Abstract
Many studies have demonstrated influences of climatic variation on a variety of ecological processes, however, its impact on the potent evolutionary force of sexual selection has largely been ignored. The intensity of sexual selection is a fundamental parameter in animal populations, which depends upon the degree of polygamy and will probably be influenced by the impact of local climatic variation upon ‘environmental potential for polygamy’. Here, we provide evidence of a direct effect of local climatic variation on the intensity of sexual selection, by showing a clear correlation between local weather conditions and inter-annual changes in the degree of polygamy in a long-term study of colonially breeding grey seals (Halichoerus grypus). Our results show that changes in local weather conditions alter the annual proportion of males contributing to the effective population size (Ne) by up to 61%. Consequently, over the ‘lifetime’ of a cohort, a broader range of individuals will contribute genetically to the next generation if local weather conditions are variable. In the context of predicted future changes in climatic variation, these findings have broad implications for population genetics of socially structured animal systems through the major influence that the degree of polygamy has upon Ne.
Keywords: climate, polygyny, reproductive success, effective population size, Halichoerus grypus
1. Introduction
The influence of climatic variation on ecological processes is well documented (Stenseth et al. 2002; Walther et al. 2002). At the population or community level, these studies focus on phenological (Stevenson & Bryant 2000; Both et al. 2004) and range shifts (Warren et al. 2001; Crick 2004) or population dynamics and interspecific interactions (Stenseth et al. 2002; Walther et al. 2002). At the individual level, the impacts on life-history traits (Nussey et al. 2005) and physiological responses (Bernardo & Spotila 2006) have been demonstrated. However, the effect of climatic variation on animal mating patterns remains largely unexplored, despite the fact that individual variation in reproductive success provides the impetus for the potent evolutionary force of sexual selection and is a primary determinant of genetically effective population size (Storz et al. 2001; Ardren & Kapuscinski 2003).
Changes in the degree of polygamy can have profound effects upon behavioural and demographic processes and therefore population genetics (Ardren & Kapuscinski 2003). Many factors affect variation in the degree of polygamy, in particular, the spatial and temporal distribution of resources and/or mates (Emlen & Oring 1977; Clutton-Brock 1989). Climatic variation clearly impinges upon the distribution of ephemeral resources, and consequently mates; we might therefore expect a clear link between local climate and the intensity of sexual selection in animal systems. However, this critical ecological process remains curiously absent from the literature on ecological effects of climatic variation (Stenseth et al. 2002; Walther et al. 2002).
Climate change has been linked to phenotypic changes in sexually selected traits (West & Packer 2002; Møller & Szép 2005), but the general importance of climatic variation to sexual selection is not yet known (West & Packer 2002). Here, we provide strong evidence of a direct effect of climatic variation on the intensity of sexual selection by examining individual variation in male mating success in a wild population of grey seals (Halichoerus grypus) in relation to changing weather conditions across nine successive breeding seasons at one colony.
Grey seals are polygynous, colonial and annual breeders with a discrete, predictable reproductive season. In the UK, adults aggregate to breed each autumn typically at remote island sites. Female dispersion patterns on the colony are determined by their pupping site preferences for fine-scale habitat features, in particular access to small pools of water necessary for behavioural thermoregulation (see electronic supplementary material). Access to preferred pupping sites confers greater success in raising their single pup (Pomeroy et al. 2001; Twiss et al. 2003). Males compete to maintain home ranges among female aggregations in order to gain copulations when females enter oestrus towards the end of lactation (Twiss et al. 2006). The spatio-temporal distribution of females is the ultimate determinant of environmental potential for polygamy (Emlen & Oring 1977) in grey seals as neither sex forage during the breeding season, but rely on stored energy reserves (blubber). Therefore, we predicted that inter-annual variation in rainfall, which determines pool availability, would affect the degree of polygyny in this system.
