Abstract
Gaze cuing, the tendency to shift attention in the direction other individuals are looking, is hypothesized to depend on a distinct neural module. One expectation of such a module is that information processing should be encapsulated within it. Here, we tested whether familiarity, a type of social knowledge, penetrates the neural circuits governing gaze cuing. Male and female subjects viewed the face of an adult male looking left or right and then pressed a keypad to indicate the location of a target appearing randomly left or right. Responses were faster for targets congruent with gaze direction. Moreover, gaze cuing was stronger in females than males. Contrary to the modularity hypothesis, familiarity enhanced gaze cuing, but only in females. Sex differences in the effects of familiarity on gaze cuing may reflect greater adaptive significance of social information for females than males.
Keywords: modularity, social cognition, attention, face, gender differences
1. Introduction
A central issue in cognitive neuroscience is the extent to which different types of information are processed in functionally distinct neural modules (Fodor 1983). One candidate module is the neural system governing gaze cuing, the tendency to shift attention in the direction other individuals are looking. Gaze cuing occurs in a fraction of a second (Friesen & Kingstone 1998; Langton & Bruce 1999), even when counter to immediate behavioural goals (Driver et al. 1999), emerges early in human development (Hood et al. 1998) and is shown by several animal species (Gomez 2005). Indeed, the spatial and temporal dynamics of gaze cuing in monkeys and humans are nearly identical (Deaner & Platt 2003). Such observations bolster the argument that gaze cuing is mediated by a dedicated and reflexive neural module which serves as the foundation for theory of mind and language (e.g. Baron-Cohen 1995).
Contextual modulations of gaze-following behaviour reported in several studies challenge this modularity hypothesis. For example, expressive faces can, under some conditions, evoke greater gaze cuing than neutral ones (Mathews et al. 2003; Hori et al. 2005; Holmes et al. 2006; but see Hietanen & Leppanen 2003). Furthermore, macaques show enhanced gaze cuing for dominant compared with subordinate monkeys (Shepherd et al. 2006). Nevertheless, the impact of these observations is limited by the fact that emotion, and possibly dominance (Keating et al. 1977), can be extracted from physical features of the face and may thus be encoded in the same feed-forward visual channel encoding eye direction. Thus, previously reported modulations of gaze cuing do not adequately test whether this system is truly encapsulated, as predicted by the modularity hypothesis. A stronger test would be to probe whether non-visual information penetrates the neural circuits controlling gaze cuing.
To do this, we tested the effects of familiarity on gaze cuing in men and women. We predicted that familiarity would enhance gaze cuing in women because they are more sensitive to social cues (Geary 1998) and show greater gaze cuing in general (Bayliss et al. 2005).
2. Material and methods
Thirty-two subjects, aged 18–39 years, participated. Seventeen were in Duke's Neurobiology Department (eight females; ‘departmental subjects’) and 15 were not, but were still affiliated with Duke (seven females). All reported normal or corrected-to-normal vision, gave written informed consent as required by the DUMC IRB, and were paid $15 for participation. To verify that departmental subjects were familiar with the gaze cue models but that non-departmental subjects were not, we administered a questionnaire showing each gaze model and asked subjects how often they had previously seen them on a 1–5 scale (1, never; 5, very often).
Stimuli were presented on a 17 in. colour LCD monitor using custom software (http://www.neurosoftware.net/). Subjects sat in a dark room with their head stabilized on a chin rest and their eyes 38 cm from the monitor. Stimuli were three images of each of six males in the Neurobiology Department: two professors (mean age 37 years), two postdoctoral associates (30) and two graduate students (32); two wore glasses and three had facial hair. Each model had one image gazing rightward, one leftward and one with eyes closed; all models faced forward. The background for each image was blackened, the image balanced for contrast and luminance and face width reduced to 94 pixels.
