Abstract
Females are usually considered to be the target of male courtship behaviour. In nature, however, social interactions rarely occur without other observers; thus, it is conceivable that some male courtship behaviours are directed not towards females, but rather towards male rivals. The northern swordtail, Xiphophorus birchmanni, is a freshwater fish found in high densities in natural streams. Males court by swimming close to and in parallel with the female, raising their large sail-like dorsal fin, and quivering briefly. Here, we show that females prefer males that display small dorsal fins to those with large ones, and that males are less aggressive to other males with large dorsal fins. Male swordtails also raise their dorsal fins more frequently when courting in the presence of other males. These results suggest that, despite female avoidance of large dorsal fins, males that raise their fin during courtship benefit by intimidating potential competitors; the intended receivers of this signal are thus males, not females. Intrasexual selection can therefore offset the forces of intersexual selection, even in a courtship display.
Keywords: mate choice, competition, sexual selection, Poeciliidae
1. Introduction
Darwin (1871) suggested that intrasexual selection, through male competition, and intersexual selection, through female mate choice, could act in unison and result in reinforcing selection on the male phenotype. An increasing number of studies, however, illustrate that this is not always the case, and that male competition and female choice can favour opposing traits (Berglund et al. 1996; Qvarnström & Forsgren 1998). For example, male cockroaches, Nauphoeta cinerea, release a social pheromone where the composition most attractive to females differs from that which confers high male social status (Moore & Moore 1999). Similarly, conflict may arise in selection on male morphological traits: in the carrion fly, Prochyliza xanthostoma, females prefer males with elongated heads, yet this trait is disadvantageous in male–male combat (Bonduriansky & Rowe 2003); and female water boatmen, Sigara falleni, favour large foreleg pala size, which is costly in male competition (Candolin 2004).
One solution to opposing intra- and intersexual selection is to simultaneously signal different things to different receivers. Cuttlefish (Sepia pharaonis) excel in this task: courting males place themselves between the female and their opponent, and display different visual patterns on each side of their body to signal to each receiver (Hanlon & Messenger 1988). Dynamic components of a courtship or agonistic display, which can be turned on or off instantaneously, offer another avenue of compromise for signalling different messages in different social situations. For example, males often display more intense signals when other males are absent and reduce the signal when they are present (reviewed in Berglund et al. 1996).
In the swordtail fish, Xiphophorus birchmanni (Poeciliidae), males display large, sail-like dorsal fins. Dorsal fin raising is a dynamic component of both courtship and agonistic displays in this species (H. S. Fisher & E. E. Rosenthal 2005 unpublished data) and is conserved across northern swordtails (Ryan et al. 1992; Ryan & Rosenthal 2001; Rosenthal et al. 2002). Here, we show, however, that female X. birchmanni prefer male stimuli with small dorsal fins over large ones. Why would males flaunt an easily concealed large dorsal fin during courtship if females avoid males with large fins? We hypothesize that large, raised dorsal fins are selected for male competition, and that because swordtails live in large groups, even during courtship, males are signalling to potential competitors. In this study, we tested males' agonistic behaviour towards animated stimuli of courting males with dorsal fins of varying size. We then paired males with females and quantified courtship in the presence of another male, another female or no observer.
2. Material and methods
Xiphophorus birchmanni were wild-caught adults collected from the Río Garces, Hidalgo, Mexico (Wong & Rosenthal 2006). Animals were maintained on a 12 : 12 h light : dark cycle and were fed TetraMin flakes and mysids.
(a) Behavioural responses to animated stimuli
Female preference tests were conducted in an aquarium (51×28×33 cm), with two 35 cm IBM CRT monitors positioned on opposite ends of the aquarium to deliver the animated sequences. We simultaneously tested the preference for the unmodified mean X. birchmanni animation, based on mean morphological and behavioural parameters for X. birchmanni sampled in the wild (Wong & Rosenthal 2006), versus the same stimulus with the dorsal fin reduced by 33%.
