Abstract
Seals may delay costly physiological processes (e.g. digestion) that are incompatible with the physiological adjustments to diving until after periods of active foraging. We present unusual profiles of metabolic rate (MR) in grey seals measured during long-term simulation of foraging trips (4–5 days) that provide evidence for this. We measured extremely high MRs (up to almost seven times the baseline levels) and high heart rates during extended surface intervals, where the seals were motionless at the surface. These occurred most often during the night and occurred frequently many hours after the end of feeding bouts. The duration and amount of oxygen consumed above baseline levels during these events was correlated with the amount of food eaten, confirming that these metabolic peaks were related to the processing of food eaten during foraging periods earlier in the day. We suggest that these periods of high MR represent a payback of costs deferred during foraging.
Keywords: diving, delayed digestion, seals, metabolic rate
1. Introduction
The foraging success of many diving predators is determined by their ability to find and process prey that is patchily distributed. They are therefore subject to all the normal constraints of patch foraging, but with an additional set of rigid, short-term constraints imposed by the need to feed at depth and load oxygen at the surface (Thompson & Fedak 2001). In combination, these constraints mean that even successful foragers are likely to spend only short periods of time in contact with their prey.
There are many published examples of how divers adjust their behaviour and physiology to maximize the time spent underwater (Kooyman 1989; Butler & Jones 1997). However, some of these adaptations are in direct conflict with the requirement to consume prey rapidly while in prey patches. For example, circulatory adjustments to diving may be incompatible with an active digestive system, such as in Weddell seals (Leptonychotes weddellii), where the blood flow to the intestines was markedly reduced during forced dives (Zapol et al. 1979) and the function and blood flow to the splanchnic organs decreased with dive duration during voluntarily diving (Davis et al. 1983).
An increase in metabolic rate (MR) after food intake is a universal phenomenon among animals; usually referred to as specific dynamic action (SDA). Increased metabolic requirements of digestion should limit foraging performance by reducing the amount of oxygen available for maintenance and swimming.
Hence, adaptations that increase foraging success may be in direct conflict with the requirements of other necessary physiological processes. One way for animals to overcome this conflict would be to defer activities that are incompatible with diving until after a foraging period (Crocker et al. 1997). To date, studies of the metabolic consequences of feeding in seals have been carried out in isolation from active foraging behaviour (Markussen et al. 1994; Rosen & Trites 1997, 2003). These studies showed that in non-diving harbour seals (Phoca vitulina) and Steller sea lions (Eumetopias jubatus), MRs increased within 30 min of feeding, increased up to twice the post-absorptive rate and remained elevated for up to 12 h. Increases of this magnitude will clearly have an effect on the duration of aerobic dives.
Although several authors have discussed the conflicting demands and potential tradeoffs between diving and food processing (McConnell et al. 1992; Crocker et al. 1997; Rosen et al. 2006), direct physiological evidence of how they might interact is scarce. Crocker et al. (1997) suggested that the occurrence of passive drift dives in elephant seals mostly at dawn was indicative of deferring food processing costs until after active foraging periods at night. Extended surface intervals (ESIs—postulated as periods of food processing) between periods of active diving have been observed in northern and southern elephant seals (Crocker et al. 1997; Bornemann et al. 2000), grey and harbour seals Sea Mammal Research Unit (SMRU), unpublished data). Conversely, products of digestion have been found in the blood of Weddell seals during diving (Davis et al. 1983), and Williams et al. (2004) found that feeding during foraging dives resulted in a 45% increase in metabolism. We have previously reported that grey seals may defer the metabolic costs of digestion while diving for food, thus maximizing dive duration while actively foraging (Sparling et al. in press b). Here, we present continuous and simultaneous records of MR, food intake, diving behaviour and heart rate collected during periods of 4–5 days, during which seals were actively diving for food in our experimental set-up, thus simulating normal foraging trips for this species. We observed short duration peaks in metabolism during extended surface periods occurring many hours after feeding. We suggest that these peaks may indicate ‘payback’ of costs deferred during periods of active diving for food.