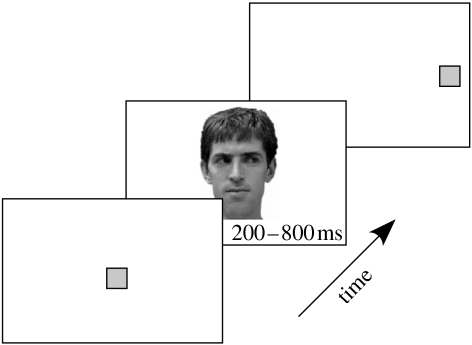
On each trial (figure 1), a yellow square (1.2°) was centrally displayed (300 or 500 ms) and then replaced by a face (5.1°) for 200, 400 or 800 ms (stimulus onset asynchrony, SOA). All the three image types (eyes left, eyes right and eyes closed) were presented randomly and with equal probability. After face offset, a 1.2° yellow target square appeared randomly 15° left or right. Participants were asked to fixate centrally and indicate the location of the target as quickly and accurately as possible with their dominant hand by pressing ‘1’ for left and ‘7’ for right on the number keypad on a computer keyboard; thus, the manual response directions, up and down, were dissociated from the indicated target direction.
Figure 1.
Target localization task. Model's gaze direction did not predict subsequent target location.
Trials were incorrect if (i) either key was pressed before the target appeared, (ii) the wrong key was pressed before the correct key, or (iii) the correct key was not pressed within 1500 ms. Correct trials were followed by a 100 ms tone and incorrect trials by a 300 ms noise and the text ‘Wrong!’ on the monitor. Twelve practice trials were followed by nine blocks of test trials. One-hundred correct trials defined a block; testing paused after each block until the subject pressed a ‘ready’ key.
For analysis, incorrect trials (2%) were eliminated and mean reaction times (RTs) were computed for each subject for congruent and incongruent trials at each SOA. RTs more than three standard deviations from each subject's overall mean were excluded. Cuing effects for each subject at each SOA were then computed by subtracting the mean RT on incongruent trials from the mean RT on congruent trials. Cuing effects served as our measure of gaze cuing. We also computed cuing effects for subjects within the Neurobiology Department when viewing faces that were categorized as either familiar or unfamiliar based on questionnaires.
3. Results
We first conducted separate repeated measures ANOVAs on the cuing effects for departmental and non-departmental subjects, with gender as a between-subjects factor (figure 2a,b). There was a significant effect of gender on cuing effects for departmental subjects (F1,15=6.4, p<0.024) but not for non-departmental subjects (F1,13=1.0, p>0.40). Moreover, cuing effects for both groups declined with increased face viewing time (SOA: departmental subjects, F2,30=12.5, p<0.0005; non-departmental subjects, F2,26=4.6, p<0.03), consistent with earlier studies (Friesen & Kingstone 1998; Langton & Bruce 1999; Deaner & Platt 2003).
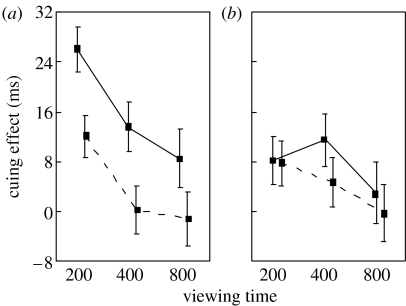
Figure 2.
Sex differences in the effect of familiarity on gaze following. Mean cuing effect (±s.e.m) for male (dashed lines) and female (solid lines) subjects from (a) within the same department (familiar) as the gaze models or (b) from outside the department (unfamiliar).
Most importantly, female departmental subjects showed the largest cuing effects, particularly at shorter SOAs. At the 200 ms SOA, cuing effects for female departmental subjects (26 ms) were on average double that of male departmental subjects (12 ms), and three times as great, on average, as either female or male non-departmental subjects (both less than 9 ms). To test the gender specificity of familiarity effects, we compared cuing effects for females based on departmental affiliation at the 200 ms SOA. Departmental females showed significantly greater cuing effects than non-departmental females (t-test: t=3.80, d.f.=13, p<0.003). In contrast, male subjects showed no effect of departmental affiliation at this SOA (t-test: t=0.86, d.f.=15, p>0.4).
