Abstract
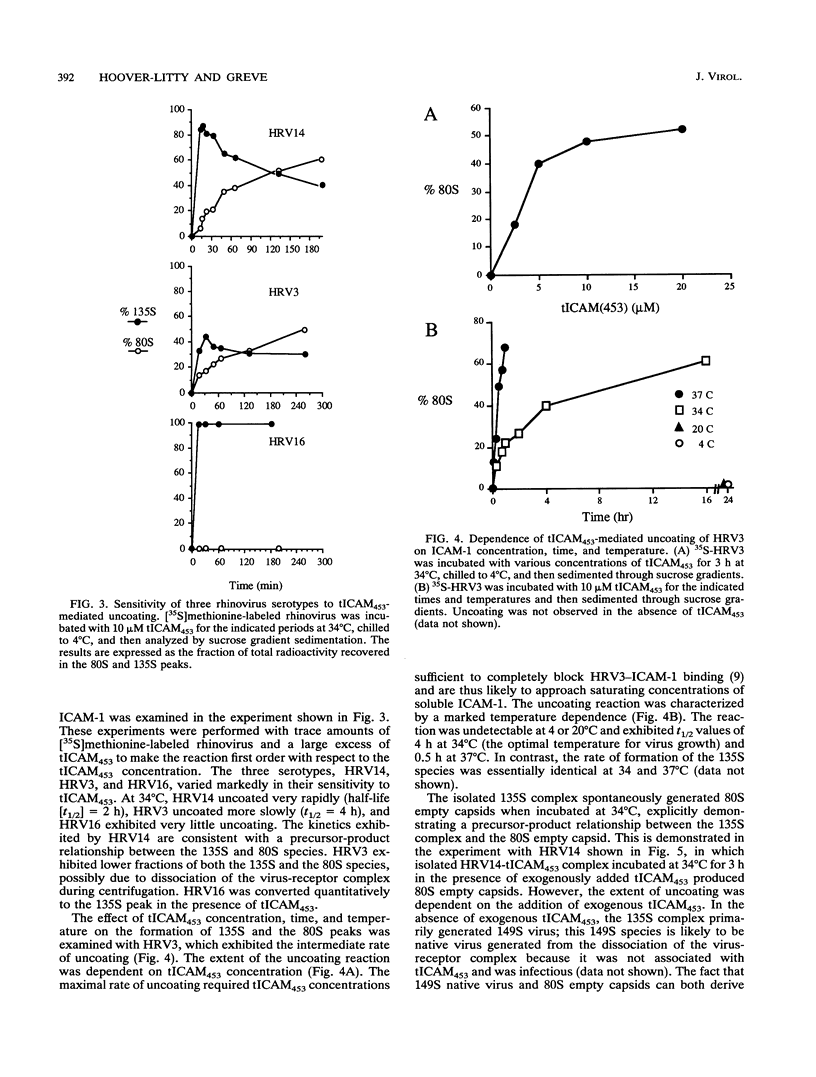
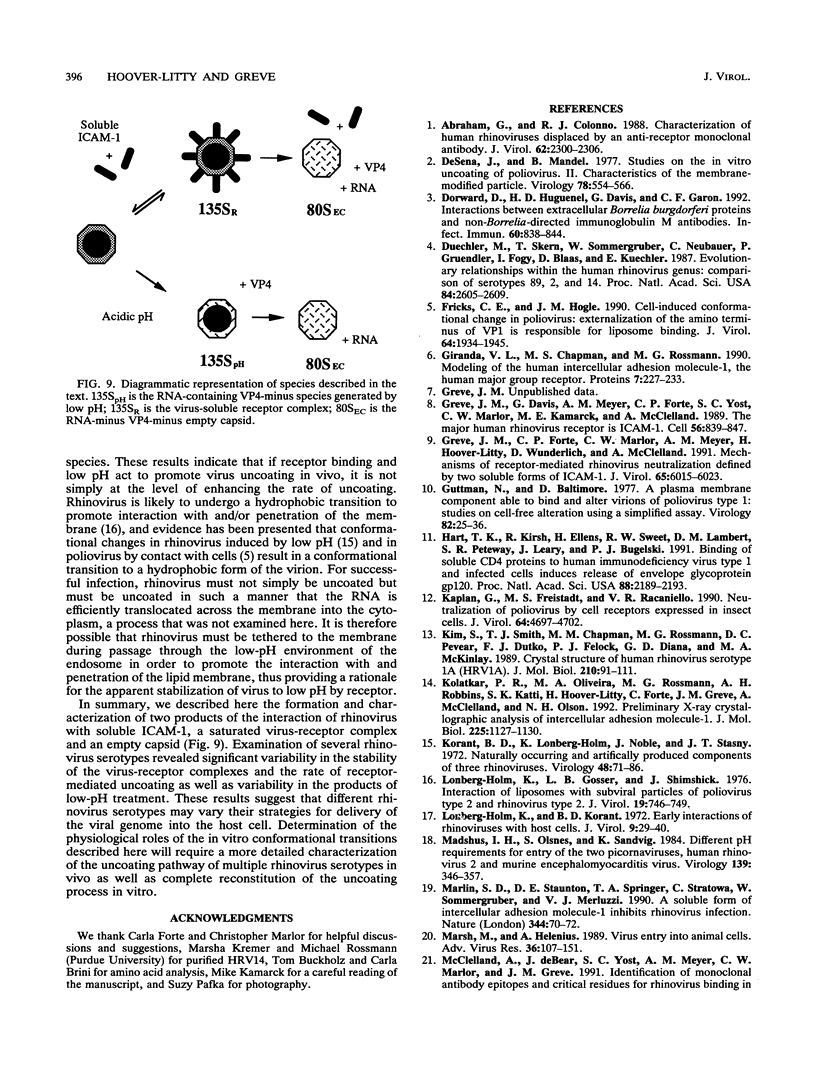
Viral receptors serve both to target viruses to specific cell types and to actively promote the entry of bound virus into cells. Human rhinoviruses (HRVs) can form complexes in vitro with a truncated soluble form of the HRV cell surface receptor, ICAM-1. These complexes appear to be stoichiometric, with approximately 60 ICAM molecules bound per virion or 1 ICAM-1 molecule per icosahedral face of the capsid. The complex can have two fates, either dissociating to yield free virus and free ICAM-1 or uncoating to break down to an 80S empty capsid which has released VP4, viral RNA, and ICAM-1. This uncoating in vitro mimics the uncoating of virus during infection of cells. The stability of the virus-receptor complex is dependent on temperature and the rhinovirus serotype. HRV serotype 14 (HRV14)-ICAM-1 complexes rapidly uncoat, HRV16 forms a stable virus-ICAM complex which does not uncoat detectably at 34 degrees C, and HRV3 has an intermediate phenotype. Rhinovirus can also uncoat after exposure to mildly acidic pH. The sensitivities of individual rhinovirus serotypes to ICAM-1-mediated virus uncoating do not correlate with uncoating promoted by incubation at low pH, suggesting that these two means of virus destabilization occur by different mechanisms. Soluble ICAM-1 and low pH do not act synergistically to promote uncoating. The rate of uncoating does appear to be inversely related to virus affinity for its receptor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Colonno R. J. Characterization of human rhinoviruses displaced by an anti-receptor monoclonal antibody. J Virol. 1988 Jul;62(7):2300–2306. doi: 10.1128/jvi.62.7.2300-2306.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sena J., Mandel B. Studies on the in vitro uncoating of poliovirus. II. Characteristics of the membrane-modified particle. Virology. 1977 May 15;78(2):554–566. doi: 10.1016/0042-6822(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Dorward D. W., Huguenel E. D., Davis G., Garon C. F. Interactions between extracellular Borrelia burgdorferi proteins and non-Borrelia-directed immunoglobulin M antibodies. Infect Immun. 1992 Mar;60(3):838–844. doi: 10.1128/iai.60.3.838-844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duechler M., Skern T., Sommergruber W., Neubauer C., Gruendler P., Fogy I., Blaas D., Kuechler E. Evolutionary relationships within the human rhinovirus genus: comparison of serotypes 89, 2, and 14. Proc Natl Acad Sci U S A. 1987 May;84(9):2605–2609. doi: 10.1073/pnas.84.9.