The playback procedure was identical to that used in previous simultaneous-choice studies using animated stimuli (Rosenthal et al. 2002; Fisher et al. 2006; Wong & Rosenthal 2006). All choice tests were conducted in an aquarium (length×width×height=75×30×30 cm) divided lengthwise into three equal sections (left, right and centre) by lines drawn on the sides of the tank. A mop of green yarn was placed in the centre section, the no-choice zone, of the tank to provide refuge for the female. Females were placed in the centre of the tank and acclimated for 10 min, then presented with 10 min of monochromatic screen, followed by simultaneous presentation of the 300 s test stimulus. Subjects were then presented with another 10 min of monochromatic screen followed by the same set of 300 s test stimuli, now switched between monitors to control for any side biases. The side from which a given stimulus was first displayed was alternated between trials. Female preference was estimated by association time, which is the standard measure of mating preferences in poeciliid fishes, including swordtails (Wong et al. 2005 and references therein). In a closely related species, Xiphophorus nigrensis, association time in laboratory trials is a strong predictor of association in open-field trials and observed mate choices in the wild (Ryan et al. 1992). Fishes that spent more than 90% of the total time in any one section of the aquarium were defined as unresponsive and excluded from the analysis.
We then repeated the procedure described above with males, instead of females, as the focal animals and recorded the number of bites and aggressive behaviours directed towards each stimulus. An aggressive display was considered a lateral orientation while quivering, a vertical head-down position, or a combination of both (Moretz & Morris 2003).
(b) Pairing experiment
Each male–female pair was housed in its own 40 l aquarium containing a net box (16.5×12×12 cm) attached to either the upper left or right corner. After pairs were acclimated for 48 h, a stimulus animal was gently placed in the net box, and we recorded the frequency of courtship displays and the number of times the focal male raised his dorsal fin for 300 s. Each pair was observed under three conditions: with a conspecific male as the stimulus animal; a conspecific female; and no animal. The order of treatments was randomized, and pairs were given 24 h between treatments.
3. Results
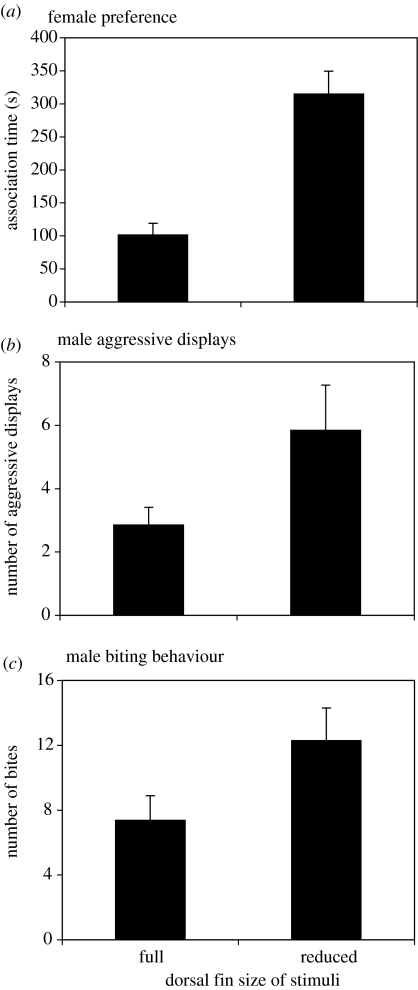
Females preferentially associated with reduced-fin male stimulus (paired t-test: t=4.59, d.f.=14, p=0.0004; figure 1a). Males similarly associated more with reduced-fin stimuli (paired t-test: t=2.98, d.f.=15, p=0.0093), directed more aggressive displays (paired t-test: t=2.16, d.f.=15, p=0.048; figure 1b) and bites (paired t-test: t=2.59, d.f.=15, p=0.021; figure 1c) towards them.
Figure 1.
Mean+s.e. of (a) female preference for, (b) male aggressive displays, or (c) bites directed towards animated stimuli of conspecific males with full dorsal fins and those reduced by 33%.
