Abstract
Novel adaptations often cause pleiotropic reductions in fitness. Under optimal conditions individual organisms may be able to compensate for, or reduce, these fitness costs. Declining environmental quality may therefore lead to larger costs. We investigated whether reduced plant quality would increase the fitness costs associated with resistance to Bacillus thuringiensis in two populations of the diamondback moth Plutella xylostella. We also measured the rate of decline in resistance on two host-plant (Brassica) species for one insect population (Karak). Population×plant species interactions determined the fitness costs in this study. Poor plant quality increased the fitness costs in terms of development time for both populations. However, fitness costs seen in larval survival did not always increase as plant quality declined. Both the fitness and the stability experiment indicated that fitness costs were higher on the most suitable plant for one population. Theoretically, if the fitness cost of a mutation interacts additively with environmental factors, the relative fitness of resistant insects will decrease with environmental quality. However, multiplicative costs do not necessarily increase with declining quality and may be harder to detect when fitness parameters are more subject to variation in poorer environments.
Keywords: Bacillus thuringiensis, fitness cost, gene X environment interaction, tritrophic interaction, Plutella, Brassica
1. Introduction
Adaptation to novel evolutionary challenges such as pesticides or new habitats often imposes a fitness cost (Crow 1957), which is a reduction in fitness relative to susceptibles in the absence of the selective agent. Fitness costs are commonly enhanced or more easily detected in stressful environments: e.g. under high competition (Kraaijeveld & Godfray 1997; Raymond et al. 2005); in low nutrient conditions (Bergelson 1994); or during overwintering (Foster et al. 1997; Carrière et al. 2001). Thus, costs may be reduced when environmental conditions are benign or when resource quality is high, perhaps because physiologically less stressed organisms may be able to rely on compensatory mechanisms to mask physiological deficiencies. Host-plant defences can represent a significant source of stress and mortality for herbivores. Moreover, the magnitude and genetics of fitness costs of resistance to the bacterium Bacillus thuringiensis (Bt) can be altered by plant defence compounds (Carrière et al. 2004) and may be greater on crop plants that are better defended against herbivore attack (Janmaat & Myers 2005). If the fitness costs of resistance to pesticides can vary predictably with food-plant defences, these characteristics may be important tools in resistance management. Conversely, high resource quality (e.g. escape from competition) may be generally important for the spread of novel adaptations through a population.
We hypothesized that fitness costs to a lepidopteran host associated with resistance to Bt should increase on better-defended food plants. Our study species, the diamondback moth, Plutella xylostella L., has repeatedly evolved resistance to Bt (Ferré & Van Rie 2002). This bacterium is widely used as a bio-pesticide (e.g. DiPel) and Bt genes expressing insect-specific toxins are commonly incorporated into genetically modified crops.
2. Material and methods
(a) Insects, plants and selection protocols
Two resistant populations of P. xylostella (Karak and Keluang) were collected from Malaysia in 2001 and 2002, respectively, from Chinese cabbage, Brassica pekinensis, farms 200 km apart that are regularly sprayed with DiPel. The strains differ in their mode of inheritance of resistance: incompletely recessive in Karak (Sayyed et al. 2004); semi-dominant in Keluang (A. Sayyed 2003, unpublished data), although the mechanism of resistance has only been described for Karak (Sayyed et al. 2004). Revertant strains, i.e. previously selected strains that had lost resistance to Bt, were produced by long-term culture on Chinese cabbage B. pekinensis; resistant strains were selected periodically with Cry1Ac (the predominant crystal toxin in DiPel) as described previously (Raymond et al. 2005). Prior to experiments, resistance levels to Cry1Ac were checked using leaf-dip bioassays (Sayyed et al. 2000).