2. Material and methods
All seals were caught at local haul-outs, held at the SMRU, St Andrews and released into the wild after 1 year. The seals were housed in outdoor seawater pools and fed on herring supplemented with vitamins. All procedures were carried out under a UK Home Office licence.
We carried out trials simulating 4–5-day-long foraging trips. One seal at a time was kept in a large pool (40×6×2.5 m) covered by aluminium mesh panels just below the water surface. Access to the surface was only available in a small breathing chamber situated in one corner of the pool. This allowed for the continual measurement of the animals' gas exchange, heart rate and diving behaviour. Animals spent alternating periods of foraging (see below) with periods of resting in water.
The simulated foraging set-up is described by Sparling et al. (in press a). Briefly, seals were trained to swim from the breathing box (the surface) to an automatic feeding device (prey patch) that was situated 80 m away. Fishes were presented over a series of dives, where the prey encounter rate remained constant within a given dive but varied between dives. Food was presented at various intervals throughout daylight hours. Oxygen consumption and carbon dioxide production during the entire trial were measured continuously using open-flow respirometry (Sparling & Fedak 2004). The system was calibrated daily using known flow rates of nitrogen (Fedak et al. 1981). Swim speed and heart-rate (HR) recorders (Wildlife Computers) were fitted to the seals. Video cameras mounted in the breathing box and above the feeder continually monitored and recorded the seal's activity.
We defined an elevated resting metabolism event (ERME) as a period of inactivity at the surface where MR was increased above the third quartile of prefeeding MR on that day. Prefeeding MR included active periods—this is why some ERMEs are within the range of prefeeding MRs. An initial analysis demonstrated that defining the ERMEs using the 75th percentile of all prefeeding metabolic rate gives results that are very close to using the 95th percentile of inactive prefeeding metabolic rate.
For each ERME, we calculated the peak MR as a multiple of prefeeding, inactive, daytime MR, duration of elevation, summed excess oxygen consumption (EOC—total oxygen consumed above prefeeding inactive levels) and, where available, average heart rate.
3. Results
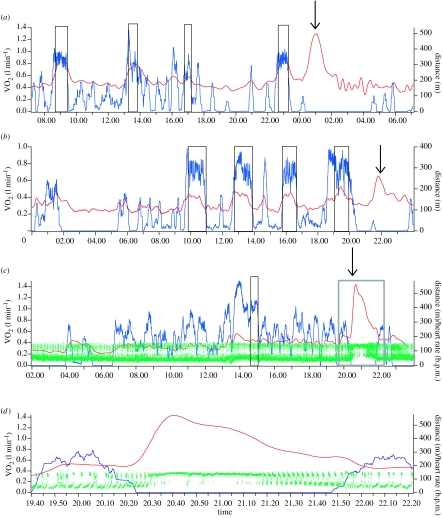
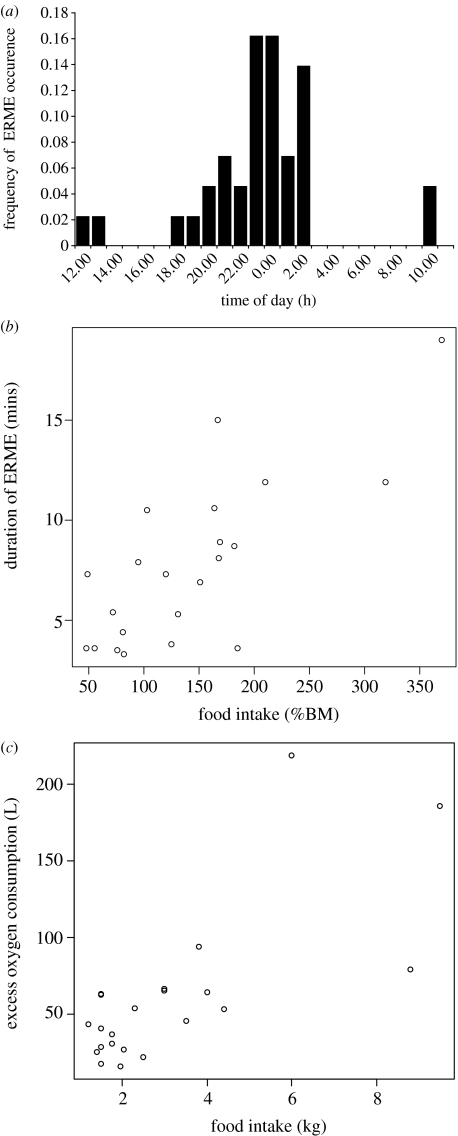
We recorded ERMEs from five grey seals—three juveniles and two adults. Table 1 shows the details of all ERMEs. Figure 1 shows some examples of ERMEs on representative traces of activity, MR and heart rate throughout 24 h periods from selected trials. Delays between feeding and the onset of ERMEs varied from 0 to 11 h after feeding (mean 4.5±3.2 h). Figure 2a shows the distribution of ERMEs throughout the day. Durations of individual ERMEs varied from 14 min to more than or equal to 3 h (101±57 min). Peak MRs ranged between 2.8 and 6.8 times (3.5±1.2) prefeeding, inactive levels.
