-like structure (see Fig. 2 ▶ for CD data). At 37°C, the protein adopts a more flexible state and thus becomes more prone to proteolytic attack (Fig. 5 ▶, top). The protein fragments indicated in the Coomassie-stained gel of Figure 5 ▶ (top, left) have been isolated to homogeneity in our previous work (Polverino de Laureto et al. 1999, 2001). In contrast, hen LYS is fully resistant to proteolytic digestion, even if proteolysis by the voracious proteinase K is conducted for 1 h at 37°C (Fig. 5 ▶ top, right).
Fig. 5.
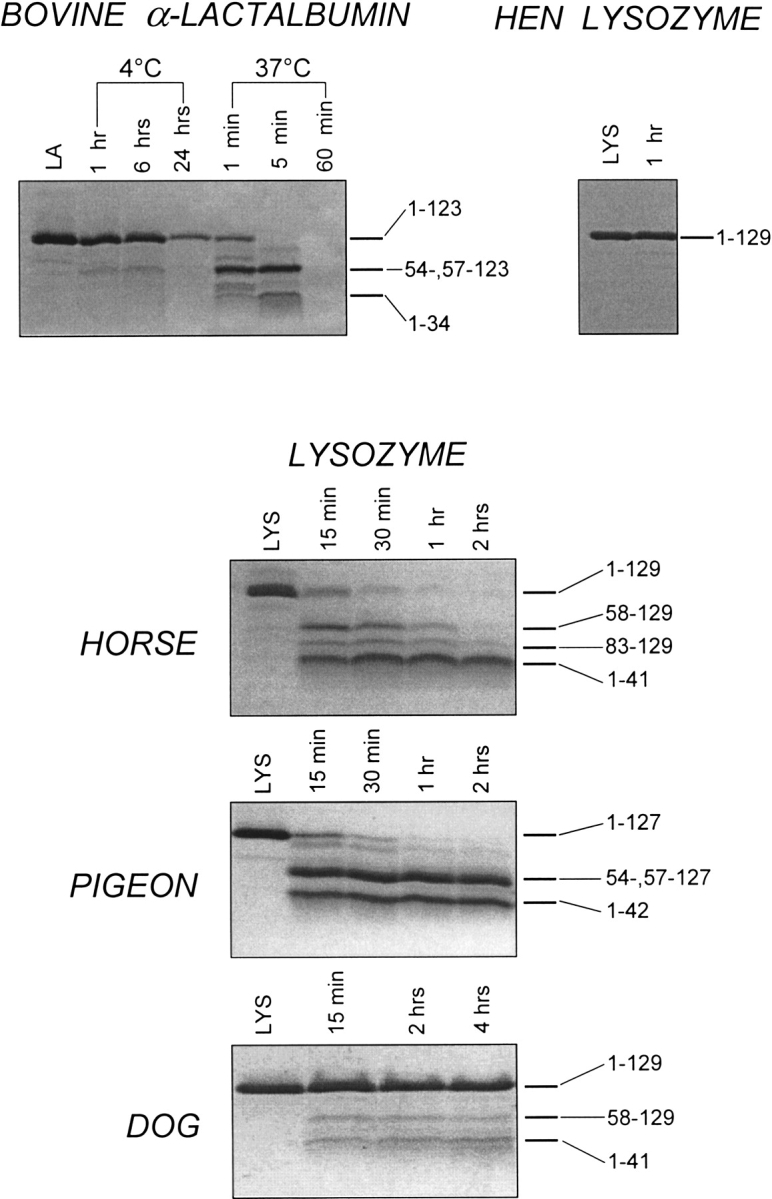
SDS-PAGE of the proteolytic digestion of hen, horse, dog, and pigeon lysozyme (LYS) and bovine α-lactalbumin (LA) by proteinase K (at pH 8.3) in the presence of EDTA. The proteolysis of bovine LA and hen LYS (top) and of horse, pigeon, and dog LYS (bottom) was conducted at 20°–22°C (enzyme-to-substrate ration [E/S], 1:500 by weight). Samples were taken from the reaction mixtures at the indicated times, mixed with aqueous TFA in order to stop the proteolysis, and then mixed with the SDS-PAGE sample buffer (Schägger and von Jagow 1987). LA and LYS refer to protein samples incubated under identical solvent conditions without added protease. Numbers in the right part of the Coomassie-stained gel refer to the identity of the protein fragments produced during proteolysis.
Conformational and dynamic features of the apo-form of the various calcium binding LYS were next investigated by the limited proteolysis approach. If the proteolysis of all LYS species is conducted in a buffer containing 1 mM CaCl2, no cleavage of the polypeptide chain is observed (data not shown). On the contrary, at 20°–22°C, the apo-proteins suffer proteolysis in a different way (Fig. 5 ▶, bottom). Mention should be given to the fact that CD measurements at 20°–22°C do not reveal major conformational transitions by depletion of calcium from the various LYS species (Fig. 2 ▶; see also Discussion).
Figure 5 ▶ shows the kinetics of proteolysis of horse milk LYS with proteinase K at 20°–22°C. This LYS species is easily digested by the protease, and after a 15-min reaction, electrophoretic bands corresponding to fragments of lower molecular mass are present in the stained gel. The protein fragments 1–41 and 58–129, as well as 83–129, were identified in the proteolytic mixture as usual (above). The major sites of cleavage occur at the level of Gln41-Ala42 and Phe57-Gln58 peptide bonds, thus producing mostly species 1–41/58–129. A minor peptide bond fission occurs at Lys82-Leu83. A comparative analysis of the time-course of proteolysis of bovine LA at 37°C and that of horse LYS at 20°–22°C reveals that the horse protein is somewhat more resistant to proteolysis than is bovine LA. This was more clearly evidenced by performing proteolysis experiments under identical conditions of solvent and temperature (data not shown).
The proteolysis of the apo-form of pigeon LYS with proteinase K is rather fast. After a 15-min reaction, a faint band of the intact protein and two bands of protein fragments are seen in the gel (Fig. 5 ▶, bottom). The two fragment bands correspond to the co-migrating fragments 54–127 and 57–127 and to fragment 1–42. In fact, by RP-HPLC it was possible to isolate from the proteolytic mixture a protein species (1–42/54–,57–127) formed by the N-terminal fragment 1–42 linked to the C-terminal fragment 54–127 or 57–127 via disulfide bridges (data not shown). The SDS-PAGE gels shown in Figure 5 ▶ reveal that the protein fragments constituting the gapped protein species (1–42/54–, 57–127) are rather resistant to further proteolysis. This contrasts with the proteolysis of bovine LA and horse LYS by proteinase K, because in these two cases, protein species with an excised chain segment located at the β-subdomain are only transiently formed during proteolysis and suffer additional cleavages. This would imply that in the absence of calcium, the gapped protein of pigeon LYS is more stable and rigid than those deriving from both bovine LA and horse LYS (see also Discussion).
Dog LYS instead is very resistant to the proteolytic attack by proteinase K, at variance from the rather easy digestion of horse and pigeon LYS. Only very faint bands of relatively large fragments are seen in the gel (Fig. 5 ▶, bottom) after prolonged incubation with proteinase K at 20°–22°C. A time-course analysis of the proteolytic mixture by both SDS-PAGE and RP-HPLC (data not shown) allowed us to conclude that very slow cleavages of dog LYS by proteinase K occur at peptide bonds Gln41-Ala42 and Phe57-Gln58.
