Abstract
Purpose
We isolated an autosomal semi-dominant cataract from our inbred SHR/OlaIpcv rat colony. Heterozygotes express pulverulent cataract with smaller eyes; homozygotes express marked microphthalmia with hypoplastic lens. We call this mutation Dca (for dominant cataract). In this study, we focus on the identification of the responsible gene.
Methods
We performed linkage mapping using 93 F2(SHR-Dca x PD) hybrids and a panel of microsatellite markers. In a separate group of animals with a SHR genetic background, we examined the lenses histologically using Epon semi-thin sections and toluidine blue staining. We also assessed the weight of the eyes as an immediate measure for microphthalmia.
Results
We mapped the Dca gene to chromosome 2, spanning 8.6 Mbp between markers D2Rat134 and D2Rat186. By sequencing the most plausible candidate gene, Gja8 (coding for connexin 50), we found a T to A transversion at codon 7, leading to a substitution of glutamine for leucin (L7Q). L7Q lies within the NH2-terminal cytosolic domain, presumably involved in voltage gating. Histology revealed disturbances in cell to cell contacts in the lens.
Conclusions
L7Q is a novel mutation in connexin 50 (Gja8), causing semi-dominant pulverulent cataracts. Dca rats can serve as a model for cataract development. A study on the properties of the mutant protein may offer an insight into the connexin channel function.
Introduction
Congenital cataract is an important cause of vision impairment and blindness, accounting to about 1/10 of childhood blindness with incidence slightly above 2/10,000 live births. Half of congenital cataract cases are believed to be inherited; most often in autosomal dominant fashion [1].
An increasing number of genes has been implicated in the genesis of cataract in humans as well as in model organisms (204 genotypes in Mouse Genome Informatics). These genes exhibit pronounced diversity with mutations ranging from the major structural constituents of the lens crystallins [2], intermediate beaded filaments phakinin and filensin [3], gap junction proteins like connexin 46 [4] and 50 (Figure 1 and [5-15]), and other membrane transport proteins like aquaporin Mip [16] to transcription factors like Pax6 [17], Maf [18], or Hsf4 [19], see also a review [20].
Figure 1.
Connexin 50 mutations known to date. The point mutations in Cx50 causing the dominant form of microphthalmia and cataract in the rat, mouse, and human are shown: G22R (Lop10 mouse) [15], R23T (human) [16], D47A (No2 mouse) [17], E48K (human) [18], S50P (L1 mouse) [19], V64A (Aey5 mouse) [20], V64G (human) [21], P88S (human) [22], P88Q (human) [23], I247M (human) [24], and R340W (Uca rat) [25]. L7Q mutation affects the NH2-terminal cytosolic portion of Cx50 (arrow).
Three different connexins are expressed in the lens, α1 (Cx43), α3 (Cx46), and α8 (Cx50) [21]. Connexin 50 or α8 connexin (Cx50), coded by the Gja8 gene (Gap junction membrane channel protein alpha-8), is expressed in the lens fiber cells as well as in the lens epithelial cells [22]. Cx50 fulfills an important role in the gap junctions in the eye. Null alleles (either generated by gene targeting [23,24] or a frameshift mutation [25]) result in recessive microphthalmia and pulverulent nuclear cataracts whereas several point mutations are associated with identical phenotype with a semi-dominant mode of inheritance (Figure 1 and [5-15]). Most of the identified point mutations are localized in the first extracellular loop, two are in the second transmembrane domain, two are in the COOH-terminal cytosolic domain, one is in the second transmembrane domain, and one is in the NH2-terminal domain (Figure 1).
Here, by using a classical positional cloning approach, we uncover a new mutation in the NH2-terminal cytosolic domain of Cx50, L7Q, which causes microphthalmia and cataract in a new mutant rat strain, SHR-Dca (for dominant cataract). This rat strain is derived from the spontaneously hypertensive rat (SHR) inbred strain; the cataract is inherited in autosomal-semi-dominant fashion. Interestingly, the NH2-terminal domain of Cx50 was reported to form part of the voltage sensor and therefore is thought to play a fundamental role in controlling the channel conductance [26].