Table 1.
Details of all ERMEs. (VO2 is rate of oxygen consumption expressed in units of l min−1.)
| seal/exp/daya | mass (kg) | lowest inactive daytime prefeeding VO2 | average active prefeeding VO2 | food intake, kg (percentage of body mass) | highest 20 min average VO2 during ERME (time of the start point) | peak MR (multiple of prefeeding baseline) | time since feeding (h) |
|---|---|---|---|---|---|---|---|
| G1Tue | 107 | 0.64 | 0.71 | 3.8 (3.5) | 1.31 (23:13) | 2.06 | 2.5 |
| G1Wed | ″ | 0.65 | 0.75 | 8.8 (8.1) | 1.65 (23:59) | 2.56 | 3 |
| G1Thu | ″ | 0.54 | 0.80 | 9.5 (8.9) | 1.73 (23:36) | 3.23 | 2.5 |
| D1Thu | 40.7 | 0.24 | 0.30 | 3.5 (8.7) | 0.81 (23:45) | 3.33 | 2.5 |
| D4Thu | 37 | 0.20 | 0.27 | 1.4 (3.8) | 0.65 (21:48) | 3.20 | 2 |
| X1Wed | 28.6 | 0.23 | 0.30 | 1.2 (4.4) | 0.60 (12:20) | 2.58 | 0 |
| X1Wed/Thu | ″ | ″ | ″ | ″ | 1.01 (01:35) | 4.37 | 9 |
| X1Thu/Fri | ″ | 0.20 | 0.29 | 4.0 (15) | 0.75 (01:16) | 3.74 | 9 |
| X2Tue | 33.3 | 0.23 | 0.31 | 2.3 (7.3) | 0.90 (22:48) | 3.87 | 4 |
| X2Tue/Wed | ″ | ″ | ″ | ″ | 0.74 (01:57) | 3.19 | 6.5 |
| X2Wed | ″ | 0.29 | 0.37 | 2.5 (7.9) | 0.62 (13:28) | 2.16 | 2.5 |
| X2Thu | ″ | 0.23 | 0.38 | 6 (19) | 0.79 (12:26) | 3.44 | 3 |
| ″ | ″ | ″ | ″ | 0.82 (22:05) | 3.75 | 2 | |
| X2Thu/Fri | ″ | ″ | ″ | ″ | 1.13 (01:58) | 4.90 | 5 |
| X3Tue | 41.2 | 0.22 | 0.34 | 1.5 (3.6) | 0.95 (20:05) | 4.27 | 5 |
| ″ | ″ | ″ | ″ | 0.87 (22:42) | 3.89 | 7.5 | |
| X3Tue/Wed | ″ | ″ | ″ | ″ | 0.76 (01:40) | 3.41 | 10.5 |
| X3Wed | ″ | 0.21 | 0.33 | 1.5 (3.6) | 1.23 (20:38) | 5.74 | 5 |
| ″ | ″ | ″ | ″ | 0.90 (21:47) | 4.22 | 6 | |
| X3Thu | ″ | 0.21 | 0.34 | 1.5 (3.6) | 1.43 (20:40) | 6.78 | 5.5 |
| X3Thu/Fri | ″ | ″ | ″ | ″ | 0.90 (01:56) | 4.50 | 11 |
| X4Wed | 45.1 | 0.22 | 0.34 | 1.5 (3.3) | 1.45 (19:24) | 6.72 | 3 |
| W1Tue | 25.3 | 0.31 | 0.41 | 1.75 (6.9) | 1.03 (12:14) | 3.27 | 0 |
| ″ | ″ | ″ | ″ | 0.68 (22:16) | 2.15 | 7 | |
| W1Wed | ″ | 0.33 | 0.46 | 3 (11.9) | 0.80 (10:23) | 2.43 | 0 |
| ″ | ″ | ″ | ″ | 0.73 (10:56) | 2.20 | 0.5 | |
| W1Wed/Thu | ″ | ″ | ″ | ″ | 0.79 (01:02) | 2.40 | 3 |
| W1Thu/Fri | ″ | 0.32 | 0.44 | 3 (11.9) | 0.74 (00:18) | 2.69 | 8 |
| W2Wed | 28.5 | 0.24 | 0.32 | 1.5 (5.3) | 0.59 (19:24) | 2.50 | 10 |
| W3Thu | 32.2 | 0.23 | 0.24 | 1.75 (5.4) | 0.73 (23:11) | 3.25 | 4 |
| A4Tue | 126.8 | 0.33 | 0.52 | 2.04 (1.6) | 0.89 (18:29) | 2.69 | 0 |
| A4Wed/Thu | ″ | 0.34 | 0.45 | 1.95 (1.5) | 1.29 (01:00) | 3.80 | 2.5 |
seal/exp/day refer to the identifying codes of each ERME, e.g. A4Thurs means seal A, experiment 4, Thursday.
Figure 1.
