Abstract
In mammals, adult neurogenesis has been extensively studied in the dentate gyrus of the hippocampus and subventricular zone. However, newly proliferated neurons have also been documented in other brain regions, including the amygdala and hypothalamus. In this review, we will examine the evidence for new neurons in the adult amygdala and hypothalamus and then discuss how environmental influences can alter cell proliferation. As some of these environmental effects may be attributed to changes in the levels of circulating hormones, we will provide evidence for estrogen-mediated cell proliferation among different species and between sexes. Finally, we will review recent data suggesting that new neurons may become functionally significant in adulthood.
Keywords: cell proliferation, social environment, steroid hormones, growth factors
INTRODUCTION
Adult neurogenesis has been documented in many brain regions in a variety of species over the past three decades. The presence of new neurons in the adult brain was first identified in the dentate gyrus of the hippocampus (DG) and the olfactory bulb (Kaplan and Hinds, 1977). Since this discovery, much research has focused on the DG, olfactory bulb, and subventricular zone (SVZ), the region in which progenitors originate and migrate to the olfactory bulb. Among the mammalian species that display adult neurogenesis in these brain areas are rats (Kaplan and Hinds, 1977), mice (Kempermann et al., 1997), hamsters (Huang et al., 1998), voles (Ormerod and Galea, 2001; Fowler et al., 2002), tree shrews (Gould et al., 1997), non-human primates (Gould et al., 1999a; Bernier et al., 2002), and even humans (Eriksson et al., 1998). The number of new neurons incorporated in the adult brain is significant; for instance, it has been estimated that adult-born neurons represent around 10–20% of the total neuronal population in the DG (Jacobs et al., 2000).
Similar to the skepticism exhibited when adult neurogenesis was first described in the 1970’s, more recent reports of new neurons in brain regions other than the DG and SVZ continue to be met with debate (Kornack and Rakic, 2001; Ming and Song, 2005). However, the most common techniques used to demonstrate new neurons in the DG and SVZ have been employed in these other brain regions with success. Such techniques include colocalization of the cell proliferation marker bromodeoxyuridine (BrdU) with phenotype-specific markers, as well as identification of doublets and triplets. Doublets and triplets are groups of 2–3 cells with small nuclei that are in close proximity to or overlapping one another; this is characteristic of cells that have recently divided or are currently undergoing cell division. While some contend that BrdU may be labeling older, mature neurons undergoing DNA repair, the appearance of BrdU-labeled cells with small nuclei, grouping as doublets or triplets, and colabeling with immature neuronal (e.g.,TuJ1) or immature glial (e.g., NG2) markers indicates that the overwhelming majority of the BrdU labeling is identifying mitotically active cells. However, appropriate controls and caution in interpretation should still be employed in BrdU studies due to limitations of the method (Taupin, 2007). Using these techniques, new neurons have been found in the amygdala and hypothalamus of voles (Fowler et al., 2002), hypothalamus of hamsters and mice (Huang et al., 1998; Kokoeva et al., 2005), amygdala and neocortex of non-human primates (Gould et al., 1999b; Bernier et al., 2002), and amygdala, hypothalamus, striatum and thalamus of rats (Pencea et al., 2001; Keihoff et al., 2006). Furthermore, in humans, multipotent neural precursors have been isolated from the amygdala, temporal and frontal cortex, and subcortical white matter, as well as the hippocampus and ventricular zone (Arsenijevic et al., 2001). Although some reports identify altered cell proliferation in these brain regions following pathological stimulation, such as ischemia, seizures or neurological disease states (Ming and Song, 2005), much of the aforementioned research documents newly proliferated neurons in control subjects and under normal physiological conditions.
While many new neurons are added to the brain during adulthood, the importance of these neurons remains questionable unless their integration within the neural network and involvement in the animal’s behavior and/or physiology can be demonstrated. New neurons in the adult brain have been shown to exhibit properties of mature neurons, including the expression of mature neuronal markers (Gould et al., 1999b; Fowler et al., 2002) and phenotype-specific neurochemicals (Benraiss et al., 2001; Kokoeva et al., 2005). In the adult hippocampus, new neurons have also been described as having axons, vesicles and synapses (Kaplan, 2001) and displaying evoked electrical propagation with synaptic transmission (Song et al., 2002b). Behaviorally, drastically minimizing the proliferation of new cells with systemic or central administration of anti-mitotic drugs results in deficits in the animal’s ability to perform tasks dependent on certain mitotic brain regions (Shors et al., 2001; Kokoeva et al., 2005).
In the current paper, we will review recent developments in the field of adult mammalian neurogenesis by focusing on the amygdala and hypothalamus – two brain regions important for social and reproductive behaviors. First, we will examine the evidence for new neuronal proliferation in these two brain regions. We will then focus on the effects of social environment in regulating neurogenesis. Since social environment may alter hormonal levels which, in turn, may underlie the changes in cell proliferation, we will examine the potential involvement of estrogen and other gonadal steroid hormones in mediating neuronal proliferation. Finally, we will conclude with a discussion of the putative functional significance of these new cells and importance within the neural network.
Adult Neurogenesis in the Amygdala and Hypothalamus
The amygdala has been implicated in a variety of social and reproductive-associated behaviors, including olfactory and pheromonal processing (Meredith, 1991), copulatory actions (Harris and Sachs, 1975), aggression (Wang et al., 1997), and social learning and memory (Kirkpatrick et al., 1994; Cahill et al., 1996). In addition to its well-known functions in homeostasis, endocrine regulation, and reproductive behaviors (Brooks, 1988), the hypothalamus also appears to be involved in pheromonal processing (Dudley et al., 1996) and social affiliation (Albert and Walsh, 1984; Gobrogge et al., 2007). Given their similarity in function, it is not surprising that the amygdala and hypothalamus are interconnected (Aizawa et al., 2004).