Methods
Animals
In this study, the following rat inbred strains were employed: SHR/OlaIpcv, RGD (Rat Genome Database) ID: 631848; PD/Cub, RGD ID: 728161; the mutant strain SHR-Dca/Cub, BN/Cub, RGD ID: 737899; CHOC/Cub, RGD ID: 737958. All experiments were performed in agreement with the Animal Protection Law of the Czech Republic (311/1997), which is in compliance with the European Community Council recommendations for the use of laboratory animals 86/609/ECC. All experiments were approved by the Charles University Animal Care Committee.
Inheritance assessment
To estimate inheritance of the cataract phenotype, we first crossed the cataract founder with the SHR/OlaIpcv control and then crossed their offspring that displayed the cataract phenotype. In the offspring of the second cross, we observed a distinct phenotype that can be described as microphthalmia. Next, we crossed the animals showing microphthalmia with the SHR/OlaIpcv control and then crossed their offspring displaying microphthalmia. The results (see Results section) allowed us to categorize the phenotype of the animals into wild-type, cataract, and microphthalmia and to assign them genotypes +/+, +/Dca, and Dca/Dca, respectively. We used the same visual categorization for linkage mapping.
Linkage mapping
SHR-Dca/Cub was crossed with PD/Cub. F1 hybrids were intercrossed, and 93 F2 hybrids were obtained. At weaning, the eye development and cataract presence was assessed by visual inspection (see in Methods section, Inheritance assessment). Genomic DNA from the tail biopsy was isolated by phenol-chloroform extraction and ethanol precipitation. Polymerase chain reaction (PCR) was performed using primers for microsatellite markers (initial density 3 markers per chromosome), and PCR products were separated using native polyacrylamide gel electrophoresis (PAGE). Genotypes were called manually. Linkage analysis was performed using MapManager QTX [27].
Gja8 sequencing
Tail genomic DNA was isolated as described for linkage mapping. Fragments of Gja8 were amplified by PCR using the following primers: Cx50_e2a F–TGG AAA GGA AGG TCA CTC CA, Cx50_e2a R–ACA GAG CTC CTC AGC CTC AC, Cx50_e2b F–TCA TCT TCG TCT CCA CTC CA, Cx50_e2b R–GAC ACA AAA GCA ACG GAC AA, Cx50_e2c F–TGT GGT GGA CTG CTT TGT GT, Cx50_e2c R–AGA AGG CAG GGT TTC TTG GT, Cx50_e2d F–ATT TCC CTT TGA CGG AGG TT, Cx50_e2d R–TTG TCA TCG GTT GTC AGC TC, Cx50_e2e F–CCA GAC GGG GAG AAA GTA GA, and Cx50_e2e R–CAG GGC AGG CAT ATG AAA CT. Primers were designed using Primer3 [28]. PCR fragments were analyzed by electrophoresis and sequenced directly using PCR primers and BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA), and the sequencing products were analyzed using ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, CA). DNA sequences were deposited in GenBank under accession numbers EU445788-EU445793.
Histological examination of the lens
Whole eyeballs were taken for light microscopic evaluation. Samples were fixed for 12 h in Karnovsky’s solution (2% formaldehyde, 2.5% glutaraldehyde in 0.08 M sodium cacodylate buffer, pH 7.4, with 20 mg CaCl2/100 ml) at 4 °C. After repeated washing in 0.1 M sodium cacodylate buffer, a prolonged dehydration in graded series of ethanol, and immersion with acetone and toluene was performed. The samples were then immersed in prepolymerized epoxy resin (Epon 812) according to routine technique and embedded in gelatine capsules. Semi-thin sections (1–2 μm) were prepared on an LKB Pyramitome or Ultrotome III (LKB, Stockholm, Sweden) and stained with toluidine blue.
Results
Dca inheritance and phenotypes
A cataract phenotype arose spontaneously in our colony of inbred SHR/OlaIpcv rats.
We crossed the founder (with cataract) to SHR. The offspring displayed either a cataract or normal phenotype in 1:1 ratio (four litters, ratio 28:26, χ2=0.07, p=0.79). Next, we intercrossed the animals with cataract and obtained animals with a normal phenotype, a cataract phenotype, and a novel phenotype that we describe as microphthalmia. The ratio was 1:2:1, respectively (six litters, ratio 13:32:17, χ2=0.58, p=0.75). Crossing animals with microphthalmia to wild type SHR yielded exclusively offspring with cataract (six litters, n=48). Crossing two animals with microphthalmia resulted in homogeneous offspring with microphthalmia (four litters, n=40). The most plausible explanation of these findings is a monogenic Mendelian inheritance in autosomal semi-dominant fashion. We speak about semi-dominant inheritance as there is a pronounced difference between the phenotype of dominant homozygotes and heterozygotes. We named this mutation, Dca (for dominant cataract). The SHR-Dca strain is coisogenic with SHR/OlaIpcv.