Examples of ERMEs (down arrows). Traces (a–c) are representative periods from different trials; the section of trace (c) indicated by the grey box is magnified in trace (d). Each trace shows the 20 min moving average of VO2 (red) and the 20 min summed distance travelled (blue). Black rectangles indicate periods of foraging and feeding. The green shades on traces (c) and (d) are the instantaneous heart rate (heart rate was not measured during the trials shown in traces (a) and (b)).
Figure 2.
(a) Time of day of ERMEs. x-Axis is centred on midnight. (b) Duration of ERMEs and daily food intake (as a percentage of body mass (BM)). (c) Amount of excess oxygen consumed during ERMEs and daily food intake.
There was a significant positive relationship between the duration of ERMEs and food intake on a particular day (linear mixed effects model with seal as a random effect, t=5.26, d.f.=16, p=0.0001). There was also a significant increase in EOC during ERMEs with increasing food intake (linear mixed effects model with seal as a random effect, t=4.90, d.f.=16, p=0.0002).
Heart rates during ERMEs were elevated to rates typical of surfacing tachycardia observed during normal diving behaviour (table 2). Rates remained elevated through most of the ERME, gradually reverting to typical bimodal apnoea/eupnoea patterns towards the end (figure 1).
Table 2.
Heart rates (HR; b.p.m.) of seals, daily average and average during ERMEs.
| seal/exp/day | daily average HR (b.p.m.) | ERME average HR (b.p.m.) |
|---|---|---|
| D1Thu | 63 | 114 |
| X1Wed | 77 | 125 |
| X1Wed/Thu | ″ | 121 |
| X1Thu/Fri | 76 | 123 |
| X2Tue | 72 | 132 |
| X2Tue/Wed | ″ | 126 |
| X2Wed | 76 | 114 |
| X2Thu | 66 | 113 |
| ″ | 120 | |
| X2Thu/Fri | ″ | 131 |
| X3Tue | 60 | 118 |
| ″ | 98 | |
| X3Tue/Wed | ″ | 90 |
| X3Wed | 57 | 130 |
| ″ | 113 | |
| X3Thu | 55 | 133 |
| X3Thu/Fri | ″ | 120 |
| X4Wed | 52 | 123 |
| W1Tue | 90 | 143 |
| ″ | 133 | |
| W1Wed | 94 | 148 |
| ″ | 143 | |
| W1Wed/Thu | ″ | 145 |
| W1Thu/Fri | 93 | 130 |
4. Discussion
Our data show that grey seals can delay the onset of SDA until many hours after feeding. In a previous study (Sparling et al. in press b), the onset of SDA could be detected during shallow feeding dives but was absent in deeper feeding dives. This could be an adaptation allowing seals to maintain longer dive durations while foraging at deep patches (compared with an observed decrease in dive durations seen at shallow depths). By extending our monitoring well beyond the period of active diving, we demonstrate that the payback of these savings for maintaining longer dives can occur many hours after periods of active foraging. Our results provide direct observations of how diving mammals may deal with the competing physiological processes required for maximizing diving ability and for food processing.