Among the first reports of neurogenesis in the adult amygdala, Bernier and colleagues (2002) identified new cells in the rostral temporal lobe of adult New World (Saimiri sciureus) and Old World (Macaca fascicularis) monkeys. BrdU-labeled cells were documented in the amygdala, piriform cortex, and adjoining inferior temporal cortex. Many of these new cells expressed the immature neuronal marker, TuJ1, or the mature neuronal markers, NeuN and MAP-2. Further reports have also demonstrated mygdaloid neurogenesis in brain injury models. For example, in adult rats, seizures induced by γ-aminobutryic acid (GABA)-A receptor antagonism result in cell death, as well as increased neurogenesis, in the amygdala (Park et al., 2006), and removal of the olfactory bulbs increases the number of new cells in the basolateral amygdala (Keihoff et al., 2006).
In the hypothalamus, the presence of adult neuronal proliferation has been examined in several rodent species. In hamsters, photoperiod appears to influence new cell number; animals housed in a short-day photoperiod exhibit more BrdU-labeled cells than those housed in a long-day photoperiod (Huang et al., 1998). In rats, intraventricular administration of brain-derived neurotrophic factor (BDNF) increases the number of new neurons in the hypothalamus (Pencea et al., 2001). More recently, (Kokoeva and colleagues 2005) have found that administration of ciliary neurotrophic factor (CNTF) enhances cell proliferation in the arcuate, ventromedial and dorsomedial nuclei of the hypothalamus. These cells express neuronal markers, as well as markers found in other mature hypothalamic neurons, such as neuropeptide Y (NPY), phosphorylated signal transducer and activator of transcription (pSTAT3), and pro-opiomelanocortin (POMC). The new neurons appeared to have complex morphologies with many arborized projections or a single process extending from the soma. In addition to its effects on neurogenesis, CNTF induced an expected decrease in body weight. Interestingly, coadministration of CNTF with the anti-mitotic drug Ara-C centrally into the lateral ventricles eliminated the proliferation of cells in the hypothalamus and prevented the long-term effects on body weight, suggesting a potential role of these new hypothalamic neurons in homeostatic function. It is important to note that for both of these studies with neurotrophic factors, the control groups receiving vehicle or saline infusions also exhibited BrdU-labeled cells in the hypothalamus, indicating that cell proliferation still occurred under the comparative “normal” conditions.
In our studies (Fowler et al., 2002; Fowler et al., 2003; Fowler et al., 2005), new neurons have been found in the cortical, medial and central nuclei of the amygdala and in the arcuate and ventromedial nuclei of the hypothalamus in prairie (Microtus ochrogaster) and meadow (M. pennsylvanicus) voles under normal physiological conditions, as well as with hormonal treatments. In addition, BrdU cells colabel with the neuronal markers TuJ1, NeuN or MAP-2, indicating neuronal phenotypes [Figure 1]. We also provide evidence that these new cells appear to proliferate locally within the amygdala (Fowler et al., 2003); BrdU cells colabeled with the early neuronal (TuJ1) or glial (NG2) markers can be identified in the amygdala and hypothalamus as early as 30 min following an acute intraperitoneal injection of BrdU. In referring to the previously established rate of cellular migration in the adult brain (Luskin and Boone, 1994), it is most probable that these neuronal and glial progenitors divided locally. This presence of TuJ1 in proliferating cells is consistent with other evidence that neuron-restricted progenitor cells can express cell-type specific markers, such as TuJ1 and doublecortin, continue to undergo self-renewal, and can differentiate into multiple neuronal phenotypes (Mayer-Proschel et al., 1997; Memberg and Hall, 1994; Brown et al., 2003; Mo et al., 2007).
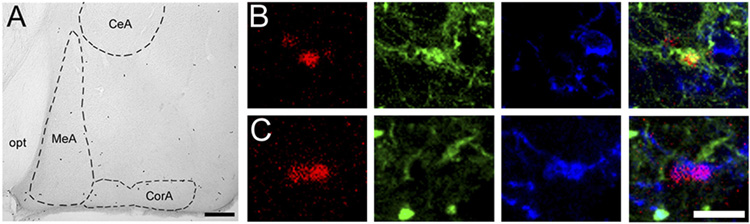
Figure 1.
[A]: Photomicrographs of newly proliferated cells in the medial (MeA) and cortical (CorA) nuclei of the amygdala in the vole brain. CeA: central nucleus of the amygdale, opt: optic tract. Scale bars = 100µm. [B & C]: Confocal laser microscope images of cells stained for BrdU (red), TuJ1 (green), NG2 (blue), and all three markers in the amygdala. BrdU and TuJ1 colocalized cells display a yellow image [B], and BrdU and NG2 colocalized cells display a purple image [C]. Scale bar = 10µm.
The DG does contain a larger number of proliferating cells than the amygdala and hypothalamus, which may be why it has been more accepted as a mitotically active region in adulthood. As a comparison, the DG contains approximately 2.6 times more cells than the amygdala and approximately 136 times more cells than the ventromedial hypothalamus in prairie voles (Fowler et al., 2005). However, the number of cells in each brain area may not be as important as the function of each individual new cell within the neural network.
Effects of Social Environment
It has been well documented that the external environment influences adult neurogenesis. In an early study, Gerd Kempermann and colleagues (1997) found that mice housed in an enriched environment displayed enhanced survival, but not proliferation, of new neurons in the DG. The enriched animals also performed better on a spatial learning task than did controls, elucidating the possible functional significance of these cells. Using similar housing conditions, these findings were then replicated in rats (Nilsson et al., 1999). In a subsequent study, mice given access to only a running wheel exhibited similar levels of neurogenesis in the DG as those in the enriched environment, suggesting that voluntary running wheel activity contributed to the enhanced neurogenesis (van Praag et al., 1999).