The common cabbage, Brassica oleracea L., var. ‘Wheelers Imperial’, and the Chinese cabbage, B. pekinensis, var. ‘One Kilo, S.B’, were used in this study at eight and five weeks old, respectively. B. oleracea has defences against lepidopteran attack (waxiness, toughness and glucosinolate levels) that are much reduced in B. pekinensis. Plants were grown in a climate-controlled glasshouse maintained at 20±1°C with supplementary lighting (400 W sodium and halide lamps: min. incident radiation 300 W m−2).
(b) Performance of revertant and resistant populations on two plant species
Cry1Ac resistant and susceptible revertant strains from the Karak and Keluang populations were used in a full-factorial experiment with plants of both Brassica species. Individual plants (n=9–10) were randomly allocated to treatments. We added 45 and 35 freshly emerged neonates to each B. oleracea and B. pekinensis plant, respectively. Mean mass and number of larvae on each plant were recorded after 5 days. After 7 days, plants were checked daily and pupae removed and weighed. Female fecundity was measured by rearing up to three individual pairs from each plant in 50 mm diameter Petri dishes (one dish per pair) with access to 20% honey solution for 5 days. The means of data from each plant were used in analyses to avoid pseudo-replication.
(c) Stability of resistance on B. oleracea and B. pekinensis
This experiment tested whether variation in plant-associated fitness costs in the Karak population would lead to different rates of decline of resistance in culture. Each replicate was initiated with 200 neonates from the resistant Karak population with four plants per replicate cage and four cages per treatment. Thereafter, each generation was propagated with 40–50 pupae. Replicates were reared in individual culture cages (at 25±1°C, 50–60% RH, 16 h photophase) for three generations. Thereafter, all populations were reared for a single generation on B. pekinensis in order to control for plant-based maternal effects and bioassayed in the following generation (N=35–40 larvae per dose and six doses).
(d) Data analysis
Statistical analysis was carried out in R (http://www.r-project.org) using analysis of variance and generalized linear modelling. Proportional data were logit-transformed and models were scaled to correct for overdispersion, where appropriate. The stability experiment was analysed with a mixed model analysis of covariance, with replicate as a random effect and bioassay dose nested within replicate. In the mixed model, mortality data were arcsine transformed and doses log-transformed.
3. Results
(a) Performance of revertant and resistant populations on two plant species
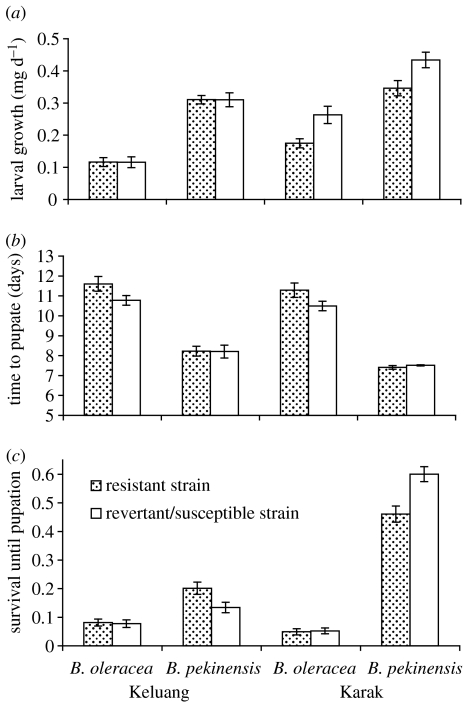
Susceptibility to Cry1Ac differed between resistant and revertant lines of the Keluang (F1,17=15.0, p<0.01) and Karak populations (F1,6=44.2, p<0.001) immediately prior to this experiment. The LC50s for the Keluang lines were 30.1 and 1.50 μg ml−1, respectively and for the Karak lines were 96.7 and 3.3 μg ml−1, respectively. Brassica oleracea was demonstrably a poorer quality host for P. xylostella: growth rates were slower and survival was reduced on this species (figure 1a–c; table 1).
Figure 1.