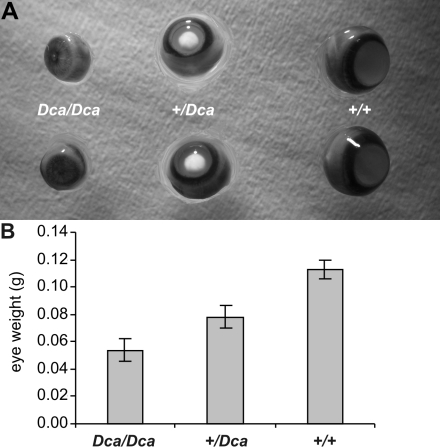
Heterozygous animals presented with well developed nuclear cataracts (of the pulverulent type), and they also had smaller eyes. Homozygotes had marked microphthalmia and the lens opacity was not so prominent (Figure 2A). Eyes of the Dca/Dca homozygotes weighed about half of the weight of the controls while the heterozygotes had an intermediate reduction in eye size (Figure 2B).
Figure 2.
Eyes of Dca rats. A: Whole eyeball preparations from mutant homozygotes (Dca/Dca), heterozygotes (+/Dca), and wild type controls (+/+) are shown. Heterozygotes show well developed nuclear cataract while the mutant homozygotes display microphthalmia. B: Eye weight is shown for each (mutant homozygotes, heterozygotes, and wild type controls). The data represent means±SEM of the average weight of the left and right eye. The differences are significant (ANOVA F=290.7, p=0, post-hoc comparison with Spjotvoll-Stoline honest significant difference test yielded p=0.00011 among all pairs). The Dca allele decreases eye size in an approximately additive manner. Dca/Dca homozygotes, n=52, 0.0538±0.0012 g; +/Dca homozygotes, n=51, 0.0781±0.0012 g; WT=+/+ (wild-type) controls, n=13, 0.1128±0.0019 g.
Dca maps to chromosome 2
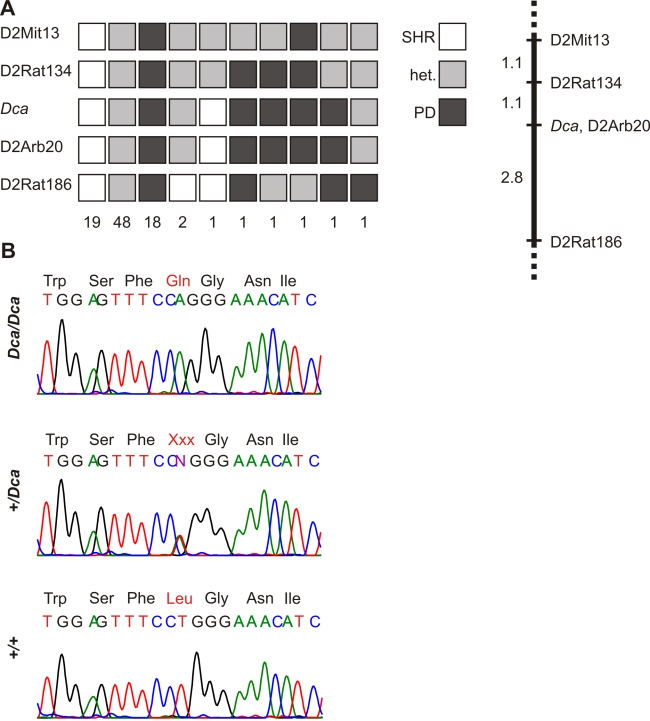
Using linkage mapping in F2 hybrids (SHR-Dca/Dca x PD, n=93), we show the mutation maps to chromosome 2 (Figure 3A). Dca is localized to 3.9 cM segment, flanked by markers D2Rat134 and D2Rat186. There is no recombination between Dca and D2Arb20. The corresponding chromosomal segment has 8.6 Mbp and contains the Gja8 gene, which encodes connexin 50 (α8 connexin, Cx50).
