Abstract
Life-history theory predicts that, in long-lived organisms, effort towards reproduction will increase with age, and research from oviparous vertebrates largely supports this prediction. In reptiles, where parental care occurs primarily via provisioning of the egg, older females tend to produce larger eggs, which in turn produce larger hatchlings that have increased survival. We conducted an experimental release study and report that maternal age positively influences offspring survivorship in the painted turtle (Chrysemys picta) and predicts offspring survival at least as well as hatchling body size does. These data suggest that, although increasing hatchling size is a major component of reproductive success in older individuals, other factors also contribute.
Keywords: life-history theory, survivorship, body size, Chrysemys picta, turtle
1. Introduction
Life-history strategies often result from constraints that limit resource allocation. These constraints lead to trade-offs, which in the case of reproductive females require that they make decisions regarding resource partitioning to reproduction and self-maintenance. How such a trade-off is resolved depends upon several factors, including the intensity of intrinsic and extrinsic mortality (Stearns 1992). Long-lived organisms are thus predicted to expend fewer resources per reproductive event and invest more in self-maintenance compared with short-lived organisms; empirical studies of long-lived taxa largely support this prediction (Congdon et al. 2003; Apanius & Nisbet 2006).
Studies of long-lived species often report positive age-dependent breeding success. Studies in birds reveal that eggs produced by older females are more likely to hatch and produce fledglings (Cam & Monnat 2000; Nisbet et al. 2002; Bogdanova et al. 2006). The increased reproductive success of older individuals is often attributed to increased egg size and/or egg number, or to improved foraging success and parental care (Curio 1983; de Forest & Gaston 1996; Christians 2002).
In reptiles, where maternal provisioning is largely limited to pre-hatch investment in eggs, reproductive success also appears to increase with age (Congdon et al. 2001). Older females produce larger eggs and, consequently, larger hatchlings (Congdon et al. 2003; Bowden et al. 2004; Wilkinson & Gibbons 2005). Larger hatchlings move more rapidly (Miller et al. 1987) and have an increased probability of survival compared with smaller conspecifics (Janzen et al. 2000; Brown & Shine 2005).
Independent of egg size, females can influence offspring phenotype through variation in resource allocation. Egg components such as lipids, proteins and water increase with female's age in birds (Bogdanova et al. 2006). In reptiles, egg components increase with increasing body size or age (Congdon & Gibbons 1985). In a recent study on the painted turtle (Chrysemys picta), we reported that eggs from older females were larger and contained more total lipid and protein, but that the proportion of these resources was constant across female ages (Harms et al. 2005).
In this study, we test the hypothesis that female age positively influences offspring fitness in the painted turtle. We conducted a field experiment to compare survivorship during simulated migration of neonates from nests to water; a critical, but brief period marked by high mortality in aquatic turtles. We predicted that offspring from older females would be larger than those from younger females and that this size difference would (i) allow them to traverse the distance between the release point and the water more rapidly and (ii) increase their survivorship. Testing the consequences for fitness of age-dependent reproductive success adds an important component to our understanding of variation in life-history strategies in long-lived organisms.
2. Material and methods
We released neonate turtles into an experimental enclosure to examine the effects of maternal age on survival during a simulated migration towards water. Painted turtle nesting was monitored on the southern portion of an island in the Mississippi River (see Bowden et al. 2004) from 26 May to 2 July 2002. Nests were excavated and the eggs weighed to the nearest 0.01 g, counted and returned to the nest. All nests were re-excavated on 21 September 2002 and the hatchlings transported to the laboratory for over-wintering. Neonatal painted turtles at this site typically over-winter in the nest (Weisrock & Janzen 1999), generally emerging in May to migrate to the water. To simulate winter conditions, hatchlings were maintained at (i) 13°C from September until late November, (ii) 5°C from late November until mid-March, and (iii) 13°C from mid-March until mid-May.