2. Material and methods
(a) Quantifying the opportunity for sexual selection
Data were gathered at the North Rona colony (Scotland) from 1996 to 2004. Behavioural observations were made from hides overlooking the study area (Twiss et al. 2006) from the 1st to 25th of October. All males present in the study area for more than 1 h were reliably identified by pelage, scarring patterns and/or brands (Twiss et al. 2006). Between 101 and 153 males were individually identified in each breeding season. The majority of these males (95%) were present for three or fewer breeding seasons and only two individual males were present for all 9 years. All observed successful copulations (where intromission was clearly achieved and the copulation proceeded, uninterrupted, to completion) were recorded (Twiss et al. 2006). Mating success for each male was defined as the number of successful copulations with females not observed copulating previously with either the same or other males. Measures of reproductive success for a subset of genotyped males correlate well with these behavioural measures of their mating success at this colony (Twiss et al. 2006). Therefore, in this study, we are confident that variation in male mating success is a reliable and accurate index of variation in reproductive success and representative of the degree of polygyny. We used these measures to compute the standardized variance in male mating success (Imales=variance/(mean mating success2)) as a measure of the opportunity for sexual selection for each breeding season (Wade & Arnold 1980).
(b) Mapping seal locations
The daily locations of all females (with identities where known), births, matings and hourly locations of all males (with identities) within the study area were recorded precisely (sub-metre accuracy) in an ARC–INFO GIS (Twiss et al. 2003, 2006). This spatial database permitted the computation of male home ranges (95% contours using kernel estimators) and measures of female density (median nearest neighbour distances). Examination of inter-diem locational shifts of known females provided data on the extent of female movement. Not all datasets were available for all nine seasons due to differing data collection priorities.
(c) Weather data
Total rainfall (mm) for October was obtained from the Meteorological Office. The closest consistent historic record is from the meteorological station at Stornoway Airport, 105 km Southwest of North Rona. There was no significant temporal autocorrelation in annual rainfall data. Rainfall measurements made by S.D.T on North Rona between 2001 and 2004 correlate with the Stornoway measurements, although the sample size is small (R2=0.94, n=4, p=0.06).
3. Results
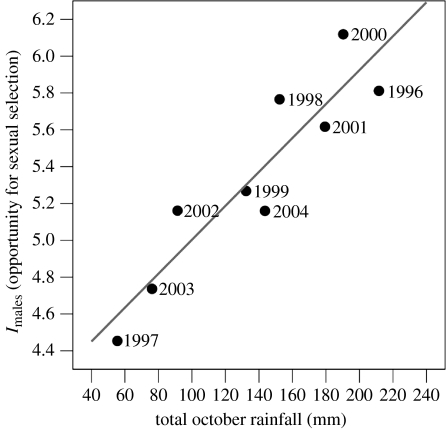
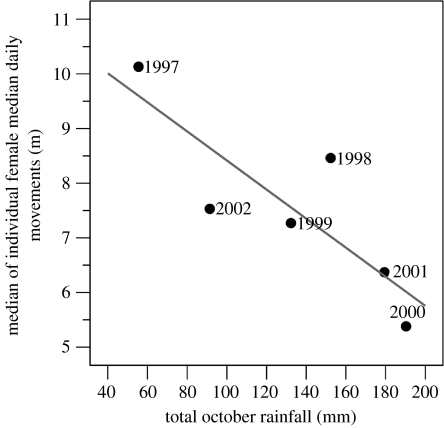
We found a highly supported positive correlation (two-tailed Spearman's rank) between the opportunity for sexual selection (Imales) and rainfall (figure 1), indicating greater polygyny when pools of water were abundant on the colony. Given that a male's ability to monopolize mating opportunities is related to operational sex ratios and the spatio-temporal distribution of females (Emlen & Oring 1977), we examined if any of these factors also exhibited inter-annual relationships with either Imales or rainfall (see electronic supplementary material) in order to discern a potential underlying mechanism. No significant correlations were found with annual measures of (i) total number of individually identified males, (ii) total number of observed successful copulations, (iii) median duration of male stay ashore, (iv) median size of male home range, (v) mean daily female: male ratio, (vi) ‘female-days’ (an index of total female numbers), and (vii) median female nearest neighbour distance (index of female dispersion patterns). However, there was a negative correlation between rainfall and female movement, such that females tended to be more mobile in drier years (figure 2), although this trend was non-significant after correction for multiple tests. However, this trend supports previous observations at this colony (Redman et al. 2001).
Figure 1.
The standardized variation in mating success among male grey seals at North Rona was more extreme in years with heavier rainfall (R=0.92, p<0.001, n=9).
Figure 2.
Female grey seals were more mobile in drier seasons (R=−0.83, p=0.042, n=6). Median of individual female median daily movements in m, which is equal to the median distance moved between successive days for each known female using their daily mapped locations, then collating these values for each year to compute an overall median for daily female movements.