Figure 3.
Identification of L7Q mutation in connexin 50 of Dca rats. A: F2 (SHR-Dca x PD) animals (n=93) are categorized according to their genotypes, with number of animals in each category below. Alleles of SHR-Dca origin are indicated in white, alleles of PD origin are in dark gray, and heterozygous state is indicated by medium gray color. The corresponding linkage map with distances in cM (Kosambi map function) is shown on the right. The Dca gene is inherited in a Mendelian fashion as semi-dominant (ratio in F2 1:2:1, microphthalmia:cataract:normal, χ2=0.96, p=0.62). Dca maps to chromosome 2. There is no recombination between Dca and D2Arb20. The critical interval containing the Dca gene is localized between D2Rat134 and D2Rat186 and spans 3.9 cM. The corresponding chromosomal segment has 8.6 Mbp. B: Sequencing of the Gja8 gene is shown. A mutation (T to A transversion in codon 7 of Cx50) results in the substitution of leucine for glutamine (L7Q) in Dca/Dca animals. +/Dca heterozygotes are also heterozygotes for L7Q.
Connexin 50 mutation L7Q
We sequenced the Gja8 coding sequence and found a T→A transversion in codon 7 in Dca/Dca homozygotes. This mutation results in a non-conservative amino acid substitution where leucine at position 7 is replaced by glutamine (L7Q). L7Q cosegregates with 10410 the mutant phenotype. Dca/Dca homozygotes (microphthalmia and hypoplastic lens) are homozygotes for L7Q. Heterozygotes +/Dca (nuclear cataract, mild microphthalmia) are heterozygotes for L7Q (Figure 3B). The coisogenic strain, SHR, and the three other inbred rat strains (BN, PD, and CHOC) do not have the mutation.
Impairment of the lens structure in Dca rats
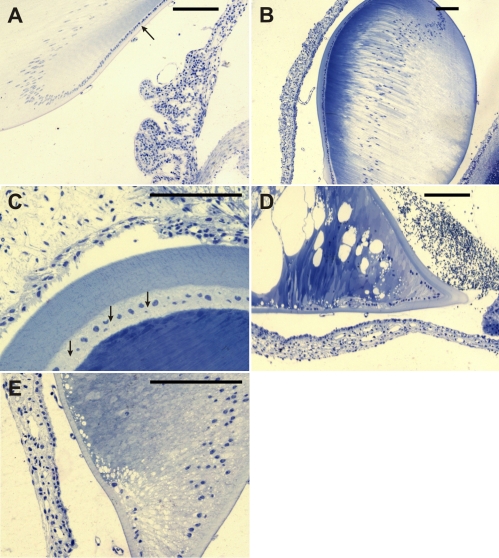
Histological examination of the lens shows relatively normal lens shape and structure in +/Dca heterozygotes (Figure 4A; +/+ control animal, 4B and C; +/Dca animals with cataract). However, the lens fibers of cataract lenses are markedly different in their stainability, and the lens capsule is irregularly thickened in comparison with control lenses. The intercellular spaces in the anterior epithelium are irregularly dilated (Figure 4C), which is in accord with the expected impairment of gap junction formation between the epithelial cells due to Cx50 mutation. In Dca/Dca homozygotes, the lens is markedly smaller (hypoplastic) and its shape is irregular (Figure 4D). The anterior epithelium is absent or is discontinuous, indicating its degeneration. Large vacuoles occur in some parts of the lens as a result of abnormal dilatation of the intercellular spaces between the fibers. In addition, thickening of the lens capsule and the differential stainability of the fibers resemble the changes seen in heterozygotes (Figure 4E).
Figure 4.
