Abstract
In a given area, plant–animal mutualistic interactions form complex networks that often display nestedness, a particular type of asymmetry in interactions. Simple ecological and evolutionary factors have been hypothesized to lead to nested networks. Therefore, nestedness is expected to occur in other types of mutualisms as well. We tested the above prediction with the network structure of interactions in cleaning symbiosis at three reef assemblages. In this type of interaction, shrimps and fishes forage on ectoparasites and injured tissues from the body surface of fish species. Cleaning networks show strong patterns of nestedness. In fact, after controlling for species richness, cleaning networks are even more nested than plant–animal mutualisms. Our results support the notion that mutualisms evolve to a predictable community-level structure, be it in terrestrial or marine communities.
Keywords: asymmetrical interactions, coevolutionary networks, coral reefs, null models
1. Introduction
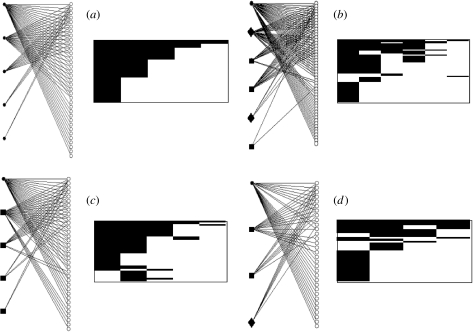
In ecological communities, each species interacts in different ways with one to several species forming networks of interacting species (Pascual & Dunne 2006). The network approach has been useful for investigating the structure and fragility of ecological interactions, and comparative studies of network structure help to uncover community-level patterns of ecological specialization in different types of interspecific interactions (Jordano et al. 2003; Vázquez & Aizen 2004; Vázquez et al. 2005; Pascual & Dunne 2006). In this context, recent studies demonstrate that the network structure of species-rich, plant–animal mutualisms is often nested, whereas antagonistic interactions are usually non-nested (Bascompte et al. 2003; Guimarães et al. 2006). Nestedness is a specific type of asymmetric interactions characterized by (i) species with many interactions form a core of interacting species, (ii) species with few interactions commonly interact only with species with many interactions and (iii) the absence of interactions between species with few interactions (figure 1).
Figure 1.
Network and matrix representations of community-level patterns of interactions between cleaners and clients. Lines and black squares represent interactions between cleaners (closed symbols and columns) and clients (open circles and rows). Cleaner fishes are represented by circles (Elacatinus) and squares (other genera), and cleaner shrimps are represented by diamonds. (a) Hypothetical, perfectly nested network, (b) Bonaire, (c) Abrolhos and (d) St Croix. Networks were drawn in Pajek (http://vlado.fmf.uni-lj.si/pub/networks/pajek/).
Nestedness is expected to appear in ecological interactions such as mutualisms between free-living species, in which the core of generalist species forms a stable set of resources, allowing the evolution of specialized lifestyles (Thompson 2005). Additionally, it was recently suggested that ecological factors such as differences in abundance among species may explain asymmetric interactions (Vázquez & Aizen 2004; Vázquez et al. 2005) and, more specifically, nestedness (Lewinsohn et al. 2006). If nestedness is indeed generated by simple coevolutionary and ecological processes such as those mentioned previously, it is expected to characterize mutualistic networks irrespective of the identity of interacting species. In fact, nestedness should be a common feature of other types of mutualisms beyond those investigated so far for plants and animals (Bascompte et al. 2003; Guimarães et al. 2006).
Here, we investigate for the first time the community-level patterns of interactions observed in cleaning symbiosis in the reef environment. In this type of mutualism, the so-called cleaners (shrimps or fishes) forage on ectoparasites, diseased or injured tissues and mucus from the body surface of fish species called clients, which in their turn get rid of unwanted material (Floeter et al. in press). Cleaning is a common and widespread type of foraging association between reef species, recorded for several animal taxa and geographical sites (Floeter et al. in press). We studied nested patterns in three cleaning networks. We specifically addressed the following questions: does nestedness characterize cleaning networks; and are the patterns of interactions observed for cleaners and clients similar to those previously observed in plant–animal mutualistic networks?