Social interaction with conspecifics significantly influences adult neurogenesis as well. These findings have been best demonstrated in voles. The female prairie vole is highly social, can be induced into behavioral estrus by male exposure, and forms selective social attachment after mating (Carter and Getz, 1993; DeVries et al., 1996; Wang et al., 1998). Therefore, the vole model provides an excellent opportunity to study the effects of environmental and endocrine changes on physiology and behavior. In our study (Fowler et al., 2002), female prairie voles that were exposed to a male for 48 hrs with mating had more BrdU-labeled cells in the amygdala and hypothalamus than did females housed in social isolation [Figure 2]. The difference within the amygdala persisted 3 weeks later, indicating positive effects on both the proliferation and survival of the new cells. The influence on cell proliferation was site specific, as group differences were not found in the DG or caudate/putamen. In a separate study, 48 hrs of mating significantly increased the number of new cells in the SVZ in female prairie voles (Smith et al., 2001). Together, these data suggest that experience with a male enhances the proliferation and/or survival of new neurons in a site-specific manner in the brain of adult female prairie voles.
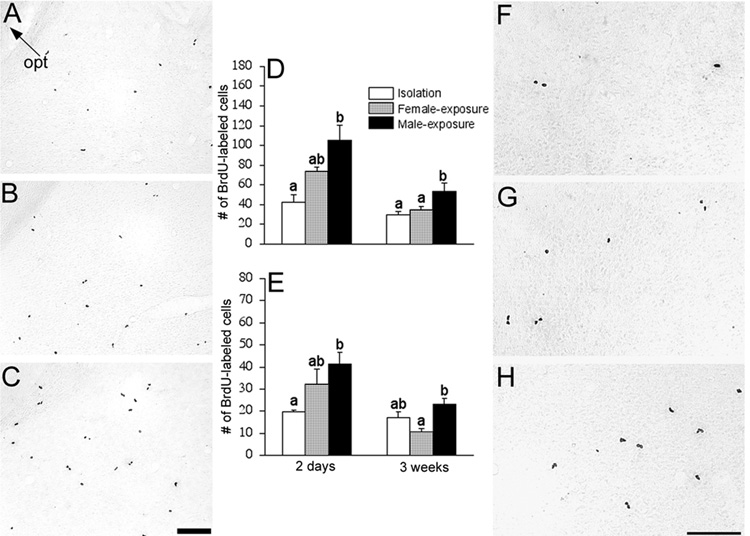
Figure 2.
Photomicrographs of BrdU-labeled cells in the amygdala [A–C] and hypothalamus [F–H]. Female prairie voles were isolated [A & F], exposed to a female [B & G], or mated and exposed to a male [C & H] for two days. Scale bars = 100µm. opt: optic tract. In the amygdala [D], two days of the male-exposure increased the number of BrdU-labeled cells and this effect was sustained three weeks later. A similar effect was also found in the hypothalamus [E]. Cell counts are presented as number of cells per section, and brain sections were anatomically matched across subjects. Letters represent the results of the post hoc test; bars with non-shared letters differed statistically from each other at p<0.05. Error bars indicate standard error of the mean.
Since the amygdala receives direct input from the olfactory bulb (Meredith, 1991), pheromonal and/or olfactory input may have contributed to cell proliferation within the amygdala. Indeed, in female prairie voles, exposure to male bedding alone for 48 hrs increased the density of BrdU-labeled cells in the amygdala, particularly in the medial and cortical nuclei, in comparison to control animals exposed to their own bedding (Liu et al., 2001a). Such effects were not found in other brain areas or when females were exposed to the bedding from other conspecific females. In addition, no such effect was found in males (Liu et al., 2007). These data indicate a stimulus-, brain region- and sex-specific effect of chemosensory cues on cell proliferation in female prairie voles.
Effects of Gonadal Steroid Hormones
The structure and function of certain brain areas depend on the levels of circulating hormones during adulthood (Garcia-Segura et al., 1994; McEwen and Alves, 1999); therefore, the influence of hormones on adult neurogenesis has become a fascinating research area. In addition to regulating reproductive and other behaviors, estrogen has been implicated in brain development, neuroprotection, and cognition (Dohler et al., 1983; McEwen et al., 1997). On the cellular level, estrogen plays a role in the proliferation (Fowler et al., 2005), survival (Leranth et al., 2000) and activation (Insel, 1990) of neurons.
The effects of estrogen on adult neurogenesis were first examined in the DG and SVZ. Fluctuations in cell proliferation across the estrus cycle have been shown in the DG of the adult female rat; the number of new cells is highest during proestrus, and ovariectomy decreases, whereas estrogen replacement restores, new cell number relative to intact controls (Tanapat et al., 1999). In the DG of female meadow voles, exposure to an acute treatment of estradiol produces a transient increase, followed by a decrease, in the number of new neurons (Ormerod and Galea, 2001). Female voles are induced ovulators and display an elevated level of estrogen following exposure to a conspecific male (Dluzen and Carter, 1979; Seabloom, 1985). In female prairie voles (Smith et al., 2001), male exposure induces an increase in the number of BrdU-labeled cells in the anterior division of the SVZ. This effect can be prevented by ovariectomy and reinstated with estradiol treatment, indicating that the effects of male exposure/mating on cell proliferation are, in part, attributable to circulating levels of estrogen.