Histological examination of the lens. Epon semi-thin sections were stained with toluidine blue. A: The control animal is shown. A single layer of the cuboidal epithelium (arrow) transforms at the equator into elongated cells representing future lens fibers. B: The lens of +/Dca heterozygotes corresponded in shape nearly completely with that of control animals. At low magnification, the most pronounced feature of the lens is marked heterogeneity in the stainability of its fibers. The lens capsule has often variable thickness and is almost regularly thicker at the anterior face. Persisting vascularization in the posterior eye chamber can be observed. C: In +/Dca heterozygotes, at the higher magnification and tangential section plane orientation, irregularly dilated intercellular spaces between anterior cuboidal epithelial cells (arrows) and newly formed lens fibers can be demonstrated. D: Severe alterations of lens development were found in homozygous Dca/Dca animals with microphthalmic eyes. The equatorial region forms a regularly sharp angle with a distinct thickening of the lens capsule. The anterior epithelium is discontinuous, and sometimes there are only isolated groups of epithelial cells visible at the anterior face of the lens. Abundant dilatations of intercellular spaces can be seen in some lens regions as great vacuolar formations. Also, stainability of lens fibers increases toward the central part. E: Higher magnification of Dca/Dca lens reveals findings similar to those in D; missing continuity of the anterior cuboidal epithelium and striking disturbances in organization and arrangement of cell to cell contacts.
Discussion
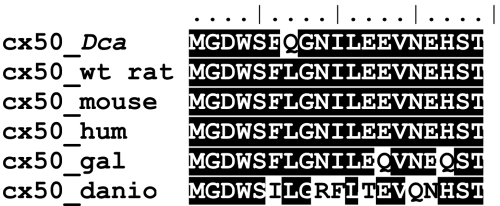
We identified the L7Q mutation by selective sequencing of the functional positional candidate Gja8. The nonrecombinant chromosome segment has 8.6 Mbp and contains at least 44 genes besides Gja8. However, none of the other genes is a solid functional candidate for cataract formation. The hypothesis, that L7Q mutation is responsible for cataract formation in SHR-Dca rats, is supported by complete cosegregation of L7Q with the mutant phenotypes. Moreover, L7Q is absent from coisogenic SHR/OlaIpcv strain as well as from 3 other unrelated rat inbred strains. The substitution resides in the NH2-terminal cytosolic fragment of Cx50 (Figure 1). Leucine in this position is conserved across major vertebrate phyla, indicating a vital role in the protein function (Figure 5). Moreover, leucine at the corresponding position is also conserved in most paralog connexins (with the exception of Cx31.1 coded by the Gjb5 gene, data not shown). Therefore, we conclude, L7Q is most probably the cause of abnormal eye development in the SHR-Dca strain, although the conclusive evidence can be brought around only by additional experiments like transgenic rescue.
Figure 5.
Conservation of NH2-terminus of Cx50. Evolutionary conservation of the NH2-terminal portion of Cx50 indicates that leucine in position 7 is invariably (100%) conserved across vertebrates (identity/similarity shading using the BLOSUM62 matrix). The sequences are as follows: Dca – sequence from the microphthalmic rat (SHR-Dca/Dca); wt_rat (Rattus norvegicus) – NP_703195.1; mouse (Mus musculus) – NP_032149.1; hum (human, Homo sapiens) – NP_005258.2; gal (chicken, Gallus gallus) – NP_990328.1; and danio (zebrafish, Danio rerio) – XP_685033.1.
Gja8 involvement in eye and lens development is well established. Recessive cataracts are associated with loss of function as demonstrated in gene targeting experiments [23,24] and also V203fs [25] while (semi) dominant cataracts are caused by different point mutations (Figure 1). The mechanism of the lens affliction stems probably from the subunit composition of connexins (and thus transport properties of gap junctions) both in the lens epithelium and fibers This concept is illustrated by replacement of Cx50 by Cx46 by homologous recombination (knock-in). Single copy Cx46 in place of Cx50 (heterozygous replacement) is able to support development of normal lens size, but results in cataract. On the other hand, When only Cx46 is expressed in place of Cx50 (homozygous replacement), the lens is clear, but its size is subnormal. [29]. The same knock-in can rescue the cataracts caused by dominant form of mutant Gja8 [9].
Gap junctions are functionally essential for multiple tissues. It is not established yet if Cx50 has any extraocular role. However, it is important to note that the L7Q mutation arises in SHR, a model of hypertension. Considering the role gap junctions play in hypertension [30], it will be interesting to investigate possible links between Cx50 and cardiovascular phenotypes.