2. Material and methods
(a) Studied communities
Most studies on cleaning symbiosis between reef animals focus on one or a few species (e.g. Sazima et al. 1999) and community-level patterns of these interactions have been largely ignored (but see Floeter et al. in press). Here, we use data on cleaners and clients in three different assemblages of reef animals in the Western Atlantic (Johnson & Ruben 1988; Wicksten 1998; Sazima 2002) and compare them with patterns found in studies of nestedness in plant–animal mutualistic networks (Bascompte et al. 2003; Guimarães et al. 2006). The three above-mentioned studies on cleaning symbiosis deal with the majority of cleaner and client species within the studied assemblages and thus are appropriate for assessing community-level patterns. Moreover, these assemblages have more than three species of cleaners each, allowing the emergence of nestedness. The studied cleaners may be grouped into four broad categories: (i) fishes cleaning through whole life cycle (Elacatinus=Gobiosoma), (ii) fishes cleaning only or mostly while juveniles (e.g. Bodianus, Pomacanthus, Thalassoma), (iii) fishes cleaning sporadically either as adults or juveniles (e.g. Chaetodon), and (iv) shrimps (e.g. Periclimenes). The studied assemblages were at (i) Abrolhos Archipelago, Western South Atlantic (hereafter Abrolhos), where five cleaners and 35 client species were examined in a total of 70 h (Sazima 2002), (ii) Bonaire, Netherlands Antilles, Caribbean (Bonaire), where six cleaners and 50 client species were examined in a total of 700 h (Wicksten 1998) and (iii) Saint Croix, US Virgin Islands, Caribbean (St Croix), where four cleaners and 32 client species were examined in a total of 110 h (Johnson & Ruben 1988). The studied areas were composed of coral reefs, and depths of studied cleaning stations varied among the three areas from 3 to 30 m.
(b) Cleaning networks
Interspecific interactions can be described as networks in which species are nodes and interactions between any species pair are depicted as links (Jordano et al. 2003). A cleaning network is defined by an adjacency matrix R describing interactions between L cleaner species and F client species in a well-defined ecological assemblage, where rij=1 if the client j is cleaned by the species i and zero otherwise. It is important to emphasize that client–cleaner interactions do not necessarily imply mutual benefits for both species. In fact, cleaner species may act as parasites in some ecological communities. Therefore, only a subset of recorded interactions in these networks is unambiguously mutualistic. Future studies should focus on the importance of exploiters of mutualisms to network structure. Here, we follow the approach already used in other interactions and consider that all interacting species are part of the mutualistic network (Jordano et al. 2003), since there is a wide gradient of mutually beneficial effects from pure mutualism to pure antagonism or amensalism, and all them potentially influence network build-up and evolution.
(c) Nestedness
The matrix R is perfectly nested if showing a progression of inclusive subsets after ordering rows and columns in decreasing totals (Lewinsohn et al. 2006, figure 1a). We follow Bascompte et al. (2003) and define the degree of nestedness, N, as N=(100−T)/100, in which T is the matrix temperature, with values ranging from 0° (perfectly nested) to 100° (perfectly non-nested). Additional details about T are provided elsewhere (Guimarães & Guimarães 2006). We used two null models to test if the degree of nestedness is expected from basic network features (Bascompte et al. 2003). The null model 1 assumes that each randomly assigned pair of cleaner and client interacts with constant probability, C, in which C is the connectance, i.e. the proportion of interactions actually observed in the network. Therefore, it tests if the observed N is higher than expected for random networks with similar number of interactions. The null model 2 assumes that the probability that a cleaner i interacts with a client j depends on the observed number of interactions of both species, such that
| (2.1) |
in which k is the observed number of interactions for the species. Therefore, the null model 2 tests if observed N is higher than expected for random networks with similar heterogeneity of interactions among species (Bascompte et al. 2003). Each community was compared with 1000 replicates generated by each null model. We only considered replicates in which all species have at least one interaction, because species without interaction lack biological meaning. All nestedness analyses were performed using ANINHADO (Guimarães & Guimarães 2006).
To assess if the observed patterns of nestedness for cleaning networks are similar to other mutualisms, we compared the values of N recorded for the three cleaning networks with those recorded for pollination (n=25), seed dispersal (n=27), ant–plant mutualisms (n=4; dataset from Bascompte et al. 2003; Guimarães et al. 2006). To study the basic aspects of network structure, we investigate how the degree of nestedness (N) is related to species richness and the ratio between the richness of two sets (animal, plant, client or cleaner) of interacting species (richness ratio). These two aspects of network structure may affect nestedness (Guimarães et al. 2006). We investigate the relationship between nestedness and these variables using multiple regression. The degree of nestedness was angular transformed and species richness and richness ratio (set with higher number of species/set with lower number of species) were log transformed to improve normality and homoscedasticity.