Discussion
The challenge of this study was to show that the simple biochemical technique of limited proteolysis, despite the many amino acid replacements occurring in the polypeptide chains of the proteins members of the LYS/LA superfamily (Fig. 1 ▶), can provide data on protein structure and stability that can be correlated with those derived from the use of CD spectroscopy, namely, a classical and convenient technique extensively used to analyze structural features and to follow conformational transitions of proteins (Kelly and Price 1997). Moreover, our proteolysis data were to be compared with the results of other numerous and recent studies conducted on LYS/LA proteins mainly using NMR and H/D exchange measurements (Morozova et al. 1995; Morozova-Roche et al. 1997; Forge et al. 1999; Kobashigawa et al. 2000) and calorimetry (Griko et al. 1995; Koshiba et al. 2000, 2001). Here, we show that indeed proteolysis data can be interpreted on the basis of CD data and existing information regarding the structure, dynamics, and stability of partly folded states that members of the LYS/LA superfamily adopt in acid solution (A-state) or on depletion of the protein-bound calcium ion at neutral pH (apo-state).
Before discussing in detail the results of the proteolysis experiments conducted on the various LYS/LA proteins, the premises for the limited proteolysis approach as used here should be considered. First of all, pepsin and proteinase K were used as proteolytic probes, because these proteases are active in acid or neutral pH, respectively, and show a very broad substrate specificity. It was anticipated that these probes would allow detection of sites of flexibility or local unfolding along the polypeptide chain of the examined proteins, as anticipated from the fact that proteolysis requires binding and adaptation of a stretch of 8 to 10 residues of a polypeptide chain to the specific stereochemistry of the active site of the protease (Schechter and Berger 1967; Fontana et al. 1997a, 1997b, 1999). Second, the LYS/LA proteins in their native state at neutral pH and, in the presence of calcium, are not attacked by the proteolytic probe (see Results), whereas in their (fully) unfolded state, they lead to many small peptides (not stained in the SDS-PAGE gels). Therefore, the relatively large protein fragments produced by limited proteolysis are those derived from the intermediate or MG state(s) of the LYS/LA proteins. The identity of protein fragments permits the identification of the sites of flexibility along the protein chain. Third, the rate of proteolysis can be related to the relative populations of the intermediates under specific experimental conditions, as well as to their overall chain flexibility that makes them more prone to proteolysis (Fontana et al. 1986, 1999). The relative contribution of these parameters in the bimolecular process of a protease attacking a protein substrate can be evaluated by considering the conformational and dynamic features of the intermediate states of the various LYS/LA proteins, as given by the results of this and previous studies.
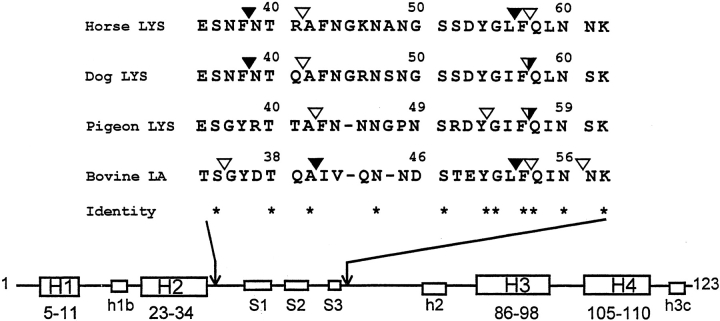
In Figure 6 ▶, the initial and main sites of limited proteolytic cleavage of LYS from different sources with pepsin and proteinase K are summarized. It is seen that cleavages are localized at the level of the small β-subdomain of the proteins (approximately from residues 34–57), as previously described for bovine LA in its A-state or apo-form (Polverino de Laureto et al. 1995, 1999, 2001). This implies that under acidic solvent conditions or on calcium removal from the holo-protein, the β-subdomain is more flexible in comparison with the rest of the polypeptide chain or even unfolded. It is the flickering region of the MG, experiencing an ensemble of many substates, which is easily attacked by a protease. Therefore, the use of proteolytic probes provides a direct evidence that the MG of LYS/LA proteins is characterized by a quite rigid core comprising the helical portions of the molecule (α-domain), whereas the remainder of the protein is rather flexible and in varying degrees of disorder (β-subdomain). This is in agreement with the current view of a bipartite structure of the MG state of LA, derived from protein dissection studies (Peng and Kim 1994; Peng et al. 1995; Wu et al. 1995), as well as from various physicochemical measurements (Schulman et al. 1997; Forge et al. 1999; Redfield et al. 1999). However, proteolytic probes provide evidence that significant differences do exist in the residual structure and dynamics of the β-subdomain in the MG states of the LYS/LA proteins (below).
Fig. 6.
(Top) Amino acid sequences of the polypeptide chain regions of members of the lysozyme (LYS)/α-lactalbumin (LA) superfamily encompassing the β-subdomain. The proteins were subjected to proteolysis in their partly folded states produced in acid or on depletion of the protein-bound calcium by EDTA at neutral pH (see text). Only the amino acid sequences of the chain region where initial proteolytic cleavages occur are shown. Closed and open arrowheads indicate sites of proteolysis by pepsin and proteinase K, respectively, and the bicolored arrowhead indicates cleavage by both pepsin and proteinase K. (Bottom) Secondary structure elements in the native structure of bovine LA (Pike et al. 1996). The four α-helices (H1 to H4) of the 123-residue chain are indicated by major boxes, and below them, the corresponding chain segments are given. The short 310-helices (h1b, 18–20; h2, 77–80; h3c, 115–118) are also shown by small boxes. The β-strands (S1, 41–44; S2, 47–50; S3, 55–56) of the β-subdomain are also shown by small boxes. (See Fig. 1 ▶ for a three-dimensional structure of bovine LA; note that in the computer-generated figure of α-LA, the short S3 β-sheet is not shown.) At variance from LA derived from other sources, the chain segment encompassing helix H4 (residues 105–110) in the crystal structure of bovine LA exhibits a variety of distinct conformers, including a distorted α-helical conformation (Pike et al. 1996).
Considering the variety of experimental conditions used to generate partly folded states or MGs of proteins belonging to the LYS/LA superfamily, a detailed comparison of results obtained in different studies can be problematic. This is particularly true for the MG attained by decalcified proteins (apo-state) at neutral pH, because the overall conformational and stability features of the apo-form can be influenced by ionic strength, temperature, and/or denaturing agent (Griko and Remeta 1999). Therefore, before attempting to correlate the results of different studies, it is relevant to exactly define the experimental conditions used to investigate the properties of a protein intermediate. Failure to define the relevant experimental conditions (e.g., ionic strength) in studies aimed to unravel molecular features of bovine LA in its MG state has already caused lively debates on definitions and properties of MG states (Ewbank et al. 1995; Okasaki et al. 1995). In the following, we will discuss separately the results of proteolysis experiments conducted on LYS/LA proteins in acid solution (A-state) or on removal of calcium at neutral pH (apo-form). Nevertheless, it is worth mentioning that the molecular features of MGs in acid at pH 2.0 or at neutral pH under (mildly) denaturing conditions (heat, ∼2 M Gdn•HCl) likely are much related, as given by the results of a variety of studies conducted over the years on LYS/LA proteins. For example, the reader is referred to the recent studies conducted on the MG state of canine LYS adopted by the protein in acid solution at pH 2.0, in the presence of 2 M Gdn•HCl (pH 4.5) or at 60°C (pH 4.5; Kobashigawa et al. 2000; Koshiba et al. 2000, 2001).