L7Q represents a new mutation occurring in the NH2-terminal cytosolic portion of Cx50. Based on literature evidence (known null alleles versus point mutations in Gja8, see Introduction), we hypothesize that L7Q is not a null allele, but the mutant protein incorporates into gap junctions and actively disrupts their functionality. This mutation in accord with other mutations in Cx50 may be invaluable for detailed study of the molecular mechanisms of the protein function and thus the function of the gap junctions. The NH2-terminal cytosolic domain is thought to form a part of the voltage sensor of the connexin channel. In particular, mutations introducing a negative charge in amino acids 2, 5, 8, 9, and 10 of Cx32 resulted in the reversal of gating polarity [26]. Thus, it will be very interesting to investigate the gating properties of the channels formed from the L7Q mutant in Cx50 (L7 in Cx50 corresponds to L6 in Cx32). L7Q may also significantly alter the gating properties but probably in a different manner since L7Q mutation does not include a charge shift.
Only few cataract models are available in the rat. These include an unknown gene mapping to chromosome 15 [31], a mutation in connexin 46 [32], and a mutation in connexin 50 [15]. The Cx50 mutation [15] results in a substitution in the COOH-terminal cytoplasmic domain of Cx50 (R340W, see also Figure 1) and differs from Dca by its variable late onset, suggesting that L7Q substitution disrupts the function of Cx50 at a functionally more important amino acid residue.
We have identified the mutation that most likely causes cataract formation in SHR-Dca rats. This finding may provide fresh insights into eye development, cataract formation, and connexin function.
Acknowledgments
This work was supported in part by Research Project MSM0021620807 from the Ministry of Education, Youth and Sports of Czech Republic
References
- 1.Francis PJ, Moore AT. Genetics of childhood cataract. Curr Opin Ophthalmol. 2004;15:10–5. doi: 10.1097/00055735-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Andley UP. Crystallins and hereditary cataracts: molecular mechanisms and potential for therapy. Expert Rev Mol Med. 2006;8:1–19. doi: 10.1017/S1462399406000111. [DOI] [PubMed] [Google Scholar]
- 3.Perng MD, Zhang Q, Quinlan RA. Insights into the beaded filament of the eye lens. Exp Cell Res. 2007;313:2180–8. doi: 10.1016/j.yexcr.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–43. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 5.Chang B, Wang X, Hawes NL, Ojakian R, Davisson MT, Lo WK, Gong XA. Gja8 (Cx50) point mutation causes an alteration of alpha 3 connexin (Cx46) in semi-dominant cataracts of Lop10 mice. Hum Mol Genet. 2002;11:507–13. doi: 10.1093/hmg/11.5.507. [DOI] [PubMed] [Google Scholar]
- 6.Willoughby CE, Arab S, Gandhi R, Zeinali S, Luk D, Billingsley G, Munier FL, Heon E. A novel GJA8 mutation in an Iranian family with progressive autosomal dominant congenital nuclear cataract. J Med Genet. 2003;40:e124. doi: 10.1136/jmg.40.11.e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steele EC, Jr, Lyon MF, Favor J, Guillot PV, Boyd Y, Church RL. A mutation in the connexin 50 (Cx50) gene is a candidate for the No2 mouse cataract. Curr Eye Res. 1998;17:883–9. doi: 10.1076/ceyr.17.9.883.5144. [DOI] [PubMed] [Google Scholar]
- 8.Berry V, Mackay D, Khaliq S, Francis PJ, Hameed A, Anwar K, Mehdi SQ, Newbold RJ, Ionides A, Shiels A, Moore T, Bhattacharya SS. Connexin 50 mutation in a family with congenital “zonular nuclear” pulverulent cataract of Pakistani origin. Hum Genet. 1999;105:168–70. doi: 10.1007/s004399900094. [DOI] [PubMed] [Google Scholar]
- 9.Xia CH, Cheung D, DeRosa AM, Chang B, Lo WK, White TW, Gong X. Knock-in of alpha3 connexin prevents severe cataracts caused by an alpha8 point mutation. J Cell Sci. 2006;119:2138–44. doi: 10.1242/jcs.02940. [DOI] [PubMed] [Google Scholar]
- 10.Graw J, Loster J, Soewarto D, Fuchs H, Meyer B, Reis A, Wolf E, Balling R, Hrabe de Angelis M. Characterization of a mutation in the lens-specific MP70 encoding gene of the mouse leading to a dominant cataract. Exp Eye Res. 2001;73:867–76. doi: 10.1006/exer.2001.1096. [DOI] [PubMed] [Google Scholar]