The amygdala and hypothalamus of many species, including rats (Pfaff and Keiner, 1973), hamsters (Wood et al., 1992) and voles (Hnatczuk et al., 1994), contain an abundance of estrogen receptors and are responsive to gonadal hormones (Insel, 1990; Coolen and Wood, 1999). In vitro, estrogen has been shown to regulate the proliferation and/or survival of new neurons in the fetal rat amygdala and hypothalamus (Arimatsu and Hatanaka, 1986; Chowen et al., 1992). As male exposure elevates serum estrogen in female voles, we tested the hypotheses that estrogen up-regulates cell proliferation in the amygdala and hypothalamus in female prairie voles and that responsiveness to estrogen varies between species with different life strategies.
Adult female prairie and meadow voles were ovarectomized and then treated with saline vehicle or estradiol, which produced species-specific effects (Fowler et al., 2005) [Figure 3]. Within the amygdala, estradiol treatment significantly increased the density of new neurons in the posterior cortical and posterior medial nuclei of the amygdala in meadow, but not prairie, voles. These differences in cell proliferation were site-specific, as treatment effects were not found in the DG or ventromedial hypothalamus (VMH) (Fowler et al., 2005). The ability of estradiol to differentially influence cell proliferation in meadow, but not prairie, voles could be partially explained by species differences in regional densities of estrogen receptor alpha (ERα) [Figure 4]. Female meadow voles displayed higher densities of ERα-labeled cells in the posterior cortical amygdala than did female prairie voles. Interestingly, a high percentage of BrdU-labeled cells in both species coexpressed ERα[Figure 4G], indicating that estrogen may exert a direct effect on cell proliferation. This conclusion appears to be further supported by data from other labs. ERα and ERβ have been localized on neural stem cells in embryonic and adult rats (Brannvall et al., 2002), and the estrogen receptor antagonist ICI 182,780 can partially block the estradiol-induced enhancement of cell proliferation in the adult female rat DG (Nagy et al., 2006). This still leaves the question of why male-exposure and mating increased cell proliferation in the amygdala of female prairie voles (Fowler, 2002), but estrogen administration alone did not exert a similar effect (Fowler, 2005). Several other factors could be responsible, including chemosensory cues (as mentioned above), social interaction, and other hormones such as prolactin. Alternatively, estradiol was administered via implanted pellet in our study (Fowler, 2005), so the natural temporal pattern of estrogen release with male-induced estrus may have been important.
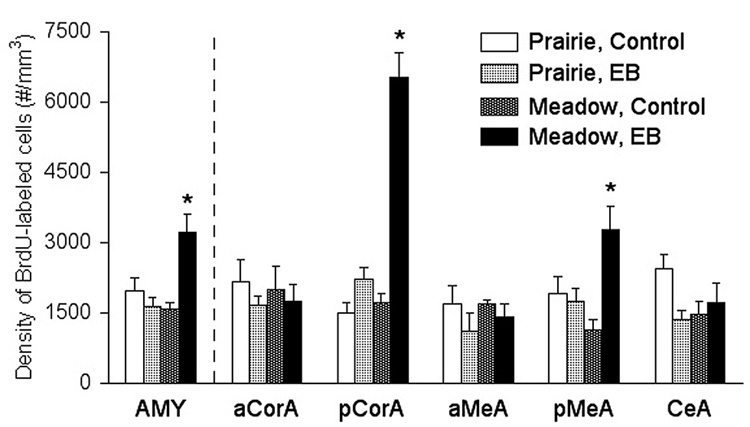
Figure 3.
Species-treatment interactions in the density of BrdU-labeled cells in the amygdala (AMY) of voles. In the posterior cortical (pCorA) and medial (pMeA) nuclei of the amygdala, EB treatment elicited a significant increase in the density of BrdU-labeled cells only in meadow voles. No species-treatment interactions were found in the anterior cortical (aCorA), anterior medial (aMeA), or central (CeA) nuclei of the amygdala. Cell counts were obtained using stereological methods and represent density counts for the entire brain area (mm3). *: p<0.05; error bars indicate standard error of the mean.
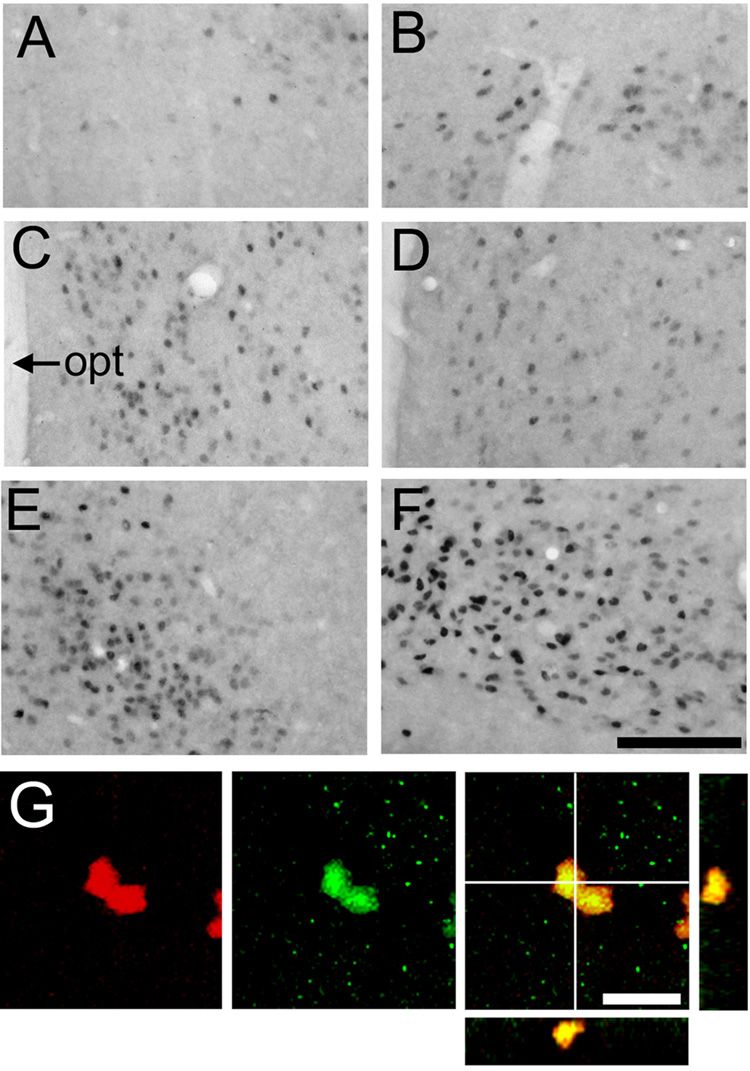
Figure 4.