The fact that ERME duration and EOC are both correlated with food intake is evidence that ERMEs are related to food processing. In addition, the fact that the majority of these periods occurred during the night when no foraging trials took place suggests that the seals delayed food processing until foraging opportunities were no longer available. This is supported by stomach temperature data from grey seals indicating limited stomach emptying during feeding periods (Labach et al. submitted).
Although we found some instances of ERMEs immediately after feeding, the majority occurred much later. This is in contrast with previous studies that consistently showed an increase in metabolism within 30 min of feeding. The duration of elevated MR was much shorter, and the magnitude of the increase was much greater in our study than reported in previous studies of SDA in non-diving seals (Markussen et al. 1994; Rosen & Trites 1997). This result highlights the importance of measuring physiological responses under ecologically relevant circumstances, and over long enough periods to ensure that there is no bias in reported responses.
It is probable that the extended surface intervals observed in wild seals (Crocker et al. 1997; Bornemann et al. 2000) are the result of periods of delayed food processing. This idea is strengthened by the observations that ESIs were highly correlated with drift dives, which are thought to be associated with food processing, in northern elephant seals (Crocker et al. 1997), and that heart rates were elevated during ESIs in harbour seals (SMRU, unpublished data).
Exactly what causes the high rates of oxygen consumption during ERMEs is unknown; however, it is likely to result from a combination of physical processing of food, transport of metabolites and processing of absorbed nutrients. The rapid breathing and high heart rates we observed suggest a rapid supply of oxygenated blood, probably to the splanchnic organs. Studies of regional blood flow during this period (Zapol et al. 1979) would be illuminating. Likewise, we do not know what happens to food between ingestion and an ERME: if it lies undigested in the gut as stomach temperature records would suggest (Labach et al. submitted), there must also be mechanisms to prevent peristalsis and release of digestive enzymes, etc. This is a promising avenue for future study.
We suggest that seals defer food processing during active foraging in situations where extended dives are advantageous. This will however constrain the absolute amounts of food that can be ingested during any particular foraging event, and may not be the optimal strategy when faced with intermittent high-density prey distributions. Not all seals defer digestion costs in all situations, e.g. Weddell seals continue to process food and suffer reduced dive durations (Davis et al. 1983; Williams et al. 2004) and benthic foraging dives in southern elephant seals were significantly shorter than travelling dives (McConnell et al. 1992). Understanding the complex interactions between diving, exercise and digestion will be essential for developing predictive models of how diving predators respond to changes in prey densities and distributions.
Acknowledgments
We thank Simon Moss for his assistance with animal care. This work was funded by the Natural Environment Research Council (NER/D/S/2003/00650).