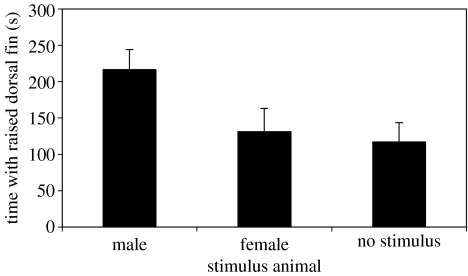
In the pairing experiments, focal males displayed an erect dorsal fin more often with a male bystander, than with a female or no bystander (Friedman's test: Fr=10.7, d.f.=8, p=0.004; Dunn's post hoc test: male versus no: z=−12.0, p<0.05, male versus female: z=0, p<0.05, no versus female: z=12.0, p>0.05; figure 2). The number of times the focal male courted the female was also influenced by the presence of a bystander (Friedman's test: Fr=6.35, d.f.=8, p=0.042): courtship was greatest with a female bystander (mean±s.e.: female 8.22±0.68, male 5.44±0.87, and no 5.67±0.93). Finally, males were not attracted to the stimulus in any treatment (Friedman's test: Fr=0.22, d.f.=8, p=0.97).
Figure 2.
Mean+s.e. of time the focal male raised his dorsal fin while paired with a female and with either a male, a female or no stimulus animal.
4. Discussion
Male X. birchmanni display large elaborate dorsal fins during courtship; here, we show that females, however, avoid suitors with such fins. Further, we demonstrate that the dorsal fin raising component of courtship is not directed at the potential mate, but rather at possible competitors. These results suggest that males are less aggressive towards rivals with large dorsal fins, and that when other males are present, they raise their dorsal fin more frequently. The evolution of a courtship signal that females avoid, particularly the one that is easily disguised, seems unlikely, except if failing to raise the dorsal fin incurs a net fitness cost. In nature, swordtail populations consist of up to hundreds of males and females within a few square metres, and mate continuously throughout the year (Morris & Ryan 1992); both potential mates and potential competitors are thus at high densities. We hypothesize that male swordtails have evolved courtship behaviour involving dorsal fin raising, not in response to pre-existing female preferences, but rather as a competitive signal to male bystanders. In X. birchmanni, the dorsal fin shows an allometric relationship with body size (Rosenthal et al. 2003), suggesting that it could function as an honest signal of fighting ability, or that it could be used to exaggerate body size.
Why should females prefer short-finned males, given that short fins signal lower competitive ability? When selecting a mate, females should strive to maximize total fitness (Kokko et al. 2003), and males that are successful in competition may not always guarantee the greatest net benefits to females (Wong 2004). There is no paternal care in swordtails, nor do males hold territories that could provide females with resources; females may, however, receive genetic benefits, less risk of transmittable diseases or physical harm by mating with less successful competitors (Qvarnström & Forsgren 1998). In Xiphophorus cortezi, barred males escalate to biting in male–male contests before non-barred males (Moretz 2005); it is possible that X. birchmanni with large dorsal fins are more aggressive to both males and females (Sih 2005). Alternatively, female avoidance of large dorsal fins may be linked to their avoidance of swords, an ornament preferred by females of other Xiphophorus species and avoided by X. birchmanni females (Wong & Rosenthal 2006). The same perceptual and evaluative mechanisms that attend to sword length may also attend to the size of the dorsal fin.
These results suggest that it is possible for a signal to evolve which has opposing effects on female attraction and male–male competition. In X. birchmanni, males with large dorsal fins enjoy a competitive advantage, but are avoided by females. Owing to the dense social environment, male swordtails are in a sense always signalling to multiple receivers, many of which are potential competitors. Here, we show that a primary component of male courtship in X. birchmanni is directed towards potential competitors, not potential mates. This study highlights the importance of investigating both intra- and intersexual selection, since each may be shaping the expression of sexually selected traits in a unique way.
Acknowledgments
We thank Francisco García de León and Juventino Ortiz for their assistance in the field. The Mexican federal government provided collection permits. The research was supported by NSF grant IOB-0447665 to G.G.R. H.S.F. was supported by a Palmer–McLeod Fellowship.