We reasoned that the effects of departmental affiliation on gaze cuing for females reflected their familiarity with the gaze models. Consistent with this hypothesis, questionnaire responses revealed that departmental females were significantly more familiar with the gaze models than were non-departmental females (repeated measures ANOVA with department as a between-subjects factor; F1,13=364.4, p<0.00001). Familiarity rankings for females were bimodally distributed (Shapiro–Wilk's W=0.68, p<0.0001), so we classified gaze models as familiar (greater than or equal to 3) or unfamiliar (less than 3) for each female. Non-departmental females were unfamiliar with all models, whereas departmental females reported 50–100% as familiar (mean 63%).
The preceding analyses strongly support the hypothesis that familiarity enhances gaze cuing in women but not men. To test this idea further, we analysed cuing effects for female and male departmental subjects as a function of familiarity. Since both these groups were generally familiar with the gaze models, we anticipated that familiarity effects on gaze cuing within the department would be small. There was a non-significant tendency for departmental females, but not males, to show stronger gaze cuing for familiar versus unfamiliar models (repeated measures ANOVA with gender as between-subjects factor; F1,14=2.0, p<0.18). This familiarity effect for females was strongest at the 400 ms SOA (18.24 ms familiar, 7.89 ms unfamiliar; post hoc least significant difference (LSD) test: p<0.07). For all but one female departmental subject, cuing effects increased with familiarity at the 400 ms SOA. Male departmental subjects showed no effect of familiarity at any SOA (post hoc LSD tests: all p's>0.18). A summary of group data may be found in table 1.
Table 1.
Group data for all conditions.
| SOA | condition | familiarity | sex | RT μ±σ | sex | RT μ±σ |
|---|---|---|---|---|---|---|
| 200 | congruent | departmental | F | 260±80 | M | 250±68 |
| 200 | incongruent | departmental | F | 284±82 | M | 260±61 |
| 200 | eyes closed | departmental | F | 272±78 | M | 262±62 |
| 400 | congruent | departmental | F | 255±70 | M | 252±65 |
| 400 | incongruent | departmental | F | 265±75 | M | 252±58 |
| 400 | eyes closed | departmental | F | 265±73 | M | 261±69 |
| 800 | congruent | departmental | F | 248±64 | M | 246±63 |
| 800 | incongruent | departmental | F | 256±74 | M | 242±59 |
| 800 | eyes closed | departmental | F | 259±71 | M | 248±64 |
| 200 | congruent | non-departmental | F | 247±57 | M | 231±62 |
| 200 | incongruent | non-departmental | F | 257±56 | M | 241±64 |
| 200 | eyes closed | non-departmental | F | 257±58 | M | 232±60 |
| 400 | congruent | non-departmental | F | 244±60 | M | 222±51 |
| 400 | incongruent | non-departmental | F | 256±62 | M | 229±56 |
| 400 | eyes closed | non-departmental | F | 252±64 | M | 229±55 |
| 800 | congruent | non-departmental | F | 248±59 | M | 223±54 |
| 800 | incongruent | non-departmental | F | 251±64 | M | 222±56 |
| 800 | eyes closed | non-departmental | F | 252±66 | M | 225±53 |
4. Discussion
This study demonstrates for the first time that familiarity accentuates gaze cuing in women. This result is interesting for two reasons. First, it shows that social knowledge can penetrate the neural circuits controlling gaze cuing. Previous studies demonstrated effects of expression and identity (Mathews et al. 2003; Hori et al. 2005; Holmes et al. 2006; Shepherd et al. 2006) but did not rule out the possibility that visual features alone were responsible. Since all subjects in the present study responded to the same images, differential gaze cuing can be attributed to social knowledge rather than image features. This finding complements previous studies showing that gaze direction influences social categorization and judgment (Macrae et al. 2002; Adams & Kleck 2005; Bayliss & Tipper 2006). Together, these observations demonstrate that the neural circuits supporting gaze cuing and those supporting other aspects of social cognition are functionally integrated.