[A–F]: Photomicrographs displaying ERα-labeling in the amygdala and ventromedial hypothalamus (VMH) in prairie (left) and meadow (right) voles. In the posterior cortical nucleus of the amygdala [pCorA; A & B] and VMH [E & F], meadow voles [B & F] had more ERα-labeled cells than did the prairie voles [A & E]. However, no species differences were found in the posterior medial nucleus of the amygdala [pMeA; C & D]. opt: optic tract. Scale bar = 200µm. [G]: Confocal laser microscope images display labeling for BrdU (red), ERα(green), and both markers in the pCorA in voles. In the right panels, the BrdU and ERα colocalized cells display a yellow image, and cross marks on the larger image indicate the location of views along the y-z axis (right) and x-z axis (below) to demonstrate 3D colocalization of BrdU and ERα. Scale bar = 5µm.
Much less work has been done to investigate the effects of hormonal status on neurogenesis in adult males. In the DG, estradiol treatment enhances the survival of new neurons in castrated male meadow voles (Ormerod et al., 2004). Interestingly, these effects of estradiol on neuronal survival appear to only occur during a period coincident with axonal extension, suggesting a hormonal influence on the process of cellular maturation. In castrated male hamsters, testosterone administration increases the volume of the medial amygdala (Cooke et al., 2002), a finding which could not be attributed to changes in soma size and suggesting the possibility of increased neurogenesis in response to testosterone. Since testosterone may be converted to dihydrotestosterone (DHT) or estrogen by aromatase, an enzyme that is found in high levels in the amygdala (Roselli et al., 1984), this finding may be due to either androgenic or estrogenic influences.
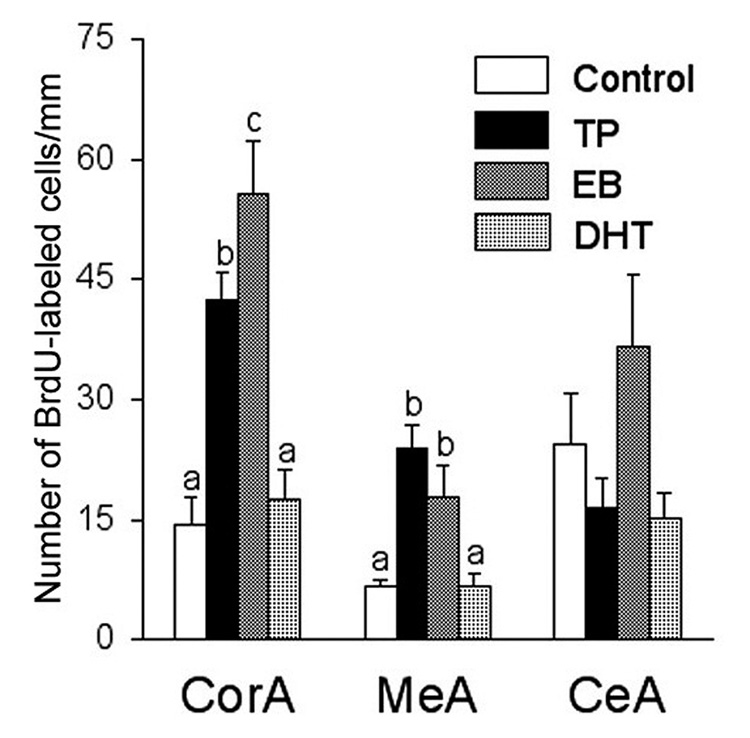
We performed a study in which castrated male meadow voles were treated with oil (control), testosterone, estradiol, or DHT (Fowler et al., 2003). Testosterone treatment increased the number of proliferating neurons in the cortical and medial nuclei of the amygdala, but not in the DG or VMH [Figure 5]. Treatment with estradiol had a similar effect, whereas DHT was ineffective. These data indicate that gonadal steroid hormones most likely act through an estrogenic-mediated mechanism to regulate adult neurogenesis in the amygdala of male meadow voles. Given the facts that new cells can be identified in the amygdala as early as 30-min following an acute BrdU injection (Fowler et al., 2003), proliferating cells contain estrogen receptors in the amygdala (Fowler et al., 2005), and a high level of aromatase is present within this brain region (Roselli et al., 1984), we may infer that the effects of both estrogen and testosterone on cell proliferation occurred locally within the amygdala.
Figure 5.
The effects of testosterone propionate (TP), estrogen benzoate (EB), or 5α- dihydrotestosterone (DHT) on the density of BrdU-labeled cells in the brain of male meadow voles. TP or EB treatment induced an increase in the density of BrdU-labeled cells in the cortical (CorA) and medial (MeA), but not central (CeA), nuclei of the amygdala. Cell counts represent the number of cells per mm2. Letters represent the results of the posthoc test; bars with non-shared letters differed statistically from each other at p<0.05. Error bars indicate standard error of the mean.