3. Results
The three analysed cleaning networks show strong nested patterns, in which species with few interactions often interact with the core of species with many interactions (Abrolhos, N=0.92; Bonaire, N=0.92; St Croix, N=0.84; figure 1). These networks were significantly more nested than expected from random interactions (null model 1, p<0.001 for all networks) or from differences in the number of interactions among species (null model 2, Abrolhos and Bonaire: p<0.001 for all networks; St Croix: p=0.007).
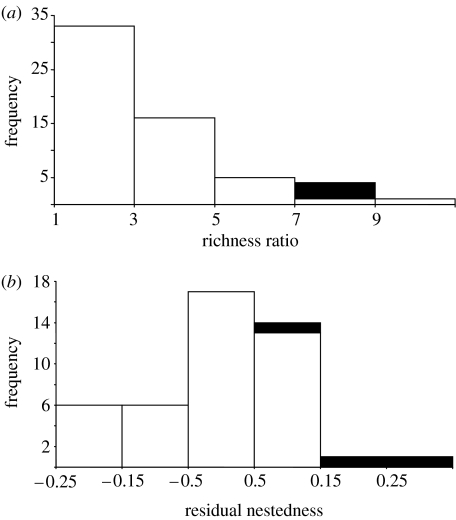
The ratio between species richness of cleaners and clients was higher than that between animals and plants in terrestrial mutualistic networks (figure 2a). In all cases, client richness was sevenfold higher than cleaner richness. However, this aspect of network structure did not affect the degree of nestedness in species-rich (more than 25 species) mutualistic networks (F=2.18, p=0.15, n=45 networks). In contrast, species richness markedly affected the degree of nestedness (F=24.12, p<0.0001). After controlling for the effects of species richness, cleaning networks show higher residual nestedness when compared with plant–animal mutualisms (figure 2b; p=0.002, randomization test, 10 000 randomizations). Thus, compared with plant–animal mutualistic networks of similar species richness, cleaning networks show more marked patterns of nested interactions.
Figure 2.
Frequency of networks per (a) richness ratio and (b) residual nestedness (see text for further details). Plant–animal mutualisms (white columns) and cleaning interactions (black columns).
4. Discussion
Cleaning interactions differ in several ways from terrestrial mutualistic networks analysed so far. For example, previously studied terrestrial mutualisms involve interactions between plants and animals, often birds and insects (Jordano et al. 2003). In contrast, the cleaning interactions in the reef environment involve a completely different set of species (fishes and shrimps) in a completely different ecosystem. Additionally, our study demonstrates that the cleaning assemblages are characterized by a few (i.e. four to six) cleaner species that maintain a highly diverse coterie of clients (typically more than 20 species). This difference in richness of cleaner and client assemblages is much higher than those observed for plants and animals in terrestrial mutualisms (Jordano et al. 2003; Vázquez & Aizen 2004). Thus, it is expected that the dynamics of cleaning symbiosis is more affected by evolutionary and ecological changes in one assemblage of species (cleaners) than plant–animal mutualisms.
In spite of differences in the component species or in basic properties of interacting assemblages, all these mutualistic interactions show strong patterns of nestedness, in which species with few interactions are linked to a core of species with many interactions. Therefore, our study broadens previous findings for interactions between terrestrial plants and animals (Bascompte et al. 2003; Guimarães et al. 2006) and indicates that mutualisms evolve to a predictable community-level structure (Thompson 2005; Lewinsohn et al. 2006), be it in terrestrial or marine assemblages.