Second, familiarity only enhanced gaze cuing in women. This finding fits a broader pattern of females showing greater social sensitivity than males in many contexts (reviewed in Geary 1998). Most germane here, females show richer face processing (Guillem & Mograss 2005), including stronger gaze cuing (Bayliss et al. 2005) and enhanced recall of faces (Herlitz & Yonker 2002). Two hypotheses could account for our results, one based on mnemonics and the other based on saliency. Specifically, enhanced recall of faces by females may facilitate processing of gaze direction (cf. O'Donnell & Bruce 2001). Alternatively, familiar faces may be relatively more salient for females than males and thus recruit greater attention to gaze direction. The salience hypothesis is consistent with the observation of enhanced gaze cuing for expressive faces in other studies (Mathews et al. 2003; Hori et al. 2005; Holmes et al. 2006).
Sex differences in processing social gaze cues could be generated by the action of sex hormones on neural circuits that process faces. Specifically, amygdala, hippocampus and orbitofrontal gyrus are all activated by perception of faces (Ishai et al. 2005) and all three regions are both sexually dimorphic in adults and highly sensitive to sex hormones in development (Goldstein et al. 2001). It has been argued that these areas form a functional circuit integrating social and emotional salience with perceptual and mnemonic processing (Vuilleumier 2002; Sabbagh 2004; Smith et al. 2006). Moreover, activation of this circuit can drive learning in the ventral visual stream (Ribeiro & Nicolelis 2004). Thus, hormonal differentiation of this circuit might ultimately produce sex differences in social attention.
Acknowledgments
We thank Jelena Ristic for discussion and assistance at early stages of this project, Sheila Roberts for technical assistance and Ben Hayden and Jeff Klein for their comments on the manuscript. This work was supported by MH066259, the Cure Autism Now Foundation and an individual NIH NRSA training fellowship (R.D.).
References
- Adams R.B, Kleck R.E. Effects of direct and averted gaze on the perception of facially communicated emotion. Emotion. 2005;5:3–11. doi: 10.1037/1528-3542.5.1.3. doi:10.1037/1528-3542.5.1.3 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. MIT Press; Cambridge, MA: 1995. Mindblindness: an essay on autism and theory of mind. [Google Scholar]
- Bayliss A.P, Tipper S.P. Predictive cues and personality judgements: should eye trust you? Psychol. Sci. 2006;17:514–520. doi: 10.1111/j.1467-9280.2006.01737.x. doi:10.1111/j.1467-9280.2006.01737.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss A.P, di Pellegrino G, Tipper S.P. Sex differences in eye gaze and symbolic cueing of attention. Q. J. Exp. Psychol. A. 2005;58A:631–650. doi: 10.1080/02724980443000124. [DOI] [PubMed] [Google Scholar]
- Deaner R.O, Platt M.L. Reflexive social attention in monkeys and humans. Curr. Biol. 2003;13:1609–1613. doi: 10.1016/j.cub.2003.08.025. doi:10.1016/j.cub.2003.08.025 [DOI] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Vis. Cogn. 1999;6:509–540. doi:10.1080/135062899394920 [Google Scholar]
- Fodor J.A. MIT Press; Cambridge, MA: 1983. The modularity of the mind. [Google Scholar]
- Friesen C.K, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychon. Bull. Rev. 1998;5:490–495. [Google Scholar]
- Geary D.C. American Psychological Association; Washington, DC: 1998. Male, female: the evolution of human sex differences. [Google Scholar]
- Goldstein J.M, Seidman L.J, Horton N.J, Makris N, Kennedy D.N, Caviness V.S, Faraone S.V, Tsuang M.T. Normal sexual dimorphism of the adult human brain assessed by in vivo magentic resonance imaging. Cereb. Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. doi:10.1093/cercor/11.6.490 [DOI] [PubMed] [Google Scholar]