Effects of Neurotransmitters and Growth Factors
Estrogen may also influence neurotransmitter and/or growth factor systems to mediate adult neurogenesis. For example, estrogen increases serotonin receptor mRNA expression in the brain (Zhou et al., 2002; Shingo and Kito, 2003). In the DG, an increase in serotonin or chronic antidepressant treatment enhances cell proliferation, whereas depletion of serotonin reduces cell proliferation (Brezun and Daszuta, 2000; Jacobs et al., 2000; Malberg et al., 2000), and a serotonin antagonist can block the estradiol-induced enhancement of cell proliferation (Banasr et al., 2001). BDNF positive cells are found in many mitotic brain areas (Castren et al., 1995; Conner et al., 1997), and in the prairie vole brain, BDNF mRNA and protein are expressed in the amygdala and hypothalamus (Liu et al., 2001b). In vitro, BDNF enhances the number and survival of new neurons derived from the SVZ (Goldman, 1998), and in vivo, BDNF infusions into the ventricles increase the number of new cells in several brain areas, including the olfactory bulb, striatum, and hypothalamus of rats (Zigova et al., 1998; Pencea et al., 2001). It has also been reported that astrocytes may secrete BDNF and induce neurogenesis in adult neural stem cells (Ikeda et al., 2001; Song et al., 2002a). Furthermore, endothelial cells can secrete BDNF and clusters of proliferating cells are found around vasculature (Leventhal et al., 1999; Palmer et al., 2000). Finally, BDNF also increases serotonin activity (Siuciak et al., 1996), and alternatively, serotonin reuptake inhibitors increase BDNF expression (Duman et al., 1997) in the rat, suggesting that BDNF and serotonin may act synergistically to regulate cellular proliferation in the adult brain.
Functional significance of the new cells
The amygdala, hypothalamus and olfactory bulb have been implicated in olfactory/pheromonal processing (Meredith, 1991; Dudley et al., 1996), social learning and memory (Albert and Walsh, 1984; Kirkpatrick et al., 1994; Cahill et al., 1996; Brennan and Keverne, 1997), and sexual and social behaviors (Harris and Sachs, 1975; Brooks, 1988; Williams et al., 1992), while the DG plays an important role in spatial learning and memory (Shors et al., 2001). Since new cells are being incorporated into these areas during adulthood, one is led to question the functional significance of the adult-born neurons. An early study demonstrated that cells produced in adulthood exhibit properties of functional neurons (Kaplan, 2001) and in vitro cultures from adult songbirds show that new neurons can become synaptically competent and develop stimulus-evoked and spontaneous action potentials (Goldman and Nedergaard, 1992). More recent in vitro studies have also shown that new cells from the adult rat hippocampus can become electrically active neurons and exhibit functional synaptic transmission (Song et al., 2002b). It remains to be determined whether new neurons in the amygdala and hypothalamus exhibit these physiological and synaptic properties of mature neurons, and until this is demonstrated, one may question whether these neurons become functionally competent. However, given the evidence from other brain areas, we have no reason to suspect that these new cells in the amygdala and hypothalamus do not mature in a similar manner.
The ability of the new neurons to contribute to adult neural processing has also been demonstrated in vivo. In mice and golden hamsters, new cells in the olfactory bulb may become activated following odor exposure (Carlen et al., 2002; Huang and Bittman, 2002). In addition, adult mice that have deficits in the migration of olfactory bulb neuronal precursors display impaired discrimination between odors (Gheusi et al., 2000), and an increase in the number of olfactory bulb neurons following odor enrichment is associated with enhanced short-term odor memory (Rochefort et al., 2002). A few studies have begun to elucidate the functional significance of estrogen-mediated neurogenesis as well. ERα and ERβ receptor knockout mice display deficiencies in social recognition (Choleris et al., 2003), possibly due to dysfunction in the amygdala. In male meadow voles, the estradiol-induced survival of new DG neurons correlates with enhanced spatial memory (Ormerod et al., 2004). Finally, treatment with an anti-mitotic drug prevents adult cell proliferation in the hypothalamus of mice and results in deficits in body weight regulation (Kokoeva et al., 2005). Similar anti-mitotic drug treatment also induces deficits in hippocampal-dependent memory formation in rats; importantly, after recovery from the drug treatment, new neurons can be produced, and trace memory acquisition is restored (Shors et al., 2001). In our pilot experiment in prairie voles, treatment with an anti-mitotic drug resulted in a reduction in the number of BrdU-labeled cells in the amygdala and an inhibition of mating-induced pair bonding, indicating a correlation between new cells in the amygdala and social behavior (C.D. Fowler, unpublished observation). One caveat of all the aforementioned studies using the anti-mitotic drug is that the AraC was administered systemically or centrally. Although the resulting effects of administration have been documented to involve the brain regions examined (e.g., body weight regulation involves the hypothalamus and pair bonding involves the amygdala), site specificity needs to be addressed in further studies.
Conclusions
In summary, neurogenesis in the adult brain has been identified in many mammalian species. The rate of proliferation and the fate of new neurons may be influenced by a variety of factors. Aspects of an animal’s external environment may induce changes in its internal physiology, which, in turn, can act on neurochemical and/or neurotransmitter systems to affect cellular proliferation and/or survival. Although many studies have characterized the factors that increase or decrease new cell numbers, the cellular mechanisms that directly act on the proliferation and/or survival have yet to be fully elucidated. Furthermore, several studies have established relationships between the presence of newly proliferated cells and behavioral/cognitive functions, but more research needs to be done to determine the exact contribution of these new neurons.