Nests used in the release experiment (i) were laid by a female that produced at least two clutches in 2002, (ii) were laid by a female that could be classified as having high nesting experience (HNE) or low nesting experience (LNE), and (iii) consisted of at least four live hatchlings to allow for at least two hatchlings/clutch to be distributed across both release distances to evaluate clutch effects. Nesting activity of this population has been monitored for the past 17 years, and we used these data to assign females to one of the two groups. The HNE group consisted of females with seven or more years of nesting experience and, based upon the time to maturation in C. picta, were a minimum of 12 years old. LNE females had three or fewer years of nesting experience and were determined to be between 5 and 7 years old (Bowden et al. 2004). Overall, 43 nests were selected to represent four different categories: LNE, first clutch (n=12); LNE, second clutch (n=11); HNE, first clutch (n=10) and HNE, second clutch (n=10). In total, 317 hatchlings were assigned to one of the four release groups: replicate 1, 40 m (n=80); replicate 1, 25 m (n=79); replicate 2, 40 m (n=79) and replicate 2, 25 m (n=79). Hatchlings were weighed to the nearest 0.01 g and carapace lengths were measured to the nearest 0.01 cm. Hatchlings were marked by clipping a single marginal scute to identify the release group, and photocopies of the plastron of each hatchling were taken for subsequent identification upon recapture (Janzen et al. 2000).
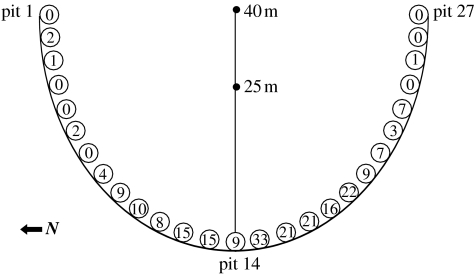
The release experiment was conducted directly across from the study site on a sand prairie on the eastern bank of the Mississippi River to eliminate interference with nesting females. A 120 m semicircle drift fence with a radius of 40 m was constructed out of 0.15 m high aluminium flashing. Pitfall traps were constructed from 1 gal plastic containers that were buried on the release side of the fence at 5 m intervals (figure 1). Release points were chosen based on the average distance from water that each female age group laid their nests in 2002 (Harms et al. 2005).
Figure 1.
Schematic of release enclosure with the number of hatchlings caught in each pitfall trap indicated.
Hatchlings were held at ambient air temperature 24 h prior to release. Two (15×15 cm) shallow depressions were dug into the sand at 40 and 25 m upslope from the fence to serve as release points (figure 1). Replicate 1 was initiated at 10.00 Central Daylight Time (CDT) on 19 May 2003 and the fence was monitored daily at 07.00, 13.00 and 19.00 CDT beginning at 19.00 CDT on 19 May 2003. Recaptured hatchlings were identified, reweighed to the nearest 0.01 g and released at their natal beach within 24 h of capture. Replicate 2 was initiated on 23 May 2003 following the same protocol. The experiment was terminated on 18 June 2003 after no hatchlings from either replicate were recaptured for 72 h.
(a) Statistical analyses
Differences in initial hatchling phenotypes were tested with an ANCOVA using initial egg mass as a covariate, while differences in recapture times were tested with an ANCOVA using hatchling carapace length (HCL) as a covariate. We examined the effects of maternal age, clutch (first versus second), release distance, HCL, hatchling mass and replicate on hatchling survival using generalized linear mixed models (Proc. GLIMMIX in SAS v. 9.0). We obtained a simplified model by using clutch identity as a random effect and iteratively removing non-significant interactions and main effects (Crawley 1993); in the end only maternal age and HCL remained. To visualize the effect of maternal age and HCL on offspring fitness, we created cubic splines (Schluter 1988) with standard errors calculated using 1000 bootstraps.
3. Results
Overall, 216 (68.1%) hatchlings were recaptured from the two replicates; we recovered 56.7% of LNE hatchlings and 79.4% of HNE hatchlings. No hatchlings were captured in either terminal pit traps, indicating that hatchlings moved towards the water and not out of the back of the enclosure (figure 1). HCL and mass at release did not significantly differ between the two replicates, but HNE hatchlings were longer than LNE hatchlings, and recovered hatchlings were longer than those that were not recaptured (table 1). For a given body length, HNE hatchlings were not significantly heavier at release than LNE hatchlings (F1,314=2.06, p=0.15). Time from release to recapture was not influenced by maternal age (ANCOVA, F2,211=0.99, p=0.32), but larger hatchlings were recaptured sooner than were smaller hatchlings (ANCOVA, F2,211=5.35, p=0.02).
Table 1.