- Gomez J.C. Species comparative studies and cognitive development. Trends Cogn. Sci. 2005;9:118–125. doi: 10.1016/j.tics.2005.01.004. doi:10.1016/j.tics.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Guillem F, Mograss M. Gender differences in memory processing: evidence from event-related potentials to faces. Brain Cogn. 2005;57:84–92. doi: 10.1016/j.bandc.2004.08.026. doi:10.1016/j.bandc.2004.08.026 [DOI] [PubMed] [Google Scholar]
- Herlitz A, Yonker J.E. Sex differences in episodic memory: the influence of intelligence. J. Clin. Exp. Neuropsychol. 2002;24:107–114. doi: 10.1076/jcen.24.1.107.970. [DOI] [PubMed] [Google Scholar]
- Hietanen J.K, Leppanen J.M. Does facial expression affect attention orienting by gaze direction cues? J. Exp. Psychol. Hum. Percept. Perform. 2003;29:1228–1243. doi: 10.1037/0096-1523.29.6.1228. doi:10.1037/0096-1523.29.6.1228 [DOI] [PubMed] [Google Scholar]
- Holmes A, Richard A, Green S. Anxiety and sensitivity to eye gaze in emotional faces. Brain Cogn. 2006;60:282–294. doi: 10.1016/j.bandc.2005.05.002. doi:10.1016/j.bandc.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Hood B.M, Willen J.D, Driver J. Adult's eyes trigger shifts of visual attention in human infants. Psychol. Sci. 1998;9:131–134. doi:10.1111/1467-9280.00024 [Google Scholar]
- Hori E, Tazumi T, Umeno K, Kamachi M, Kobayashi T, Ono T, Nishijo H. Effects of facial expression on shared attention mechanisms. Physiol. Behav. 2005;84:397–405. doi: 10.1016/j.physbeh.2005.01.002. doi:10.1016/j.physbeh.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Ishai A, Schmidt C.F, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res. Bull. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. doi:10.1016/j.brainresbull.2005.05.027 [DOI] [PubMed] [Google Scholar]
- Keating C.F, Mazur A, Segall M.H. Facial gestures which influence the perception of status. Soc. Psychol. Q. 1977;40:374–378. [Google Scholar]
- Langton S.R.H, Bruce V. Reflexive visual orienting in response to the social attention of others. Vis. Cogn. 1999;6:541–567. doi:10.1080/135062899394939 [Google Scholar]
- Macrae C.N, Hood B.M, Milne A.B, Rowes A.C, Mason M.F. Are you looking at me? Eye gaze and person perception. Psychol. Sci. 2002;3:460–464. doi: 10.1111/1467-9280.00481. doi:10.1111/1467-9280.00481 [DOI] [PubMed] [Google Scholar]
- Mathews A, Fox E, Yiend J, Calder A. The face of fear: effects of eye gaze and emotion on visual attention. Vis. Cogn. 2003;10:823–835. doi: 10.1080/13506280344000095. doi:10.1080/13506280344000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell C, Bruce V. Familiarisation with faces selectively enhances sensitivity to changes to the eyes. Perception. 2001;30:755–764. doi: 10.1068/p3027. doi:10.1068/p3027 [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Nicolelis M.A. Reverberation, storage, and postsynaptic propagation of memories during sleep. Learn. Mem. 2004;11:686–696. doi: 10.1101/lm.75604. doi:10.1101/lm.75604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh M.A. Understanding orbitofrontal contributions to theory-of-mind reasoning: implications for autism. Brain Cogn. 2004;55:209–219. doi: 10.1016/j.bandc.2003.04.002. doi:10.1016/j.bandc.2003.04.002 [DOI] [PubMed] [Google Scholar]
- Shepherd S.V, Deaner R.O, Platt M.L. Social status gates social attention in monkeys. Curr. Biol. 2006;16:R119–R120. doi: 10.1016/j.cub.2006.02.013. doi:10.1016/j.cub.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Smith A.P.R, Stephan K.E, Rugg M.D, Dolan R.J. Task and content modulate amygdala–hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. doi:10.1016/j.neuron.2005.12.025 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. Facial expression and selective attention. Curr. Opin. Psychiatry. 2002;15:291–300. doi:10.1097/00001504-200205000-00011 [Google Scholar]