A-state of LYS/LA proteins
A comparative analysis of Coomassie-stained SDS-PAGE of Figure 4 ▶ clearly reveals that the rates of proteolysis of horse, pigeon, and dog LYSs by pepsin at pH 2.0 are intermediate between those of bovine LA and hen LYS. In fact, whereas LA is fully digested in few minutes and hen LYS is fully resistant, the other LYS species instead require up to hours for their digestion. From the data shown in the gels, it is possible to propose the following ranking for the rate of proteolysis: bovine LA > horse LA > pigeon LYS |L: dog LYS. The results of proteolysis experiments using pepsin are in agreement with CD data (Figs. 2 and 3 ▶ ▶), because the CD spectrum of hen LYS does not change in acid solution, whereas that of bovine LA displays a strong reduction of the dichroic signals in the near-UV region (250 to 310 nm), implying a major disruption of tertiary structure. Also the near-UV CD spectra of horse and pigeon LYS in acid are different from those observed at neutral pH, but with these two proteins, the dichroic activity in acid is much more significant than that of bovine LA, indicating that important elements of tertiary structure are maintained in horse and pigeon LYS. Also, the CD signal near 230 nm, deriving from Cotton effects of aromatic chromophores (Strickland 1974; Woody 1978, 1994) and observed in the native state of both LA and LYS species, is retained in the CD spectrum of dog LYS in acid solution, although is strongly diminished in the spectra of bovine LA, horse LYS, and pigeon LYS. Therefore, CD data indicate that dog LYS is a most stable protein to acid-mediated unfolding, approaching hen LYS in its stability. Indeed, the native-like and rigid structure of dog LYS at pH 2.0 renders this protein quite resistant to peptic digestion, and even after a 5-h reaction, most of the protein remains undigested (Fig. 4 ▶, bottom). However, because CD data do not provide evidence of a major conformational transition for dog LYS at pH 2.0, the question arises if in acid solution this protein actually adopts a MG state. In a recent study, Koshiba et al. (2001) examined the pH-dependent thermal unfolding behavior of dog LYS by differential scanning calorimetry. It was shown that there is a cooperative thermal unfolding transition from the native to an intermediate or MG state for dog LYS at acidic pH. The melting temperature Tm was measured in the pH range 1.95 to 4.50, and for example, the figures for Tm were 28.40° and 20.54°C at pH 3.0 and 2.74, respectively. At pH 1.95, a figure for Tm could not be measured, because the protein was mostly in the intermediate state at this very acidic pH (see also Koshiba et al. 2000). Therefore, calorimetric data indicate that the actual conformational state of dog LYS during proteolysis by pepsin at pH 2.0 and 20°–22°C is that of an intermediate.
Considering that limited proteolysis occurs at sites of enhanced mobility along the polypeptide chain or regions of local unfolding (Fontana et al. 1986, 1997a, 1997b, 1999), it seems relevant to try to correlate the results of this study with those obtained using the more conventional technique of amide hydrogen exchange (Woodward 1994; Roder 1989; Englander 2000). Indeed, this technique provides information regarding the global and local unfolding events of a globular protein and has been extensively used for the analysis of the molecular features of MGs of several members of the LYS/LA superfamily. In these studies, the H/D exchange properties of the native state have been compared with those of the A-state in acid solution. The results of these analyses were expressed in terms of protection factors (P), defined as the ratio of intrinsic and experimentally determined rates of H/D exchange of the amide proton. Therefore, P-values along the polypeptide chain provide a clear-cut indication of the dynamics of sites or regions of a protein (Englander et al. 1997).
We are fortunate that H/D exchange data have been reported for the MG state in acid (A-state) of bovine LA (Forge et al. 1999), horse LYS (Morozova et al. 1995; Morozova-Roche et al. 1997), and dog LYS (Kobashigawa et al. 2000), whereas similar data are not yet available for pigeon LYS. Briefly, with bovine LA at pH 2.0, the significantly protected amide hydrogen atoms are located in the α-domain of the protein, whereas those of the β-subdomain residues (among them T48, E49, and N56) are no longer protected against exchange. The H/D exchange data obtained with bovine LA at pH 2.0 parallel those obtained with human LA (Schulman et al. 1997; Redfield et al. 1999) and indicate that the β-subdomain of native LA is highly flexible or largely disrupted in the A-state, in agreement with the view that the MG state of LA has a bipartite structure given by a structured α-domain and a disordered β-subdomain (Peng and Kim 1994; Peng et al. 1995; Wu et al. 1995). Indeed, limited proteolysis of bovine LA in acid solution by pepsin occurs at the level of the flexible or unfolded β-subdomain (Fig. 6 ▶; Polverino de Laureto et al. 1995, 1997, 2001).
The challenge of this study is to possibly correlate also the different rates of peptic digestion among the protein members of the LYS/LA superfamily (Fig. 4 ▶). In a recent study, the protein structure and dynamics of dog LYS were examined by H/D exchange in conjunction with two-dimensional NMR measurements (Kobashigawa et al. 2000). The main conclusion of this study was that the α-helices of dog LYS are more stabilized than in bovine LA or horse LYS. The H/D exchange data of dog LYS in the MG state at pH 2.0 and 25°C revealed that there are 64 amide hydrogens that are highly protected, whereas there are only 17 in horse LYS at pH 2.0 and 5°C (Morozova-Roche et al. 1997). Moreover, the protection factors at pH 2.0 are two orders of magnitude larger for dog LYS in respect to those of horse LYS, implying that the dog protein is more rigid than the horse one. In the MG of dog LYS at pH 2.0, at variance from bovine LA and horse LYS, also several amides in the β-sheet region of the protein were protected from exchange. Indeed, the protected amides in dog LYS were those of residues F43, D53, Y54, L59, and D60, these being all located in the three β-strands of the protein. The conclusion reached by Kobashigawa et al. (2000) was that the β-subdomain is not unstructured in acid solution at pH 2.0, but likely remains similar to that observed in the native state at neutral pH. Therefore, that dog LYS is almost fully resistant to proteolysis by pepsin in acid solution, whereas bovine LA and LYS from horse milk are easily digested (Fig. 4 ▶), is in full agreement with H/D exchange data.
The molecular features of pigeon LYS are not yet investigated in such a detail as those of bovine LA, horse LYS, and dog LYS. Perhaps, the relatively lower interest on this protein species derives from the fact that pigeon LYS does not easily form a partly folded state. The near-UV CD spectrum of pigeon LYS at neutral pH is much similar to that of bovine LA in terms of both shape and intensity of dichroic signals (Figs. 2 and 3 ▶ ▶). However, whereas in acid solution the dichroic activity in the 250- to 310-nm region of bovine LA is strongly reduced, that of pigeon LYS changes slightly in terms of intensity, but it retains most of its overall features. Because near-UV CD spectra of proteins can be consider a fingerprint of the specific and rigid interactions of aromatic chromophores within the native globular fold (Strickland 1974), CD data therefore indicate that, at variance form bovine LA, substantial native-like interactions are retained in pigeon LYS at pH 2.0. However, evidence for a distinct intermediate with MG characteristics has been obtained when pigeon LYS was exposed to stronger acid conditions as pH 1.0, but the maximum population of the intermediate at this pH was only 31% at 36°C (Haezebrouck et al. 1998). Therefore, the highly ordered and native-like state of pigeon LYS at pH 2.0 renders this protein much more resistant to peptic digestion than it is bovine LA (see SDS-PAGE gels in Fig. 4 ▶).