Descriptive statistics for (a) all hatchlings (pre-release) and (b) those recaptured by female age. (Numbers represent means±s.d.m.)
| (a) | ||
|---|---|---|
| pre-release (n=317) | LNE females (n=157) | HNE females (n=160) |
| carapace length (mm) | 24.89±1.59 | 27.00±1.21 |
| mass (g) | 3.65±0.67 | 4.38±0.52 |
| (b) | ||
| recaptures (n=216) | LNE females (n=89) | HNE females (n=127) |
| carapace length (mm) | 25.16±1.53 | 27.00±1.23 |
| mass (g) | 3.71±0.66 | 4.38±0.54 |
| time to recapture (h) | 227±109 | 193±107 |
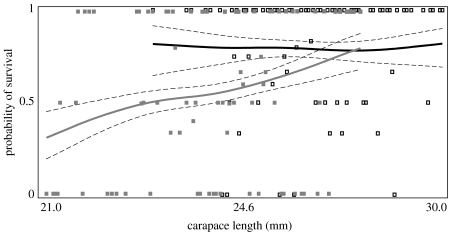
Hatchling recovery was not affected by mass, clutch, release distance or replicate. Maternal age and HCL were significant predictors of survival (age: F1,310=5.44, p=0.02; HCL: F1,310=3.91, p=0.05). If we constrain our analysis to only the area of HCL overlap (24.4–28.0 mm), we still detected a significant positive effect of maternal age (F1,227=6.32, p=0.01; figure 2).
Figure 2.
Probability of survival based on cubic spline analysis of HCL. Solid squares represent LNE hatchlings and open squares represent HNE hatchlings. Solid lines represent the cubic spline for LNE (grey) or HNE (black) hatchlings, and the dashed lines are ±1 s.e.m.
4. Discussion
We report here that neonate survivorship during a critical life stage increases with maternal age in the painted turtle, a long-lived vertebrate. We also found that longer hatchlings were more likely to reach the drift fence and did so more rapidly than shorter hatchlings. Importantly, analyses that focused on similar-sized hatchlings produced by HNE and LNE females confirmed these results. Coupled with the finding that relative offspring mass did not vary with female age, the observed differences in survivorship might have more to do with changes in egg quality (i.e. total egg components, yolk steroids, etc.) or nest-site parameters (i.e. nest location, depth, etc.) as females mature than simply with increased egg or hatchling size.
In a previous paper, we hypothesized that LNE females, which nest closer to the water than HNE females, might do so either to offset the migration disadvantage their smaller offspring face or to increase their own survival by reducing the risk of dehydration or predation while on land (Harms et al. 2005). Although we cannot directly eliminate the size disadvantage hypothesis, this scenario seems unlikely because survivorship of LNE hatchlings was similar for both release distances. Instead, LNE females probably nest closer to water to ensure their own survival, a strategy consistent with other long-lived species that preferentially invest in self-maintenance early in life (Ricklefs & Wilkelski 2002; Apanius & Nisbet 2006).
Although apparently entailing more costs than benefits for long-lived species, early reproduction is the norm rather than the exception (Curio 1983). Early reproductive bouts may prepare a female for future reproductive success through increased experience, and thus selection may favour early reproduction even when the likelihood of immediate success is diminished.
Acknowledgments
We thank the students who assisted with the 2002 and 2003 field seasons and the Army Corp of Engineers for permission to work at Thomson Causeway. Animals were collected under permits NH02-0073 and NH03-0073 from the Illinois DNR and Special Use Permits 32576-02024 and 32576-03014 from the USFWS. Research was conducted in accordance with Iowa State University Care and Use of Animals Protocol no. 1-2-5036-J. Funding was provided by NSF grants IBN0080194 to F.J.J. and IBN0212935 to F.J.J. and R.M.B.