Although caution is needed due to our small sample, our results suggest that cleaning networks may be more nested than terrestrial mutualisms with similar species richness. Thus, cleaning networks are even more asymmetric than plant–animal mutualisms in terms of the specificity of their interactions. Future studies should investigate the relative importance of ecological and evolutionary processes that might lead to the nested pattern. Symmetric interactions (i.e. reciprocal levels of specificity among interacting partner species) in plant–animal networks may be a result of the evolutionary history constraining interactions (Lewinsohn et al. 2006). Thus, we hypothesize that cleaning mutualisms may be less affected by phylogenetic constraints than other mutualisms. Consequently, convergence (Thompson 2005) and the variation in local assemblage composition to differences in abundances (Lewinsohn et al. 2006) would act more freely to generate a highly nested structure such as that in the present study. Clients and cleaners may select their partners preferring some species or individuals over others (e.g. Sazima et al. 1999). In fact, recent macroecological analyses support this idea and indicate that abundance, together with client's diet, size and behaviour, may play a key role on the patterns of interactions among cleaners and clients (Floeter et al. in press).
Acknowledgments
We thank the IBAMA (M. Skaff) for permission to study at Abrolhos; the Brazilian Navy (A. S. Silveira) and Abrolhos Turismo (R. Morais) for their logistic support. A. Valido, D. Vázquez, P. Jordano, J. N. Thompson and an anonymous reviewer provided their comments to the manuscript. J. Bascompte and V. Rico-Gray provided information about mutualistic networks. This work was supported by FAPESP.
References
- Bascompte J, Jordano P, Melián C.J, Olesen J.M. The nested assembly of plant–animal mutualistic networks. Proc. Natl Acad. Sci. USA. 2003;100:9383–9387. doi: 10.1073/pnas.1633576100. doi:10.1073/pnas.1633576100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter, S. R., Vázquez, D. P. & Grutter, A. S. In press. The macroecology of marine cleaning mutualisms. J. Anim. Ecol [DOI] [PubMed]
- Guimarães P.R, Guimarães P. Improving the analyses of nestedness for large sets of matrices. Environ. Model. Software. 2006;21:1512–1513. doi:10.1016/j.envsoft.2006.04.002 [Google Scholar]
- Guimarães P.R, Rico-Gray V, Dos Reis S.F, Thompson J.N. Asymmetries in specialization in ant–plant networks. Proc. R. Soc. B. 2006;273:2041–2047. doi: 10.1098/rspb.2006.3548. doi:10.1098/rspb.2006.3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W.S, Ruben P. Cleaning behavior of Bodianus rufus, Thalassoma bifasciatum, Gobiosoma evelynae, and Periclimenes pedersoni along a depth gradient at Salt River Submarine Canyon, St. Croix. Environ. Biol. Fishes. 1988;23:225–232. doi:10.1007/BF00004913 [Google Scholar]
- Jordano P, Bascompte J, Olesen J.M. Invariant properties in coevolutionary networks of plant–animal interactions. Ecol. Lett. 2003;6:69–81. doi:10.1046/j.1461-0248.2003.00403.x [Google Scholar]
- Lewinsohn T.M, Prado P.I, Jordano P, Bascompte J, Olesen J.M. Structure in plant–animal interaction assemblages. Oikos. 2006;113:174–184. doi:10.1111/j.0030-1299.2006.14583.x [Google Scholar]
- Pascual M, Dunne J.A. Oxford University Press; Oxford, UK: 2006. Ecological networks: linking dynamics in food webs. [Google Scholar]
- Sazima C. 2002 Atividade de limpeza de duas espécies sintópicas de peixes limpadores e diversidade de seus clientes em Abrolhos, Bahia. Zoology MSc thesis, UNESP, Rio Claro.
- Sazima I, Moura R.L, Sazima C. Cleaning activity of juvenile angelfish, Pomacanthus paru, on the reefs of the Abrolhos Archipelago, western South Atlantic. Environ. Biol. Fishes. 1999;56:399–407. doi:10.1023/A:1007531925845 [Google Scholar]
- Thompson J.N. Chicago University Press; Chicago, IL: 2005. The geographic mosaic of coevolution. [Google Scholar]
- Vázquez D.P, Aizen M.A. Asymmetric specialization: a pervasive feature of plant–pollinator interactions. Ecology. 2004;85:1251–1257. [Google Scholar]
- Vázquez D.P, Poulin R, Krasnov B.R, Shenbrot G.I. Species abundance and the distribution of specialization in host–parasite interaction networks. J. Anim. Ecol. 2005;74:946–955. doi:10.1111/j.1365-2656.2005.00992.x [Google Scholar]
- Wicksten M.K. Behaviour of cleaners and their client fishes at Bonaire, Netherlands Antilles. J. Nat. Hist. 1998;32:13–30. [Google Scholar]