Calcium-free forms of LYS/LA proteins
The apo-state of the LYS/LA proteins does not necessarily correspond to a MG, because only at (very) low ionic strength and/or in the presence of moderate concentrations of a denaturing agent (e.g., Gdn•HCl) or on heating, some of the LYS/LA proteins adopt a partly folded or MG state. In agreement with previous studies (Griko and Remeta 1999), the apo-form of bovine LA at 4°C is fully native, as given by far- and near-UV CD data (Fig. 2 ▶). Different figures for Tm from the native to the MG state of bovine LA in the presence of salt have been reported (from 15° to 42°C; Xie et al. 1991; Griko et al. 1995; Veprintsev et al. 1997; Griko and Remeta 1999). We have monitored the thermal unfolding process of apo-LA in 10 mM Tris•HCl/0.1 M KCl (pH 7.5), by recording the CD signals at 270 nm on heating the protein sample. From the cooperative transition curve for the native ↔ MG transition a Tm of 34°C was evaluated (data not shown). More recently, both X-ray (Chrysina et al. 2000) and NMR analyses (Wijesinha-Bettoni et al. 2001) clearly showed that the conformation of apo-LA in the presence of salt (e.g., 0.5 M NaCl) at neutral pH is much similar to that of the calcium-loaded protein. Therefore, it was of interest to study the effect of temperature on the proteolysis of apo-LA, hoping that the proteolytic probe could monitor the native ↔ MG transition of the protein.
Proteolysis of the apo-form of bovine LA by proteinase K has been conducted at 4° and 37°C, and the pattern and kinetics of proteolysis was determined by both SDS-PAGE (Fig. 5 ▶) and RP-HPLC (data not shown). Proteolysis at 4°C was very slow, and even after 24 h, some intact LA is still present in the proteolysis mixtures, and only small peptides are the products of proteolysis (not stained in the SDS-PAGE gel and earlier eluted from the RP-HPLC column; data not shown). At 37°C, bovine LA is instead cleaved at few sites along its 123-residue chain. These results are interpreted in terms of a native-like structure of apo-LA at 4°C, almost as fully resistant to the proteolysis as the holo-protein. We propose that at 4°C, the native ↔ unfolded equilibrium of the protein substrate dictates the rate of proteolysis, and thus, the proteolytic probe can discriminate between the native species resistant to proteolysis and the unfolded species, which is easily digested at many sites along the polypeptide chain. On the other hand, at 37°C the population of the partly folded state or MG is much enhanced and becomes the actual substrate of limited proteolysis. Hen LYS does not form a MG at neutral pH and is fully resistant to proteolysis (Fig. 5 ▶, top).
The SDS-PAGE gels displayed in Figure 5 ▶ indicate that horse and pigeon LYSs are readily cleaved by proteinase K at neutral pH at 20°–22°C, whereas dog LYS is instead almost fully resistant to proteolysis (Fig. 5 ▶, bottom). It should be stressed that natively folded, calcium-loaded forms of these LYS species are not attacked by the proteolytic probe. Therefore, the relatively large protein fragments produced by proteolysis derive from the intermediate or MG state(s) of the apo-form of the protein substrate(s). As for bovine LA, it is the equilibrium between the native and MG state that actually dictates and controls the rate of proteolysis (see also above). To compare proteolysis data of horse and dog LYSs with those of bovine LA, we have to consider that also these two LYS species in their apo-form undergo a native ↔ MG transition on heating. Indeed, in analogy to bovine LA, horse and dog LYSs at pH 4.5, in the absence of calcium, display a thermal unfolding transition from the native to the MG state characterized by a Tm of 39° and 41.5°C, respectively. However, the MG of horse LYS is known to be much more native-like than the MG of LA and stabilized by some specific side-chain interactions (Van Dael et al. 1993; Morozova-Roche et al. 1997). Apparently, the MG state of horse LYS is a specific thermodynamic state that is distinct from both the native and unfolded states, because the thermal transition from the MG to the thermally unfolded state is characterized by a Tm of 66.4°C at pH 4.5 and accompanied by a large enthalpy increase (Van Dael et al. 1993; Griko et al. 1995; Koshiba et al. 2000). In a recent study, Koshiba et al. (2000) compared the stabilities of horse and dog LYS at pH 4.5 in the absence of calcium and found that the thermal transition of the MG of the dog protein to the unfolded state is characterized by a Tm of 86.9°C, that is, ∼20°C higher than that of horse LYS. The conclusion of this study was that the MG of dog LYS is extraordinarily more stable than that of all other members of the LYS/LA superfamily and that it is stabilized by a number of native-like interactions. On the other hand, the MG of bovine LA shows a diffuse thermal transition without a heat absorption peak in the scanning calorimetry measurements (Yutani et al. 1992), indicating that the MG state of LA is thermodynamically indistinguishable form the unfolded state. Therefore, the limited proteolysis data do agree with the fact that the MG state of horse LYS is more native-like than that of bovine LA, because horse LYS is more resistant to proteolysis than bovine LA (see Results). Moreover, it is the stable and rigid structure of the MG of dog LYS that prevents the proteolytic attack of this protein by proteinase K at neutral pH (Fig. 5 ▶, bottom).
Available evidence indicates that the apo-form of pigeon LYS does not show a stable intermediate at neutral pH and unfolds by a two-state mechanism in both heat- or Gdn•HCl-mediated unfolding (Nitta et al. 1993; Haezebrouck et al. 1998; Kikuchi et al. 1998). Both apo-form and calcium-loaded pigeon LYS unfold at pH 4.5 in a cooperative process with a Tm of 63° and 76°C, respectively (Haezebrouck et al. 1998). However, kinetic experiments of refolding from a Gdn•HCl-mediated unfolded protein species provided evidence that a transient intermediate with significant amounts of secondary structure is formed at pH 6.0 (Haezebrouck et al. 1998). Proteolysis of the apo-form of pigeon LYS by proteinase K leads to the excision from the protein of the chain segment 43–54 or 43–57, leading to the gapped protein species 1–42/54–,57–127, which is rather resistant to further proteolysis (see the SDS-PAGE gel of Fig. 5 ▶, bottom). We interpret these proteolysis data as indicating that the removal of calcium from the holo-form of pigeon LYS at 20°–22°C leads to a localized change in structure and dynamics at the level of chain segment 43–54/57. We may propose that the apo-form of pigeon LYS adopts a native-like MG or highly ordered MG (Redfield et al. 1994; Morozova et al. 1995; Laidig and Daggett 1996). Of note, this local conformational change on going from the holo- to the apo-form is barely detected by near-UV CD data, because only the minimum of negative ellipticity near 230 nm is slightly reduced in the apo-form in respect to that observed with the holo-protein (Fig. 3 ▶).
Concluding remarks
Partly folded states (or MGs) of proteins are extensively investigated in many laboratories using a variety of experimental and theoretical approaches (Arai and Kuwajima 2000). The aims of these studies are to contribute to an understanding of the protein folding problem, as well as to the elucidation of the mechanism(s) of protein aggregation (inclusion bodies, amyloidosis), because it has been demonstrated that aggregation occurs via partly folded states (Fink 1998). One of the most thoroughly studied MGs is that attained by LA at pH 2.0 (Kuwajima 1989, 1996). As a matter of fact, the molecular features of LA in acid (A-state) served to define the key properties of the MG as a folding intermediate possessing a stable secondary structure, but lacking the tertiary contacts of the native state. As a result of many studies conducted on a variety of model proteins using several experimental techniques and approaches, the notion of the MG itself has evolved substantially. The initial definition of MG (Ptitsyn 1987, 1995) as a highly dynamic, slightly expanded, and global state of the entire polypeptide chain of a protein retaining the secondary structure, but lacking the fixed specific tertiary contacts of the native protein, does not pertain to all MG species so far investigated. Nowadays, there is a common belief that the MG is not an universal stable intermediate in protein folding, because a great variety of partly folded or MG states of proteins exists, ranging from those much similar to the native protein characterized by a native-like fold and specific side-chain structure to those highly dynamic and unfolded and thus approaching the fully denatured state (Privalov 1996; Fink et al. 1998; Uversky 1998; Uversky et al. 1998; Arai and Kuwajima 2000).