ACKNOWLEDGMENTS
We are grateful to Dr. Xixi Jia for her contribution to the research reviewed from our lab and to Kim Young for her critical reading of the manuscript. Our research described in this review was supported by NIH grants: MH-64352 (CDF), MH-58616, MH-66734, and DA-19627 (ZXW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa H, Sato Y, Maekawa M, Fujikawa H, Hirata T, Yuasa S. Development of the amygdalohypothalamic projection in the mouse embryonic forebrain. Anat Embryol. 2004;208:249–264. doi: 10.1007/s00429-004-0399-9. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Walsh ML. Neural systems and the inhibitory modulation of agonistic behavior: a comparison of mammalian species. Neurosci Biobehav Rev. 1984;8:5–24. doi: 10.1016/0149-7634(84)90017-4. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y, Hatanaka H. Estrogen treatment enhances survival of cultured fetal rat amygdala neurons in a defined medium. Brain Res. 1986;391:151–159. doi: 10.1016/0165-3806(86)90017-9. [DOI] [PubMed] [Google Scholar]
- Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Deglon N, Kostic C, Zurn A, Aebischer P. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol. 2001;170:48–62. doi: 10.1006/exnr.2001.7691. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur J Neurosci. 2000;12:391–396. doi: 10.1046/j.1460-9568.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- Brooks CM. The history of thought concerning the hypothalamus and its functions. Brain Res Bull. 1988;20:657–667. doi: 10.1016/0361-9230(88)90075-5. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci U S A. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Cassidy RM, Brismar H, Smith GA, Enquist LW, Frisen J. Functional integration of adult-born neurons. Curr Biol. 2002;12:606–608. doi: 10.1016/s0960-9822(02)00771-6. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL. Monogamy and the prairie vole. Scientific American. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- Castren E, Thoenen H, Lindholm D. Brain-derived neurotrophic factor messenger RNA is expressed in the septum, hypothalamus and in adrenergic brain stem nuclei of adult rat brain and is increased by osmotic stimulation in the paraventricular nucleus. Neuroscience. 1995;64:71–80. doi: 10.1016/0306-4522(94)00386-j. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson J, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: A study with oxytocin and estrogen receptor-α and -β knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowen JA, Torres-Aleman I, Garcia-Segura LM. Trophic effects of estradiol on fetal rat hypothalamic neurons. Neuroendocrinology. 1992;56:895–901. doi: 10.1159/000126321. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Hegstrom CD, Breedlove SM. Photoperiod-dependent response to androgen in the medial amygdala of the Siberian hamster, Phodopus sungorus. J Biol Rhythms. 2002;17:147–154. doi: 10.1177/074873002129002438. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Testosterone stimulation of the medial preoptic area and medial amygdala in the control of male hamster sexual behavior: redundancy without amplification. Behav Brain Res. 1999;98:143–153. doi: 10.1016/s0166-4328(98)00063-1. [DOI] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad Sci U S A. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Carter CS. Ovarian hormones regulating sexual and social behaviors in female prairie voles, Microtus ochrogaster. Physiol Behav. 1979;23:597–600. doi: 10.1016/0031-9384(79)90063-5. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Coquelin A, Hines M, Davis F, Shryne JE, Shryne JE, Gorski RA. Hormonal influence on sexual differentiation of rat brain anatomy. In: Balthaazart J, Prove E, Gilles R, Balthaazart J, Prove E, Gilles R, editors. Hormones and behavior in higher vertebrates. Berlin: Springer-Verlag; 1983. pp. 194–203. [Google Scholar]
- Dudley CA, Rajendren G, Moss RL. Signal processing in the vomeronasal system: modulation of sexual behavior in the female rat. Crit Rev Neurobiol. 1996;10:265–290. doi: 10.1615/critrevneurobiol.v10.i3-4.10. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Freeman ME, Wang Z. Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol. 2003;57:257–269. doi: 10.1002/neu.10273. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-α in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489:166–179. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Chowen JA, Parducz A, Naftolin F. Gonadal hormones as promoters of structural synaptic plasticity: cellular mechanisms. Prog Neurobiol. 1994;44:279–307. doi: 10.1016/0301-0082(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA. Adult neurogenesis: from canaries to the clinic. J Neurobiol. 1998;36:267–286. [PubMed] [Google Scholar]
- Goldman SA, Nedergaard M. Newly generated neurons of the adult songbird brain become functionally active in long-term culture. Brain Res Dev Brain Res. 1992;68:217–223. doi: 10.1016/0165-3806(92)90063-3. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang ZX. Anterior hypothalamic neural activation and neurochemical association with aggression in pair bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999a;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999b;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Harris VS, Sachs BD. Copulatory behavior in male rats following amygdaloid lesions. Brain Res. 1975;86:514–518. doi: 10.1016/0006-8993(75)90906-3. [DOI] [PubMed] [Google Scholar]
- Hnatczuk OC, Lisciotto CA, DonCarlos LL, Carter CS, Morrell JI. Estrogen receptor immunoreactivity in specific brain areas of the prairie vole (Microtus ochrogaster) is altered by sexual receptivity and genetic sex. J Neuroendocrinol. 1994;6:89–100. doi: 10.1111/j.1365-2826.1994.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Bittman EL. Olfactory bulb cells generated in adult male golden hamsters are specifically activated by exposure to estrous females. Horm Behav. 2002;41:343–350. doi: 10.1006/hbeh.2002.1767. [DOI] [PubMed] [Google Scholar]