Performance of Cry1Ac resistant and revertant/susceptible P. xylostella from two populations (Karak and Keluang) on the plant species B. oleracea and B. pekinensis. Data are mean±s.e. Standard errors for survival are based on the normal approximation to the binomial.
Table 1.
Summary of ANOVAs exploring the factors affecting five fitness components of Cry1Ac resistant and revertant susceptible populations of P. xylostella. (Two insect populations (Karak and Keluang) and two host-plant species (B. oleracea and B. pekinensis) were used in this experiment, n=9–10 plants per treatment. Key to significance levels: *p<0.05; **p<0.01; ***p<0.001.)
| fitness component | larval growth rate | survival till late larva | development time | pupal weight | survival till pupation | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| source of variation | d.f. | F | d.f. | F | d.f. | F | d.f. | F | d.f. | F |
| plant species | 1 | 165.1*** | 1 | 55.6*** | 1 | 312.4*** | 1 | 37.9*** | 1 | 144.5*** |
| population | 1 | 42.5*** | 1 | 4.45* | 1 | 10.75** | 1 | 13.0*** | 1 | 22.7*** |
| resistance | 1 | 8.10** | 1 | 0.09 | 1 | 4.41* | 1 | 0.44 | 1 | 0.09 |
| plant×popn | 1 | 0.62 | 1 | 26.0*** | 1 | 0.88 | 1 | 9.76** | 1 | 56.1*** |
| plant×resist | 1 | 0.02 | 1 | 0.005 | 1 | 5.27* | 1 | 0.002 | 1 | 0.01 |
| resist×popn | 1 | 9.27** | 1 | 0.18 | 1 | 0.07 | 1 | 1.26 | 1 | 2.66 |
| resist×plant×popn | 1 | 0.001 | 1 | 1.84 | 1 | 0.01 | 1 | 1.82 | 1 | 4.07* |
| residuals | 73 | 77 | 70 | 68 | 71 | |||||
Fitness costs did not consistently increase on the poorer quality plant species. Karak insects showed reduced larval growth rates relative to revertant susceptibles on both species (table 1; figure 1a). Only development time showed increasing fitness costs with declining plant quality across both populations (table 1; figure 1b). Resistance affected survival to pupation differently for the two populations, there being a significant three-way interaction between resistance, cabbage species and population (table 1; figure 1c). Karak larvae showed fitness costs in terms of survival on the high-quality plant (B. pekinensis), whereas Keluang larvae showed the opposite pattern, i.e. improved survival of resistant larvae on B. pekinensis (figure 1c).
For the other fitness components, there was no detectable difference between resistant and susceptible sub-populations (table 1). There were insufficient data to analyse the fecundity of Karak insects feeding on B. oleracea, owing to poor survival to pupation (figure 1c). However, resistance did not influence the fecundity of Keluang insects (F1,25=0.2, p>0.05) nor the fecundity of Karak insects reared on B. pekinensis (F1,17=0.29, p>0.05).
(b) Stability of resistance on B. oleracea and B. pekinensis in the Karak strain
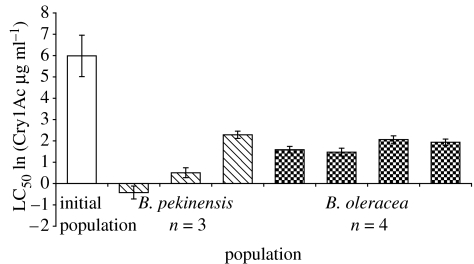
We expected that resistance would decline more rapidly on the plant species (B. pekinensis) on which Karak showed higher relative fitness costs in terms of survival (figure 1c). One B. pekinensis replicate went extinct in this experiment, leaving four replicates on B. oleracea and three on B. pekinensis. As predicted, Cry1Ac mortality was highest in the populations cultured on B. pekinensis (plant species treatment, mixed model ANCOVA, d.f.=1,6, likelihood ratio 5.65, p=0.017). Inspection of the 95% confidence limits of the LC50s confirms that resistance fell faster in two out of the three B. pekinensis replicates relative to the B. oleracea replicates (figure 2).