The MGs of LYS/LA proteins share the characteristic of having a flexible or disrupted β-subdomain, whereas the α-domain is much more stable and rigid and retains a native-like overall fold (Peng and Kim 1994; Wu et al. 1995; Schulman et al. 1997; Forge et al. 1999; Redfield et al. 1999). The mobile region of the MG is much more prone to the proteolytic attack, and therefore, proteolytic probes can detect conformational and dynamic features of the MG states of the various LYS/LA proteins. However, significant differences do exist in terms of dynamics or degree of local unfolding of the β-subdomain within the MG states of the LYS/LA proteins, because rates of proteolysis are much different. Overall, the relative mobility of the β-subdomain in intermediates of LYS/LA proteins probed here by the limited proteolysis approach correlates well with the molecular and dynamic features of the MG states of protein members of the LYS/LA superfamily previously deduced from NMR and, in particular, H/D exchange measurements (Morozova-Roche et al. 1997; Forge et al. 1999; Kobashigawa et al. 2000). Therefore, present data emphasize the utility of limited proteolysis experiments for analyzing protein structure and dynamics, even in the special and difficult case of the ensemble of MG states (Fontana et al. 1997a). Specific advantages of limited proteolysis experiments are that they can be used to specifically probe the flexible regions of a protein molecule (Fontana et al. 1986) and, if used in combination with mass spectrometric techniques for analyzing protein digests, require very minute amounts of protein sample (Fontana et al. 1997a,b, 1999; Spolaore et al. 2001).
Materials and methods
Materials
Bovine LA, hen egg-white LYS, porcine pepsin, and proteinase K from Tritirachium album were obtained from Sigma and used without further purification. Samples of LYS from equine (Noppe et al. 1996) and canine (Mizuguchi et al. 1998) milk and from egg-white of pigeon (Haezebrouck et al. 1998) were prepared to homogeneity following methods previously described. All other chemicals were of analytical reagent grade and were obtained from Sigma or Fluka.
Circular dichroism measurements
CD spectra were recorded in nitrogen atmosphere on a Jasco J-710 spectropolarimeter equipped with a thermostated cell holder. Far- and near-UV CD spectra were recorded using a 1-mm and 5-mm pathlength quartz cell in the far- and near-UV regions, respectively. The mean residue ellipticity [θ] (deg × cm2 × dmole−1) was calculated from the formula [θ] = (θobs/10) × (MRW/lc), where θobs is the observed ellipticity in deg; MRW, the mean residue molecular weight (molecular weight of the protein divided by the number of the amino acids); l, the optical pathlength in centimeters; and c, the protein concentration in grams per milliliter. CD spectra were recorded at 20°–22°C with a protein concentration of 0.4 to 0.7 mg/mL and 0.05 to 0.08 mg/mL in the near- and far-UV region, respectively, in 0.1 M Tris•HCl/0.1 M KCl (pH 7.5), containing 5 mM CaCl2 or 1 mM EDTA, and in 0.01 M HCl/0.1 M KCl (pH 2.0). All CD measurements were corrected by subtracting the buffer spectra. Protein concentrations were determined by absorption measurements at 280 nm on a double-beam Lambda 2 spectrophotometer (Perkin Elmer). Extinction coefficients at 280 nm were 2.35 mg−1cm−1 for horse LYS (Desmet et al. 1989), 2.32 mg−1cm−1 for dog LYS (Kikuchi et al. 1998), 2.68 mg−1cm−1 for hen egg-white LYS (Canfield 1963), and 2.01 mg−1cm−1 for bovine LA (Kuwajima and Sugai 1978). In the case of pigeon LYS, the extinction coefficient of 2.05 mg−1cm−1 was calculated from the amino acid composition of the protein according to Gill and von Hippel (1989).
Proteolytic digestion of LA and LYS
Limited proteolysis of protein samples was performed at pH 2.0 with pepsin or at pH 8.3 with proteinase K in the presence of EDTA essentially following procedures previously described (Polverino de Laureto et al. 1995, 1999, 2001). The reactions were conducted at 20°–22°C at a protein concentration of ∼1 mg/mL using an E/S ratio of 1:500. Proteolysis was stopped by alkalinization of the protein mixture with aqueous ammonia (pepsin) or by acidification with 4% TFA in water (proteinase K). The time-course digestions were followed by SDS-PAGE under reducing conditions using the Tricine buffer systems (Schägger and von Jagow 1987). The proteolytic mixtures were also analyzed by RP-HPLC on a Vydac C18 column (150 × 4.6 mm, 5μm; The Separations Group) eluted at a flow rate of 0.6 mL/min with a linear gradient of acetonitrile containing 0.1% TFA from 5% to 22% in 5 min and from 22% to 50% in 17 min. The effluent of the column was monitored by absorbance measurements at 226 nm. The identity of proteolytic fragments was established on the basis of their apparent molecular mass given by SDS-PAGE, N-terminal sequencing and MALDI mass spectrometry and by comparing these data with the known amino acid sequences of the various proteins. For other experimental details, see also previous work from our laboratory (Polverino de Laureto et al. 1995, 1999, 2001).
Amino acid sequencing and mass determination
Automated N-terminal sequencing was performed with an Applied Biosystems protein sequencer (model 477A) equipped with an on-line analyzer (model 120A) of phenylthiohydantoin-derivatives of amino acids. Standard manufacturer's procedures and programs were used with minor modifications. Mass determinations of proteolytic fragments were obtained using a time-of-flight (TOF) MALDI mass spectrometer (model Kompact Maldi-1; Kratos-Shimadzu). α-Cyano-4-hydroxycinnamic acid dissolved in acetonitrile and 0.1% aqueous TFA (2:3 ratio, by volume) was used as matrix, whereas bovine insulin was used as a standard for instrument calibration. Raw data were analyzed by the software Kompact provided by Kratos.
Acknowledgments
This work was supported by the Italian National Council of Research (CNR) in the framework of Biotechnology Project and by the Ministry of University and Technological Research (MURST; PRIN-2000).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
LA, bovine α-lactalbumin
LYS, lysozyme
A-state, low-pH form of LA
apo-LA and apo-LYS, calcium-depleted forms of LA and LYS
CD, circular dichroism
E/S, enzyme-to-substrate ratio
H/D, hydrogen/deuterium
Gdn•HCl, guanidine hydrochloride
MG, molten globule
TFA, trifluoroacetic acid
Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl) ethyl]glycine
MALDI, matrix-assisted laser-desorption ionization
PAGE, polyacrylamide gel electrophoresis
SDS, sodium dodecyl sulfate
RP, reverse-phase
HPLC, high-pressure liquid chromatography
TOF, time-of-flight
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0205802.
Enzymes: Pepsin (EC 3.4.23.1); proteinase K (EC 3.4.21.64).