References
- Apanius V, Nisbet I.C.T. Serum immunoglobulin G levels are positively related to reproductive performance in a long-lived seabird, the common tern (Sterna hirundo) Oecologia. 2006;147:12–23. doi: 10.1007/s00442-005-0238-6. doi:10.1007/s00442-005-0238-6 [DOI] [PubMed] [Google Scholar]
- Bogdanova M.I, Nager R.G, Monaghan P. Does parental age affect offspring performance through differences in egg quality? Funct. Ecol. 2006;20:132–141. doi:10.1111/j.1365-2435.2006.01088.x [Google Scholar]
- Bowden R.M, Harms H.K, Paitz R.T, Janzen F.J. Does egg size vary with demographic stage because of a physiological constraint? Funct. Ecol. 2004;18:522–529. doi:10.1111/j.0269-8463.2004.00861.x [Google Scholar]
- Brown G.P, Shine R. Female phenotype, life history, and reproductive success in free-ranging snakes (Tropidonophis mairii) Ecology. 2005;86:2763–2770. [Google Scholar]
- Cam E, Monnat J.Y. Stratification based on reproductive state reveals contrasting patterns of age-related variation in demographic parameters in the kittiwake. Oikos. 2000;90:560–574. doi:10.1034/j.1600-0706.2000.900314.x [Google Scholar]
- Christians J.K. Avian egg size: variation within species and inflexibility within individuals. Biol. Rev. 2002;77:1–26. doi: 10.1017/s1464793101005784. [DOI] [PubMed] [Google Scholar]
- Congdon J.D, Gibbons J.W. Egg components and reproductive characteristics of turtles: relationships to body size. Herpetologica. 1985;41:194–205. [Google Scholar]
- Congdon J.D, Nagle R.D, Kinney O.M, van Loben Sels R.C. Hypotheses of aging in a long-lived vertebrate, Blanding's turtle (Emydoidea blandingii) Exp. Gerontol. 2001;36:813–827. doi: 10.1016/s0531-5565(00)00242-4. doi:10.1016/S0531-5565(00)00242-4 [DOI] [PubMed] [Google Scholar]
- Congdon J.D, Nagle R.D, Kinney O.M, van Loben Sels R.C, Quinter T, Tinkle D.W. Testing hypotheses of aging in long-lived painted turtles (Chrysemys picta) Exp. Gerontol. 2003;38:765–772. doi: 10.1016/s0531-5565(03)00106-2. doi:10.1016/S0531-5565(03)00106-2 [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Blackwell Scientific Publications; Oxford, UK: 1993. GLIM for ecologists. [Google Scholar]
- Curio E. Why do young birds reproduce less well? Ibis. 1983;125:400–404. [Google Scholar]
- de Forest L.N, Gaston A.J. The effect of age on timing of breeding and reproductive success in the Thick-billed Murre. Ecology. 1996;77:1501–1511. doi:10.2307/2265547 [Google Scholar]
- Harms H.K, Paitz R.T, Bowden R.M, Janzen F.J. Age and season impact resource allocation to eggs and nesting behavior in the painted turtle. Physiol. Biochem. Zool. 2005;78:996–1004. doi: 10.1086/432920. doi:10.1086/432920 [DOI] [PubMed] [Google Scholar]
- Janzen F.J, Tucker J.K, Paukstis G.L. Experimental analysis of an early life-history stage: selection on size of hatchling turtles. Ecology. 2000;81:2290–2304. doi:10.2307/177115 [Google Scholar]
- Miller K, Packard G.C, Packard M.J. Hydric conditions during incubation influence locomotor performance of hatchling snapping turtles. J. Exp. Biol. 1987;127:401–412. [Google Scholar]
- Nisbet I.C.T, Apanius V, Friar M.S. Breeding performance of very old common terns. J. Field Ornithol. 2002;73:117–124. [Google Scholar]
- Ricklefs R.E, Wilkelski M. The physiology/life-history nexus. Trends Ecol. Evol. 2002;17:462–468. doi:10.1016/S0169-5347(02)02578-8 [Google Scholar]
- Schluter D. Estimating the form of natural selection on a quantitative trait. Evolution. 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. doi:10.2307/2408904 [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Weisrock D.W, Janzen F.J. Thermal and fitness-related consequences of nest location in painted turtles (Chrysemys picta) Funct. Ecol. 1999;13:94–101. doi:10.1046/j.1365-2435.1999.00288.x [Google Scholar]
- Wilkinson L.R, Gibbons J.W. Patterns of reproductive allocation: clutch and egg size variation in three freshwater turtles. Copeia. 2005;2005:868–879. doi:10.1643/0045-8511(2005)005[0868:PORACA]2.0.CO;2 [Google Scholar]