Figure 2.
The susceptibility to the Bt toxin Cry1Ac after three generations of culture on two different plants species. Data are LC50s for individual replicates in each treatment (N=3 or 4), with 95% confidence limits and the LC50 of the population used to start the experiment. Data are calculated from ln-transformed doses.
4. Discussion
Contrary to our expectation, the lower quality of plants did not consistently increase the fitness costs of resistance to Bt in P. xylostella. Larval development time was extended for both resistant Karak and Keluang populations on the better-defended host. However, fitness costs acting on survival depended on interactions between population and environment, as found previously (Carrière et al. 2004; Raymond et al. 2005), and for one population (Karak) increased on the higher-quality plants. While population-level effects were tested with very low statistical power (n=2) in the performance experiment, these results reflect our experience of the stability of resistance in these two populations in culture and formal experiments (figure 2). Independent mutations conferring resistance in different strains could be responsible for the variation in fitness costs. Alternatively, differences in genetic background can also alter pleiotropic fitness costs (Bohannan et al. 1999).
A mechanistic explanation of different patterns shown by different performance parameters (survival and development time) with respect to resource quality is beyond the scope of this paper. However, some simple theory can illustrate how different models of fitness costs can make very different predictions for the impact of resource quality on fitness costs. If fitness costs act additively, the proportional reduction in fitness is independent of resource quality. Thus, the fitness of homozygous susceptibles is WSS=1−q, where q is the impact of resource quality on fitness, and the fitness of homozygous resistant individuals is WRR=1−q−c, where c is the cost of resistance. If c+q>1, WRR is assumed to be zero. Under a multiplicative model, the effects of resistance are a fixed proportion of the effects of resource quality, thus WRR=(1−q)(1−c). The relative fitness of resistant individuals is WRR/WSS in all cases. Using data from these experiments, it can be seen that the two different models make very different predictions (table 2).
Table 2.
The impact of resource quality (q) and additive or multiplicative fitness costs on the relative fitness of resistant and susceptible strains. (Fitness calculated as per discussion, see §4.)
| c-fitness cost | additive costs q=0.4 | additive costs q=0.6 | additive costs q=0.9 | multiplicative costs (all q>0) |
|---|---|---|---|---|
| 0.05 | 0.92 | 0.83 | 0.5 | 0.95 |
| 0.1 | 0.83 | 0.67 | 0 | 0.90 |
| 0.15 | 0.75 | 0.50 | 0 | 0.85 |
| 0.2 | 0.67 | 0.33 | 0 | 0.80 |
| 0.25 | 0.58 | 0.17 | 0 | 0.75 |
| 0.3 | 0.50 | 0 | 0 | 0.7 |
There are no a priori reasons for expecting either the multiplicative or the additive model to be true for the fitness costs associated with novel adaptations. With a multiplicative model, only additional costs (i.e. an increase in c with declining quality) will alter relative fitness. Differences in fitness at lower resource quality may also be harder to resolve if poor resource quality leads to lower sample size and reduced statistical power. With P. xylostella, fitness costs for survival of Cry1Ac resistant individuals have only been identified on B. oleracea plants significantly older than those used in this study (four months and above; Raymond et al. 2005; Raymond et al. 2006, unpublished data). Older plants are increasingly better defended against insect attack (Verkerk & Wright 1994), suggesting that values of c may vary within species with only subtle variations in resource quality. If there is an additive component to fitness costs, a decline in resource quality will automatically increase the difference in relative fitness between genotypes. Greater reductions in resource quality will also lead to larger differences in relative fitness, as found for Bt-resistant Trichoplusia ni feeding on a range of species (Janmaat & Myers 2005).
Acknowledgments
The Biotechnology and Biological Sciences Research Council (grant D15960) funded this study.