References
- Arai, M. and Kuwajima, K. 2000. Role of the molten globule state in protein folding. Adv. Protein Chem. 53 209–282. [DOI] [PubMed] [Google Scholar]
- Blanch, E.W., Morozova-Roche, L.A., Hecht, L., Noppe, W., and Barron, L.D. 2000. Raman optical activity characterization of native and molten globule state of equine lysozyme: Comparison with hen lysozyme and bovine α-lactalbumin. Biopolymers 57 235–248. [DOI] [PubMed] [Google Scholar]
- Canfield, R.E. 1963. The amino acid sequence of egg-white lysozyme. J. Biol. Chem. 238 2698–2707. [PubMed] [Google Scholar]
- Chamberlain, A.K. and Marqusee, S. 2000. Comparison of equilibrium and kinetic approaches for determining protein folding mechanisms. Adv. Protein Chem. 53 283–328. [DOI] [PubMed] [Google Scholar]
- Chaudhuri, T.K., Horij, K., Yoda, T., Arai, M., Nagata, S., Terada, T.P., Uchiyama, H., Ikura, T., Tsumoto, K., Kataoka, K., Mutsushima, M., Kuwajima, K., and Kumagai, I. 1999. Effect of the extra N-terminal methionine residue on the stability and folding of recombinant α-lactalbumin expressed in Escherichia coli. J. Mol. Biol. 285 1179–1194. [DOI] [PubMed] [Google Scholar]
- Chaudhuri, T.K., Arai, M., Terada, T., Ikura, T., and Kuwajima, K. 2000. Equilibrium and kinetic studies on folding of the authentic and recombinant forms of human α-lactalbumin by circular dichroism spectroscopy. Biochemistry 39 15643–15651. [DOI] [PubMed] [Google Scholar]
- Chen, Y.H., Yang, J.T., and Chau, K.H. 1974. Determination of the helix and β form of proteins in aqueous solution by circular dichroism. Biochemistry 13 3350–3359. [DOI] [PubMed] [Google Scholar]
- Chrysina, E.D., Brew, K., and Acharya, K.R. 2000. Crystal structures of apo- and holo-bovine α-lactalbumin at 2.2 Å resolution reveal an effect of calcium on inter-lobe interaction. J. Biol. Chem. 275 37021–37029. [DOI] [PubMed] [Google Scholar]
- Desmet, J., Van Dael, H., Van Cauwelaert, F., Nitta, K., and Sugai, S. 1989. Comparison of the binding of Ca2+ and Mn2+ to bovine α-lactalbumin and equine lysozyme. J. Inorg. Biochem. 37 185–191. [DOI] [PubMed] [Google Scholar]
- Dill, K.A. and Chan, H.S. 1997. From Levinthal to pathways to funnels. Nat. Struct. Biol. 4 10–19. [DOI] [PubMed] [Google Scholar]
- Dobson, C.M. 1994. Solid evidence for molten globules. Curr. Biol. 4 636–640. [DOI] [PubMed] [Google Scholar]
- Dolgikh, D.A., Gilmanshin, R.I., Brazhnikov, E.V., Bychkova, V.E., Semisotnov, G.V., Venyaminov, S.Y., and Ptitsyn, O.B. 1981. α-Lactalbumin: Compact state with fluctuating tertiary structure? FEBS Lett. 136 311–315. [DOI] [PubMed] [Google Scholar]
- Eliezer, D., Yao, J., Dyson, J., and Wright, P.E. 1998. Structural and dynamic characterization of partially folded states of apomyoglobin and implications for protein folding. Nat. Struct. Biol. 5 148–155. [DOI] [PubMed] [Google Scholar]
- Engelhard, M. and Evans, P.A. 1995. Kinetics of interaction of partially folded proteins with a hydrophobic dye: Evidence that molten globule character is maximal in early folding intermediates. Protein Sci. 4 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander, S.W. 2000. Protein folding intermediates and pathways studied by hydrogen exchange. Annu. Rev. Biophys. Biomol. Struct. 29 213–238. [DOI] [PubMed] [Google Scholar]
- Englander, S.W., Mayne, L., Bai, Y., and Sosnick, T.R. 1997. Hydrogen exchange: The modern legacy of Lindenstrom-Lang. Protein Sci. 6 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, P.A. and Radford, S.E. 1994. Probing the structure of folding intermediates. Curr. Opin. Struct. Biol. 4 100–106. [Google Scholar]
- Ewbank, J.J., Creighton, T.E., Hayer-Hartl, M.K., and Hartl, F.U. 1995. What is the molten globule? Nat. Struct. Biol. 2 10–11. [DOI] [PubMed] [Google Scholar]
- Fink, A.L. 1995. Compact intermediate states in protein folding. Annu. Rev. Biomol. Struct. 24 495–522. [DOI] [PubMed] [Google Scholar]
- ———. 1998. Protein aggregation: Folding aggregates, inclusion bodies and amyloid. Folding Des. 3 R9–R23. [DOI] [PubMed] [Google Scholar]
- Fink, A.L., Oberg, K.A., and Seshadri, S. 1998. Discrete intermediate versus molten globule models for protein folding: Characterization of partially folded intermediates of apomyoglobin. Folding Des. 3 19–25. [DOI] [PubMed] [Google Scholar]
- Fontana, A., Fassina, G., Vita, C., Dalzoppo, D., Zamai, M., and Zambonin, M. 1986. Correlation between sites of limited proteolysis and segmental mobility in thermolysin. Biochemistry 25 1847–1851. [DOI] [PubMed] [Google Scholar]
- Fontana, A., Polverino de Laureto, P., and De Filippis V. 1993. Molecular aspects of proteolysis of globular proteins. In: Protein stability and stabilization (eds. W. der Tweel, A. Harder, and M. Buitelaar), pp. 101–110. Elsevier Sci. Publ., Amsterdam.
- Fontana, A., Polverino de Laureto, P., De Filippis, V., Scaramella, E., and Zambonin, M. 1997a. Probing the partly folded states of proteins by limited proteolysis. Folding Des. 2 R17–R26. [DOI] [PubMed] [Google Scholar]
- ———. 1999. Limited proteolysis in the study of protein conformation. In Proteolytic enzymes: Tools and targets (eds. E.E. Sterchi and W. Stöcker), pp. 257–284, Springer Verlag, Heidelberg, Germany.