- 11.Zheng JQ, Ma ZW, Sun HM. A heterozygous transversion of connexin 50 in a family with congenital nuclear cataract in the northeast of China. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005;22:76–8. [PubMed] [Google Scholar]
- 12.Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet. 1998;62:526–32. doi: 10.1086/301762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora A, Minogue PJ, Liu X, Reddy MA, Ainsworth JR, Bhattacharya SS, Webster AR, Hunt DM, Ebihara L, Moore AT, Beyer EC, Berthoud VM. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet. 2006;43:e2. doi: 10.1136/jmg.2005.034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polyakov AV, Shagina IA, Khlebnikova OV, Evgrafov OV. Mutation in the connexin 50 gene (GJA8) in a Russian family with zonular pulverulent cataract. Clin Genet. 2001;60:476–8. doi: 10.1034/j.1399-0004.2001.600614.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita S, Furumoto K, Nobukiyo A, Kamohara M, Ushijima T, Furukawa T. Mapping of A gene responsible for cataract formation and its modifier in the UPL rat. Invest Ophthalmol Vis Sci. 2002;43:3153–9. [PubMed] [Google Scholar]
- 16.Shiels A, Bassnett S. Mutations in the founder of the MIP gene family underlie cataract development in the mouse. Nat Genet. 1996;12:212–5. doi: 10.1038/ng0296-212. [DOI] [PubMed] [Google Scholar]
- 17.Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994;7:463–71. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson RV, Perveen R, Kerr B, Carette M, Yardley J, Heon E, Wirth MG, van Heyningen V, Donnai D, Munier F, Black GC. Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet. 2002;11:33–42. doi: 10.1093/hmg/11.1.33. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto M, Izu H, Seki K, Fukuda K, Nishida T, Yamada S, Kato K, Yonemura S, Inouye S, Nakai A. HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J. 2004;23:4297–306. doi: 10.1038/sj.emboj.7600435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graw J. Congenital hereditary cataracts. Int J Dev Biol. 2004;48:1031–44. doi: 10.1387/ijdb.041854jg. [DOI] [PubMed] [Google Scholar]
- 21.White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol. 1994;125:879–92. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahm R, van Marle J, Prescott AR, Quinlan RA. Gap junctions containing alpha8-connexin (MP70) in the adult mammalian lens epithelium suggests a re-evaluation of its role in the lens. Exp Eye Res. 1999;69:45–56. doi: 10.1006/exer.1999.0670. [DOI] [PubMed] [Google Scholar]
- 23.White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998;143:815–25. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129:167–74. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- 25.Ponnam SP, Ramesha K, Tejwani S, Ramamurthy B, Kannabiran C. Mutation of the gap junction protein alpha 8 (GJA8) gene causes autosomal recessive cataract. J Med Genet. 2007;44:e85. doi: 10.1136/jmg.2007.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys J. 2000;79:2403–15. doi: 10.1016/S0006-3495(00)76485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manly KF, Cudmore RH, Jr, Meer JM. Map Manager QTX, cross-platform software for genetic mapping. Mamm Genome. 2001;12:930–2. doi: 10.1007/s00335-001-1016-3. [DOI] [PubMed] [Google Scholar]
- 28.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Wittinghan FJ, Sellitto C, Li L, Gong X, Brink PR, Mathias RT, White TW. Dominant cataracts result from incongruous mixing of wild-type lens connexins. J Cell Biol. 2003;161:969–78. doi: 10.1083/jcb.200303068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figueroa XF, Isakson BE, Duling BR. Vascular gap junctions in hypertension. Hypertension. 2006;48:804–11. doi: 10.1161/01.HYP.0000242483.03361.da. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama M, Amano S, Tsuji A, Sasahara M, Serikawa T, Ihara N, Matsuda M, Hazama F, Handa J. Genetic analysis of cataract in Ihara epileptic rat. Mamm Genome. 2001;12:207–11. doi: 10.1007/s003350010263. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida M, Harada Y, Kaidzu S, Ohira A, Masuda J, Nabika T. New genetic model rat for congenital cataracts due to a connexin 46 (Gja3) mutation. Pathol Int. 2005;55:732–7. doi: 10.1111/j.1440-1827.2005.01896.x. [DOI] [PubMed] [Google Scholar]