References
- Bergelson J. The effects of genotype and the environment on costs of resistance in lettuce. Am. Nat. 1994;143:349–359. doi:10.1086/285607 [Google Scholar]
- Bohannan B.J.M, Travisano M, Lenski R.E. Epistatic interactions can lower the cost of resistance to multiple consumers. Evolution. 1999;53:292–295. doi: 10.1111/j.1558-5646.1999.tb05355.x. doi:10.2307/2640942 [DOI] [PubMed] [Google Scholar]
- Carrière Y, Ellers-Kirk C, Patin A.L, Sims M.A, Meyer S, Liu Y.B, Dennehy T.J, Tabashnik B.E. Overwintering cost associated with resistance to transgenic cotton in the pink bollworm (Lepidoptera: Gelechiidae) J. Econ. Entomol. 2001;94:935–941. doi: 10.1603/0022-0493-94.4.935. [DOI] [PubMed] [Google Scholar]
- Carriere Y, Ellers-Kirk C, Biggs R, Higginson D.M, Dennehy T.J, Tabashnik B.E. Effects of gossypol on fitness costs associated with resistance to Bt in pink bollworm. J. Econ. Entomol. 2004;97:1710–1718. doi: 10.1603/0022-0493-97.5.1710. [DOI] [PubMed] [Google Scholar]
- Crow J.F. Genetics of insect resistance to chemicals. Annu. Rev. Entomol. 1957;2:227–246. doi:10.1146/annurev.en.02.010157.001303 [Google Scholar]
- Ferré J, Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2002;47:501–533. doi: 10.1146/annurev.ento.47.091201.145234. doi:10.1146/annurev.ento.47.091201.145234 [DOI] [PubMed] [Google Scholar]
- Foster S.P, Harrington R, Devonshire A.L, Denholm I, Clark S.J, Mugglestone M.A. Evidence for a possible fitness trade-off between insecticide resistance and the low temperature movement that is essential for survival of UK populations of Myzus persicae (Hemiptera: Aphididae) Bull. Entomol. Res. 1997;87:573–579. [Google Scholar]
- Janmaat A.F, Myers J.H. The cost of resistance to Bacillus thuringiensis varies with the host plant of Trichoplusia ni. Proc. R. Soc. B. 2005;272:1031–1038. doi: 10.1098/rspb.2004.3040. doi:10.1098/rspb.2004.3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijeveld A.R, Godfray H.C.J. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. doi:10.1038/38483 [DOI] [PubMed] [Google Scholar]
- Raymond B, Sayyed A.H, Wright D.J. Genes and environment interact to determine the fitness costs of resistance to Bacillus thuringiensis. Proc. R. Soc. B. 2005;272:1519–1524. doi: 10.1098/rspb.2005.3103. doi:10.1098/rspb.2005.3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyed A.H, Haward R, Herrero S, Ferré J, Wright D.J. Genetic and biochemical approach for characterization of resistance to Bacillus thuringiensis toxin Cry1Ac in a field population of the diamondback moth, Plutella xylostella. Appl. Environ. Microbiol. 2000;66:1509–1516. doi: 10.1128/aem.66.4.1509-1516.2000. doi:10.1128/AEM.66.4.1509-1516.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyed A.H, Raymond B, Ibiza-Palacios M.S, Escriche B, Wright D.J. Genetic and biochemical characterization of field evolved resistance to Bacillus thuringiensis toxin Cry1Ac in diamondback moth, Plutella xylostella. Appl. Environ. Microbiol. 2004;70:7010–7017. doi: 10.1128/AEM.70.12.7010-7017.2004. doi:10.1128/AEM.70.12.7010-7017.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk R.H.J, Wright D.J. Interactions between the diamondback moth, Plutella xylostella L. and glasshouse and outdoor-grown cabbage cultivars. Ann. Appl. Biol. 1994;125:477–488. [Google Scholar]