- Huang L, DeVries GJ, Bittman EL. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J Neurobiol. 1998;36:410–420. doi: 10.1002/(sici)1097-4695(19980905)36:3<410::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ikeda O, Murakami M, Ino H, Yamazaki M, Nemoto T, Koda M, Nakayama C, Moriya H. Acute up-regulation of brain-derived neurotrophic factor expression resulting from experimentally induced injury in the rat spinal cord. Acta Neuropathol (Berl) 2001;102:239–245. doi: 10.1007/s004010000357. [DOI] [PubMed] [Google Scholar]
- Insel TR. Regional induction of c-fos-like protein in rat brain after estradiol administration. Endocrinology. 1990;126:1849–1853. doi: 10.1210/endo-126-4-1849. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Kaplan MS. Environmental complexity stimulates visual cortex neurogenesis: death of a dogma and a research career. Trends Neurosci. 2001;24:617–620. doi: 10.1016/s0166-2236(00)01967-6. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Keihoff G, Becker A, Grecksch G, Bernstein HG, Wolf G. Cell proliferation is influenced by bulbectomy and normalized by imipramine treatment in a region-specific manner. Neuropsychopharmacology. 2006;31:1165–1176. doi: 10.1038/sj.npp.1300924. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): behavioral and anatomical specificity. Behav Neurosci. 1994;108:501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- Leranth C, Roth RH, Elswoth JD, Naftolin F, Horvath TL, Redmond DE., Jr Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: Implications for Parkinson's disease and memory. J Neurosci. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Fowler CD, Meredith M, Wang ZX. 2001 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, 2001; 2001a. Chemosensory cues affect adult neurogenesis in female, but not male, prairie voles. Program No. 587.4. [Google Scholar]
- Liu Y, Curtis JT, Fowler CD, Meredith M, Wang ZX. Chemosensory cues affect adult neurogenesis in the amygdala of prairie voles in a sex-specific manner. Soc Behav Neuroendocrin Abs, Pacific Grove, CA. 2007 in press. [Google Scholar]
- Liu Y, Fowler CD, Young LJ, Yan Q, Insel TR, Wang ZX. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J Comp Neurol. 2001b;433:499–514. doi: 10.1002/cne.1156. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Boone MS. Rate and pattern of migration of lineally-related olfactory bulb interneurons generated postnatally in the subventricular zone of the rat. Chem Senses. 1994;19:695–714. doi: 10.1093/chemse/19.6.695. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48:S8–15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- Memberg SP, Hall AK. Dividing neuron precursors express neuron-specific tubulin. J Neurobiol. 1994;27:26–43. doi: 10.1002/neu.480270104. [DOI] [PubMed] [Google Scholar]
- Meredith M. Sensory processing in the main and accessory olfactory systems: comparisons and contrasts. J Steroid Biochem Mol Biol. 1991;39:601–614. doi: 10.1016/0960-0760(91)90258-7. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Ann Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mo Z, Moore AR, Filipovic R, Ogawa Y, Kazuhiro I, Antic SD, Zecevic N. Human cortical neurons originate from radial glia and neuron-restricted progenitors. J Neurosci. 2007;27:4132–4145. doi: 10.1523/JNEUROSCI.0111-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy AI, Ormerod BK, Mazzucco C, Galea LA. Estradiol-induced enhancement in cell proliferation is mediated through estrogen receptors in the dentate gyrus of adult female rats. Drug Dev Res. 2006;66:142–149. [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA. Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience. 2001;102:369–379. doi: 10.1016/s0306-4522(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT-Y, Galea LA. Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience. 2004;128:645–654. doi: 10.1016/j.neuroscience.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Park JH, Cho H, Kim H, Kim K. Repeated brief epileptic seizures by pentylenetetrazole cause neurodegeneration and promote neurogenesis in discrete brain regions of freely moving adult rats. Neuroscience. 2006;140:673–684. doi: 10.1016/j.neuroscience.2006.02.076. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Ellinwood WE, Resko JA. Regulation of brain aromatase activity in rats. Endocrinology. 1984;114:192–200. doi: 10.1210/endo-114-1-192. [DOI] [PubMed] [Google Scholar]
- Seabloom RW. Tamarin RH. Biology of new world Microtus. Shippensburg, PA: American Society of Mammalogists; 1985. Endocrinology; pp. 685–724. [Google Scholar]
- Shingo AS, Kito S. Estrogen induces insulin-like growth factor-1 mRNA expression in the immortalized hippocampal cell: determination by quantitative real-time polymerase chain reaction. Neurochem Res. 2003;28:1379–1383. doi: 10.1023/a:1024900616704. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Boylan C, Fritsche M, Altar CA, Lindsay RM. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 1996;710:11–20. doi: 10.1016/0006-8993(95)01289-3. [DOI] [PubMed] [Google Scholar]
- Smith MT, Pencea V, Wang Z, Luskin MB, Insel TR. Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Horm Behav. 2001;39:11–21. doi: 10.1006/hbeh.2000.1630. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens C, Gage F. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002a;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002b;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hulihan T, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young LJ, De Vries GJ, Insel TR. Voles and vasopressin: a review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Prog Brain Res. 1998;119:483–499. doi: 10.1016/s0079-6123(08)61589-7. [DOI] [PubMed] [Google Scholar]
- Williams JR, Slotnick BM, Kirkpatrick BW, Carter CS. Olfactory bulb removal affects partner preference development and estrus induction in female prairie voles. Physiol Behav. 1992;52:635–639. doi: 10.1016/0031-9384(92)90390-n. [DOI] [PubMed] [Google Scholar]
- Wood RI, Brabec RK, Swann JM, Newman SW. Androgen and estrogen concentrating neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1992;596:89–98. doi: 10.1016/0006-8993(92)91536-n. [DOI] [PubMed] [Google Scholar]
- Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res. 2002;100:75–83. doi: 10.1016/s0169-328x(02)00134-1. [DOI] [PubMed] [Google Scholar]
- Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]