References
- Berglund A, Bisazza A, Pilastro A. Armaments and ornaments: an evolutionary explanation of traits of duel utility. Biol. J. Linn. Soc. 1996;58:385–399. doi:10.1006/bijl.1996.0043 [Google Scholar]
- Bonduriansky R, Rowe L. Interactions among mechanisms of sexual selection on male body size and head shape in a sexually dimorphic fly. Evolution. 2003;57:2046–2053. doi: 10.1111/j.0014-3820.2003.tb00384.x. doi:10.1554/03-047 [DOI] [PubMed] [Google Scholar]
- Candolin U. Opposing selection on a sexually dimorphic trait through female choice and male competition in a water boatman. Evolution. 2004;58:1861–1864. doi: 10.1111/j.0014-3820.2004.tb00470.x. doi:10.1554/04-058 [DOI] [PubMed] [Google Scholar]
- Darwin C. Murray; London, UK: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- Fisher H.S, Wong B.B.M, Rosenthal G.G. Alteration of the chemical environment disrupts communication in a freshwater fish. Proc. R. Soc. B. 2006;273:1187–1193. doi: 10.1098/rspb.2005.3406. doi:10.1098/rspb.2005.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon R.T, Messenger J.B. Adaptive coloration in young cuttlefish (Sepia officianalis L.): the morphology and development of body patterns and their relation to behaviour. Phil. Trans. R. Soc. B. 1988;320:437–487. [Google Scholar]
- Kokko H, Brooks R, Jennions M.D, Morley J. The evolution of mate choice and mating biases. Proc. R. Soc. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. doi:10.1098/rspb.2002.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A.J, Moore P.J. Balancing sexual selection through opposing mate choice and male competition. Proc. R. Soc. B. 1999;266:711–716. doi:10.1098/rspb.1999.0694 [Google Scholar]
- Moretz J.A. Aggression and fighting ability are correlated in the swordtail fish Xiphophorus cortezi: the advantage of being barless. Behav. Ecol. Sociobiol. 2005;59:51–57. doi:10.1007/s00265-005-0008-9 [Google Scholar]
- Moretz J.A, Morris M.R. Evolutionary labile responses to a signal of aggressive intent. Proc. R. Soc. B. 2003;270:2271–2277. doi: 10.1098/rspb.2003.2510. doi:10.1098/rspb.2003.2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M.R, Ryan M.J. Breeding cycles in natural populations of Xiphophorus nigrensis, X. multilineatus, and X. pygmaeus. Copeia. 1992;1992:1074–1077. doi:10.2307/1446640 [Google Scholar]
- Qvarnström A, Forsgren E. Should females prefer dominant males? Trends Ecol. Evol. 1998;13:498–501. doi: 10.1016/s0169-5347(98)01513-4. [DOI] [PubMed] [Google Scholar]
- Rosenthal G.G, Wagner W.E.J, Ryan M.J. Secondary loss of preference for swords in the pygmy swordtail Xiphophorus nigrensis (Pisces: Poeciliidae) Anim. Behav. 2002;63:37–45. doi:10.1006/anbe.2001.1887 [Google Scholar]
- Rosenthal G.G, de la Rosa Reyna X.F, Kazianis S, Stephens M.J, Morizot D.C, Ryan M.J, García de León F.J. Dissolution of sexual signal complexes in a hybrid zone between the swordtails Xiphophorus birchmanni and X. malinche (Poeciliidae) Copeia. 2003;2:299–307. doi:10.1643/0045-8511(2003)003[0299:DOSSCI]2.0.CO;2 [Google Scholar]
- Ryan M.J, Pease C.M, Morris M.R. A genetic polymorphism in the swordtail Xiphophorus nigrensis: testing the prediction of equal fitnesses. Am. Nat. 1992;139:21–31. doi:10.1086/285311 [Google Scholar]
- Ryan M.J, Rosenthal G.G. Variation and selection in swordtails. In: Dugatkin L.A, editor. Model systems in behavioral ecology. Princeton University Press; Princeton, NJ: 2001. pp. 133–148. [Google Scholar]
- Sih A. The mix matters: behavioral types and group dynamics in water striders. Behaviour. 2005;142:1417–1431. doi:10.1163/156853905774539454 [Google Scholar]
- Wong B.B.M. Superior fighters make mediocre fathers in the Pacific blue-eyed fish. Anim. Behav. 2004;67:583–590. doi:10.1016/j.anbehav.2003.08.015 [Google Scholar]
- Wong B.B.M, Rosenthal G.G. Female disdain for swords in a swordtail fish. Am. Nat. 2006;167:136–140. doi: 10.1086/498278. doi:10.1086/498278 [DOI] [PubMed] [Google Scholar]
- Wong B.B.M, Fisher H.S, Rosenthal G.G. Species recognition by male swordtails via chemical cues. Behav. Ecol. 2005;16:818–822. [Google Scholar]