4. Discussion
We have shown here that the opportunity for sexual selection varies in response to climatic variation in this colony of grey seals, with a reduced degree of polygyny in drier seasons. Furthermore, our data identify a probable underlying mechanism for this relationship. The increased mobility of females in drier seasons reduces the ability of dominant males to monopolize matings, allowing a greater number of less dominant males to gain matings.
Why are females more mobile in drier seasons? Female grey seals exhibit a high degree of inter-annual site fidelity on North Rona, returning to within a median distance of 39 m from their previous pupping sites (Pomeroy et al. 2005). Having pupped, females tend to be ‘tied’ to the pupping location for the remainder of their stay due to the limited mobility of pups. If the season subsequently proves to be dry with few pools, females without access to pools are forced to commute, sometimes relatively long distances to access this important resource, and then return to nurse their pups (Redman et al. 2001).
Autumnal weather patterns in the UK are projected to change over this century with significant increases in inter-annual variability of precipitation and temperature (Hulme et al. 2002). Although many studies have examined the potential responses of a variety of ecological processes to such climate change scenarios (Stenseth et al. 2002; Walther et al. 2002), very few have explored the impact on sexual selection (Møller & Szép 2005; Spottiswoode et al. 2006). Our findings demonstrate changes in individual behaviour that lead to a shift in the distribution of individual reproductive success (degree of polygyny) over time within a population. Such individual level responses are potentially rapid and plastic, and have wide-ranging consequences.
The degree of polygyny is a primary determinant of genetically effective population size (Ne), and therefore changes in the variance of reproductive success can have profound consequences for the behavioural, demographic (including extinction risk) and evolutionary dynamics of populations (Storz et al. 2001; Laporte & Charlesworth 2002; Ardren & Kapuscinski 2003). Increases in polygyny will decrease the diversity of a population in isolation, since Ne is ‘reduced by an increased male fertility variance’ (Laporte & Charlesworth 2002). Temporal variation of Ne within a wild vertebrate population has only recently been shown (Ardren & Kapuscinski 2003). We have found, for the first time to our knowledge, inter-annual variation in the degree of polygyny, with its consequent impact on Ne, in relation to climatic variation. In our study, increased rainfall enhances the degree of polygyny, while dry years allow a greater number of previously unsuccessful males to sire offspring. A total of 23 males gained at least one copulation in the wettest year, compared with 37 in the driest year, representing a 61% increase in the number of males contributing to Ne. While the precise relationship in other polygamous populations or species may differ, the important conclusion is that over the ‘lifetime’ of a cohort, a broader range of individuals will contribute genetically to the next generation if local weather conditions are variable, than expected from the variance in mating success within a single breeding episode. This subtle effect of changing climatic variability has widespread implications, as relatively few animal populations have mating patterns in which the level and form of polygamy are not intimately linked to resource distribution (Clutton-Brock 1989).
Acknowledgments
This work was funded by a NERC grant and postdoctoral fellowships. V.P. was supported by an NSERC postgraduate scholarship. We thank R. Harcourt, S. Moss, P. Redman and S. Ruddell for their help in the field, and S. A. Richards for his helpful comments on the manuscript.
Supplementary Material
Background information for study site and species and additional details of analyses
References
- Ardren W.R, Kapuscinski A.R. Demographic and genetic estimates of effective population size (Ne) reveals genetic compensation in steelhead trout. Mol. Ecol. 2003;12:35–49. doi: 10.1046/j.1365-294x.2003.01705.x. doi:10.1046/j.1365-294X.2003.01705.x [DOI] [PubMed] [Google Scholar]
- Bernardo J, Spotila J.R. Physiological constraints on organismal response to global warming: mechanistic insights from clinally varying populations and implications for assessing endangerment. Biol. Lett. 2006;2:135–139. doi: 10.1098/rsbl.2005.0417. doi:10.1098/rsbl.2005.0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both C, et al. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. B. 2004;271:1657–1662. doi: 10.1098/rspb.2004.2770. doi:10.1098/rspb.2004.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T.H. Mammalian mating systems. Proc. R. Soc. B. 1989;236:339–372. doi: 10.1098/rspb.1989.0027. [DOI] [PubMed] [Google Scholar]
- Crick H.Q.P. The impact of climate change on birds. Ibis. 2004;146:48–56. doi:10.1111/j.1474-919X.2004.00327.x [Google Scholar]
- Emlen S.T, Oring L.W. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. doi:10.1126/science.327542 [DOI] [PubMed] [Google Scholar]
- Hulme, M. et al 2002 Climate Change Scenarios for the United Kingdom. The UKCIP02 Scientific Report. Tyndal Centre for Climate Change Research, School of Environmental Sciences, University of East Anglia.