References
- Bornemann H, Kreyscher S, Ramdohr S, Martin T, Carlini A, Sellmann L, Plotz J. Southern elephant seal movements and Antarctic sea ice. Antarct. Sci. 2000;12:3–15. [Google Scholar]
- Butler P.J, Jones D.R. Physiology of diving of birds and mammals. Physiol. Rev. 1997;77:837–899. doi: 10.1152/physrev.1997.77.3.837. [DOI] [PubMed] [Google Scholar]
- Crocker D.E, Le Boeuf B.J, Costa D.P. Drift diving in female northern elephant seals: implications for food processing. Can. J. Zool. 1997;75:27–39. [Google Scholar]
- Davis R.W, Castellini M.A, Kooyman G.L, Maue R. Renal glomerular filtration rate and hepatic blood flow during voluntary diving in Weddell seals. Am. J. Physiol. 1983;245:743–748. doi: 10.1152/ajpregu.1983.245.5.R743. [DOI] [PubMed] [Google Scholar]
- Fedak M.A, Rome L, Seeherman H.J. One-step N2 dilution technique for calibrating open-circuit VO2 measuring systems. J. Appl. Physiol. 1981;51:772–776. doi: 10.1152/jappl.1981.51.3.772. [DOI] [PubMed] [Google Scholar]
- Kooyman G.L. Springer; Berlin, Germany: 1989. Diverse divers. [Google Scholar]
- Labach, H., Sparling, C. E., Gallon, S. L. & Thompson, D. Submitted. Stomach temperature is not a reliable indicator of meal size in grey seals.
- Markussen N.H, Ryg M, Oritsland N.A. The effect of feeding on the metabolic rate in harbour seals (Phoca vitulina) J. Comp. Physiol. 1994;164:89–93. doi: 10.1007/BF00301648. [DOI] [PubMed] [Google Scholar]
- McConnell B.J, Chambers C, Fedak M.A. Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the southern-ocean. Antarct. Sci. 1992;4:393–398. [Google Scholar]
- Rosen D.A.S, Trites A.W. Heat increment of feeding in Steller sea lions, Eumetopias jubatus. Comp. Biochem. Physiol. 1997;118A:877–881. doi: 10.1016/s0300-9629(97)00039-x. doi:10.1016/S0300-9629(97)00039-X [DOI] [PubMed] [Google Scholar]
- Rosen D.A.S, Trites A.W. No evidence for bioenergetic interaction between digestion and thermoregulation in Steller sea lions (Eumetopias jubatus) Physiol. Biochem. Zool. 2003;76:899–906. doi: 10.1086/378140. doi:10.1086/378140 [DOI] [PubMed] [Google Scholar]
- Rosen, D. A. S., Winship, A. J. & Hoopes L. A. 2006 Thermal and digestive constraints to foraging behavior in marine mammals. Fisheries Centre Working Paper #2006-17, University of British Columbia, Vancouver, Canada. [DOI] [PMC free article] [PubMed]
- Sparling C.E, Fedak M.A. Metabolic rates of freely diving grey seals. J. Exp. Biol. 2004;207:1615–1624. doi: 10.1242/jeb.00952. doi:10.1242/jeb.00952 [DOI] [PubMed] [Google Scholar]
- Sparling, C. E., Georges, J. Y., Gallon, S. L., Fedak, M. A. & Thompson, D. In press a How long does a dive last? Foraging decisions by breath hold divers in a patchy environment—a test of a simple model. Anim. Behav.
- Sparling, C. E., Georges, J. Y., Thompson, D., Gallon, S. L., Brookes, K. & Fedak, M. A. In press b Do grey seals defer the heat increment of feeding to maximize dive duration? In European Research on Cetaceans 18 (ed. P. G. H. Evans), Proceedings of the European Cetacean Society.
- Thompson D, Fedak M.A. How long should a dive last: a simple model of foraging decisions by breath-hold divers in a patchy environment. Anim. Behav. 2001;61:287–296. [Google Scholar]
- Williams T.E, Fuiman L.A, Horning M, Davis R.W. The cost of foraging by a marine predator, the Weddell seal Leptonychotes weddellii: pricing by the stroke. J. Exp. Biol. 2004;207:973–982. doi: 10.1242/jeb.00822. doi:10.1242/jeb.00822 [DOI] [PubMed] [Google Scholar]
- Zapol W.M, Liggins G.C, Schneider R.C, Qvist J, Snider M.T, Creasy R.K, Hochachka P.W. Regional blood flow during simulated diving in the conscious Weddell seal. J. Appl. Physiol. 1979;47:968–973. doi: 10.1152/jappl.1979.47.5.968. [DOI] [PubMed] [Google Scholar]