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks C. E., Hogle J. M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990 May;64(5):1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giranda V. L., Chapman M. S., Rossmann M. G. Modeling of the human intercellular adhesion molecule-1, the human rhinovirus major group receptor. Proteins. 1990;7(3):227–233. doi: 10.1002/prot.340070304. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Forte C. P., Marlor C. W., Meyer A. M., Hoover-Litty H., Wunderlich D., McClelland A. Mechanisms of receptor-mediated rhinovirus neutralization defined by two soluble forms of ICAM-1. J Virol. 1991 Nov;65(11):6015–6023. doi: 10.1128/jvi.65.11.6015-6023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman N., Baltimore D. A plasma membrane component able to bind and alter virions of poliovirus type 1: studies on cell-free alteration using a simplified assay. Virology. 1977 Oct 1;82(1):25–36. doi: 10.1016/0042-6822(77)90029-0. [DOI] [PubMed] [Google Scholar]
- Hart T. K., Kirsh R., Ellens H., Sweet R. W., Lambert D. M., Petteway S. R., Jr, Leary J., Bugelski P. J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Freistadt M. S., Racaniello V. R. Neutralization of poliovirus by cell receptors expressed in insect cells. J Virol. 1990 Oct;64(10):4697–4702. doi: 10.1128/jvi.64.10.4697-4702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. S., Smith T. J., Chapman M. S., Rossmann M. C., Pevear D. C., Dutko F. J., Felock P. J., Diana G. D., McKinlay M. A. Crystal structure of human rhinovirus serotype 1A (HRV1A). J Mol Biol. 1989 Nov 5;210(1):91–111. doi: 10.1016/0022-2836(89)90293-3. [DOI] [PubMed] [Google Scholar]
- Kolatkar P. R., Oliveira M. A., Rossmann M. G., Robbins A. H., Katti S. K., Hoover-Litty H., Forte C., Greve J. M., McClelland A., Olson N. H. Preliminary X-ray crystallographic analysis of intercellular adhesion molecule-1. J Mol Biol. 1992 Jun 20;225(4):1127–1130. doi: 10.1016/0022-2836(92)90110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Noble J., Stasny J. T. Naturally occurring and artificially produced components of three rhinoviruses. Virology. 1972 Apr;48(1):71–86. doi: 10.1016/0042-6822(72)90115-8. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Gosser L. B., Shimshick E. J. Interaction of liposomes with subviral particles of poliovirus type 2 and rhinovirus type 2. J Virol. 1976 Aug;19(2):746–749. doi: 10.1128/jvi.19.2.746-749.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Korant B. D. Early interaction of rhinoviruses with host cells. J Virol. 1972 Jan;9(1):29–40. doi: 10.1128/jvi.9.1.29-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H., Olsnes S., Sandvig K. Different pH requirements for entry of the two picornaviruses, human rhinovirus 2 and murine encephalomyocarditis virus. Virology. 1984 Dec;139(2):346–357. doi: 10.1016/0042-6822(84)90380-5. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Staunton D. E., Springer T. A., Stratowa C., Sommergruber W., Merluzzi V. J. A soluble form of intercellular adhesion molecule-1 inhibits rhinovirus infection. Nature. 1990 Mar 1;344(6261):70–72. doi: 10.1038/344070a0. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland A., deBear J., Yost S. C., Meyer A. M., Marlor C. W., Greve J. M. Identification of monoclonal antibody epitopes and critical residues for rhinovirus binding in domain 1 of intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7993–7997. doi: 10.1073/pnas.88.18.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Neubauer C., Frasel L., Kuechler E., Blaas D. Mechanism of entry of human rhinovirus 2 into HeLa cells. Virology. 1987 May;158(1):255–258. doi: 10.1016/0042-6822(87)90264-9. [DOI] [PubMed] [Google Scholar]
- Noble J., Lonberg-Holm K. Interactions of components of human rhinovirus type 2 with Hela cells. Virology. 1973 Feb;51(2):270–278. doi: 10.1016/0042-6822(73)90427-3. [DOI] [PubMed] [Google Scholar]
- Pevear D. C., Fancher M. J., Felock P. J., Rossmann M. G., Miller M. S., Diana G., Treasurywala A. M., McKinlay M. A., Dutko F. J. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol. 1989 May;63(5):2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. J Biol Chem. 1989 Sep 5;264(25):14587–14590. [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Erickson H. P., Springer T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990 Apr 20;61(2):243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Heath G. F. Factors affecting the growth of Rhinovirus 2 in suspension cultures of L132 cells. J Gen Virol. 1970 Jan;6(1):15–24. doi: 10.1099/0022-1317-6-1-15. [DOI] [PubMed] [Google Scholar]