- Fontana, A., Zambonin, M., De Filippis, V., Bosco, M., and Polverino de Laureto, P. 1995. Limited proteolysis of cytochrome c in trifluoroethanol. FEBS Lett. 362 266–270. [DOI] [PubMed] [Google Scholar]
- Fontana, A., Zambonin, M., Polverino de Laureto, P., De Filippis, V., Clementi, A., and Scaramella, E. 1997b. Probing the conformational state of apomyoglobin by limited proteolysis. J. Mol. Biol. 266 223–230. [DOI] [PubMed] [Google Scholar]
- Forge, V., Wijesinha, R.T., Balbach, J., Brew, K., Robinson, C.V., Redfield, C., and Dobson, C.M. 1999. Rapid collapse and slow structural reorganization during the refolding of bovine α-lactalbumin. J. Mol. Biol. 288 673–688. [DOI] [PubMed] [Google Scholar]
- Gill, S.G. and von Hippel, P.H. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182 319–326. [DOI] [PubMed] [Google Scholar]
- Griko, Y.V. and Remeta, D.P. 1999. Energetics of solvent and ligand-induced conformational changes in α-lactalbumin. Protein Sci. 8 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griko, Y.V., Freire, E., Privalov, G., Van Dael, H., and Privalov, P.L. 1995. The unfolding thermodynamics of c-type lysozymes: A calorimetric study of the heat denaturation of equine lysozyme. J. Mol. Biol. 252 447–459. [DOI] [PubMed] [Google Scholar]
- Grobler, J.A., Ramakrishna, R.K., Pervaiz, S., and Brew, K. 1994. Sequences of two highly divergent canine type c lysozymes: Implications for the evolutionary origins of the lysozyme/α-lactalbumin superfamily. Arch. Biochem. Biophys. 313 360–366. [DOI] [PubMed] [Google Scholar]
- Haezebrouck, P., Noyelle, K., and Van Dael, H. 1998. Equilibrium and kinetic folding of pigeon lysozyme. Biochemistry 37 6772–6780. [DOI] [PubMed] [Google Scholar]
- Hubbard, S.J. 1998. The structural aspects of limited proteolysis of native proteins. Biochim. Biophys. Acta 1382 191–206. [DOI] [PubMed] [Google Scholar]
- Hurley, W.L. and Schuler, L.A. 1987. Molecular cloning and nucleotide sequence of a bovine α-lactalbumin cDNA. Gene 61 119–122. [DOI] [PubMed] [Google Scholar]
- Ikeguchi, M., Kuwajima, K., Mitani, M., and Sugai, S. 1986a. Evidence for identity between the equilibrium unfolding intermediate and a transient folding intermediate: A comparative study of the folding reactions of α-lactalbumin and lysozyme. Biochemistry 25 6965–6972. [DOI] [PubMed] [Google Scholar]
- Ikeguchi, M., Kuwajima, K., and Sugai, S. 1986b. Ca2+-Induced alteration in the unfolding behaviour of α-lactalbumin. J. Biochem. 99 1191–1201. [DOI] [PubMed] [Google Scholar]
- Jennings, P.A. and Wright, P.E. 1993. Formation of a molten globule intermediate early in the kinetic folding pathway of apomyoglobin. Science 262 892–896. [DOI] [PubMed] [Google Scholar]
- Kataoka, M., Kuwajima, K., Tokunaga, F., and Goto, Y. 1997. Structural characterization of α-lactalbumin by solution X-ray scattering. Protein Sci. 6 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, S.M. and Price, N.C. 1997. The application of circular dichroism to study of protein folding and unfolding. Biochim. Biophys. Acta 1338 161–185. [DOI] [PubMed] [Google Scholar]
- Kikuchi, M., Kawano, K., and Nitta, K. 1998. Calcium-binding and structural stability of echidna and canine milk lysozymes. Protein Sci. 7 2150–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, P.S. and Baldwin, R.L. 1990. Intermediates in the folding reactions of small proteins. Annu. Rev. Biochem. 59 631–660. [DOI] [PubMed] [Google Scholar]
- Kobashigawa, Y., Demura, M., Koshiba, T., Kumaki, Y., Kuwajima, K., and Nitta, K. 2000. Hydrogen exchange study of canine milk lysozyme: Stabilization mechanism of the molten globule. Proteins 40 579–589. [DOI] [PubMed] [Google Scholar]
- Koshiba, T., Yao, M., Kobashigawa, Y., Demura, M., Nakagawa, A., Tanaka, I., Kuwajima, K, and Nitta, K. 2000. Structure and thermodynamics of the extraordinary stable molten globule state of canine milk lysozyme. Biochemistry 39 3248–3257. [DOI] [PubMed] [Google Scholar]
- Koshiba, T., Kobashigawa, Y., Demura, M., and Nitta, K. 2001. Energetics of the three-state unfolding of a protein: Canine milk lysozyme. Protein Eng. 14 967–974. [DOI] [PubMed] [Google Scholar]
- Kronman, M.J. 1989. Metal-ion binding and the molecular conformational properties of α-lactalbumin. Crit. Rev. Biochem. Mol. Biol. 24 565–667. [DOI] [PubMed] [Google Scholar]
- Kuwajima, K. 1989. The molten globule state as a clue for understanding the folding and cooperativity of globular protein structure. Proteins 6 87–103. [DOI] [PubMed] [Google Scholar]
- ———. 1996. The molten globule state of α-lactalbumin. FASEB J. 10 102–109. [DOI] [PubMed] [Google Scholar]
- Kuwajima, K., and Arai, M., eds. 1999. Old and new views of protein folding. Elsevier, Amsterdam, The Netherlands.
- Kuwajima, K. and Sugai, S. 1978. Equilibrium and kinetics of the thermal unfolding of α-lactalbumin: The relation to its folding mechanism. Biophys. Chem. 8 247–254. [DOI] [PubMed] [Google Scholar]
- Kuwajima, K., Nitta, K., Yoneyama, M., and Sugai, S. 1976. Three-state denaturation of α-lactalbumin by guanidine hydrochloride. J. Mol. Biol. 106 359–373. [DOI] [PubMed] [Google Scholar]
- Laidig, K.E. and Daggett, V. 1996. Molecular dynamics simulation of apocytochrome b562: The highly ordered limit of molten globules. Folding Des. 1 335–346. [DOI] [PubMed] [Google Scholar]
- Matthews, C.R. 1995. Pathways of protein folding. Annu. Rev. Biochem. 62 653–683. [DOI] [PubMed] [Google Scholar]
- McKenzie, H.A. and Shaw, D.C. 1985. The amino acid sequence of equine milk lysozyme. Biochem. Int. 10 23–31. [PubMed] [Google Scholar]
- Menéndez-Arias, L., Gavilanes, J.G., and Rodriguez, R. 1985. Amino acid sequence around the cisteine residues of pigeon egg-white lysozyme: Comparative study with other type c lysozymes. Comp. Biochem. Physiol. 4 639–642. [DOI] [PubMed] [Google Scholar]
- Mizuguchi, M., Arai, M., Ke, Y., Nitta, K., and Kuwajima, K. 1998. Equilibrium and kinetics of the folding of equine lysozyme studied by circular dichroism spectroscopy. J. Mol. Biol. 283 265–277. [DOI] [PubMed] [Google Scholar]
- Mizuguchi, M., Masaki, K., and Nitta, K. 1999. The molten globule state of a chimera of human α-lactalbumin and equine lysozyme. J. Mol. Biol. 8 1137–1148. [DOI] [PubMed] [Google Scholar]
- Morozova, L.A., Haynie, D.T., Arico-Muendel, C.C., Van Dael, H., and Dobson, C.M. 1995. Structural bases of the stability of a lysozyme molten globule. Nat. Struct. Biol. 2 871–875. [DOI] [PubMed] [Google Scholar]
- Morozova-Roche, L.A., Arico-Muendel, C.C., Haynie, D.T., Emelyanenko, V.I., Van Dael, H., and Dobson, C.M. 1997. Structural characterization and comparison of the native and A-states of equine lysozyme. J. Mol. Biol. 268 903–921. [DOI] [PubMed] [Google Scholar]
- Nitta, K. and Sugai, S. 1989. The evolution of lysozyme and α-lactalbumin. Eur. J. Biochem. 182 111–118. [DOI] [PubMed] [Google Scholar]
- Nitta, K., Tsuge, H., and Iwamoto, H. 1993. Comparative study of the stability of the folding intermediates of the calcium-binding lysozymes. Int. J. Peptide Protein Res. 41 118–123. [DOI] [PubMed] [Google Scholar]