- Laporte V, Charlesworth B. Effective population size and population subdivision in demographically structured populations. Genetics. 2002;162:501–519. doi: 10.1093/genetics/162.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller A.P, Szép T. Rapid evolutionary change in a secondary sexual character linked to climatic change. J. Evol. Biol. 2005;18:481–495. doi: 10.1111/j.1420-9101.2004.00807.x. doi:10.1111/j.1420-9101.2004.00807.x [DOI] [PubMed] [Google Scholar]
- Nussey D.H, Clutton-Brock T.H, Albon S.D, Pemberton J, Kruuk L.E.B. Constraints on plastic responses to climate variation in red deer. Biol. Lett. 2005;1:457–460. doi: 10.1098/rsbl.2005.0352. doi:10.1098/rsbl.2005.0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy P.P, Worthington-Wilmer J, Amos W, Twiss S.D. Reproductive performance links to fine scale spatial patterns of female grey seal relatedness. Proc R. Soc. B. 2001;268:711–717. doi: 10.1098/rspb.2000.1422. doi:10.1098/rspb.2000.1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy P.P, Redman P.R, Ruddell S.J.S, Duck C.D, Twiss S.D. Breeding site choice fails to explain interannual associations of female grey seals. Behav. Ecol. Sociobiol. 2005;57:546–556. doi:10.1007/s00265-004-0882-6 [Google Scholar]
- Redman P, Pomeroy P.P, Twiss S.D. Grey seal maternal attendance patterns are affected by water availability on North Rona, Scotland. Can. J. Zool. 2001;79:1073–1079. doi:10.1139/cjz-79-6-1073 [Google Scholar]
- Spottiswoode C.N, Tøttrup A.P, Coppack T. Sexual selection predicts advancement of avian spring migration in response to climate change. Proc. R. Soc. B. 2006;273:3023–3029. doi: 10.1098/rspb.2006.3688. doi:10.1098/rspb.2006.3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth N.C, Mysterud A, Ottersen G, Hurrell J.W, Chan K.-S, Lima M. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. doi:10.1126/science.1071281 [DOI] [PubMed] [Google Scholar]
- Stevenson I.R, Bryant D.M. Climate change and constraints on breeding. Nature. 2000;406:366–367. doi: 10.1038/35019151. doi:10.1038/35019151 [DOI] [PubMed] [Google Scholar]
- Storz J.F, Bhat H.R, Kunz T.H. Genetic consequences of polygyny and social structure in an Indian fruit bat, Cynopterus sphinx. II. Variance in male mating success and effective population size. Evolution. 2001;55:1224–1232. doi: 10.1111/j.0014-3820.2001.tb00642.x. doi:10.1554/0014-3820(2001)055[1224:GCOPAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Twiss S.D, Duck C, Pomeroy P.P. Grey seal (Halichoerus grypus) pup mortality not explained by local breeding density on North Rona, Scotland. J. Zool. Lond. 2003;259:83–91. [Google Scholar]
- Twiss S.D, Pomeroy P.P, Graves J.A, Poland V.F. Finding fathers—spatio-temporal analysis of paternity assignment in grey seals (Halichoerus grypus) Mol. Ecol. 2006;15:1939–1953. doi: 10.1111/j.1365-294X.2006.02927.x. doi:10.1111/j.1365-294X.2006.02927.x [DOI] [PubMed] [Google Scholar]
- Wade M.J, Arnold S.J. The intensity of sexual selection in relation to male sexual behaviour, female choice, and sperm precedence. Anim. Behav. 1980;28:446–461. doi:10.1016/S0003-3472(80)80052-2 [Google Scholar]
- Walther G.-R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.-M, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Warren M.S, et al. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature. 2001;414:65–69. doi: 10.1038/35102054. doi:10.1038/35102054 [DOI] [PubMed] [Google Scholar]
- West P.M, Packer C. Sexual selection, temperature and the lion's mane. Science. 2002;297:1339–1343. doi: 10.1126/science.1073257. doi:10.1126/science.1073257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Background information for study site and species and additional details of analyses