- Noppe, W., Hanssens, I., and De Cuyper, M. 1996. Simple two-step procedure for the preparation of highly active pure equine milk lysozyme. J. Chromat. Sect. A 719 327–331. [DOI] [PubMed] [Google Scholar]
- Ohgushi, M. and Wada, A. 1983. Molten globule state: A compact form of globular proteins with mobile side-chains. FEBS Lett. 164 21–24. [DOI] [PubMed] [Google Scholar]
- Okasaki, A., Ikura, T., and Kuwajima, K. 1995. Reply to Ewbank et al. 1995. Nat. Struct. Biol. 2 10–11.7719845 [Google Scholar]
- Peng, Z.-y. and Kim, P.S. 1994. A protein dissection study of a molten globule. Biochemistry 33 2136–2141. [DOI] [PubMed] [Google Scholar]
- Peng, Z.-y., Wu, L.C., and Kim, P.S. 1995. Local structural preferences in the α-lactalbumin molten globule. Biochemistry 34 3248–3252. [DOI] [PubMed] [Google Scholar]
- Permyakov, E.A. and Berliner, L.J. 2000. α-Lactalbumin: Structure and function. FEBS Lett. 473 269–274. [DOI] [PubMed] [Google Scholar]
- Pike, A.C.W., Brew, K., and Acharya, K.R. 1996. Crystal structures of guinea-pig, goat and bovine α-lactalbumin highlight the enhanced conformational flexibility of regions that are significant for its action in lactose synthase. Structure 4 691–703. [DOI] [PubMed] [Google Scholar]
- Plaxco, K.W. and Dobson, C.M. 1996. Time-resolved biophysical methods in the study of protein folding. Curr. Opin. Struct. Biol. 6 630–636. [DOI] [PubMed] [Google Scholar]
- Polverino de Laureto, P., De Filippis, V., Di Bello, M., Zambonin, M., and Fontana, A. 1995. Probing the molten globule state of α-lactalbumin by limited proteolysis. Biochemistry 34 12596–12604. [DOI] [PubMed] [Google Scholar]
- Polverino de Laureto, P., Scaramella, E., Frigo, M., Gefter Wondrich, F., De Filippis, V., Zambonin, M., and Fontana, A. 1999. Limited proteolysis of bovine α-lactalbumin: Isolation and characterization of protein domains. Protein Sci. 8 2290–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverino de Laureto, P., Vinante, D., Scaramella, E., Frare, E., and Fontana, A. 2001. Stepwise proteolytic removal of the β-subdomain in α-lactalbumin: The protein remains folded and can form the molten globule in acid solution. Eur. J. Biochem. 268 4324–4333. [DOI] [PubMed] [Google Scholar]
- Privalov, P.L. 1996. Intermediate states in protein folding. J. Mol. Biol. 258 707–725. [DOI] [PubMed] [Google Scholar]
- Ptitsyn, O.B. 1987. Protein folding: Hypotheses and experiments. J. Protein Chem. 6 273–293. [Google Scholar]
- ———. 1995. Molten globule and protein folding. Adv. Protein Chem. 47 83–229. [DOI] [PubMed] [Google Scholar]
- Ptitsyn, O.B., Pain, R.H., Semisotnov, G.V., Zerovnik, E., and Razgulyaev, O.I. 1990. Evidence for a molten globule state as a general intermediate in protein folding. FEBS Lett. 262 20–24. [DOI] [PubMed] [Google Scholar]
- Raschke, T.M. and Marqusee, S. 1997. The kinetic folding intermediate of ribonuclease H resembles the acid molten globule and partially unfolded molecules detected under native conditions. Nat. Struct. Biol. 4 298–304. [DOI] [PubMed] [Google Scholar]
- Redfield, C., Smith, R.A.G., and Dobson, C.M. 1994. Structural characterization of a highly-ordered molten globule. Nat. Struct. Biol. 1 23–29. [DOI] [PubMed] [Google Scholar]
- Redfield, C., Schulman, B.A., Milhollen, M.A., Kim, P.S., and Dobson, C.M. 1999. α-Lactalbumin forms a compact molten globule in the absence of disulfide bonds. Nat. Struct. Biol. 6 948–952. [DOI] [PubMed] [Google Scholar]
- Roder, H. 1989. Structural characterization of protein folding intermediates by proton NMR and hydrogen exchange. Methods Enzymol. 176 446–473. [DOI] [PubMed] [Google Scholar]
- Roder, H. and Colón, W. 1997. Kinetic role of early intermediates in protein folding. Curr. Opin. Struct. Biol. 7 15–28. [DOI] [PubMed] [Google Scholar]
- Schägger, H. and von Jagow, G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166 368–379. [DOI] [PubMed] [Google Scholar]
- Schechter, I., and Berger, A. 1967. On the size of the active site in proteases: Papain. Biochem. Biophys. Res. Commun. 27 157–162. [DOI] [PubMed] [Google Scholar]
- Schulman, B.A., Kim, P.S., Dobson, C.M., and Redfield, C. 1997. A residue-specific NMR view of the non-cooperative unfolding of a molten globule. Nat. Struct. Biol. 4 630–634. [DOI] [PubMed] [Google Scholar]
- Spolaore, B., Bermejo, R., Zambonin, M, and Fontana, A. 2001. Protein interactions leading to conformational changes monitored by limited proteolysis: Apo form and fragments of horse cytochrome c. Biochemistry 40 9460–9468. [DOI] [PubMed] [Google Scholar]
- Strickland, E.H. 1974. Aromatic contribution to circular dichroism spectra of proteins. CRC Crit. Rev. Biochem. 5 113–175. [DOI] [PubMed] [Google Scholar]
- Uversky, V.N. 1998. How many molten globule states there exist? Biofizika (Moscow) 43 416–421. [PubMed] [Google Scholar]
- Uversky, V.N., Karnoup, A.S., Segel, D., Seshadry, S., Doniach, S., and Fink, A.L. 1998. Anion-induced folding of staphylococcal nuclease: Characterization of multiple partially folded intermediates. J. Mol. Biol. 278 879–894. [DOI] [PubMed] [Google Scholar]
- Van Dael, H., Haezebrouck, P., Morozova, L., Arico-Muendel, C., and Dobson, C.M. 1993. Partially folded states of equine lysozyme: Structural characterization and significance for protein folding. Biochemistry 32 11886–11894. [DOI] [PubMed] [Google Scholar]
- Veprintsev, D.B., Permyakov, S.E., Permyakov, E.A., Rogov, V.V., Cawtherm, K.M., and Berliner, L.J. 1997. Cooperative thermal transitions of bovine and human apo-α-lactalbumins: Evidence for a new intermediate state. FEBS Lett. 412 625–628. [DOI] [PubMed] [Google Scholar]
- Veprintsev, D.B., Narayan, M., Permyakov, S.E., Uversky, V.N., Brooks, C.L., Cherskaya, A.M., Permyakov, E.A., and Berliner, L.J. 1999. Fine tuning of the N-terminus of a calcium-binding protein: α-Lactalbumin. Proteins 37 65–72. [DOI] [PubMed] [Google Scholar]
- Wijensiha-Bettoni, R., Dobson, C.M., and Redfield, C. 2001. Comparison of the structural and dynamical properties of holo and apo bovine α-lactalbumin by NMR spectroscopy. J. Mol. Biol. 307 885–898. [DOI] [PubMed] [Google Scholar]
- Woody, R.W. 1978. Aromatic side chain contributions to the far-ultraviolet dichroism of peptides and proteins. Biopolymers 17 1451–1467. [Google Scholar]
- ———. 1994. Contributions of tryptophan side chains to the far-ultraviolet circular dichroism of proteins. Eur. Biophys. J. 23 253–262. [DOI] [PubMed] [Google Scholar]
- Woodward, C.K. 1994. Hydrogen exchange rates and protein folding. Curr. Opin. Struct. Biol. 4 112–116. [DOI] [PubMed] [Google Scholar]
- Wu, L.C., Peng, Z.-y., and Kim, P.S. 1995. Bipartite structure of the α-lactalbumin molten globule. Nat. Struct. Biol. 2 281–286. [DOI] [PubMed] [Google Scholar]
- Xie, D., Bhakuni, V., and Freire, E. 1991. Calorimetric determination of the energetics of the molten globule intermediate in protein folding: Apo-α-lactalbumin. Biochemistry 30 10673–10678. [DOI] [PubMed] [Google Scholar]
- Yutani, K., Ogasahara, K., and Kuwajima, K. 1992. Absence of the thermal transition in apo-α-lactalbumin in the molten globule state: A study by differential scanning microcalorimetry. J. Mol. Biol. 228 347–350. [DOI] [PubMed] [Google Scholar]